Bovine papillomaviruses: their role in the aetiology of cutaneous tumours of bovids and equids
Abstract
Bovine papillomavirus (BPV) is perhaps the most extensively studied animal papillomavirus. In cattle BPVs induce benign tumours of cutaneous or mucosal epithelia, called papillomas or warts. Cattle papillomas are benign tumours and generally regress without eliciting any serious clinical problems in the host, but occasionally persist and provide the focus for malignant transformation to squamous cell carcinoma, as in the case of cancer of the urinary bladder and cancer of the upper alimentary canal. BPV is the only papillomavirus that jumps species: the virus also infects equids, and gives rise to fibroblastic tumours called sarcoids. Sarcoids very rarely regress, more often they persist and can be locally aggressive. These tumours are the most common dermatological tumour of equids worldwide.
The purpose of this review is to discuss the biology of BPV, the biology of bovine tumours and equine sarcoids, and present the current understanding of BPV in tumour pathogenesis in its natural host, cattle, and in its heterologous host, equids. Finally, the use of anti-BPV vaccines as a therapy for equine sarcoids will be discussed. Only limited information on the clinical or pathological aspects of either bovine or equine tumours will be provided as this subject has been extensively addressed previously.
Sources of Funding
See acknowledgements.
Conflict of Interest
No conflict of interest has been declared.
Papillomaviruses: introduction
Papillomaviruses (PVs) are strictly species specific and even in experimental conditions do not infect any other host than their natural one.1 The only known case of cross-species infection is the infection of horses and other equids by bovine papillomavirus (BPV)-1 and more rarely BPV-2.
The study of BPV in cattle has led to the understanding of virus natural history, the direct link between virus infection and neoplasia, the relationship between virus and host and environment, the host immune response to the virus and to the development of antipapillomavirus vaccines. Studies in cultured cells have shed light on the molecular biology of the virus and the function of its proteins.2 However, although BPV has been recognized as a likely cause of equine sarcoids more than half a century ago,3 the study of BPV infection in horses and its role in the pathogenesis of the tumour have been lagging behind, and only recently systematic investigations of BPV in sarcoid have been attempted.4–6
The authors will review first the biology of BPV, with particular attention to its role in cattle papillomas and to those aspects relevant to equine sarcoid, then describe the equine sarcoid, examine the role of BPV in sarcoid development and finally give an indication of the future direction of research into BPV and sarcoids.
The biology of BPV – heterogeneity of BPV
Ten BPVs (BPV-1–10) have been characterized,7–11 which fall into four genera on the basis of their biology and their genome homology12 BPV-1 and BPV-2, which belong to the δ papillomavirus genus, are commonly defined as fibropapillomaviruses. That is, viruses that infect both the epithelium and the underlying derma, giving rise to fibropapillomas. BPV-3, BPV-4, BPV-6, BPV-9 and BPV-10, belonging to the genus ξ, are epitheliotropic viruses that infect only the epithelium and induce true papillomas. BPV-5 and BPV-8 are members of the ɛ PV genus and appear to have a dual pathology, causing both fibropapillomas and epithelial papillomas,13 and their genomes appear to share homologuey with both ξ BPVs and δ BPVs.9,14 On the basis of phylogenetic analyses, it has been proposed that BPV-7 be assigned to a new genus (Table 1).11 Further additional novel BPVs have been reported,15 which await further characterization. The general term BPV will be used to describe the virus, and the BPV type will be specified when needed.
| δ papillomaviruses: (fibropapillomas) | BPV-1: face, teats, penis |
| BPV-2: face, back | |
| ɛ papillomaviruses: (mixed) | BPV-5: teats, udders |
| BPV-8: teats, skin | |
| ξ papillomaviruses: (papillomas) | BPV-3: back |
| BPV-4: upper GI tract | |
| BPV-6: teats, udders | |
| BPV-9: teats | |
| BPV-10: teats | |
| Novel genus: | BPV-7: skin, teats |
The virion, the viral genome and viral transcripts
The virion
The BPV virion has a constant morphology and structure independent of the site or type of lesion. It is a nonenveloped icosahedral structure of 55–60 nm diameter, which forms paracrystalline arrays in the nucleus of infected cells; it contains the double-stranded covalently closed circular DNA complexed with cellular histones. The virion is composed of the major L1 and minor L2 capsid proteins. The detailed structure of the BPV virion has been determined recently and an atomic model has been generated, which shows that the C-terminus of L1 and the N-terminus of L2 are exposed on the surface of the virion and are thus likely to have a role in infection and immunogenicity.16,17
The viral genome
The genome of BPV is a double-stranded closed circle of approximately 8000 nucleotides. The genome is divided into three canonical regions: a long control region (LCR, containing the cis-regulatory elements necessary for the replication and transcription of the viral DNA), the region containing the early genes (encoding nonstructural proteins) and the region containing the late genes (encoding the structural proteins) (Fig. 1).
