Biolistic co-transformation of the nuclear and plastid genomes
Summary
Particle gun-mediated (so-called ‘biolistic’) transformation represents a universal genetic transformation technology that is widely applied in nearly all groups of organisms. The mechanism of how accelerated DNA-coated particles, after their entry into the cell, deliver the foreign DNA to the target compartment is not known. Here we have studied this process in plants by performing co-transformation experiments with vectors targeted to two different cellular compartments, the nucleus and the plastids (chloroplasts). We find that coating of particles with both plastid and nuclear transformation vectors can result in co-transformation of chloroplasts and the nucleus. In contrast, mixing of particles coated individually with the vectors does not produce co-transformed plants. Our data suggest that a single DNA-coated particle can transform more than one compartment of the plant cell, opening up the possibility to generate doubly transgenic plants in one step. Importantly, co-transformation can also be obtained in the absence of selection, thus providing a method to produce marker-free transgenic genomes. In addition, our findings raise the possibility of occasional inadvertent co-transformation of two genomes and, therefore, have important implications for the molecular characterization and regulation of transgenic plants.
Introduction
Particle gun-mediated (‘biolistic’) transformation makes use of physical processes to transfer DNA into living cells. The method is independent of viruses or bacteria, so that limitations related to pathogen–host relationships (as, for example, in Agrobacterium-mediated transformation or adenovirus-mediated transfection) do not exist. Owing to the absence of biological constraints (at least until the transforming DNA has entered the cell), biolistic transformation is also not limited to specific cell types, species or genotypes. Moreover, there are no special vector requirements and foreign DNA of any size, sequence or conformation can be delivered into cells by the biolistic process (Altpeter et al., 2005).
Due to the simple physical principle it is based on, biolistic transformation represents a universal genetic transformation technology and has successfully been used in nearly all groups of organisms (Klein et al., 1987; Johnston et al., 1988; Schiedlmeier et al., 1994; Altpeter et al., 2005). In eukaryotes, it does not only allow the transformation of the nuclear genome, but also provides the unique opportunity of stably transforming the small genomes of the two DNA-containing cell organelles, mitochondria and plastids (Boynton et al., 1988; Johnston et al., 1988; Svab et al., 1990; Randolph-Anderson et al., 1993).
The presence of two or even three genomes in eukaryotic cells and the physical nature of the biolistic process offer the possibility of transforming more than one cellular compartment in a single experiment. In yeast mitochondrial transformation, this approach is used to preselect transformants for a nuclear auxotrophic marker followed by selection for restoration of respiratory activity (Johnston et al., 1988; Bonnefoy and Fox, 2007). It is not clear how DNA could be delivered simultaneously to two cellular compartments. It seems exceedingly unlikely that two particles hit one and the same cell, with one of them delivering DNA into the nucleus and the other one into an organelle due to the very low proportion of hit cells in a biolistic experiment. Alternatively, a single particle could deliver DNA into two compartments, perhaps, even without penetrating both target compartments. In this scenario, the transforming DNA would be stripped off the particle upon penetration of the cell and subsequent gene delivery would occur through active or passive DNA uptake mechanisms, as shown to exist, for example, in plant mitochondria (Koulintchenko et al., 2003).
In plants, there is a good correlation between nuclear transformation and the physical presence of a particle in the nucleus. However, a small fraction of transiently transformed cells that expressed the reporter gene β-glucuronidase were reported to lack a detectable particle in the cell (Hunold et al., 1994). We, therefore, reasoned that gene delivery may be possible without the DNA-coated particle becoming stuck in the target compartment and, perhaps, even without physically hitting the target compartment. If this situation were the case, biolistic transformation of plant cells could result in occasional co-transformation of two compartments, a possibility that is usually not considered and usually not rigorously tested for in transgenic experiments employing particle bombardment.
Here we have investigated the possibility of simultaneous co-transformation of two genomes in the plant cell in biolistic transformation experiments. We show that coating of a particle with DNA constructs destined for two different genomes in the plant cell can produce plastid–nuclear co-transformed plants, whereas mixing of particles coated individually with the vectors does not produce co-transformed plants. Our findings provide a method for rapidly generating doubly transgenic plants with genetic alterations in both the nucleus and the chloroplast. In addition, our results shed new light on the mechanisms underlying the biolistic transformation process and have important implications for the molecular analyses required to characterize transgenic plant lines.
