The vacuolar transport of aleurain-GFP and 2S albumin-GFP fusions is mediated by the same pre-vacuolar compartments in tobacco BY-2 and Arabidopsis suspension cultured cells
Summary
Soluble proteins reach vacuoles because they contain vacuolar sorting determinants (VSDs) that are recognized by vacuolar sorting receptor (VSR) proteins. Pre-vacuolar compartments (PVCs), defined by VSRs and GFP-VSR reporters in tobacco BY-2 cells, are membrane-bound intermediate organelles that mediate protein traffic from the Golgi apparatus to the vacuole in plant cells. Multiple pathways have been demonstrated to be responsible for vacuolar transport of lytic enzymes and storage proteins to the lytic vacuole (LV) and the protein storage vacuole (PSV), respectively. However, the nature of PVCs for LV and PSV pathways remains unclear. Here, we used two fluorescent reporters, aleurain-GFP and 2S albumin-GFP, that represent traffic of lytic enzymes and storage proteins to LV and PSV, respectively, to study the PVC-mediated transport pathways via transient expression in suspension cultured cells. We demonstrated that the vacuolar transport of aleurain-GFP and 2S albumin-GFP was mediated by the same PVC populations in both tobacco BY-2 and Arabidopsis suspension cultured cells. These PVCs were defined by the seven GFP-AtVSR reporters. In wortmannin-treated cells, the vacuolated PVCs contained the mRFP-AtVSR reporter in their limiting membranes, whereas the soluble aleurain-GFP or 2S albumin-GFP remained in the lumen of the PVCs, indicating a possible in vivo relationship between receptor and cargo within PVCs.
Introduction
Plant cells contain functionally and morphologically distinct vacuoles: protein storage vacuoles (PSVs) and lytic vacuoles (LVs) (Paris et al., 1996; Neuhaus and Rogers, 1998; Surpin and Raikhel, 2004). Soluble vacuolar proteins such as storage proteins and hydrolytic enzymes reach vacuoles because they contain vacuolar sorting determinants (VSDs) that can be recognized by specific vacuolar sorting receptor (VSR) proteins. BP-80 was the first VSR protein identified in pea (Pisum sativum) that recognized the VSD Asn-Pro-Ile-Arg (NPIR) of cysteine protease aleurain (Kirsch et al., 1994). Similarly, the major seed storage protein 2S albumin was transported to PSV by AtVSR1 in Arabidopsis seeds (Shimada et al., 2003), whereas the transport of phaseolin and chitinase to a PSV-like compartment was achieved by the AtRMR1 receptor protein in Arabidopsis cells (Park et al., 2005) and in tobacco cells (Park et al., 2007).
Multiple vesicular transport pathways leading to vacuoles have also been demonstrated in various plant cells. For example, BP-80 and its cargo proaleurain were believed to reach the LV via clathrin-coated vesicles (CCVs) and the Golgi apparatus in plant cells (Jiang and Rogers, 1998, 2003). In contrast, the transport of storage proteins to PSVs in developing pumpkin cotyledons was shown to be mediated by the PV72 receptor, and the endoplasmic reticulum (ER)-derived precursor-accumulating (PAC) vesicles bypassing the Golgi apparatus (Shimada et al., 1997, 2002; Hara-Nishimura et al., 1998). Similar to mammalian cells and yeast, the transport of vacuolar proteins to LVs and PSVs in plant cells is also believed to be involved in an intermediate pre-vacuolar compartment (PVC) (Bethke and Jones, 2000; Lam et al., 2005; Tse et al., 2004).
The PVCs are membrane-bound intermediate organelles that serve dual roles in mediating protein traffic between the Golgi apparatus or the trans-Golgi network (TGN) and the vacuole: receiving proteins from the Golgi apparatus for further delivery to the vacuole, and recycling receptors back to the Golgi apparatus for further selective transport (Jiang and Rogers, 2003). PVCs can be defined and identified by their morphological structure, the presence of specific VSRs and their cargo proteins (Lam et al., 2005; Mo et al., 2006; Tse et al., 2004). Several proteins have been used as markers to define plant PVCs, including the pea BP-80 (Paris et al., 1997), the Arabidopsis AtELP (Ahmed et al., 1997), the Arabidopsis AtPEP12p (da Silva Conceicao et al., 1997) and VSRs (Li et al., 2002). Using VSRs and the BP-80 reporter as PVC markers, multivesicular bodies (MVBs) were identified as PVCs via immunogold electron microscope in tobacco BY-2 cells (Miao et al., 2006; Tse et al., 2004), as well as in germinating mung bean seeds (Wang et al., 2007).
The Arabidopsis genome contains seven VSR homologs (AtVSR1–AtVSR7), for which there is relatively little information about their individual subcellular localization and function in plants (Neuhaus and Paris, 2005). When the seven GFP fusions with the transmembrane domain (TMD) and cytoplasmic tail (CT) of individual AtVSR1–AtVSR7 were expressed in transgenic tobacco BY-2 cells, the seven GFP-AtVSR fusions were found to localize to PVCs, because organelles marked by GFP-AtVSR largely co-localized with the endogenous VSR proteins, as detected by the VSRat-1 antibodies (Miao et al., 2006). As TMD and CT were sufficient and specific for VSR targeting in plant cells (Jiang and Rogers, 1998), these results indicated that the seven AtVSRs were likely to be localized to PVCs in Arabidopsis, even though the final proof needs to come from a future study of individual AtVSRs in Arabidopsis cells or plants under the control of the 35S constitutive promoter, or under the control of the VSR promoters. In addition, because the seven GFP-AtVSR fusions were expressed individually in the transgenic tobacco BY-2 cells used for the subcellular co-localization study, using VSR antibodies (Miao et al., 2006) that cross-react with several endogenous VSR proteins (Tse et al., 2004; Wang et al., 2007), it is not clear whether all of these seven GFP-AtVSR fusions (and the seven AtVSRs) are localized to the same PVC populations; this can only be addressed via the co-expression of multiple any fluorescent protein (XFP)-AtVSR fusions in the same plant cells.
We are interested in several biological questions that are related to PVC-mediated protein trafficking in plant cells. For example, will the seven XFP-AtVSR fusions be localized to the same or distinct PVC populations in plant cells? Do distinct PVC populations co-exist in the same plant cells that are defined by different XFP-AtVSR fusions? Do vacuolar proteins of hydrolytic enzymes and storage proteins reach the same or distinct PVCs on their route to vacuoles? In this study, we have addressed these questions using our recently developed transient expression systems of tobacco BY-2 and Arabidopsis protoplasts (Miao and Jiang, 2007). When transiently expressed together in pairs in protoplasts of tobacco BY-2 and Arabidopsis cells, the seven XFP-AtVSR fusions were found to be largely co-localized as punctate PVCs in untreated cells or as enlarged/vacuolated PVCs in wortmannin-treated cells. When the two soluble fluorescent reporter proteins, 2S albumin-GFP and aleurain-GFP, that represent the PSV and LV transport pathways in plants, respectively, were co-expressed with the PVC marker mRFP-AtVSR, they were found to be co-localized to the same punctate PVCs in BY-2 and Arabidopsis protoplasts. In wortmannin-treated cells, the vacuolated PVCs contain mRFP-AtVSR reporters in their limiting membrane, whereas the soluble aleurain-GFP or 2S albumin-GFP remained in the lumen of the enlarged PVCs, indicating the possible in vivo relationship or topology of the receptor/cargo within the PVC. Furthermore, co-localization between pairs of GFP-AtVSRs and Ara6 or Ara7/Rha1 demonstrated that the plant Rab5 homologs were also localized to PVC/MVB in both BY-2 and Arabidopsis protoplasts, an equivalence of the late endosome in plant cells.