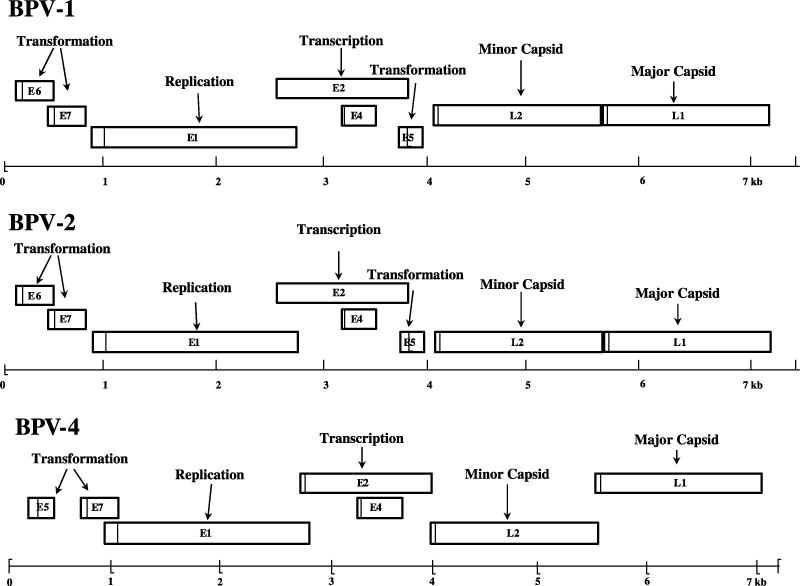
Diagrammatic genomic organization of BPV-1, BPV-2 and BPV-4. The viral genomes are represented as linear, with the open reading frames (ORFs) as rectangles. E indicates the early ORFs and L the late ORFs. The initiation codon is indicated by a vertical line. The function of the proteins encoded by the individual ORFs is indicated.
Transcriptional control
The BPV LCR contains 12 sites that interact with the E2 protein. E2 is crucial in the life cycle of BPV; it is both a replication factor for viral DNA and the major viral transcription regulator, and its binding to the LCR activates or represses transcription of the viral genes.18–20 In addition to E2, a number of cellular transcription factors interact with the LCR, either promoting or inhibiting transcription of the viral genes. The result of these interactions is a finely tuned circuit where positive and negative, viral and cellular controlling elements all contribute to the spatially and temporally regulated expression of the viral genes. When this balance is destabilized, transcription of the viral genes, and therefore expression of the viral proteins, increases and the outcome is often cell transformation to the neoplastic state.
Viral transcripts
Expression of the viral genes takes place through a complex pattern of RNA splicing events that process mature mRNA from polycistronic precursor RNA.21,22 As a general rule, the late transcripts encoding the structural proteins are found only in the more differentiated layers of warts and other productive lesions, whereas the RNAs encoding the early proteins are found both in warts and in transformed cells.
Function of BPV transforming proteins
Early proteins
BPV encodes three oncoproteins, E5, E6 and E7. E5 is the major viral oncoprotein followed by E6, with a more modest role played by E7.
E6 oncoprotein
BPV E6 is a transcriptional activator; it interacts with and inhibits the transcription coactivator CBP/p300 and in so doing also inhibits the function of the tumour suppressor p53.23 E6 binds the focal adhesion protein paxillin and blocks the interaction between paxillin and several other focal adhesion proteins, including vinculin and focal adhesion kinase.24 Disruption of focal adhesions underlies the observed anchorage-independent growth and disruption of the actin cytoskeleton induced by E6. Furthermore, E6 interacts with AP-1, the trans-Golgi network-specific clathrin adaptor complex, and this interaction is likely to affect cellular processes involving clathrin-mediated trafficking pathway.25 Despite its importance, the ξ BPVs do not have an E6 gene, which has been substituted with an E5-like gene (see succeeding discussion).
E7 oncoprotein
BPV E7 cooperates with E5 as well as E6 in cell transformation, as judged by anchorage-independent growth of mouse cells.26 The E7 protein of human papillomavirus (HPV) type 16 (the virus associated with cervical carcinoma) interacts with and inhibits the tumour suppressor p105Rb. This inhibition leads to unscheduled cell proliferation. Contrary to HPV-16 E7, E7 of δ genus BPVs lacks the canonical p105Rb-binding domain LXCXE27 and it is not yet clear how it contributes to cell transformation.
E5 oncoprotein
Whereas in the δ BPVs the E5 gene overlaps with the E2 gene, in the ξ BPVs the E5 gene has replaced the E6 gene (Fig. 1; it has to be noted that in some ξ BPVs, the E5-like gene is defined as E8).9 The E5 protein is very hydrophobic with a high leucine content; it is localized in the cell endomembrane compartments, particularly the Golgi apparatus, and binds the 16 k ductin/subunit c of the vacuolar H+ ATPase.28,29 This interaction results in inhibition of gap junction intercellular communication and lack of acidification of endosomes and Golgi apparatus.28,30
Gap junctions are channels for small molecular weight secondary messengers, important in the homeostatic control in a tissue; if a transformed cell is released from the control of the surrounding normal cells, it can proliferate freely and give rise to an expanding transformed clone. The lack of gap junction communication in papillomavirus-transformed cells probably contributes to cell transformation by isolating the newly infected cell from the surrounding normal cells, thus allowing other transforming events to become established.