Results
Selection of plastid–nuclear co-transformants
We used two transformation vectors targeting two different genomes in the plant cell: the nuclear genome and the plastid (chloroplast) genome to explore the possibility of co-transforming two genomes with a single DNA-coated particle (Figure 1a). The nuclear transformation vector, pNKY, contains the kanamycin resistance gene nptII and the gene for the yellow fluorescent protein (YFP). Both transgenes are driven by nuclear (eukaryotic-type) expression signals (Figure 1a). The plastid transformation vector, pPSG, contains the spectinomycin resistance gene aadA (Svab and Maliga, 1993) and the gene for the green fluorescent protein (GFP), both of which are under the control of plastid (prokaryotic-type) expression signals (Stegemann and Bock, 2009; Figure 1a). Plastid–nuclear co-transformation experiments in tobacco (Nicotiana tabacum) were performed by coating gold particles with a mixture of pNKY and pPSG and subsequently selecting for resistance to: (i) kanamycin; (ii) spectinomycin; or (iii) kanamycin + spectinomycin. These experiments will be referred to subsequently as plasmid mixture experiments. To also test the possibility of obtaining co-transformation by two particles hitting the same cell, gold particles were coated separately with pNKY and pPSG and mixed immediately prior to bombardment (particle mixture experiment). For all four sets of experiments, approximately 3000 bombarded leaf pieces were subjected to antibiotic selection (Table 1).
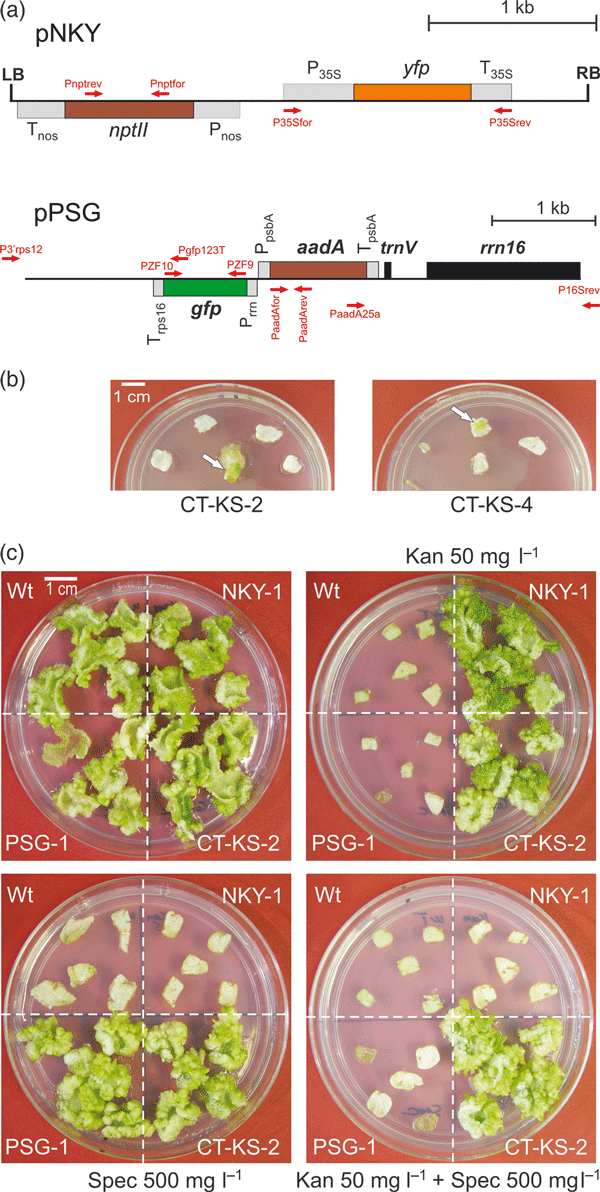
Plastid–nuclear co-transformation experiments.(a) Physical maps of the transformation vectors used in this study (Stegemann and Bock, 2009). Genes above the line are transcribed from the left to the right, genes below the line are transcribed in the opposite direction. Nuclear transformation vector pNKY contains the kanamycin resistance gene nptII (driven by the nos promoter and terminator sequences, Pnos and Tnos) and the fluorescent reporter gene yfp (driven by the CaMV 35S promoter and terminator sequences, P35S and T35S). Plastid transformation vector pPSG contains the spectinomycin resistance gene aadA (driven by the plastid psbA promoter and terminator sequences, PpsbA and TpsbA) and the fluorescent reporter gene gfp (driven by the plastid rRNA operon promoter and the rps16 terminator, Prrn and Trps16). The location and orientation of primers used for the molecular characterization of transgenic lines are indicated by arrows.(b) Selection of plastid–nuclear co-transformants on medium containing both spectinomycin and kanamycin. Two co-transformants, line CT-KS-2 and line CT-KS-4, are exemplarily shown.(c) Resistance tests of putative plastid–nuclear co-transformants. Leaf pieces from a wild-type plant (Wt), a nuclear transformant generated with vector pNKY (line NKY-1), a plastid transformant generated with vector pPSG (line PSG-1) and a co-transformant (line CT-KS2) were exposed to regeneration medium without antibiotics (upper left panel), with kanamycin (Kan; upper right panel), spectinomycin (Spec; lower left panel) or both kanamycin and spectinomycin (lower right panel). Antibiotic concentrations are indicated.