Results
Establishment of a transient expression system using protoplasts of tobacco BY-2 and Arabidopsis cultured cells
We have previously demonstrated that GFP fusions with the TMD and CT of the seven Arabidopsis VSR proteins (termed GFP-AtVSR1–GFP-AtVSR7 fusions) were localized to PVCs in transgenic tobacco BY-2 cells, because these GFP fusions co-localized with VSR antibodies, and because organelles marked by GFP fusions became vacuolated in response to treatment with wortmannin (Miao et al., 2006). However, in these studies, the seven GFP-AtVSR fusions were individually studied without direct comparison between each other in the same transgenic cells, as there could be different tobacco VSRs with overlapping localization that could be indistinguishable by the antibody. To facilitate a quick study on pairs of XFP fusion proteins in the same living cells, we have recently developed a transient expression system using protoplasts derived from tobacco BY-2 and Arabidopsis suspension cultured cells (Miao and Jiang, 2007). This approach provides a quick and reliable tool in studying the subcellular localization of various fluorescent fusions in plant living cells.
To find out the nature of the PVCs defined by the seven XFP-AtVSR fusions in the same living cells, we first modified these GFP fusion constructs from the binary vectors (Miao et al.., 2006) into the transient expression vector pBI221, and made various monomeric red fluorescent protein (mRFP)-AtVSR fusions (Figure S1) by replacing the GFP with mRFP so that pairs of reporters can be compared directly in the same cells after transient co-expression. We then carried out a transient expression study using protoplasts derived from tobacco BY-2 cells. As shown in Figure 1, when the known pairs of PVC markers, GFP-BP-80 and mRFP-AtVSR2, were transiently co-expressed together in BY-2 protoplasts, these two reporters were largely co-localized (Figure 1a). In contrast, when the Golgi marker Man1-GFP was transiently co-expressed with the PVC marker mRFP-AtVSR2, these two markers were mostly separated from each other (Figure 1b). In addition, in BY-2 protoplasts treated with wortmannin, the wortmannin-induced vacuolated PVCs marked by the GFP-BP-80 remained separated from the Golgi marker Man1-mRFP (Figure 1c). These results are consistent with our previous studies in which individual GFP-AtVSR fusions were found to be co-localized with the endogenous tobacco VSR proteins to PVCs in stably transformed tobacco BY-2 cell lines (Miao et al., 2006).
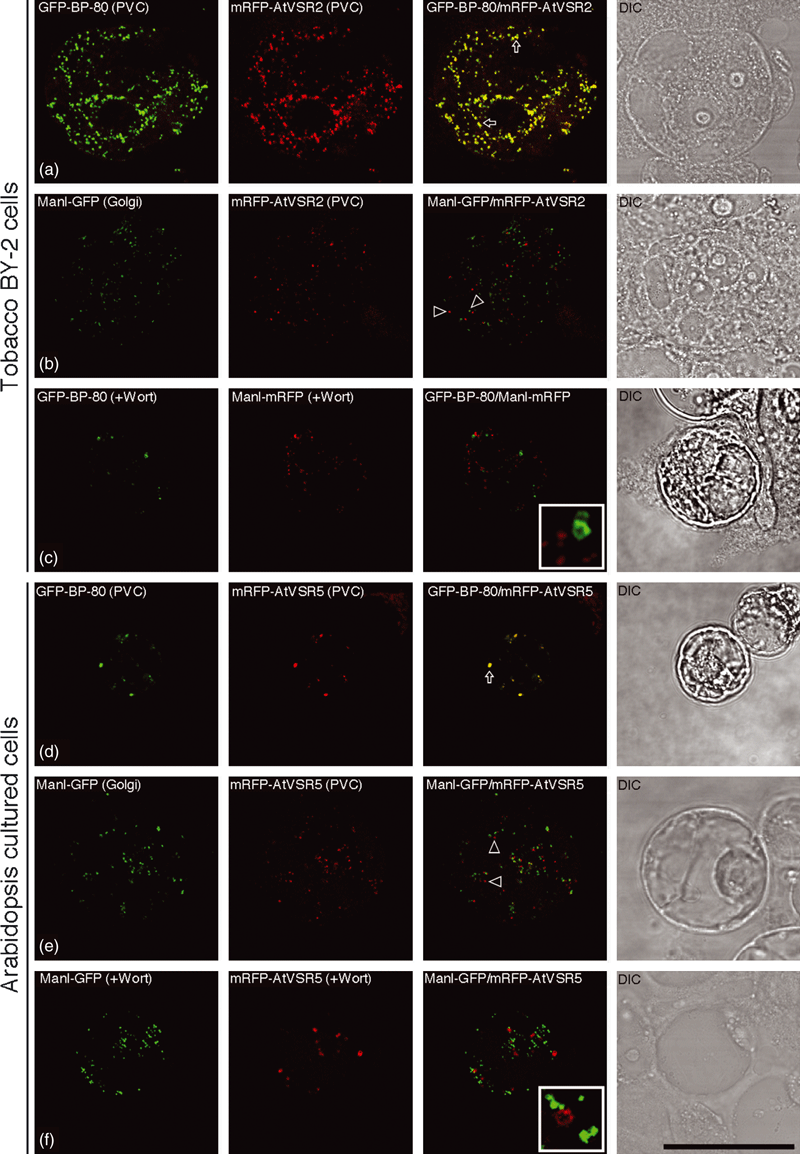
Establishment of a transient expression system for expressing GFP fusions using protoplasts from tobacco BY-2 and Arabidopsis cultured cells.(a) The pre-vacuolar compartment (PVC) markers GFP-BP-80 reporter (green) and mRFP-AtVSR2 reporter (red) co-localized together in punctate PVCs when they were co-expressed together in the protoplasts of BY-2 cells. Arrows indicated examples of co-localization between these two reporters.(b) The Golgi reporter Man1-GFP (green) was separated from the PVC marker mRFP-AtVSR2 (red) when they were co-expressed together in the protoplasts of BY-2 cells. Open arrowheads indicate examples of separation between these two reporters.(c) Protoplasts of BY-2 cells co-expressing GFP-BP-80 and Man1-mRFP were treated with wortmannin (Wort) at 16.5 μm for 1 h before confocal imaging, in which the vacuolated PVCs marked by GFP-BP-80 were separated from the unchanged Golgi labeled by Man1-mRFP.(d) The PVC reporters GFP-BP-80 (green) and mRFP-AtVSR5 (red) co-localized to punctate PVCs in Arabidopsis protoplasts. Arrows indicated examples of co-localization between these two reporters.(e) Golgi organelles marked by the Man1-GFP reporter (green) were separated from the PVC reporter mRFP-AtVSR5 (red) in Arabidopsis protoplasts. Open arrowheads indicate examples of separation between these two reporters.(f) Arabidopsis protoplasts co-expressing Man1-GFP (green) and mRFP-AtVSR5 (red) were treated with wortmannin (Wort) at 16.5 μm for 1 h before confocal imaging, in which the vacuolated PVCs marked by mRFP-AtVSR5 were separated from the unchanged Golgi labeled by Man1-GFP.DIC: differential interference contrast. Scale bar: 50 μm.
