Rubisco activase is a key regulator of non-steady-state photosynthesis at any leaf temperature and, to a lesser extent, of steady-state photosynthesis at high temperature
Summary
The role of Rubisco activase in steady-state and non-steady-state photosynthesis was analyzed in wild-type (Oryza sativa) and transgenic rice that expressed different amounts of Rubisco activase. Below 25°C, the Rubisco activation state and steady-state photosynthesis were only affected when Rubisco activase was reduced by more than 70%. However, at 40°C, smaller reductions in Rubisco activase content were linked to a reduced Rubisco activation state and steady-state photosynthesis. As a result, overexpression of maize Rubisco activase in rice did not lead to an increase of the Rubisco activation state, nor to an increase in photosynthetic rate below 25°C, but had a small stimulatory effect at 40°C. On the other hand, the rate at which photosynthesis approached the steady state following an increase in light intensity was rapid in Rubisco activase-overexpressing plants, intermediate in the wild-type, and slowest in antisense plants at any leaf temperature. In Rubisco activase-overexpressing plants, Rubisco activation state at low light was maintained at higher levels than in the wild-type. Thus, rapid regulation by Rubisco activase following an increase in light intensity and/or maintenance of a high Rubisco activation state at low light would result in a rapid increase in Rubisco activation state and photosynthetic rate following an increase in light intensity. It is concluded that Rubisco activase plays an important role in the regulation of non-steady-state photosynthesis at any leaf temperature and, to a lesser extent, of steady-state photosynthesis at high temperature.
Introduction
There is an urgent need to not only increase global crop productivity and yield, but also to make crop plants more tolerant to the projected increases in temperature. As photosynthesis is one of the most important but heat-sensitive metabolic process of plants, it would be one of the factors targeted for possible improvement for crop yield. It has been reported that inhibition of photosynthesis by moderate heat stress (e.g. 40°C) could be due to the deactivation of Rubisco (for a review, see Portis, 2003; Salvucci and Crafts-Brandner, 2004c). The activation state of Rubisco is regulated by Rubisco activase (Portis, 2003), which may fail to maintain a high Rubisco activation state at high temperature because of its thermolability (Crafts-Brandner and Salvucci, 2000; Salvucci and Crafts-Brandner, 2004a,b). Rubisco activase is a nuclear-encoded chloroplast enzyme that is usually present as two isoforms in most species studied: a large isoform of 45–48 kDa (α form) and a small isoform of 41–43 kDa (β form) (Zhang and Komatsu, 2000; Portis, 2002; Spreitzer and Salvucci, 2002). The α form is regulated by the ATP/ADP ratio and redox state in the chloroplast, whereas the β form is non-redox-regulated (Zhang and Portis, 1999; Zhang et al., 2002). Rubisco activase facilitates carbamylation and the maintenance of Rubisco activity by removing inhibitors such as tight-binding sugar phosphates from Rubisco catalytic sites in an ATP dependent manner (Spreitzer and Salvucci, 2002; Portis, 2003; Parry et al., 2008). Recently, enhanced thermostability of Rubisco activase in Arabidopsis has been shown to improve CO2 assimilation rates and plant growth under heat stress (Kurek et al., 2007; Kumar et al., 2009). Sage et al. (2008) proposed that Rubisco activase may be an important factor determining the response of boreal plants to global warming based on studies with the dominant species in the boreal forest of North America. Thus, genetic manipulation of Rubisco activase could provide the means to improve photosynthetic thermotolerance in crops.
On the other hand, another factor that might contribute to the observed inhibition of photosynthesis at high temperatures is the down-regulation of Rubisco activase in response to a limitation in some other process (Sharkey, 2005; Sage and Kubien, 2007). For example, triose phosphate utilization limitation at moderately high temperature may result in lowered ATP/ADP ratios (Sharkey, 1989). Moreover, moderately high temperature reduced the pH component of the trans-thylakoid proton motive force (Zhang et al., 2009) and lowered electron flow (Schrader et al., 2004). Such reductions in electron transport could reduce the stromal redox state (Ruuska et al., 2000; Yamori et al., 2011b). A lower ATP/ADP ratio or stromal redox state can result in a decreased Rubisco activase activity. The dependence of the Rubisco activation state and the light-saturated photosynthetic rate on the Rubisco activase content has been investigated with transgenic plants expressing different amounts of Rubisco activase. Previous studies found that lowering the Rubisco activase content had little effect on the Rubisco activation state or the light-saturated photosynthetic rate unless the amount of Rubisco activase is reduced to about 10% of the wild-type levels at 25°C (Mate et al., 1993, 1996; Jiang et al., 1994). At moderately high temperatures, Rubisco activation state decreased only slightly with reductions in Rubisco activase content, but the temperature response of Rubisco activation was not strongly dependent on Rubisco activase content, suggesting that other processes might also modulate Rubisco activation (Yamori and von Caemmerer, 2009). Thus, the primary reason for the inhibition of photosynthesis at moderately high temperatures remains unknown. It might be possible that the mechanism depends on the plant species and growth conditions (Yamori and von Caemmerer, 2009; Yamori et al., 2010b, 2011a).