| Particle preparation | Primary selection | Selected leaf pieces | Primary resistant shootsa | Kan resistant in additional regeneration | Spec resistant in additional regeneration | Spec + Strep resistant in additional regeneration | Kan + Spec resistant in additional regeneration | Transgenic lines confirmed by PCR | YFP positive | GFP positive |
|---|---|---|---|---|---|---|---|---|---|---|
| Plasmid mixture | Kan | 2784 | 130 | 106 | 0 | n.a. | 0 | 106 (nptII) | 55b | 0 |
| Spec | 2952 | 74 | 1 | 74 | 45 | 1 | 44 (aadA) 1 (nptII + aadA) | 1 | 45 | |
| Kan + Spec | 3396 | 4 | 3 | 3 | 3 | 3 | 3 (nptII + aadA) | 3 | 3 | |
| Particle mixture | Kan + Spec | 3180 | 0 | – | – | – | – | – | – | – |
- n.a., not analyzed; Kan, kanamycin; Spec, spectinomycin; Strep, streptomycin.
- aNumbers include escapes and spontaneous spectinomycin-resistant mutants.
- bNumber of YFP-positive lines (evidenced by microscopy) is lower than number of nptII-positive lines (assayed by PCR), because some transformants may have incorporated the nptII gene, but not the yfp gene into the nuclear genome. Also, YFP fluorescence may be below the detection limit in some transgenic lines.
Selection on medium containing either kanamycin or spectinomycin resulted in a large number of antibiotic-resistant candidate transformants, as expected (Table 1). Double selection for kanamycin and spectinomycin in the particle mixture experiment yielded no resistant cell lines, in agreement with the theoretical consideration that the probability of two particles hitting the same cell is close to zero. Interestingly, double selection in the plasmid mixture experiment produced three strongly resistant cell lines (Figure 1b and Table 1; an initially obtained fourth line was later identified as false positive; see below).
Analysis of transgenic lines
Tissue explants from all primary antibiotic-resistant lines were subjected to additional regeneration tests on selective medium to eliminate escapes and spontaneous spectinomycin-resistant mutants (Bock, 2001; Svab and Maliga, 1993; Table 1). Resistance tests of the lines obtained by double selection in the plasmid mixture experiment on media with combinations of antibiotics confirmed that all three lines displayed both strong kanamycin resistance (conferred by the nuclear nptII marker gene) and strong spectinomycin/streptomycin resistance (conferred by the plastid aadA marker gene; Figure 1c; Table 1). Molecular analyses employing polymerase chain reactions (PCR) with transgene-specific primer pairs revealed that the lines harbored all four transgenes (nptII, yfp, aadA and gfp), demonstrating that they were indeed co-transformed (Figure 2a; CT-KS lines: co-transformants obtained by selection for kanamycin and spectinomycin). PCR assays using primers that bind outside of the flanking plastid DNA sequences in the transformation vector (Figure 1a) demonstrated integration of the transgenes into the plastid genome via homologous recombination, as expected (Figure 2b).