The inhibition of acidification of endosomes and Golgi apparatus leads to the disruption of cellular protein processing and sorting, resulting in the retention and recycling of undegraded activated growth factor receptors from endosomal compartments.31,32 Additionally, BPV E5 interacts directly with and activates the PDGF receptor.33,34 Therefore, the mitogenic response is potentiated, directly, by E5 interacting with the PDGF receptor, and, indirectly, by E5 interacting with the 16 k protein, and in so doing inhibiting receptor down-regulation. Moreover, E5 activates several protein kinases involved in the control of cell cycle, thus causing a general dysregulation of the normally tightly controlled programme of cell proliferation.35–37
E5 and MHC class I down-regulation
The major histocompatibility complex class I (MHC I) plays a critical role in immune surveillance as it is responsible for the presentation of antigenic peptides to effector T cells. The complex consists of heavy chain (HC), β2-microglobulin and peptide, and is transported from the endoplasmic reticulum through the Golgi apparatus to the plasma membrane.38
A recently discovered function of BPV E5 is the down-regulation of MHC I.39–41 Regulation of MHC I by BPV E5 takes place at multiple levels: transcription of the MHC I HC gene is reduced, the MHC I HC peptide is degraded40 and the MHC I complex is sequestered in the Golgi cisternae and is irreversibly prevented from reaching the cell surface.41,42 Retention of MHC I in the Golgi cisternae is the result of at least two events. First, to the E5-induced alkalinization of the Golgi apparatus as a similar reduction of surface MHC I is observed in cell treated with ionophores, which prevents the acidification of endomembranes,41 and secondly, to a direct physical interaction between E5 and MHC I HC.42 These observations strongly support the view that BPV E5 helps the establishment of a successful infection not only through cell transformation but also by down-regulating MHC I, and thus allows the infected cells to evade host immunosurveillance.
In vivo expression of viral proteins
Transforming proteins
The distribution of BPV E5 and E7 proteins has been studied in bovine warts and papillomas.26,39,43,44 E5 is expressed in the cytoplasm of both basal and superficial differentiating keratinocytes. This localization supports the hypothesis that E5 is needed during the early stages of viral infection, whereas its presence in the more differentiated layers suggests an involvement of E5 in the late stages of the virus life cycle. Important for the notion that E5 has a role in the establishment of infection is the observation that MHC I is not expressed in cells that express E5.39
BPV-1 E7 is only observed in the cytoplasm and nucleoli of cells in the basal and lower spinous layers,26 whereas BPV-4 E7 is found also in the differentiated layers.44 The significance of the cytoplasmic localization of BPV-4 E7 in the differentiated layers is unclear, but it has been suggested that E7's frequent coexpression with E5 in the lower layers serves to modulate the cellular response of basal epithelial cells to E5.26
Structural proteins
The structural proteins L1 and L2 are expressed in the nucleus of the differentiated keratinocytes.44 During virion morphogenesis, L2 binds to viral DNA, favouring its encapsidation (45 and references herein). L1 and possibly also L2 mediate virus attachment to the cell receptor.16 Both proteins encode virus-neutralizing epitopes and both have been successfully used as vaccines.46
BPV and benign disease in cattle
The natural history of BPV infection has been extensively studied and reviewed,2,47,48 and the pathology of the warts/papillomas induced by BPV will be only briefly described here.
Infection by δ BPVs (fibropapillomaviruses) leads to fibropapillomas, whereas infection by (epitheliotropic) ξ BPVs induces epithelial papillomas without fibroblast involvement (Table 17,11). ɛ BPV has a dual pathology.13 Of note is the finding that the teats and udders of cows are subjected to infection by many different types of BPVs: BPV-1 and BPV-5–10 (Table 17,11), and additional types still to be properly classified.15
Normally, the papillomas regress as a result of a cell-mediated immune response49 but some animals are unable to reject the infection and succumb to widespread cutaneous or mucosal involvement. These forms of papillomatosis are problematic and of economic significance. Although it is believed that persistent papillomatosis is caused by immunosuppression, this is documented only in cases of BPV-4 infection of the upper GI tract.50,51 In animals chronically immunosuppressed by the ingestion of bracken fern (Pteridium aquilinum), the BPV-4-induced papillomas often progress to squamous cell carcinomas (see succeeding discussions).50
BPV and cancer in cattle
Co-factors, either environmental or genetic, are needed for papillomavirus-induced lesions to progress to squamous cell cancer. Bracken fern has been identified as a major environmental cofactor in BPV-induced carcinogenesis in cattle. Bracken fern contains immunosuppressants and a number of mutagens. Chronic bracken-induced immunosuppression causes a drop in circulating lymphocytes, and even during periods of bracken withdrawal the lymphocyte count remains very low.