As we had studied the Arabidopsis VSR fusion proteins, we next wanted to find out if the same results would be obtained in Arabidopsis protoplasts. Thus, we also performed a similar transient expression study using protoplasts derived from the Arabidopsis suspension cultured PSB-D cells. Similar results were obtained when similar pairs of PVC or Golgi markers were transiently expressed in Arabidopsis protoplasts, where mRF-AtVSR5 was largely co-localized with the PVC marker GFP-BP-80 (Figure 1d), but was separate from the Golgi marker Man1-GFP (Figure 1e). Similarly, in wortmannin-treated Arabidopsis protoplast, the Golgi marker Man1-GFP was largely separated from the enlarged PVCs labeled by mRFP-AtVSR5, when these two markers were transiently co-expressed together (Figure 1f). These results demonstrate that protoplasts derived from either tobacco BY-2 cells or from the Arabidopsis suspension cultured cells are suitable for studying the subcellular localization of various GFP-tagged fusion proteins using this transient co-expression system.
The seven AtVSR fusion reporters were co-localized to the same PVCs in pairs
The seven GFP-AtVSR proteins were previously found to be largely co-localized with the PVC marker VSRat-1 antibodies in confocal immunofluorescence in transgenic tobacco BY-2 cells (Miao et al., 2006). However, as these GFP-AtVSR proteins were not compared directly in the same cell, we are not sure if all the seven GFP-AtVSR fusions will localize to the same or distinct PVC populations in plant cells. To address this question, we next performed transient expression of pairs of XFP-AtVSR fusions in either BY-2 or Arabidopsis protoplasts. As shown in Figure 2, when co-expressed together in tobacco BY-2 protoplasts, GFP-AtVSR7 and mRFP-AtVSR6 were largely co-localized either to the punctate PVCs in untreated cells (Figure 2a) or to the vacuolated PVCs in wortmannin-treated cells (Figure 2b). Similarly, GFP-AtVSR3 and mRFP-AtVSR5 were largely co-localized either to punctate PVC organelles in untreated cells (Figure 2c) or to vacuolated PVCs in wortmannin-treated cells (Figure 2d), when they were co-expressed together in Arabidopsis protoplasts. These results indicated that the tested pairs of XFP-AtVSR fusions were likely to be co-localized to the same PVC populations in both tobacco BY-2 and Arabidopsis cultured cells. As identical results were obtained from tobacco BY-2 and Arabidopsis cells, and that the seven XFP-AtVSR fusions were derived from Arabidopsis VSR sequences, we thus used either BY-2 or Arabidopsis protoplasts for further study.

G/RFP-AtVSR reporters were co-localized to the same pre-vacuolar compartments (PVCs) in protoplasts of BY-2 and Arabidopsis cells.(a) Pairs of PVC reporters GFP-AtVSR7 (green) and mRFP-AtVSR6 (red) were co-localized together to punctate PVCs in the protoplasts of tobacco BY-2 cells.(b) Pairs of PVC reporters GFP-AtVSR7 (green) and mRFP-AtVSR6 (red) were co-localized together to vacuolated PVCs in wortmannin-treated (16.5 μm for 1 h) protoplasts of tobacco BY-2 cells.(c) Pairs of PVC reporters GFP-AtVSR3 (green) and mRFP-AtVSR5 (red) were co-localized together to punctate PVCs in Arabidopsis protoplasts.(d) Pairs of PVC reporters GFP-AtVSR3 (green) and mRFP-AtVSR5 (red) were co-localized together to vacuolated PVCs in wortmannin-treated (16.5 μm for 1 h) Arabidopsis protoplasts.DIC: differential interference contrast. Scale bar: 50 μm.
To further find out if all the seven XFP-AtVSR fusions co-localized to the same PVC populations, we also carried out transient expression studies via the co-expression of pairs of XFP-AtVSR in both tobacco BY-2 and Arabidopsis protoplasts. As shown in Figures S2 and S3, similar to previous results shown in 1, 2, all tested pairs of PVC markers were found to be largely co-localized to punctate PVCs in both BY-2 and Arabidopsis protoplasts (Figures S2 and S3). For example, when co-expressed together in pairs in tobacco BY-2 protoplasts, the known PVC marker GFP-BP-80 co-localized with both mRFP-AtVSR3 and mRFP-AtVSR4 (Figure S2a,b), whereas XFP-AtVSR3 was found to be largely co-localized with XFP-AtVSR1, XFP-AtVSR5 or XFP-AtVSR7 (Figure S2c–e). Similarly, when transiently expressed together in pairs in Arabidopsis protoplasts, GFP-BP-80 co-localized with mRFP-AtVSR2, mRFP-AtVSR4 and mRFP-AtVSR6 (Figure S3a–c), whereas mRFP-AtVSR6 was found to be largely co-localized with GFP-AtVSR1 and GFP-AtVSR7 (Figure S3d,e). Taken together, all of the results obtained from both BY-2 and Arabidopsis cells thus far (1, 2, S2 and S3) demonstrated that the seven XFP-AtVSR fusions were most likely to be localized to the same PVC populations when they were transiently expressed in BY-2 and Arabidopsis protoplasts in this study.
Vacuolar transport of aleurain-GFP and 2S albumin-GFP reached the same PVCs in Arabidopsis cultured cells
Vacuolar proteins are believed to traffic through PVCs prior to reaching vacuoles in plant cells (Jiang and Rogers, 2003; Lam et al., 2007b). In addition, multiple vesicular transport pathways leading to LVs or PSVs have been demonstrated in vegetative or suspension cultured cells, even though the morphology of PSVs in these cell types remains elusive (Jiang and Rogers, 1998, 2003; Park et al., 2005). For example, in mesophyll cells, GFP fusions containing the C-terminal VSD of tobacco chitinase A or the N-terminal VSD of barley aleurain reached vacuoles via the PSV and LV pathways, respectively (Di Sansebastiano et al., 1998, 2001). Similarly, the storage protein phaseolin was trafficked through a PSV pathway in Arabidopsis leaves (Park et al., 2005).