Research into finding ways to increase crop yields has focused on improving steady-state photosynthesis (Evans and von Caemmerer, 2011). However, leaves in natural plant canopies experience a highly variable light environment over the course of a day due to changes in leaf angle, cloud cover, and overshadowing canopy cover. By contrast, transgenic plants have not yet been used to clarify the limiting step of non-steady-state photosynthesis. When the light intensity on a leaf is increased suddenly after a prolonged period of low light or darkness, the rate of photosynthesis rises gradually over several minutes and approaches a new steady state (Pearcy, 1990). This phenomenon has been termed ‘photosynthetic induction’. Experiments with antisense tobacco plants that contain reduced activase levels showed that the rate of Rubisco activation following an increase in light intensity was linearly related to the amount of Rubisco activase (Mott et al., 1997; Hammond et al., 1998). Thus, it has been suggested that Rubisco activity would be an important limitation to photosynthesis under non-steady-state conditions caused by changes in light intensity (Seemann et al., 1988; Woodrow and Mott, 1989; Mott and Woodrow, 1993). There are few studies that address improvements of non-steady-state photosynthesis, although plants are often exposed to fluctuating light conditions (i.e. cloudy weather or understory environments).
If Rubisco activase is the main limitation for steady or non-steady-state photosynthesis, it would be valuable to improve the activity of Rubisco activase in various crop plants. Alternatively, if other factors, including electron transport capacity, are the primary limitations, efforts to improve Rubisco activase activity would not have any impact on photosynthetic performance and plant growth. Thus, it is important to examine the regulation of Rubisco activation state at steady-state and/or non-steady-state conditions, and at various leaf temperatures. To elucidate the role of Rubisco activase in regulating Rubisco activity at: (i) the steady-state; as well as (ii) the non-steady-state; following an increase in light intensity at several leaf temperatures, transgenic rice with increased levels of maize Rubisco activase and antisense plants with reduced levels of Rubisco activase have been examined.
Results
Physiological components of leaf photosynthesis
Rubisco activase content differed among wild-type, transgenic plants that overexpressed maize Rubisco activase and transgenic plants that expressed antisense Rubisco activase (Figure 1). However, the contents of several components of the photosynthetic machinery, which include nitrogen, Rubisco, chlorophyll and cytochrome f, were similar among these lines (Table 1). We therefore assumed that the differences in photosynthetic properties among these lines are primarily the result of the differences in Rubisco activase content and/or differences in the thermostability of Rubisco activase between rice and maize.
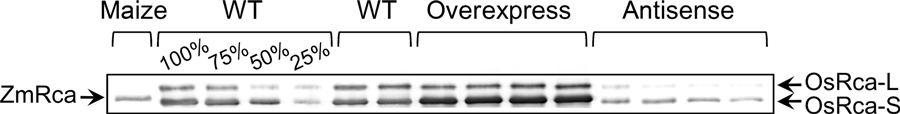
Western blot analysis in leaves of wild-type (WT) rice, plants overexpressing maize Rubisco activase, and antisense plants with low Rubisco activase expression levels. Protein extracts, loaded on an equal leaf area basis, were separated by SDS-PAGE; the series of dilutions relative to WT is indicated. Western blots were probed with polyclonal antibodies raised against rice Rubisco activase and visualized using alkaline phosphatase conjugated to a secondary antibody.
| Nitrogen (mmol m−2) | Rubisco (μmol m−2) | Chls a + b (mmol m−2) | Cytochrome f (%) | |
|---|---|---|---|---|
| Wild-type | 104.4 ± 2.5 | 3.26 ± 0.12 | 0.611 ± 0.029 | 100 ± 2.9 |
| Overexpress | 107.2 ± 2.5 | 3.13 ± 0.12 | 0.622 ± 0.014 | 97.2 ± 4.8 |
| Antisense | 97.0 ± 1.4 | 3.43 ± 0.11 | 0.605 ± 0.021 | 93.9 ± 6.5 |
- The amount of nitrogen, Rubisco, chlorophyll per unit leaf area was quantified. The cytochrome f content is shown as a percentage relative to wild-type levels. Data represent means ± standard error (SE), n = 4–5. There were no significant differences in the photosynthetic components among wild-type, activase-overexpressing, and antisense plants (Turkey–Kramer’s multiple comparison test, P < 0.05).