Cancer of the upper GI tract
Cases of upper and lower GI tract cancer are found at a high frequency in areas where cattle graze on bracken-infested land.52,53 The carcinomas of the upper GI tract derive from BPV-4-induced papillomas. The contribution of viral, immunological and chemical factors in progression of papillomas to carcinomas was first established in the field52 and then in experimental conditions.54 Healthy cattle infected with BPV-4 reject their papillomas caused by a cell-mediated immune response.49 In contrast, in bracken-eating immunosuppressed cattle, the papillomas spread throughout the upper GI tract, do not undergo regression and progress to cancer.54
These epidemiological and molecular studies have allowed the determination of the events that take place during carcinogenesis. In addition to the action of the viral oncoproteins (see previous discussion), the number of the cellular receptors for epidermal growth factor increases, the ras gene is activated, and the p53 gene is mutated.48 These transforming events, involving cellular genes, are ascribed to the bracken fern mutagens, but, although this has been proved in vitro,55–58 it remains to be established in vivo. In support of a role for bracken mutagens in carcinogenesis, two of the most potent mutagens of the plant (quercetin and ptaquiloside) are found in urine, serum and milk of bracken-eating cattle.59,60
Cancer of the urinary bladder
In addition to cancer of the upper GI tract, bracken-eating cattle develop chronic enzootic haematuria, urinary bladder cancers and chromosomal abnormalities.52,60–64 Urinary bladder cancer comprises two main types, carcinoma of the urothelium and haemangioendotheliomas of the subjacent capillaries. Often the two types of tumour occur together in the same bladder. The involvement of bracken and BPV in bladder carcinogenesis has been recognized for a long time,65,66 more recently the virus–bracken interaction has been reproduced experimentally,67 and a carcinogenic process, similar in outline to the one established for BPV-4, has been recognized. BPV-1/2 infects the epithelium of the urinary bladder (the modality of infection of the urinary bladder is still unknown) and establishes an abortive infection, with no production of virus. The viral oncoprotein E5 is expressed,33,68,69 the ras gene is activated,46,70 the PDGF receptor is activated and complexed with E533 and expression of the tumour suppressor locus fragile histidine tetrads (FHIT) is down-regulated.61 The immunosuppression induced by bracken fern prevents tumour rejection, and the fern mutagens contribute to genome destabilization.
Equine sarcoids: definition and clinical aspects
Sarcoids are locally invasive fibroblastic skin tumours and represent the most common equine neoplasm, with reported prevalence rates ranging from 12.9% to 67% of all equine tumours.71 Sarcoids can occur anywhere on an animal's body although some sites are more commonly affected than others; often these are sites of skin exposed to trauma or skin damage. Tumours may exist as single or multiple lesions, and animals with several hundred tumours are not uncommon. The gross appearance of sarcoids can vary and up to six clinical types are recognized. Occult sarcoids present as hairless circular areas of skins; verrucous lesions exhibit a wart-like appearance; fibroblastic sarcoids present as ulcerated fleshy masses; nodular sarcoids consist of firm masses lying underneath the skin and mixed sarcoids show a combination of features of verrucous, fibroblastic and nodular sarcoids.72 The sixth form or malignant/malevolent sarcoid form is a more recently described clinical type, which can show extensive invasion and infiltration of lymphatics,72 a feature that is not observed in the other types, although localized infiltration and invasion is common in all sarcoids. Although some lesions can remain quiescent for many years, the milder forms of the disease (occult and verrucous) can undergo transformation to more advanced types (fibroblastic and malignant) especially following trauma.72–74 Histopathologically, however, the different types of lesions are often difficult to distinguish as they exhibit similar histological changes including dermal proliferation of spindle-shaped fibroblasts forming whorls, epidermal hyperplasia and hyperkeratosis and rete peg formation.75
Currently there is no effective therapy for sarcoids. Commonly employed treatment options include immunomodulation, cryotherapy and excision; however, 20–50% of lesions recur following surgical intervention.74,76 The presence of sarcoid lesions at pre-purchase veterinary examination often leads to the animal being ‘failed’ and/or causes insurance companies to place significant exclusion clauses in the cover provided in so-called loss-of-use and veterinary fee policies. Thus, equine sarcoids have a noticeable impact on equine health and welfare, and present a significant financial burden for owners. This is particularly so in developing countries where donkeys are a large part of the agrarian economy. To date, no sex predilection has been reported; however, an increased incidence has been reported in younger animals and the crude incidence of sarcoid in donkeys has been estimated at 0.6 cases/animal/year.77–80
Equine sarcoids: evidence to support a viral aetiology
The early transmission studies were the first to demonstrate the viral aetiology of sarcoids. The first report dates back to 1939 when Montpellier et al.81 demonstrated sarcoid autotransmission in a mule using fresh tumour material. This was corroborated by Olson in 1948,82 who showed that autotransmission was only successful when skin was scarified and could not be achieved using topical, intradermal or subcutaneous inoculations. Voss (1969)83 transmitted equine sarcoids by both autologous and heterologous transfer of whole tumour tissue and cell-free extracts and again reported that subcutaneous and intradermal inoculations were unsuccessful.