As the seven XFP-AtVSR fusions were found to co-localize to the same PVC populations when they were transiently expressed in tobacco BY-2 or Arabidopsis protoplasts, we next wanted to find out if the vacuolar reporter proteins, known to reach vacuoles via the lytic or storage pathways, respectively, would reach the same or distinct PVCs on their path to vacuoles in suspension cultured cells. Two reporters were used to address this question: the lytic pathway marker aleurain-GFP and the storage pathway marker 2S albumin-GFP. The vacuolar trafficking of aleurain was mediated by BP-80 for reaching the lytic vacuole (Ahmed et al., 2000; Humair et al., 2001; Jiang and Rogers, 2003; Kirsch et al., 1994), whereas the PSV targeting of 2S albumin was mediated by AtVSR1/AtELP in Arabidopsis (Craddock et al., 2008; Fuji et al., 2007; Shimada et al., 2003). As the VSD for the 2S albumin was not known, we thus used the full-length cDNA of the Arabidopsis 2S albumin to make the 2S albumin-GFP fusion.
As shown in Figure 3, when transiently expressed in Arabidopsis protoplasts, the lytic vacuole marker aleurain-GFP was largely co-localized with the PVC marker mRFP-AtVSR2 to punctate PVC organelles (Figure 3a). Similarly, aleurain-GFP was largely co-localized with other PVC markers, mRFP-AtVSR4 or mRFP-AtVSR6, when they were co-expressed together in Arabidopsis protoplasts (data not shown). Interestingly, in wortmannin-treated Arabidopsis protoplasts co-expressing aleurain-GFP and the PVC marker mRFP-AtVSR2, the signals for the soluble aleurain-GFP were found mainly inside the vacuolated PVCs, with mRFP-AtVSR signals being found on their limiting membrane (Figure 3b,e). Similar results were obtained when the PSV marker 2S albumin-GFP was co-expressed together with the PVC marker mRFP-AtVSR5 in Arabidopsis protoplasts, where 2S albumin-GFP was largely co-localized with mRFP-AtVSR5 (Figure 3c) or mRFP-AtVSR6 (data not shown) to punctate PVCs, but the signals for 2S albumin-GFP were found mainly inside the enlarged PVCs in wortmannin-treated cells (Figure 3d,f). As mRFP-AtVSR2 and mRFP-AtVSR5 largely co-localized with the same PVC marker GFP-BP-80 (Figure 1) when they were co-expressed in the same cells, we can thus conclude that both aleurain-GFP and 2S albumin-GFP were localized to the same PVC populations on their path to vacuoles, whereas GFP signals were found inside the vacuoles at later stages (18–24 h) after transformation (Figure S4) in Arabidopsis protoplasts. In addition, similar results were obtained in tobacco BY-2 cells, in which the aleurain-GFP and 2S albumin-GFP were also found to be co-localized with the same PVC marker mRFP-AtVSR2 (Figure S5). Detailed analysis on the vacuolated PVCs in wortmannin-treated Arabidopsis protoplasts co-expressing mRFP-AtVSR and aleurain-GFP or 2S albumin-GFP further illustrated the possible in vivo relationship between a soluble cargo protein (as represented by either aleurain-GFP or 2S albumin-GFP) and its integral membrane sorting receptor (as represented by mRFP-AtVSR) within a PVC, where the detection of the luminal cargo might result from the acidic pH of PVC that would abolish the possible cargo–receptor interaction, thus causing the release of the cargo from its membrane receptor (Figure 4). When distinct fluorescent signals were measured across the enlarged PVCs in wortmannin-treated cells (Figure 4a), the green signals from either aleurain-GFP or 2S albumin-GFP followed a typical Gaussian curve (Figure 4b), where the mRFP-AtVSR signals reached two peaks (Figure 4b, red curve) on either side of the aleurain-GFP or 2S albumin-GFP signals (Figure 4b, green curve). Therefore, because of the limited resolution of confocal microscopy, only vacuolated or enlarged PVCs will allow the visualization of the possible relationship between the lumenal soluble cargo protein (or other soluble proteins) and its peripheral integral membrane receptor protein within PVC (Figure 4c).
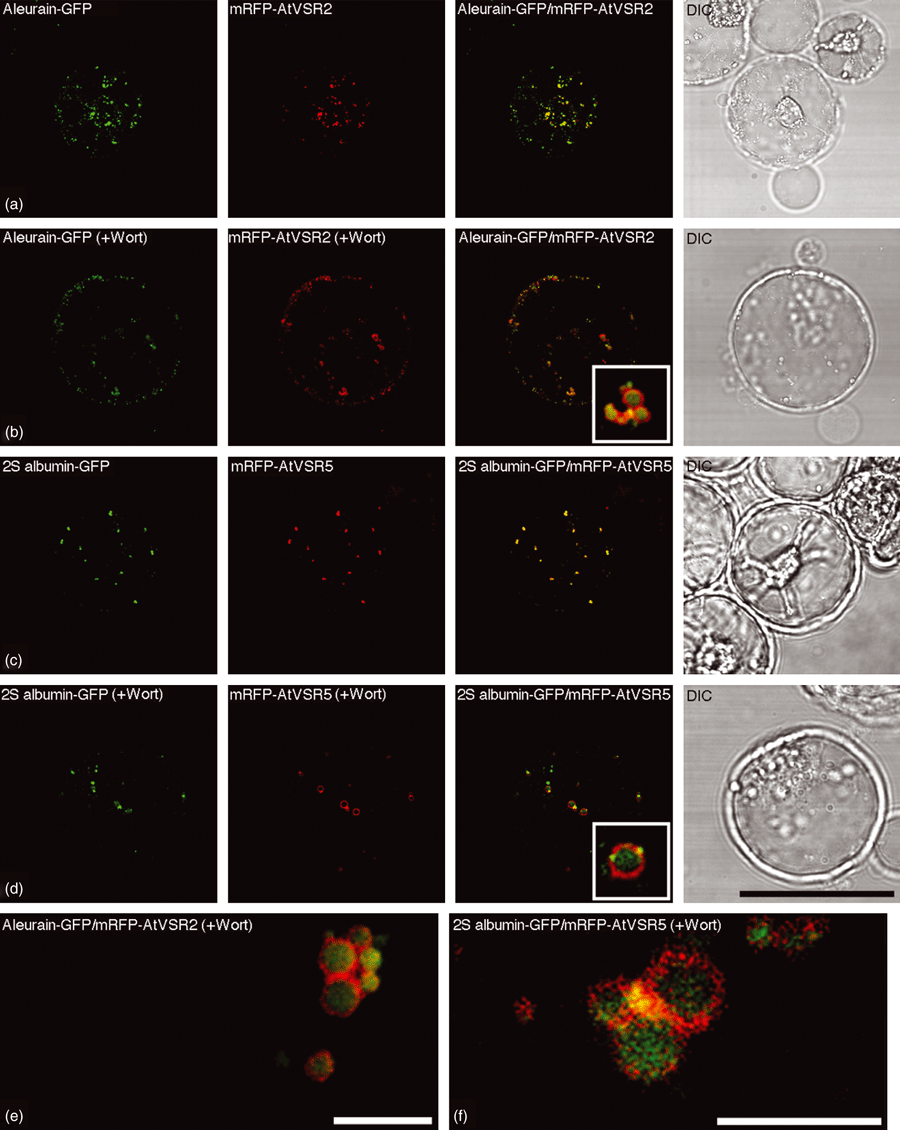
Aleurain-GFP and 2S albumin-GFP reached the same pre-vacuolar compartments (PVCs) in Arabidopsis protoplasts.(a, b) Aleurain-GFP (green) and the PVC marker mRFP-AtVSR2 (red) were co-localized to punctate PVCs in Arabidopsis protoplasts (a) or were co-localized inside the vacuolated PVCs in wortmannin-treated (16.5 μm for 1 h) Arabidopsis protoplasts (b) when they were transiently co-expressed together in Arabidopsis protoplasts.(c, d) 2S albumin-GFP (green) and the PVC marker mRFP-AtVSR5 (red) were co-localized to punctate PVCs in Arabidopsis protoplasts (c) or were co-localized inside the vacuolated PVCs in wortmannin-treated Arabidopsis protoplasts (d) when they were transiently co-expressed together in Arabidopsis protoplasts.(e, f) Relationship or interaction between the protein cargo aleurain-GFP/2S (e) or albumin-GFP (f) and the PVC marker mRFP-AtVSR2 in selective enlarged PVCs in wortmannin-treated Arabidopsis protoplasts. DIC: differential interference contrast. Scale bars: (a–d), 50 μm; (e, f), 5 μm.