Role of Rubisco activase in limiting steady-state photosynthesis at high light intensity
At 15 and 25°C, a large reduction (i.e. more than 70%) of the Rubisco activase content in antisense plants was required before CO2 assimilation rate at 250 μmol mol−1 CO2 concentration at high light (A250) or the Rubisco activation state were decreased (Figure 2). Thus, overexpression of maize Rubisco activase did not lead to an increase in A250 nor the Rubisco activation state below 25°C. On the other hand, at 40°C, A250 and Rubisco activation state slightly decreased with a decrease in Rubisco activase content in the antisense plants. In addition, A250 and the Rubisco activation state in Rubisco activase-overexpressing plants were slightly greater than in wild-type plants (Figure 2). Effect of Rubisco activase content on steady-state photosynthesis and Rubisco activation state was similar between at 250 μmol mol−1 and at 390 μmol mol−1 CO2 concentration (Figure S1). Temperature responses of the average values for CO2 assimilation rate and Rubisco activation state at 250 or 390 μmol mol−1 CO2 concentrations are shown in Figure S2. At high temperature, overexpression of Rubisco activase had a small stimulatory effect on Rubisco activation state and CO2 assimilation rate.
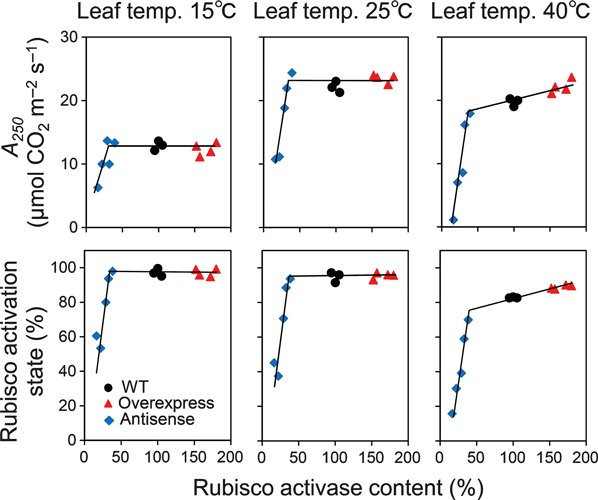
CO2 assimilation rate at 1500 μmol photons m−2 s−1 and 250 μmol mol−1 CO2 concentration (A250) and the Rubisco activation state as a function of Rubisco activase contents. A250 and Rubisco activation state was shown at leaf temperatures of 15, 25 and 40°C. Rubisco activase content in all genotypes including plants that overexpress maize activase were determined by immunoblotting with polyclonal antibodies raised against rice activase. Therefore, the detection of maize Rubisco activase can be less sensitive compared with rice Rubisco activase, leading to underestimate the content for maize Rubisco activase. Wild-type: black circle, activase-overexpressing plants: red triangle, antisense plants: blue diamond.
CO2 response curves of CO2 assimilation rate (A), chloroplast electron transport rates estimated from chlorophyll fluorescence (ETR) and non-photochemical quenching (NPQ) were analyzed at high light (Figure 3). At 15°C and 25°C, CO2 response of A, ETR and NPQ were similar between wild-type and activase-overexpressing plants. At 40°C, A, ETR and NPQ at high CO2 concentrations were also similar between wild-type and activase-overexpressing plants, whereas activase-overexpressing plants had greater A and ETR, but lower NPQ at low CO2 concentrations than wild-type plants. In antisense plants, A and ETR were lower, but NPQ was higher than in wild-type and activase-overexpressing plants, irrespective of CO2 concentration or leaf temperature.
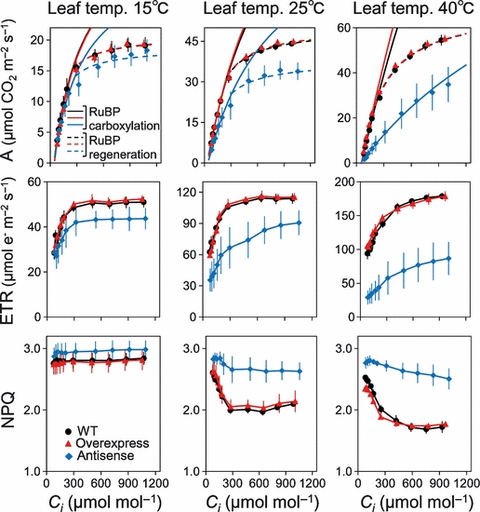
CO2 response of CO2 assimilation rate (A), electron transport rate estimated from chlorophyll fluorescence (ETR) and non-photochemical quenching (NPQ) under 1500 μmol photons m−2 s−1 at 15, 25 and 40°C. Rubisco-limited A (Ac: solid line) was estimated from eqn 1 in Experimental procedures, whereas RuBP regeneration-limited A (Ar: dotted line) was estimated from eqn 2 in Experimental procedures. Wild-type: black circle, activase-overexpressing plants: red triangle, antisense plants: blue diamond. Data represent means ± standard error (SE), n = 4–5.