The original description of equine sarcoid by Jackson (1936)84 classified the lesion together with bovine and canine papillomas because of their clinical appearance and pathology. The work by Olson and Cook (1951)3 was the first attempt to demonstrate an association with bovine papillomaviruses. Olson and Cook showed that intradermal inoculation of bovine wart material into horses could give rise to sarcoid-like lesions; however, most of the lesions underwent spontaneous regression, which is rarely seen in natural sarcoids. One animal however, developed a recurrent tumour, compatible with sarcoid. Inoculation of horses with material from this tumour reproduced similar recurrent tumours. Ragland and Spencer (1969)85 also reported the growth of sarcoid-like lesions following intradermal inoculation. Interestingly, in one study, cattle inoculated with sarcoid extracts (both intradermally and topical application to scarified skin) did not develop lesions.86
Role of BPV in sarcoids
Although the studies described in the previous discussion do not provide a causative role, there are now many reports in the literature showing that BPV (type 1 and less commonly, type 2) is involved in the pathogenesis of the sarcoid with the vast majority of tumours harbouring episomal (nonintegrated into the cell genome) BPV DNA.87–101 In situ hybridization has consistently shown that viral DNA is localized to the fibroblast nuclei and is absent from epithelial cells.102,103 Despite the consistent finding of BPV DNA in sarcoids, viral particles have not been demonstrated in lesions, and the infection is considered to be nonproductive.88
BPV DNA is undetected in other equine skin lesions including melanomas, papillomas and squamous cell carcinomas,99 although we and others have shown that BPV-1/2 DNA can be detected in some cases of equine inflammatory skin disease;91,99,101 however, the significance of this observation remains unknown.
It has to be noted that an equine papillomavirus (EqPV), different from BPV, has been found in equine papillomas.104
BPV sequence variants in sarcoids
Studies by Trenfield et al. (1985)90 and Angelos et al. (1991)91 were the first to provide evidence for sequence variation within the BPV genome in sarcoids, and to suggest that the virus may not be BPV but closely related to BPV. Several additional studies have confirmed these findings93,95,99 and it is now well established that sarcoids are associated with distinct variants of BPV. In a recent study by our group, we have detected variation within the LCR of BPV-1.5 The BPV-1 LCR from 35 equine sarcoids and from 14 BPV-1-positive bovine tumours was sequenced. The most common variant, LCR II, was present in 66% of all equine infections but was not found in cattle infections. Functional analyses showed that sequence differences observed in the variant LCR II have a discernible effect on LCR functionality in equine cells but not in bovine cell.5 We have also documented that the variants detected in equine sarcoids show increases in codon usage in equine cells.4,5,99 This is interesting, particularly for the E5 gene as these variants would be expected to be translated more efficiently in equine cells and therefore increase the cell transformation efficiency. These data support the hypothesis that ‘equine-specific’ BPV-1 genomes are able to preferentially infect and be maintained within equids.
Comparative aspects of BPV infection in cattle and equids
The molecular biology of BPV-1 infection in equine sarcoids resembles that of BPV-2 infection of the urinary bladder in cattle: in both cases, infection is abortive and nonproductive, early proteins, including the major oncoprotein E5, are consistently expressed,33,68,99,105,106 providing clear evidence that the presence of the viral DNA is not accidental; late proteins are occasionally detected97 but no viral particles have been observed,88 suggesting that the infection is aborted at a late stage. However, there are differences in viral copy number between bovine bladder cancer and equine sarcoids. We have recently shown that although genome copy number can vary, infection in the horse is maintained at a significantly higher genome copy number (0.1 to 284 viral genome equivalent (g.e.) per cell) than in the BPV-induced bladder cancers of cattle (from 0.3 to 1.8 g.e.) (Table 2).107
| No. of tumours | No. of genomes per cell | ||||
|---|---|---|---|---|---|
| Mean | Median | Range | |||
| Equine sarcoids | BPV-1 | 11 | 82.08 | 50 | 0.1–284 |
| Bovine bladder cancers | BPV-2 | 5 | 0.5 | 0.3 | 0.5–1.8 |
BPV latency
Like many viruses, BPVs can establish a latent infection. The viral genome can be often found in normal epithelia of cattle and equids with no clinical sign of disease,98,100,108 both in tumour-bearing and in clinically normal hosts. Normal epithelia are the accepted site of latent infection, and indeed the reactivation of BPV at sites of trauma suggests that viral DNA is present in these sites in latent form, and that damage of the epithelium, possibly through production of inflammatory cytokines and stimulation of cell proliferation, induces expression of viral genes, leading to papilloma formation.108 However, epithelia may not be the only site of latent papillomavirus in cattle. BPV DNA is present in episomal form in circulating lymphocytes of cattle (F. Roperto, University of Naples, personal communication)108,109 and latent BPV infection of lymphocytes has been experimentally established in cattle.64 Contrary to the finding of BPV DNA in bovine peripheral blood cells, we have previously failed to detect BPV DNA in blood samples obtained from horses.110 A recent study, however, has shown that BPV-DNA is present in the blood of affected horses.111
Interestingly, papillomavirus virus-like particles (VLPs) have been shown in vitro to interact with a variety of immune cells, including dendritic cells, B cells, monocytes and macrophages, and it has been hypothesized that this interaction is likely to be important in the immune response to PV capsids.112 These observations suggest the hypothesis that blood cells not only represent another site of latent virus, but also that the relationship between PV and immune cells can modulate the host immune response to virus.