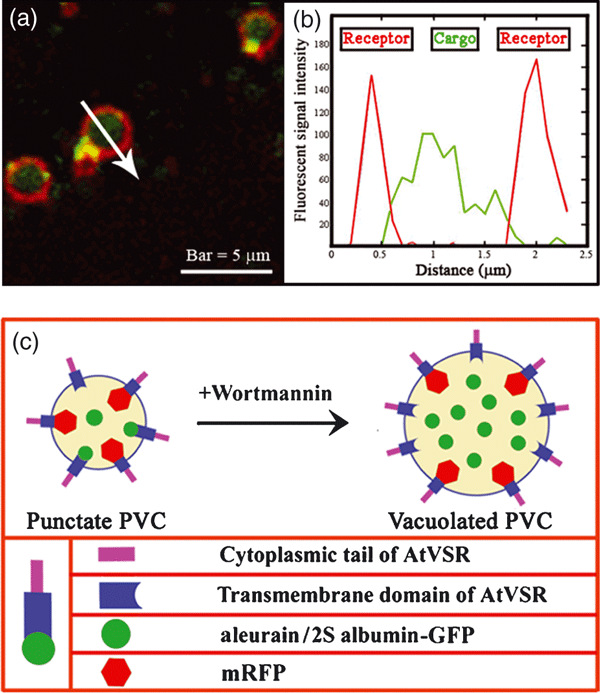
Distribution of soluble cargo reporter and membrane receptor in vacuolated pre-vacuolar compartments (PVCs) of Arabidopsis protoplasts.(a) High magnification of selected enlarged PVCs in wortmannin-treated Arabidopsis protoplasts, showing the internal distribution of aleurain-GFP or 2S albumin-GFP inside the vacuolated PVCs containing mRFP-AtVSR on their limiting membrane.(b) The fluorescence profile across an enlarged PVC, as indicated by the arrow shown in panel (a). The y-axis shows the color values of the signals of aleurain-GFP or 2S albumin-GFP (green curve) and mRFP-AtVSR (red curve) along the arrow. The x-axis shows the distance in μm.(c) A schematic working model showing the relationship among soluble cargo proteins (as represented by aleurain-GFP or 2S albumin-GFP), endogenous Arabidopsis thaliana vacuolar sorting receptor (AtVSR) proteins and AtVSR reporters (as represented by mRFP-AtVSR fusions) within the PVCs of Arabidopsis cells before and after wortmannin treatment.
To further find out if other well-characterized soluble vacuolar reporter proteins would reach the same PVC population in cultured cells, we also co-expressed the GFP-chitinase together in pairs with the PVC reporter mRFP-AtVSR in Arabidopsis protoplasts. However, the results obtained were not conclusive, because the expressed GFP-chitinase exhibited both ER (probably due to overexpression) and punctate patterns, where the punctate signals were co-localized with mRFP-AtVSR to PVCs. In wortmannin-treated cells, the punctate GFP-chitinase was also found inside the enlarged PVC, as seen for the aleurain-GFP and 2S albumin-GFP fusions (data not shown). It would therefore be very interesting in a future study to find out if all of the other known soluble vacuolar reporter proteins, including the RFP-AFVY and the barley lectin fusion GFP-lectin, would reach the same PVC population on their way to vacuoles in tobacco BY-2 and Arabidopsis cultured cells. In addition, it would also be interesting to use the VSR promoters to control the expression of AtVSR-GFP fusions in transgenic plants to obtain novel information about the expression and localization of VSRs in plants. However, care must be taken in such a study in the future, as the seven AtVSRs were shown to be spatially expressed in Arabidopsis at the mRNA level (Laval et al., 2003).
Endosomal and PVC markers co-localized together in plant suspension cultured cells
Several Rab proteins have been shown to localize to PVCs or endosomes in plant cells. For example, the Rab5-related GTPase Rha1/AtRabF2a was found to co-localize with VSR to PVCs in Arabidopsis cells (Lee et al., 2004; Sohn et al., 2003), whereas Ara6/AtRabF1 and Ara7/AtRabF2b were shown to localize to endosomes, because they co-localized with the internalized endosomal marker FM4-64 in Arabidopsis cells (Ueda et al., 2001, 2004). Interestingly, Rha1/AtRabF2a, Ara6/AtRabF1 and Ara7/AtRabF2b were all localized to MVB/PVC in Arabidopsis roots via an immunogold EM study using high-pressure frozen/freeze-substituted samples (Haas et al., 2007). However, no direct comparison among these markers expressed in the same cells has been made thus far. In addition, as the secretory TGN also serves as an early endosome (EE) in the endocytic pathway (Dettmer et al., 2006; Lam et al., 2007a), we have thus proposed that the PVCs/MVBs are late endosomes enriched with VSR, Rha1/AtRabF2a, Ara6/AtRabF1 and Ara7/AtRabF2b proteins in the same plant cells (Lam et al., 2007b). A direct comparison among all of these markers expressed in the same cell is thus necessary to verify this hypothesis.