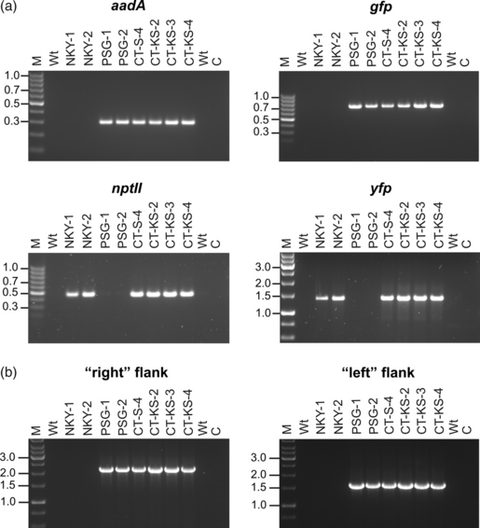
Analysis of plastid–nuclear co-transformants.(a) PCR assays to test for the presence of all four transgenes in plastid–nuclear co-transformants. The transgene assayed for is given above each ethidium bromide-stained agarose gel. Wt: wild type; M: DNA size marker (fragment sizes given in kb); C: buffer control.(b) PCR assay to test for integration of the transgenes into the plastid genome by homologous recombination. Transgene-specific primers were combined with primers located outside of the flanking plastid sequences in the transformation vector (see Experimental Procedures and Figure 1a). ‘Left flank’ and ‘right flank’ are labeled according to Figure 1(a).
Analysis of GFP and YFP fluorescences and their subcellular localizations by confocal laser-scanning microscopy revealed GFP fluorescence in the chloroplast and YFP fluorescence in the nucleocytosolic compartment in all CT-KS lines (Figure 3 and Table 1). These data indicated that both fluorescent reporter genes are expressed in the targeted compartments and provided additional strong evidence for plastid–nuclear co-transformation.
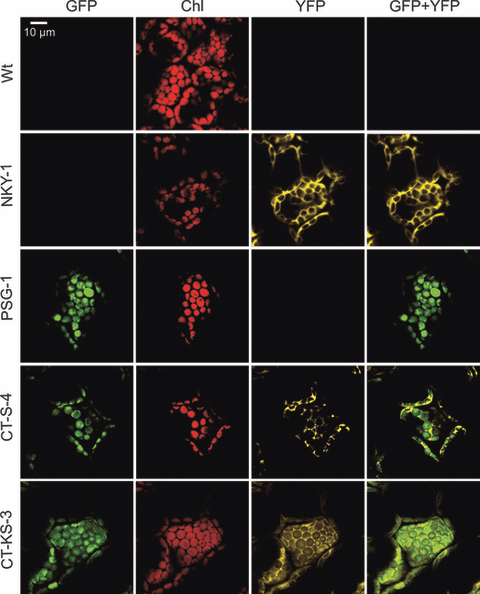
Analysis of expression and subcellular localization of the fluorescent reporter proteins.The wild type (Wt), an NKY nuclear transformant, a PSG plastid transformant and two plastid–nuclear co-transformants were comparatively assayed for GFP fluorescence, chlorophyll fluorescence (Chl), YFP fluorescence and the overlay of GFP and YFP fluorescences (GFP + YFP). One of the co-transformants shown was obtained by spectinomycin selection (CT-S-4), the other by selection for spectinomycin and kanamycin resistance (CT-KS-3). Cells of the two co-transformants exhibit both GFP fluorescence in the chloroplast and YFP fluorescence in the nucleocytosolic compartment, strongly suggesting that plastid–nuclear co-transformation has occurred.
Co-transformation in the absence of selection
We next wanted to test if plastid–nuclear co-transformants are also obtained if the primary transgenic line is selected only on one antibiotic, as normally done in transformation experiments. To this end, we analyzed all transgenic lines obtained by kanamycin selection or spectinomycin selection in our plasmid mixture experiments. While from 106 (nuclear-transgenic) lines selected on kanamycin, none was positive for the plastid transgenes, one out of 45 (plastid-transformed) lines selected on spectinomycin also harbored the two nuclear transgenes nptII and yfp and displayed nucleocytosolic YFP fluorescence in addition to the chloroplast GFP fluorescence (Table 1; Figure 2). This line is referred to as CT-S (co-transformant obtained by selection for spectinomycin). Identification of plastid–nuclear co-transformed lines by kanamycin selection may be somewhat less likely than by spectinomycin selection, because: (i) plastid transformation frequency is usually lower than nuclear transformation frequency; and (ii) the plastid genome occurs in high copy numbers and segregates randomly in the absence of selection pressure. This situation can result in loss of the transformed plastid genomes during subsequent cell divisions in the absence of spectinomycin selection (Lutz and Maliga, 2008; Rogalski et al., 2008).