The in vivo catalytic turnover rate of Rubisco, which was modeled from A250 and the carbamylated active Rubisco content according to Yamori and von Caemmerer (2009), was similar among wild-type, overexpressing plants and antisense plants at any temperatures tested (Table S1). This finding demonstrates that the differences in Rubisco activation state quantitatively accounted for differences in observed changes in CO2 assimilation rate between wild-type and transgenic plants.
Role of Rubisco activase in limiting non-steady-state photosynthesis after an increase or decrease in light intensity
To investigate the role of Rubisco activase in the regulation of non-steady-state photosynthesis, changes in photosynthetic parameters were measured at 250 μmol mol−1 CO2 concentration when A250 was limited by Rubisco activity under high light (Woodrow and Mott, 1989; Woodrow et al., 1996; Mott et al., 1997). When rice leaves maintained for a period under low light of 60 μmol photons m−2 s−1 were subjected to high light of 1500 μmol photons m−2 s−1, A250 or ETR250 rapidly increased during the first few minutes, and then increased more slowly to a final steady-state rate (Figure 4). The initial rate after the application of the high light intensity was different among wild-type, Rubisco activase-overexpressing, and antisense plants. The speed by which A250 and ETR250 change following an increase in light intensity were much faster in Rubisco activase-overexpressing plants compared with in wild-type, and the level of NPQ in overexpressing plants tended to be lower than in wild-type. On the other hand, in antisense plants, the speed by which A250 and ETR250 change following an increase in light intensity was slower than in wild-type, and NPQ in antisense plants was greater than in wild-type. These results indicate that during the first few minutes after a change in light intensity, activase-overexpressing plants would be able to use more light energy to drive electron transport to generate ATP and NADPH without dissipation of excess energy. On the other hand, antisense plants would have to dissipate a large amount of light energy that could not be used for CO2 assimilation.
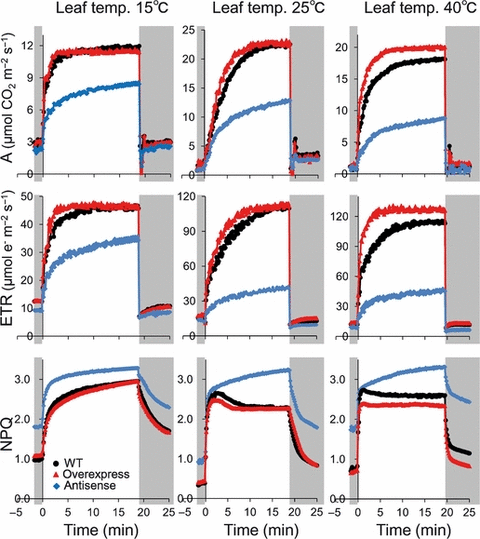
Response of CO2 assimilation rate (A), electron transport rate estimated from chlorophyll fluorescence (ETR) and non-photochemical quenching (NPQ) at 15, 25 and 40°C after an increase or decrease in light intensity. To measure the response of photosynthesis at 250 μmol mol−1 CO2 concentration to an increased or decreased light intensity, plants were first allowed to reach a steady-state rate of photosynthesis at 1500 μmol photons m−2 s−1 for 30 min, and were subsequently placed for 40 min at the initial low level of 60 μmol photons m−2 s−1. The leaves were then re-irradiated at the high light intensity of 1500 μmol photons m−2 s−1 and photosynthetic measurements were recorded. After the analysis of the time course of photosynthetic activation, the leaves were again placed at an initial low level of 60 μmol photons m−2 s−1 and photosynthetic measurements were recorded. Wild-type: black circle, activase-overexpressing plants: red triangle, antisense plants: blue diamond. Values are the mean; n = 4–5.
The intercellular CO2 concentration (Ci) changed very little during the first 10 min after an increase in light intensity. However, to account for effects of changes in Ci, A250 was normalized to a constant Ci of 250 μmol mol−1 (Figure S3, Woodrow and Mott, 1989). These normalized data were plotted as the natural logarithm of the difference between the final steady-state rate and the rate at each time point (Figure S3). The linear portion of this semi-logarithmic plot reflects an exponential phase versus time, in which photosynthesis is considered to be primarily limited by Rubisco activity (Woodrow and Mott, 1989). Therefore, the rate constant for Rubisco activation can be deduced from the slope of the linear phase of the photosynthetic induction obtained with gas-exchange analysis. The apparent rate constant for photosynthetic induction in wild-type was greater than in antisense plants, but was lower than the rate in overexpressing plants (Figure 5). This situation indicated that wild-type plants show a faster rate of Rubisco activation than antisense plants, but a slower rate of Rubisco activation than overexpressing plants. The relaxation time, which is an indication of the time required to complete activation of photosynthesis, was then calculated from the rate constant for photosynthetic induction (Woodrow and Mott, 1989). At any leaf temperature, the relaxation times in wild-type plants were shorter than in antisense plants and longer than in overexpressing plants (Table S2), which indicated that the rate to reach the final steady state in wild-type was faster than in antisense plants and slower than in overexpressing plants.