Equine sarcoids: disease transmission mechanisms
In cattle, BPV is transmitted by contact between animals or contact with fomites.113 Currently, it is not known whether BPV infection of horses is transmitted from one horse to another, whether the disease is transmitted from cattle to horses or how BPV can cause infection in a non-natural host. As stated previously, inoculation of horses of cattle wart material can induce sarcoid-like lesions; however, one study reported that inoculation of cattle with sarcoid material did not result in warts.73 The occurrence of epizootics of equine sarcoids in herds of horses and donkeys has also been reported.78,114
The absence of viral particles has led to the speculation that the disease may be the result of a transformed infectious cell line in analogy with canine transmissible venereal tumour.115,116 Our recent microsatellite analysis of sarcoid tumours and matched blood samples shows that the genotype of the tumour-derived DNA is identical to the host genotype.117 This demonstrates that the disease is not caused by an infectious heterologous cell line.
In an attempt to investigate animal to animal transmission, we have recently performed sequence analyses of BPV-1 genomes isolated from sarcoids from four donkeys. The animals were housed in pairs: in each pair one animal was ‘sarcoid free’ and one animal was ‘sarcoid affected’. In both pairs the sarcoid-free donkeys developed sarcoids. Tumours from all four donkeys were examined for the presence of BPV-1 variation. As shown in Fig. 2, the viral sequences within each pair were identical but distinct between the pairs, providing strong evidence for animal to animal transmission (our unpublished observations).
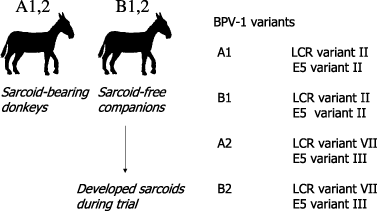
Disease transmission of equine sarcoids. Four donkeys were housed in pairs: in each pair one animal was ‘sarcoid free’ and one animal was ‘sarcoid affected’. In both pairs the ‘sarcoid free’ donkey developed sarcoids. Tumours from all four donkeys were examined for the presence of BPV-1 variation. The viral sequences within each pair were identical but distinct between the pairs, providing strong evidence for animal to animal transmission. Sequence data is from.5,99
Interestingly, there is preliminary evidence that face flies (Musca autumnalis) may be able to act as vectors in disease transmission.118 In this study, BPV DNA was detected in face flies collected from sarcoid affected animals but not from flies isolated from unaffected controls, suggesting that flies may act as vectors for the transmission of BPV. This is an important finding in terms of disease management but requires further evaluation.
Equine sarcoids: genetic predisposition
For the development of malignant tumours in cattle, viral infection alone is not sufficient and BPV requires cofactors. The environmental cofactor of BPV in carcinogenesis of the urinary bladder cancer has been identified in the presence of bracken fern in the pasture.52,61,62,67 As BPV-1 DNA can be detected in normal equine skin,98,100 BPV-1 infection appears to be necessary but not sufficient for sarcoid development. No environmental cofactor has been identified for BPV and equine sarcoid but the genetic background of the host is important. A study examining the association of sarcoid development with breed showed that the frequency of sarcoids in Quarter horses was nearly twice that of Thoroughbreds. In contrast, the frequency of sarcoids in Standardbreds was less than half that of Thoroughbred horses,119 supporting a genetic predisposition to the disease. A familial predisposition to equine sarcoid has also been described.114,120 Several studies have shown a strong association between risk of sarcoid development and specific equine MHC alleles. A significant association between MHC class II haplotype W3 and MHC class I haplotype B1 has been reported in Thoroughbred horses.121 Similarly, the MHC class II W13 haplotype was shown to be strongly associated with sarcoids in Swedish halfbreds122 Swiss, French and Irish Warmbloods horses.123 A further study also showed an association between increased recurrence of sarcoids following surgery in horses with the class II W13 haplotype, and association between early onset of sarcoids and the class I A5 haplotype.124
Immune response to BPV
Viruses and their hosts are continuously fighting each other. Viruses must be able to overcome the host immune response to replicate themselves; nevertheless, despite the viral evasion of immune surveillance, eventually the host mounts an effective immune response and virus and virus-infected cells are eliminated.
The immune response of cattle to BPV is surprisingly poor.125 Animals may carry massive tumours, actively producing virus in large quantities, but cattle do not respond easily to BPV antigens during the course of infection, and anti-BPV antibodies are seldom detected. The failure of the immune system to recognize either incoming virus or progeny virus is because of the fact that the virus life cycle is restricted to the epithelium and therefore is not in contact with the immune system.126,127 This interpretation is supported by the fact that field animals with ulcerated and bleeding tumours do have high titres of natural anti-BPV antibodies, and good antibody responses can be obtained after intramuscular inoculation with purified virus or viral proteins, confirming that only when the papilloma is damaged, or a threshold of unknown nature is reached via immunization, viral antigens come into contact with immune cells.125 Weak T- and B-cell responses to capsid proteins or to the transforming protein E7 can be observed in some animals at later stages of infection and appear to be associated with papilloma rejection.126
During rejection of papillomas, large masses of activated lymphocytes accumulate in the underlying derma. In these clusters, CD4+ lymphocytes are the predominant subtype, whereas γδ and CD8+ lymphocytes predominate in the basal layer and among the keratinocytes.49,126 The contribution of the individual lymphocyte subtypes to papilloma regression remains to be established.