Stable inheritance of co-transformed genomes
To provide additional genetic evidence for plastid–nuclear co-transformation and, at the same time, confirm integration of the pNKY vector into the nuclear genome and integration of the pPSG vector into the chloroplast genome, regenerated plants were grown to maturity, selfed and also reciprocally crossed with wild-type plants. Inheritance assays were then performed by germinating seeds on antibiotic-containing medium (Figure S1). Plastid genes and transgenes display a uniparentally maternal mode of inheritance (Mogensen, 1996; Ruf et al., 2007; Hagemann, 2010), whereas nuclear transgenes are inherited according to Mendel’s laws. When seeds were germinated in the presence of spectinomycin, all crosses with CT-S or CT-KS lines as maternal parent yielded uniformly resistant seedlings (Figure S1). In contrast, crosses between wild-type plants and CT-S or CT-KS lines as paternal parent gave rise to seedlings that were uniformly sensitive to spectinomycin, demonstrating that the aadA transgene is excluded from pollen transmission and, thus confirming the location of the aadA transgene in the plastid genome. When seeds were plated on medium with kanamycin, the nuclear antibiotic resistance gene nptII showed the expected Mendelian segregation irrespective of the directionality of the cross (Figure S1), which confirmed its localization in the nuclear genome.
Discussion
In this work, we have investigated the possibility to achieve co-transformation of two physically isolated genomes of the plant cell, the nuclear genome and the chloroplast genome. Our data demonstrate that particle gun-mediated transformation can indeed result in plastid–nuclear co-transformation. Importantly, co-transformation can be obtained even in the absence of double selection, indicating that in standard transformation experiments inadvertent co-transformation of two genomes can occur. Although co-transformation of two compartments (nucleus and mitochondrion) has been achieved by plasmid mixture experiments in yeast (Johnston et al., 1988), co-transformation in the absence of selection has, to our knowledge, not been demonstrated before. In fact, yeast mitochondrial transformation even appears to be dependent upon prior selection for a nuclear marker (Johnston et al., 1988). Another important difference between the yeast system and our work is that, in yeast, the selection for co-transformation is performed in two successive steps: after an initial selection for nuclear transformation, the transgenic clones are replica plated and exposed to the second selection for mitochondrial transformation (Johnston et al., 1988; Bonnefoy and Fox, 2007).
Based on the co-transformation frequency determined in this work, it appears likely that, for example, a significant fraction of the plastid transformants described in the literature (in the range of at least 2–3%) contain an additional copy of the transgene cassette in the nucleus. This has important implications for the molecular characterization of transgenic plants, which is required to: (i) draw reliable conclusions about the phenotypic effects of transgenes; and/or (ii) meet the regulatory demands for commercialization. Inadvertent introduction of a transgene into a second genome of the plant cell can even result in active expression from the unintended location. Although plastid transgenes are usually equipped with promoters taken from endogenous plastid genes, at least some of these promoters can also be active in the nucleus. For example, the frequently used plastid psbA promoter is known to be also active in the nucleus (Cornelissen and Vandewiele, 1989). RFLP experiments using restriction enzyme combinations suitable to distinguish between plastid and nuclear integrants and/or outcrossing of the transgenic chloroplasts followed by analysis of a larger number of progeny plants for the presence of nuclear transgene copies by PCR (Stegemann et al., 2003) would be suitable experiments to exclude the possibility of inadvertent co-transformation of two genomes.
Our findings also suggest that, in biolistic transformation experiments, a single particle can deliver foreign genes to more than one compartment of the plant cell. This indicates that delivery of a DNA-coated particle into the target compartment is not required for transformation to occur. Previous work using transient nuclear transformation with a particle inflow gun (whereas we used the PDS-1000/He gun model) had shown that most of the transiently transformed (β-glucuronidase-expressing) cells carried a particle in their nucleus. However, there was also a small fraction of transiently transformed cells that expressed the reporter gene, but did not have a detectable particle in the nucleus (Hunold et al., 1994), suggesting that at least transient nuclear transformation is possible without a retained bullet. However, it is known that only a small fraction of transiently transformed cells possesses the potential to develop into stable transformation events. For obvious reasons, the relationship between particle-free cells and stable genetic transformation is not amenable to direct experimental investigation. Our data presented here cannot definitively exclude the possibility that either nuclear or plastid transformation require entry of the DNA-loaded particle into the target compartment. However, the observation of plastid–nuclear co-transformation in plasmid mixture experiments (but not in particle mixture experiments) suggests that at least one of the two stable transformation types must be independent of a retained bullet.