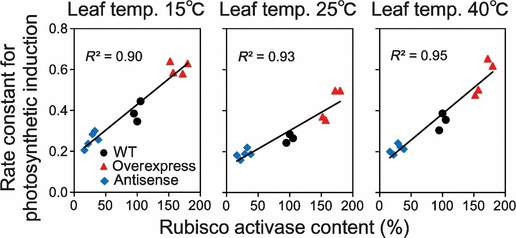
Rate constant for photosynthetic induction as a function of Rubisco activase content at 15, 25 and 40°C. The rate constant for photosynthetic induction was estimated from the response of CO2 assimilation rate after an increase in light intensity (Figure 4). The rates of photosynthesis were normalized to a Ci of 250 μmol mol−1 in order to account for the effects of changes in Ci (Woodrow and Mott, 1989). These normalized data were then plotted as the natural logarithm of the difference between the final steady-state rate (Af) and the rate (A) at each time point (Figure S3). The slope of the linear phase indicates an apparent rate constant for photosynthetic induction. A greater rate constant for photosynthetic induction indicates a faster rate of Rubisco activation state after an increase in light intensity (Woodrow and Mott, 1989). The relaxation time, which is an indication of the time required to complete activation of photosynthesis, was then calculated from the rate constant for photosynthetic induction (Woodrow and Mott, 1989; Table S2). Wild-type: black circle, activase-overexpressing plants: red triangle, antisense plants: blue diamond. Values are the mean; n = 4–5.
Changes in Rubisco activation state following an increase in light intensity were analyzed after exposure of a leaf to high light for various time periods (Figure 6). Two to four min after exposure to high light, the Rubisco activation state in overexpressing plants was greater than in the wild-type, irrespective of the leaf temperature. In addition, the Rubisco activation state reached the steady state substantially faster in overexpressing plants than in wild-type. After 20 min, Rubisco activation state in wild-type and overexpressing plants remained constant, but continued to increase for a much longer time period in antisense plants. Thus, a final steady-state rate was achieved much faster in overexpressing plants than in wild-type and slower in antisense plants than in wild-type (Figure 6). The increase in Rubisco activation state with time after an increase in light intensity was approximately linear for the first 4 min (Figure 6). The slope of a linear regression fitted to this region showed that the rate of Rubisco activation after an instantaneous increase in light intensity was 13.0% greater in overexpressing plants than in wild-type plants, and was 58.1% smaller in antisense plants than in wild-type. These are similar trends to those observed for the light activation of A250 and ETR250 (Figure 4).
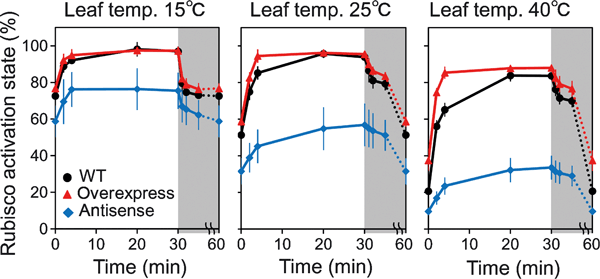
Response of the Rubisco activation state at 15, 25 and 40°C after an increase or decrease in light intensity. The light treatment is the same as that in Figure 4. Rubisco activation state was measured at: (i) the initial low level of 60 μmol photons m−2 s−1; (ii) 2 min after a rapid increase in light intensity at 1500 μmol photons m−2 s−1; (iii) 4 min after a rapid increase in light intensity at 1500 μmol photons m−2 s−1; (iv) 20 min after a rapid increase in light intensity at 1500 μmol photons m−2 s−1; (v) 30 min after a rapid increase in light intensity at 1500 μmol photons m−2 s−1; (vi) 1 min after a rapid decrease in light intensity at 60 μmol photons m−2 s−1; (vii) 2 min after a rapid decrease in light intensity at 60 μmol photons m−2 s−1; (viii) 5 min after a rapid decrease in light intensity at 60 μmol photons m−2 s−1; and (ix) 30 min after a rapid decrease in light intensity at 60 μmol photons m−2 s−1. Wild-type: black circle, activase-overexpressing plants: red triangle, antisense plants: blue diamond. Data represent means ± standard error (SE), n = 4–5.