We therefore tested if mRFP-Rha1, mRFP-Ara7 or Ara6-mRFP would co-localize with the PVC/MVB marker GFP-BP-80 (Tse et al., 2004) in both BY-2 and Arabidopsis protoplasts. As shown in 5, 6, when transiently expressed together with the PVC marker GFP-BP-80 in either BY-2 protoplasts or Arabidopsis protoplasts, all individual mRFP-Rha1, mRFP-Ara7 or Ara6-mRFP reporters (red) were largely co-localized with the PVC marker GFP-BP-80 (green) to typical punctate PVC organelles in untreated cells (Figure 5), and to the limiting membranes of the vacuolated PVCs in wortmannin-treated cells (Figure 6). These results demonstrated that the tested Rab5 homologs were localized to the same VSR-positive PVCs/MVBs in both tobacco BY-2 and Arabidopsis cultured cells. In addition, the punctate signals of mRFP-Ara7 (Figure S6a) and mRFP-AtVSR2 (Figure S6b) were largely separated from the TGN/EE/PM marker YFP-SCAMP. Such PVC/MVB localization of Rab5 homologs is consistent with the immunogold EM localization study in Arabidopsis root cells, where antibodies for Ara6, Rha1 and Ara7 labeled MVBs specifically on their limiting membranes (Haas et al., 2007). As the internalized endosomal marker FM4-64 reached the SCAMP-positive EE/TGN prior to the VSR-positive PVC/MVB (Lam et al., 2007a; Tse et al., 2004), the Rab5-positive PVC/MVB thus also serve as a late endosome in the endocytic pathway in plant cells.
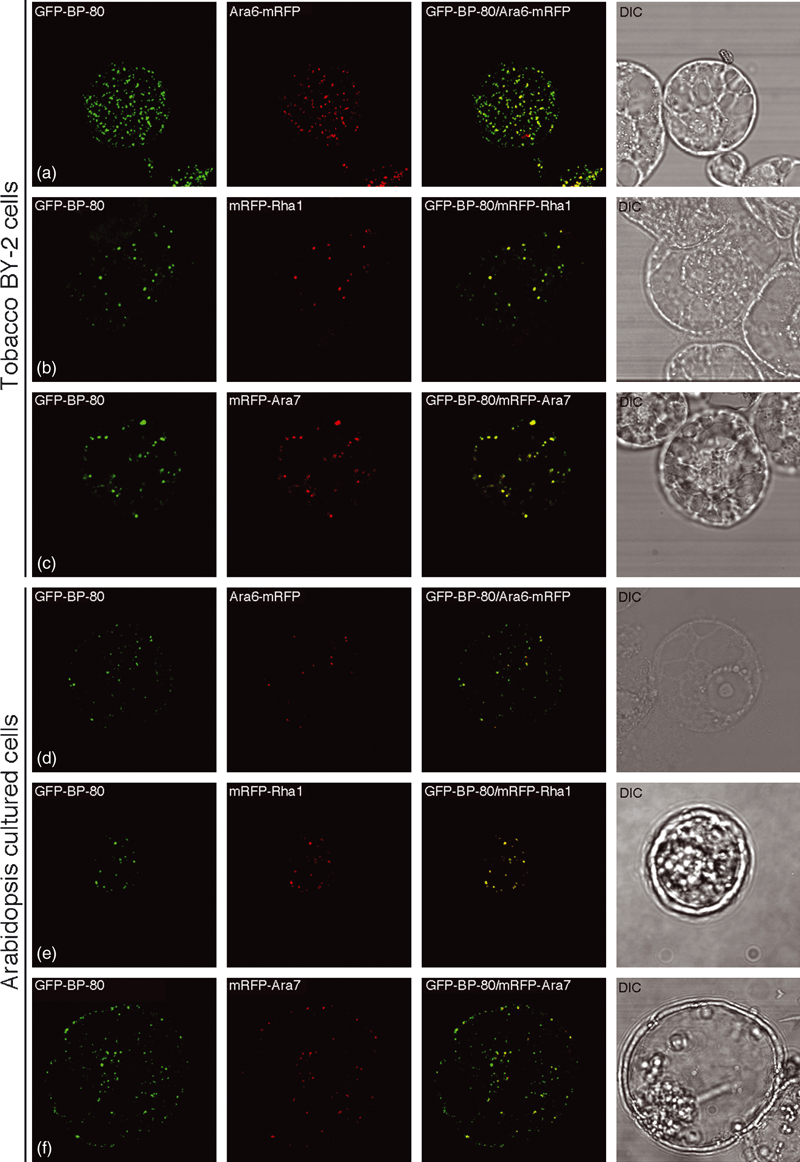
The endosomal markers Ara6, Ara7 and Rha1 were co-localized together with the pre-vacuolar compartment (PVC) marker GFP-BP-80 to the same PVCs in protoplasts of BY-2 and Arabidopsis cells.Pairs of the PVC reporter GFP-BP-80 (green) and endosomal marker Ara6-mRFP, mRFP-Rha1 or mRFP-Ara7 (red) were co-expressed in protoplasts of BY-2 and Arabidopsis cells, as indicated, followed by confocal image collection. The yellow color indicates the co-localization of the two reporters. DIC: differential interference contrast. Scale bar: 50 μm.
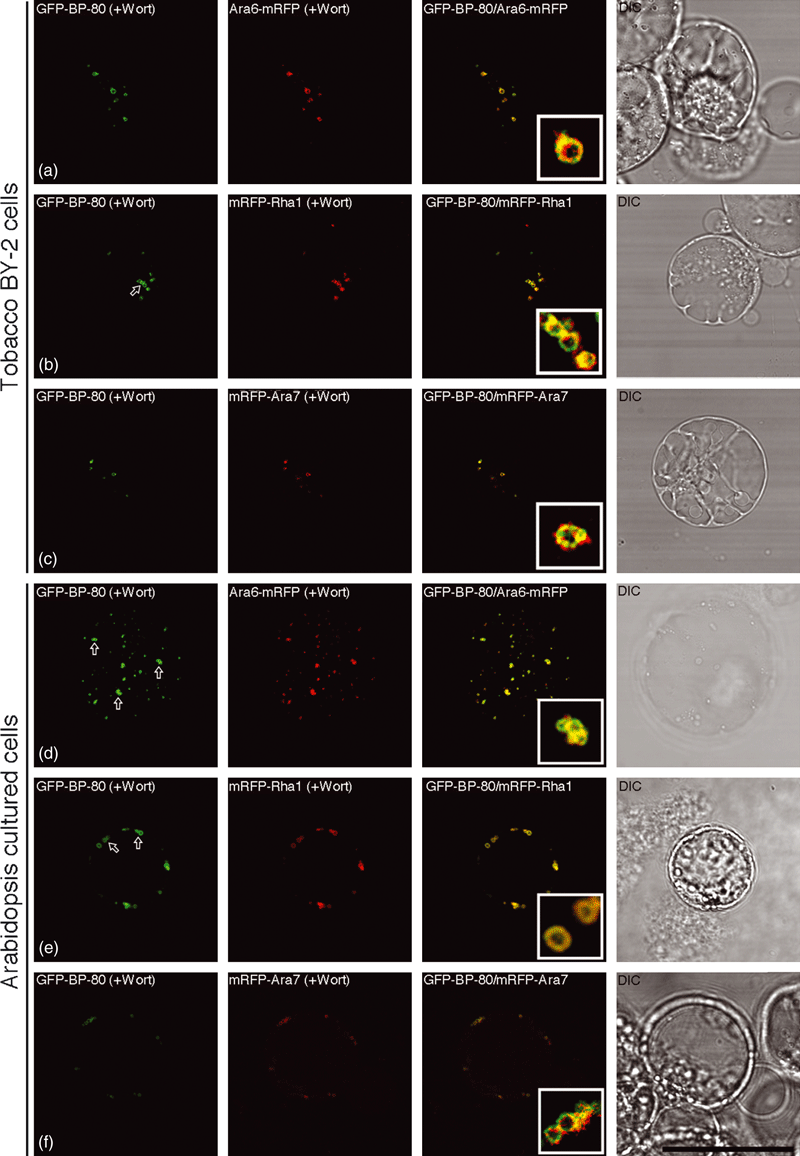
The endosomal markers Ara6, Ara7 and Rha1 were co-localized together with the pre-vacuolar compartment (PVC) marker GFP-BP-80 to the same vacuolated PVCs in wortmannin-treated protoplasts of BY-2 and Arabidopsis cells.Pairs of the PVC reporter GFP-BP-80 (green) and endosomal marker Ara6-mRFP, mRFP-Rha1 or mRFP-Ara7 (red) were co-expressed in protoplasts of BY-2 and Arabidopsis cells, as indicated, followed by wortmannin (Wort) treatment at 16.5 μm for 1 h before confocal image collection. The yellow color indicates the co-localization of the two reporters. Arrows indicate examples of a possible homotypic fusion of vacuolar sorting receptor (VSR)-marked PVCs in response to wortmannin treatment. DIC: differential interference contrast. Scale bar: 50 μm.