The possibility to perform a one-step co-transformation of two different genomes in the cell provides a unique and fast method for all applications that require the combined expression of transgenes in the nucleus and the chloroplasts. This situation is, for example, the case for the two-component systems that have been designed to make the expression of plastid genes and transgenes inducible (McBride et al., 1994; Lössl et al., 2005; Surzycki et al., 2007). One-step plastid–nuclear co-transformation as described here is significantly faster than sequential transformation of the two genomes or combination of lines transformed separately by crossing. Compared with sequential transformation, more leaf samples need to be bombarded to obtain co-transformation. However, as plastid transformation becomes more and more efficient (Maliga, 2004), the time advantage of co-transformation is likely to outstrip the requirement for larger-scale transformation experiments.
Finally, the possibility to obtain co-transformation in the absence of selection for a nuclear marker gene provides an elegant method to generate marker-free transgenic plants. Although several methods for the post-transformation removal of nuclear selectable marker genes have been described, each of them has its problems and limitations (Ebinuma et al., 2001; Hare and Chua, 2002). For example, site-specific recombinases, representing the most commonly used tools for selectable marker excision, do not always act in a strictly site-specific manner and can recognize cryptic target sites (with weak sequence similarity) in the genome (e.g., Corneille et al., 2003). Also, the procedures involved are time-consuming requiring specialized vector design and several rounds of crosses. Employing a co-transformation strategy, in which the selection marker is placed into the plastid genome, provides an elegant method to generate clean marker-free nuclear-transgenic plants that do not even carry a footprint of the removed selection marker (e.g., a loxP copy remaining in the genome after CRE-mediated recombinational marker excision). As plastids are maternally inherited in most crops, a simple cross using the co-transformed plant as pollen donor will subsequently eliminate the transgenic plastid genome.
In theory, our co-transformation strategy could also be used to obtain organelle transformation in the absence of selection. However, we believe that this will be much more difficult due to the high ploidy level and random segregation of organellar genomes. In the absence of selection, the transformed plastid genome copy is unlikely to displace all the many wild-type plastid genomes that are still present in the cell (Zoschke et al., 2007) and, in most instances, will simply be lost due to random genome sorting.
In summary, our data: (i) reveal new insights into the mechanism of biolistic transformation; (ii) provide a fast method for the one-step co-transformation of two different genomes in the plant cell (by direct selection for co-transformation); (iii) offer a strategy for producing marker-free transgenic plants (by performing co-transformation in the absence of selection); and (iv) raise new issues concerning the required molecular characterization of transgenic plants.
Experimental Procedures
Plant material and growth conditions
Tobacco (Nicotiana tabacum cv. Petit Havana) plants were grown under aseptic conditions on agar-solidified Murashige and Skoog (MS) medium (Murashige and Skoog, 1962) containing 30 g L−1 of sucrose. After 4 weeks of growth, young leaves were harvested and used for biolistic transformation. Regenerated shoots from transgenic lines were rooted and propagated on MS medium. To obtain seeds, transgenic plants were transferred to soil and grown to maturity under standard greenhouse conditions.