When rice leaves maintained under high light of 1500 μmol photons m−2 s−1 were subjected to low light of 60 μmol photons m−2 s−1, A250 and ETR250 rapidly decreased to a relatively low and constant level (Figure 4). The response of A250 and ETR250 following this decrease in light intensity was similar among wild-type, overexpressing, and antisense plants. However, there were differences in the response of the Rubisco deactivation state (Figure 6). At any leaf temperature, overexpressing plants maintained a higher level, and antisense plants a lower level, of Rubisco activation state, after a decrease in light intensity as well as at the onset of the high light treatment. In the present study, we used transgenic plants that overexpress the β form of maize Rubisco activase, which is non-redox regulated (Zhang et al., 2002). Thus, at low light, the Rubisco activation state would be maintained at a higher level in transgenic plants compared with the level in the wild-type (Figure 6).
Discussion
At any leaf temperatures, the speed by which Rubisco activation state and CO2 assimilation change following an increase in light intensity changed markedly faster in plants overexpressing maize Rubisco activase compared to wild-type plants (4, 6). In addition, these parameters changed more slowly in antisense plants with reduced levels of Rubisco activase (4, 6). In contrast to this role of Rubisco activase in the regulation of photosynthesis following changes in light intensity, relatively large variations in Rubisco activase had little effects on steady-state photosynthesis below 25°C (Figure 2). Only at high temperature (40°C) could an effect of Rubisco activase on the steady-state photosynthesis be distinguished (Figure 2). Thus, we conclude that Rubisco activase plays an important role in the regulation of non-steady-state photosynthesis at all leaf temperatures and, to a lesser extent, has an effect on steady-state photosynthesis at moderately high temperatures.
Below moderate leaf temperatures (i.e. 25°C), the observed effect of reduced activase levels on steady-state photosynthesis is consistent with previous studies, which showed that the light-saturated CO2 assimilation rate in antisense tobacco plants (Nicotiana tabacum) with reduced activase was similar to that in wild-type unless the activase content was markedly reduced (Jiang et al., 1994; Mate et al., 1996; Eckardt et al., 1997; He et al., 1997; Yamori and von Caemmerer, 2009). On the other hand, at high temperature of 40°C, Rubisco activation state and CO2 assimilation rate at 40°C decreased slightly with more modest decreases in Rubisco activase content as has been observed in the present study (Yamori and von Caemmerer, 2009; Figure 2). It has also been reported that CO2 assimilation rates and plant growth were improved under heat stress in transgenic Arabidopsis expressing thermotolerant Rubisco activase isoforms (Kurek et al., 2007; Kumar et al., 2009). These results support the view that Rubisco activase activity would partly limit steady-state Rubisco activation and, therefore, the CO2 assimilation rates at high temperatures. However, it should be noted that the extent of contribution of Rubisco activase to the regulation of steady-state photosynthesis at high temperature could be different depending on growth temperature (Yamori and von Caemmerer, 2009) and/or plant species (Yamori et al., 2010b, 2011a).
In contrast to the effects on steady-state photosynthesis, Rubisco activase played an important role in the regulation of non-steady-state photosynthesis at any leaf temperature. The contribution of Rubisco to the photosynthetic induction response can be determined by the rates of Rubisco activation and deactivation. The rate of Rubisco activation following an increase in light intensity was slower in wild-type than in activase-overexpressing plants, and was more rapid in wild-type than in antisense plants (Figure 6). On the other hand, there were only small differences in the rate of Rubisco deactivation after a decrease in light intensity among wild-type, activase-overexpressing and antisense plants. However, the Rubisco activation state at low light was different among wild-type, activase-overexpressing and antisense plants. Because antisense plants had a lower Rubisco activation state at the onset of the high light treatment, the photosynthetic induction in these plants was more limited by the rate of the change in Rubisco activation state compared with wild-type. On the other hand, the higher activation state in activase-overexpressing plants at the onset of high light allowed for a faster increase in CO2 assimilation rate compared to the wild-type. Taken together, the present study indicates that rapid regulation of the Rubisco activation state by Rubisco activase following an increase in light intensity and/or the maintenance of a high Rubisco activation state under low light conditions could contribute to greater potential for increases in CO2 assimilation rate following an increase in light intensity.
Activation of Rubisco following an increase in light intensity is typically complete within 10 min (Woodrow and Mott, 1989). Leaves within natural plant canopies experience highly variable light environments over the course of a day due to changes in leaf angle, cloud cover, and overshadowing canopy cover. For species growing in environments where a substantial fraction of the total daily irradiance is received as sunflecks (e.g. understory environments), CO2 assimilation rates might rarely approach steady state. Therefore, there would be some advantage for such species to have a relatively high amount of Rubisco activase to: (i) activate Rubisco more rapidly following an increase in light intensity; and/or (ii) maintain a higher activation state of Rubisco at low light, as activation of Rubisco limits CO2 assimilation during photosynthetic induction. However, it should be noted that maintaining high levels of Rubisco activation under low light might come at a cost due to ATP hydrolysis by Rubisco activase (Portis, 2002, 2003). Further research is necessary to elucidate the importance of Rubisco activation/deactivation at low light.