Discussion
Pre-vacuolar compartments are believed to mediate protein trafficking between the Golgi apparatus, late Golgi or TGN and vacuoles in eukaryotic cells. Soluble proteins reach the LVs and PSVs via multiple vesicular transport pathways in plant cells (Neuhaus and Rogers, 1998). MVBs were first identified as PVCs for the LV pathway in tobacco BY-2 cells, as defined by the presence of VSRs and the BP-80 reporter (Li et al., 2002; Tse et al., 2004). These VSR-marked PVCs can also be identified by their vacuolation response to wortmannin treatment (Miao et al., 2006; Tse et al., 2004). However, it is not known if plant cells would contain distinct PVC populations for the lytic vacuole and protein storage vacuole pathways in either vegetative cells or seeds (Jiang and Rogers, 2003). In this study, using transient expression of protoplasts, we demonstrated that the seven XFP-VSR1–XFP-VSR7 fusions co-localized to the same PVCs when they were transiently expressed in pairs in protoplasts of both BY-2 and Arabidopsis cells. Furthermore, both aleurain-GFP and 2S albumin-GFP were co-localized with the PVC marker XFP-VSR, indicating their identical PVC localization. All of these results indicate that XFP-VSR, aleurain-GFP and 2S albumin-GFP localize to the same PVC populations in the protoplasts of BY-2 and Arabidopsis suspension cultured cells.
Single or distinct PVC population in suspension cultured cells?
As the BP-80 reporter containing the TMD and CT of BP-80 co-localized with the endogenous VSR proteins in transgenic tobacco cells (Jiang and Rogers, 1998; Tse et al., 2004), a similar reporter system was thus used to study the subcellular localization of the seven Arabidopsis VSR proteins (Miao et al., 2006), in which all of the seven GFP fusions with TMD and CT of individual AtVSR1–AtVSR7s were found to localize to PVCs in transgenic tobacco BY-2 cells (Miao et al., 2006). Such PVC localization was collectively defined by three criteria: co-localization with VSR antibodies, vacuolation in response to wortmannin treatment and persistence of a punctate pattern in the presence of a low concentration of brefeldin A (BFA) (5–10 μg ml−1; Tse et al., 2004; Miao et al., 2006; Lam et al., 2007a,b). The PVC localization of GFP-AtVSR fusions is likely to reflect the subcellular localization of AtVSRs in Arabidopsis, even though such a conclusion needs to be confirmed in transgenic Arabidopsis plants expressing the reporter or epitope-tagged AtVSRs in future studies. However, as the VSR-at-1 antibodies cross-react with several VSR isoforms (Miao et al., 2006; Tse et al., 2004; Wang et al., 2007), and because individual GFP-AtVSR fusions were compared with VSR antibodies for co-localization, it was not clear whether all of these seven GFP-AtVSR fusions were localized to the same PVC populations in transgenic tobacco BY-2 cells (Miao et al., 2006).
The current study gave an answer to this unsolved question by co-expression of XFP-AtVSR pairs in protoplasts. When various combinations of pairs of XFP-AtVSR fusions were transiently expressed together in the protoplasts of BY-2 cells or Arabidopsis cells, they co-localized to the same BP-80-positive PVCs, with typical punctate patterns in untreated cells, or to the limiting membranes of the vacuolated PVCs in wortmannin-treated cells (1, 2, S2, and S3). Therefore, all of the seven XFP-AtVSR1–XFP-AtVSR7 fusions co-localized to the same PVC populations when they were transiently co-expressed in BY-2 or Arabidopsis protoplasts. The identical PVC localization of AtVSR1-7 may reflect the major functional sites for VSRs in various cell types in plants, even though the expression of AtVSRs was found to be spatially regulated in Arabidopsis (Laval et al., 2003), and that different members of AtVSRs were shown to function in transporting storage proteins or hydrolytic enzymes in Arabidopsis (Ahmed et al., 2000; Craddock et al., 2008; Fuji et al., 2007; Shimada et al., 2003). However, in Arabidopsis mesophyll cells, unidentified PVCs for PSVs, which were distinct from the VSR-positive lytic PVCs, were suggested to play roles in mediating protein sorting to PSVs through the Golgi complex (Park et al., 2005). Thus, we cannot exclude the possibility that distinct PVCs for the PSV pathway may exist in different cell types, including seeds (Hinz et al., 2007; Jiang and Rogers, 2003; Park et al., 2005, 2007).
Dual roles of XFP-AtVSR-positive PVCs in cultured cells?
Multiple transport pathways are responsible for sorting proteins to LVs and PSVs in plant cells (Jiang and Rogers, 2003). In this regard, distinct PVCs for LVs and PSVs may exist in plants. Using VSRs and BP-80 reporters as PVC markers (Li et al., 2002), MVBs were first identified as PVCs in tobacco BY-2 cells (Tse et al., 2004). As the trafficking of BP-80 reporters represented the LV pathway in tobacco cells (Jiang and Rogers, 1998), the identified MVBs were thus considered to be lytic PVCs (Li et al., 2002; Tse et al., 2004). Similarly, in germinating mung bean seeds, MVBs were also found to contain VSRs for transporting hydrolytic enzymes to PSVs for protein degradation, to provide amino acids needed for seedling growth (Wang et al., 2007). Interestingly, a joint MVB was recently shown to be responsible for transporting both lytic enzymes and storage proteins in Arabidopsis embryo cells (Hinz et al., 2007; Otegui et al., 2006), even though further sorting of hydrolytic enzymes and storage proteins from the PVCs/MVBs into either LVs or PSVs could also occur.