The poor immune response to BPV is likely to be the main reasons for the persistence of infection: even in immunocompetent hosts, the papillomas persist for many months before regression takes place. It has recently become clear that, in addition to the passive immunoescape as a result of the viral life cycle being confined to the epithelium, papillomaviruses have evolved ways of hiding from the host immune system.126 Among these there is down-regulation of MHC I by E5 (see previous discussion).
Currently little is known about the immune response to sarcoids in equids. The recognized association between sarcoid prevalence and the MHC class I and class II haplotypes121–124 confirm that presentation of antigenic peptides to T cells is important in mediating protection. Specific MHC class II alleles may be associated with an impaired immune response to BPV. Associations between certain MHC class II genes and the development of tumours induced in rabbits by CRPV128 and in human cervical carcinoma associated with HPV types 16 or 18 are recognized.129,130
In most PV infections, regression of lesions occurs following activation of the host immune response. However, several immune evasion mechanisms that may contribute to persistence and progression of PV-associated disease have also been described.126 Sarcoids are nonregressing, in contrast to many other lesions caused by PV infection suggesting that expression of the BPV proteins in equine cells may evoke similar immune evasion mechanisms. However, sarcoid regression can be stimulated in animals with a moderate tumour burden by intralesional inoculation of BCG131 or by the application of immunomodulatory compounds132 both of which stimulate local cellular immunity with macrophage activation and subsequent release of cytokines that enhance both humoral and cell-mediated immunity. Therapeutic vaccination with autologous tumour cells can induce clearance of recurrent sarcoid lesions133 correlating with early results showing that experimentally induced sarcoids also regress.82 The observation that, under appropriate conditions, sarcoids can be induced to regress, further supports that the persistence of naturally occurring equine sarcoids may be caused by an impaired immune response. Recently, E5, the major oncoprotein of BPV, has been shown to cause retention of MHC class I molecules within the Golgi apparatus and thus prevents transport of MHC class I complex to the cell surface both in vitro and in vivo.39–42 We have recently shown that BPV-1 E5 is also able to down-regulate the expression of equine MHC class I (Marchetti et al., unpublished observations). MHC I is required for the presentation of viral antigens to CD8+ T lymphocytes for the elimination of infected cells. Therefore, the E5 protein may provide a direct way in which the virus avoids the immune system also in horses.
BPV vaccines
Prophylactic vaccines in cattle
Prophylactic (preventative) vaccination was first achieved with BPV-2 virions, and virus-neutralizing antibodies were elicited by vaccination with BPV-2 L1 protein.46 Following these earlier studies, BPV-2 and BPV-4 were chosen as emblematic for skin and mucosal papillomavirus, respectively, and because, as described previously, both are implicated in cancer. Also in the case of these two viruses, L1 elicits neutralizing antibodies and confers protection from infection. Additionally, full protection is achieved with BPV-4 L2, and specifically with the N-terminus of the protein.134–136 These results are consistent with the observation that the N-terminus of L2 is exposed on the surface of the BPV-1 virion137 and therefore accessible by the immune system. Interestingly, the BPV-4 L2 epitopes are homologuous to and cross-react with epitopes identified in the L2 protein of several genital HPVs,46 leading to the testable prediction that vaccines based on HPV L2 proteins may elicit cross-type protective immunity in humans.
Papillomavirus capsid proteins can self-assemble in empty VLPs when expressed in eukaryotic cells. BPV-4 VLPs containing either the L1 and L2 proteins or only the L1 protein are very potent prophylactic vaccines.138 Protective immunity against BPV can therefore be achieved either with L2 alone or with L1 alone.
Therapeutic vaccines in cattle
Therapeutic (curative) vaccines, designed to induce regression of premalignant lesions, have been developed based on the L2 protein of BPV-2, or on the E7 protein of BPV-4.46 In both cases, regression is accompanied by infiltration of lymphocytes and macrophages, indicative of a cell-mediated immune response, similar to that observed in naturally regressing papillomas. However, it is not known whether the cellular immune response in vaccinated calves is directed against the same antigens that mediate natural papilloma rejection.
Therapeutic vaccine in equids
The presence of viral antigens in equine sarcoids presents the opportunity to evaluate anti-BPV vaccination strategies. To this end we vaccinated sarcoid-bearing donkeys in a placebo-controlled trial using chimeric VLPSs (cVLPs) comprising BPV-1 L1 and E7 proteins.139 The choice of vaccine was determined by the fact that L1 provides antibody protection and E7 stimulates tumour regression. Sarcoid regression was observed in 50% of vaccinated animals (four of eight), more frequently than in placebo-treated animals (two of nine). Conversely, tumour progression was observed in only one vaccinated donkey whereas in control animals, three showed progression. In a parallel study,140 sarcoid-bearing horses were vaccinated with an identical cVLP vaccine. Sarcoid regression was observed also in this trial and many sarcoids remained stationary. Taken together, our data suggest that cVLPs are a promising therapeutic vaccine.
Conclusions
The BPV system has been ground-breaking in the recognition of the oncogenic nature of the virus, the elucidation of the relationship between virus and cofactors and the development of antiviral vaccines. Although not all the facets of infection in cattle can be applied to infection in horses, comparative pathology studies between BPV in bovids and equids have contributed in no small measure to the understanding of equine sarcoids. There are still many things to be learned from BPV, which, in addition to a greater knowledge of the fundamental pathobiology of equine sarcoids, have the potential to provide intervention measures for this problematic tumour.