It has been demonstrated that the rate of photosynthetic induction is faster in shade plants than in sun plants (Ögren and Sundin, 1996), as shade plants of forest understory environment are likely to experience the most variable light conditions (e.g. sunflecks; Pearcy, 1990). The results indicated that the slow response of sun plants was the result of lower rates of both Rubisco activation and stomatal opening. In addition, Ernstsen et al. (1997) showed that impatiens (Impatiens walleriana) showed no acclimation response of rate of Rubisco activation and deactivation irrespective of growth-light conditions (i.e. under sun, fluctuating light, and shade conditions), whereas basil (Ocimum basilicum) modulated the rate of Rubisco deactivation in response to growth-light conditions. This situation suggests that differences in Rubisco deactivation rate are important in the adaptation of the light conditions in basil. Moreover, the initial rate of Rubisco activation following an increase in light intensity, estimated by the method of Mott et al. (1997), differs between plant species: for example, 3.5–4.5 μmol Rubisco sites m−2 min−1 for Arabidopsis (Mott et al., 1997), 3.5–5.0 μmol Rubisco sites m−2 min−1 for tobacco (Hammond et al., 1998) and 8–10 μmol Rubisco sites m−2 min−1 for rice (Fukayama et al., 1998; this study). Thus, inherent ability for the photosynthetic induction response could be different depending on plant species. For the first step of photosynthetic induction, (e.g. within 2 min) the chloroplast electron transport system would be the major limiting factor (Pearcy, 1990). In particular, cyclic electron transport (Tikkanen et al., 2010) and water-water cycle (Makino et al., 2002) play an important role in this phase of photosynthetic induction. As both electron flows generate ΔpH across the thylakoid membranes, it is possible that they function as an ATP supplier for Rubisco activase during the early phase of photosynthetic induction. Therefore, it would be interesting to analyze differences in the ratio of Rubisco to Rubisco activase content as well as the role of the electron transport system in influencing photosynthetic regulation, depending on prevailing light conditions and plant species.
Considering sunlight events may last for hours in more open canopies with no cloud cover, CO2 assimilation rates would approach steady state. Under these conditions, different biochemical processes would be the major limiting factors affecting steady-state CO2 assimilation at high light (e.g. Rubisco and chloroplast electron transport, Yamori et al., 2006a, 2010b, 2011b). Thus, the optimal allocation of protein between Rubisco and Rubisco activase depends on the growth conditions, because of the interaction between Rubisco and Rubisco activase under both steady-state and non-steady-state conditions. Under fluctuating light conditions, a greater allocation of nitrogen to Rubisco activase would be advantageous for total daily carbon fixation, whereas under constant light, a smaller allocation of nitrogen to Rubisco activase would be advantageous. However, under high temperature, the situation would be slightly more complex, as under such conditions Rubisco activase may somewhat limit the steady-state CO2 assimilation rate (Figure 2). Thus, considering the enormous variety of natural light environments, an optimal allocation of nitrogen to Rubisco activase would be different depending on the prevailing light environment and/or growth temperature. Furthermore, an additional role for activase has been hypothesized in protecting the thylakoid protein synthesis machinery under heat stress (Rokka et al., 2001; Chen et al., 2010). Therefore, future research should consider the importance of the multiple functions of Rubisco activase.
Conclusion
Below moderate leaf temperature (e.g. 25°C), Rubisco activase was not a limiting factor for the steady-state Rubisco activation state and CO2 assimilation rate, whereas at higher temperatures, Rubisco activase was a limiting factor. However, the enhancement of photosynthesis by an increased Rubisco activase expression was small. On the other hand, Rubisco activase was important for achieving rapid activation of Rubisco and, therefore, higher rates of CO2 assimilation, following an increase in light intensity at any leaf temperature. Thus, it is concluded that Rubisco activase plays an important role in the regulation of non-steady-state photosynthesis at any leaf temperature and, to a lesser extent, of steady-state photosynthesis at moderately high temperatures. Our results indicate that a selective enhancement of Rubisco activase capacity could result in more rapid enhanced rates of Rubisco activation and CO2 assimilation after an increase in light intensity. This situation would be especially advantageous in natural environments where irradiance often fluctuates.
Experimental procedures
Plant materials and growth conditions
Rice (Oryza sativa L. cv Nipponbare) plants and the T1 progeny of several primary transformants with reduced amounts of Rubisco activase (Masumoto et al., 2012) and the T2 progeny of several primary transformants with overexpressing maize Rubisco activase (Fukayama et al., 2012) were grown hydroponically in an environmentally controlled growth chamber. The chamber for all the plants was operated with a day/night temperature of 25/22°C, a photosynthetically photon flux density (PPFD) of 600 μmol m−2 s−1, a 12 h photoperiod and a CO2 concentration of 1500 μmol mol−1. The high CO2 concentration for the plant growth was selected in order to minimize differences in the growth rates of plants and the capacity of CO2 assimilation at the growth condition (Yamori and von Caemmerer, 2009). The hydroponic solution used for rice was previously described by Yamori et al. (2011a), and a nitrogen concentration of 3.0 mm was used (1.5 mm NH4NO3). The solution was renewed once a week.