Nuclear and plastid transformation and selection of transgenic plant lines
The transformation vectors used in this study have been described previously (Stegemann and Bock, 2009). For biolistic transformation, young tobacco leaves were bombarded with plasmid DNA-coated 0.6 μm gold particles (Bio-Rad, http://www.bio-rad.com) using the DuPont PDS-1000/He biolistic gun with the Hepta Adaptor setup (Bio-Rad). For co-transformation experiments, two sets of DNA-coated gold particles were prepared. In the plasmid mixture experiments, plasmids pNKY and pPSG were mixed in a 1:1 ratio and the mixture was used to coat the particles. In the particle mixture experiments, samples of gold particles were coated individually with plasmids pNKY and pPSG and the particles were mixed in a 1:1 ratio immediately before bombardment. After biolistic bombardment, the leaves were cut into small pieces, which were placed onto the surface of the appropriate selective regeneration medium. For kanamycin selection, 50 mg L−1 of kanamycin was added to the regeneration medium. For spectinomycin selection, the regeneration medium was supplemented with 500 mg L−1 spectinomycin. For double selection, 500 mg L−1 of spectinomycin and 50 mg L−1 of kanamycin were added to the regeneration medium. Ten shots (using the Hepta Adaptor setup and bombarding a Petri dish fully covered with tobacco leaves) were performed per experiment and selection scheme. Spontaneous spectinomycin-resistant mutants were eliminated by double resistance tests on regeneration medium containing both spectinomycin and streptomycin (500 mg L−1 each; Svab and Maliga, 1993; Bock, 2001). Tissue samples from antibiotic-resistant calli or regenerating shoots were transferred onto fresh selection medium for elimination of escapes, further propagation of nuclear transformants and isolation of homoplasmic transplastomic tissue (Svab and Maliga, 1993; Bock, 2001).
DNA isolation and polymerase chain reactions
Total plant DNAs were extracted from fresh leaf tissue by a cetyltrimethyl ammoniumbromide (CTAB)-based method (Doyle and Doyle, 1990). For transgene detection, samples of total DNA were amplified in an Eppendorf thermal cycler using GoTaq® Flexi DNA polymerase (Promega, http://www.promega.com) and gene-specific primer pairs. The standard PCR program was 35 cycles of 45 sec at 94°C, 45 sec at 56–58°C, and 1–2 min at 72°C with a 4 min extension of the first cycle at 94°C and a 4 min final extension at 72°C. For specific detection of the reporter and selectable marker genes, the following synthetic oligonucleotides were used as primers: aadA: PaadAfor (5′-CGCCGAAGTATCGACTCA-3′) and PaadArev (5′-TCGCGCTTAGCTGGATAAC-3′); nptII: Pnptfor (5′-GAGGCAGCGCGGCTATC-3′) and Pnptrev (5′-GCGGTCCGCCACACCCA-3′); gfp: PZF9 (5′-TTTTCATATGAGTAAAGGAGAAGAACTT-3′) and PZF10 (5′-TTTTATTAATGATTAGTTCATCCATGCC-3′); yfp: P35Sfor (5′-GACCAAAGGGCTATTGAGAC-3′) and P35Srev (5′-CGGGGGATCTGGATTTTAGTAC-3′). To confirm transgene integration into the plastid genome by homologous recombination, transgene-specific primers were combined with primers binding to chloroplast genome sequences outside of the two flanking regions of plastid DNA present in the transformation vector (Figure 1a). The corresponding primer pairs were PaadA25a (5′-AGATCACCAAGGTAGTCGGCAA-3′)/P16Srev (5′-GCACCTTCCAGTACGGCTAC-3′) giving a PCR product of 2162 bp and Pgfp123T (5′-CAACCATTACCTGTCCAC-3′)/P3′rps12 (5′-CGAAGAGTAACTAGGACCA-3′) yielding a PCR product of 1608 bp.
Confocal laser-scanning microscopy
GFP and YFP fluorescences and their subcellular localization were determined by confocal laser-scanning microscopy (TCS SP5; Leica, http://www.leica-microsystems.com) using an argon laser for excitation (at 488 nm) and a 500–510 nm filter for detection of GFP fluorescence, a 514–527 nm filter for detection of YFP fluorescence and a 610–700 nm filter for detection of chlorophyll fluorescence.
Inheritance assays
To determine the inheritance patterns of the selectable marker genes, seeds were produced by selfing transgenic plants and by crossing them reciprocally to wild-type plants. Surface-sterilized seed samples were then germinated on synthetic medium in the presence or absence of antibiotics. Maternal inheritance of the plastid marker gene aadA was assayed by germination in the presence of spectinomycin; Mendelian inheritance of the nuclear marker gene nptII was determined by germination on kanamycin-containing medium.
Acknowledgements
We thank Drs. Mark Lohse and Daniel Karcher for providing plasmids, Claudia Hasse and Stefanie Seeger for help with plant transformation and Sandra Stegemann (all MPI-MP) for help with confocal laser-scanning microscopy. This research was supported by the Max Planck Society.