Acknowledgements
The authors would like to acknowledge support from the Medical Research Council, the Association for International Cancer Research, the World Cancer Research Funds, Cancer Research UK, Horserace Betting Levy Board, Home of Rest for Horses (now Horse Trust), Pet Plan Charitable Trust and The Donkey Sanctuary. MSC is a Fellow of CR UK.
References
Résumé Le papillomavirus bovin (BPV) est peut être le papillomavirus animal le plus étudié. Chez les vaches, les BPVs sont responsables de tumeurs bénignes cutanées ou des épithélia, appelés papillomes ou verrues. Les papillomes sont des tumeurs bénignes qui régressent généralement spontanément sans provoquer de problème sérieux chez l’hôte, mais qui peuvent occasionnellement persister et se transformer en carcinome épidermoïde comme dans le cas des cancers de la vessie ou du tractus digestif. Les BPVs sont les seuls virus qui peuvent passer d’espèce à espèce et qui infecte également les chevaux en provoquant des tumeurs fibroblastiques appelées sarcoides. Les sarcoides régressent très rarement et le plus souvent persistent et peuvent être localement agressifs. Il s’agit des tumeurs les plus fréquentes chez le cheval dans le monde entier.
Le but de cette revue est de discuter la biologie des BPV, la biologie des tumeurs bovines et des sarcoides du cheval et de présenter les données actuelles de la pathogénie des tumeurs liées aux BPV chez leur hôte naturel, les bovins et chez les équidés. Finalement, l’utilisation de vaccins à base de BPV comme traitement des sarcoïdes équins est discuté. Seules des données limitées sur les aspects cliniques et pathologiques des tumeurs bovines et équines seront développés car ce sujet a déjàété traité en détail auparavant.
Resumen El papilomavirus bovino es posiblemente el papilomavirus animal más estudiado. En el ganado vacuno produce tumores benignos del epitelio cutáneo y de mucosas llamados papilomas o verrugas. Los papilomas bovinos son benignos y generalmente desaparecen sin producir ningún problema clínico de seriedad en el hospedador, pero ocasionalmente persisten y constituyen un foco para transformación maligna a carcinoma de células escamosas, como sucede en el caso del cancer de la vejiga de la orina y el cancer del canal alimentario superior. El virus del papiloma bovino es el único papilomavirus capaz de pasar a otras especies: el virus también infecta équidos dando lugar a tumores fibroblásticos llamados sarcoides. Los sarcoides raramente desaparecen de forma espontánea y con más frecuencia persisten y pueden ser producir invasión a nivel local. Estos tumores son los tumores de piel más comunes en équidos de todo el mundo.
El propósito de esta revisión es discutir la biología del virus de papiloma bovino, la biología de los tumores bovinos y de los sarcoides equinos, y presentar los conocimientos actuales del virus de papiloma bovino en la patogenia de los tumores en su hospedador natural, el ganado bovino, y en los hospedadores heterológos, équidos. Finalmente se discute el uso de vacunas frente al virus como terapia para los sarcoides equinos. Solo se comentaran brevemente aspectos de la clínica y patología del los tumores bovinos y equinos ya que esos aspectos se han discutido en profundidad anteriormente.
Zusammenfassung Das bovine Papillomavirus (BPV) ist wahrscheinlich das am meisten untersuchte tierische Papillomavirus. Bei Rindern verursachen BPVs gutartige Tumoren der kutanen oder mukösen Epithelien, die Papillomas oder Warzen genannt werden. Rinderpapillome sind gutartige Tumoren, die sich normalerweise ohne ernsthafte klinische Probleme beim Wirt auszulösen, zurückbilden. Manchmal bleiben sie jedoch bestehen und bilden den Herd für eine maligne Umwandlung zum Plattenepithelkarzinom, wie im Falle des Krebs der Harnblase und bei Krebs des oberen Verdauungstraktes. BPV ist das einzige Papillomavirus, welches die Spezies wechselt: das Virus infiziert auch Einhufer und löst fibroplastische Tumoren aus, die Sarkoide genannt werden. Sarkoide bilden sich sehr selten zurück, häufiger bleiben sie bestehen und können lokal aggressiv sein. Diese Tumoren sind weltweit die häufigsten Hauttumoren der Einhufer.
Der Zweck dieser Review war es die Biologie von BPV, sowie die Biologie der bovinen Tumoren und der equinen Sarkoide zu diskutieren, und das momentane Verständnis von BPV bei der Pathogenese der Tumoren beim natürlichen Wirt, dem Rind, und bei seinem heterologen Wirt, den Einhufern, zu präsentieren. Letztendlich wird die Verwendung von anti-BPV Vakzinen als Therapie für die equinen Sarkoide diskutiert. Es wird nur wenig Information über die klinischen und pathologischen Aspekte sowohl der bovinen wie auch der equinen Tumoren vorgestellt, da dieses Thema schon früher umfangreich behandelt worden war.