Gas-exchange and Chl fluorescence measurements
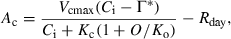 (1)
(1)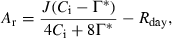 (2)
(2)Chlorophyll a fluorescence was also determined simultaneously with gas exchange during the temperature response measurements, using an attached portable Chl fluorometer (Mini-PAM, Waltz, Effeltrich, Germany; http://www.walz.com). The quantum yield of photosystem II [ΦPSII = (Fm′ – F′)/Fm′] and non-photochemical quenching [NPQ = (Fm – Fm′)/Fm′] were calculated. The electron transport rate (ETR) was calculated as ETR = 0.5 × absI × ΦPSII, where 0.5 is the fraction of absorbed light reaching PS II and absI is absorbed irradiance taken as 85% of the incident irradiance (Genty et al., 1989).
For the investigation of the time course of photosynthetic activation, rice leaves were illuminated for 30 min with high light (1500 μmol photons m−2 s−1) and were subsequently placed for 40 min at an initial low level of 60 μmol photons m−2 s−1. The leaves were then re-irradiated with high light (1500 μmol photons m−2 s−1), and gas-exchange measurements were recorded at intervals of 10 s for 25 min after the light intensity was increased. For investigation of the time course of photosynthetic deactivation, the photosynthetic rate in leaves was measured over time following a decrease in light intensity from 1500 to 60 μmol photons m−2 s−1, after the analysis of the time course of photosynthetic activation.
The A was normalized to a Ci of 250 μmol mol−1, to account for changes in A resulting from changes in Ci. Normalization assumed that the relationship between A and Ci is linear and passes through the CO2 compensation point (Woodrow and Mott, 1989). The CO2 compensation point was determined from A–Ci curves at several leaf temperatures in all the species (Table S1). Relaxation time was calculated following the method described by Woodrow and Mott (1989). The A was plotted as the natural logarithm of the difference between the final steady-state rates and the rate at each time point. The apparent rate constant of photosynthetic activation was determined from the slope of the linear phase that occurred from 0.5 to 5.0 min after the increase of light intensity. The slope of the linear phase, a few minutes after the change of light intensity, indicates the apparent rate constant for photosynthetic induction (Woodrow et al., 1996). The relaxation time was calculated as the reciprocal of the apparent rate constant. Thus, this value is an indication of the time required to complete activation of photosynthesis.
Determinations of chlorophyll, nitrogen, cytochrome f, Rubisco and Rubisco activase
Immediately after the measurements of gas exchange, leaf samples were taken and immersed in liquid nitrogen and stored at −80°C until determinations of photosynthetic components. The contents of leaf nitrogen, chlorophyll and Rubisco were quantified as described by Yamori et al. (2011a). Proteins were separated by SDS-PAGE and transferred to a polyvinylidene difluoride membrane. Rubisco activase was quantified by immunoblotting with anti-activase antibody (Fukayama et al., 2012). The content of cytochrome f, which is considered to be a key rate-limiting step of electron transport (Yamori et al., 2011b), was quantified by immunoblotting with anti-cytochrome f antibody (Yamori et al., 2011c).
Rubisco activation state
For analysis of the time course of Rubisco activation after illumination, leaf samples were cut from a single leaf at various times following illumination, rapidly frozen in liquid N2 and stored at −80°C. For analysis of Rubisco activation state under steady-state conditions, samples used for the Rubisco activation assay were collected from a leaf equilibrated under steady-state conditions in a gas-exchange chamber for at least 20 min. Rubisco activation state in leaf samples was determined with NADH-oxidation by measuring the initial extractable Rubisco activity and expressing it as a fraction of the total Rubisco activity after activation with MgCl2 and CO2 (Yamori et al., 2006b, 2011a).
Acknowledgements
We would like to thank Dr D. Tholen for valuable comments on the manuscript. This work was supported by grants from the Japan Society for the Promotion of Science (Postdoctoral Fellowships to W.Y. and Scientific Research No. 20380041 to A.M. and No. 20580014 to H.F.), from the Ministry of Education, Culture, Sports, Science and Technology, Japan (Scientific Research on Innovative Area No. 21114006 to A.M. and No. 22114511 to H.F.) and from the Ministry of Agriculture, Forestry, and Fisheries of Japan (Genomics for Agricultural Innovation, GPN 0007).