In this study, when transiently expressed together in tobacco BY-2 and Arabidopsis cells, either the storage protein reporter 2S albumin-GFP or the hydrolytic enzyme reporter aleurain-GFP were found to co-localize with the PVC reporter mRFP-AtVSR in punctate PVC organelles in untreated cells (Figures 3 and S5), or inside the lumen of the vacuolated PVCs in wortmannin-treated cells, where mRFP-AtVSRs were mainly localized on the limited membrane of PVCs (Figure 3), showing a possible in vivo cargo–receptor relationship within PVCs. These results indicated that both lytic enzymes and storage proteins are likely to be localized to the same VSR-positive PVCs/MVBs in these cultured cells. Therefore, the transport of lytic enzymes and storage proteins to the central vacuole is likely to be mediated by the same VSR-positive PVC/MVB populations in BY-2 and Arabidopsis cultured cells. These results are in line with results obtained in Arabidopsis embryo cells where MVBs were found to contain both lytic enzymes and storage proteins (Otegui et al., 2006). In addition, the fact that VSR-positive PVCs mediate vacuolar transport of both aleurain-GFP and 2S albumin-GFP in cultured cells is also consistent with several recent studies. For example, in the seeds of an Arabidopsis knock-out mutant lacking the AtVSR1 protein (Craddock et al., 2008; Fuji et al., 2007; Shimada et al., 2003), both endogenous vacuolar proteins and the heterologously expressed vacuolar reporter proteins were found to be non-specifically secreted outside of the cells in transgenic Arabidopsis plants lacking AtVSR1. In addition, the expression of various GFP-tagged tonoplast intrinsic proteins (TIPs), under the control of their own TIP promoters, suggested the existence of a single vacuole population in Arabidopsis (Hunter et al., 2007). As the vacuolar transport of all these proteins are affected by the VSR proteins (Craddock et al., 2008; Hunter et al., 2007; Otegui et al., 2006; Wang et al., 2007), it is thus reasonable to suggest that these proteins may traffic via the same VSR-positive PVCs on their route to vacuoles in plant cells. It will therefore be interesting in a future study to find out if other known non-VSR-transported vacuolar proteins would reach the same VSR-positive PVCs prior to reaching vacuoles in plants.
The homotypic fusion of PVC may contribute membranes needed for wortmannin-induced vacuolation of PVCs
Wortmannin, an inhibitor of phosphatidylinositol-3-kinase (Arcaro and Wymann, 1993; Kjeken et al., 2001), has been a useful tool in studying protein trafficking and organelle dynamics in eukaryotes. Wortmannin induced the vacuolation of multivesicular compartments in human cells (Fernandez-Borja et al., 1999; Houle and Marceau, 2003), as well as the PVCs/MVBs in tobacco BY-2 cells (Miao et al., 2006; Tse et al., 2004). Such wortmannin-induced dilation of PVCs may represent a general mechanism in plants, because such a response has been observed in various plant cell types, including pea, Arabidopsis and tobacco (Miao et al., 2006). However, relatively little is known about the underlying molecular mechanism. Three mechanisms have been proposed to contribute to the membranes needed for the rapid enlargement or vacuolation of PVCs/MVBs in response to wortmannin treatment in plant cells (Lam et al., 2007b), including: (i) homotypic fusions of PVCs/MVBs during wortmannin treatment; (ii) wortmannin-induced fusions between TGNs/early endosomes and PVCs/MVBs, as seem for SCAMP-positive TGNs in BY-2 cells (Lam et al., 2007a); and (iii) fusions between the internal vesicles of PVCs/MVBs and the limiting membranes of PVCs/MVBs (Lam et al., 2007b).
Two indirect evidences from this study support the hypothesis that wortmannin induced homotypic fusions of PVCs/MVBs for their enlargement. First, the cargo fusion proteins, 2S albumin-GFP or aleurain-GFP, were largely co-localized with the PVC marker mRFP-AtVSR as punctate patterns when they were transiently co-expressed together in either BY-2 or Arabidopsis protoplasts. Interestingly, in wortmannin-treated cells, the soluble cargo proteins were found in the lumen of the vacuolated PVCs, whereas the integral membrane protein mRFP-AtVSR remained in the limiting membranes of PVCs: a result demonstrating the possible relationship between cargo and receptor within PVCs. In addition, the fluorescent intensity of either 2S albumin-GFP or aleurain-GFP remained constant inside the enlarged PVCs (e.g. Figure 3b) in wortmannin-treated cells, as compared with punctate PVCs (e.g. Figure 3a) in untreated cells, an evidence indirectly supporting the scenario of the wortmannin-induced homotypic fusion of PVCs for vacuolation in plant cultured cells (Figure 4c). Otherwise, the original fluorescent signals of 2S albumin-GFP or aleurain-GFP would be dramatically diluted, and became much weaker inside the enlarged PVCs upon wortmannin treatment. Second, the average numbers of the punctate PVC organelles was gradually decreased as enlarged PVCs appeared. In addition, visible fusions between or among XFP-tagged PVCs were also observed (Figure 6d, as indicated by arrows for examples). In general, the punctate PVC numbers were reduced by more than 70% within 2 h of wortmannin treatment, which induced the appearance of less than 30% as many enlarged PVCs (e.g. comparing Figure 5 with Figure 6).
The PVCs/MVBs are late endosomes
The endosomal system comprises early, intermediate and late endosomes in mammalian cells. Specific Rab GTPases were identified to target corresponding endosomal compartments, such as Rab4, Rab5, Rab11 and Rab21 (Simpson et al., 2004; Sonnichsen et al., 2000). Plants contain three mammalian Rab5 homologs, Ara7/RabF2b, Rha1/RabF2a and the plant-unique Ara6/RabF1, that are also localized to endosomal compartments in various plant cell types because they are co-localized with the internalized endosomal marker FM4-64 (Ueda et al., 2001, 2004). Confocal immunofluorescent studies demonstrated that Rha1/AtRabF2a and Ara7/AtRabF2b were co-localized with VSRs to PVCs in Arabidopsis mesophyll cells (Lee et al., 2004; Sohn et al., 2003), where VSR-positive PVCs were identified as MVBs in BY-2 cells (Li et al., 2002; Tse et al., 2004), and in germinating mung bean seeds (Wang et al., 2007). Interestingly, immunogold EM studies showed that Rha1/AtRabF2a, Ara6/AtRabF1 and Ara7/AtRabF2b were localized to MVBs in Arabidopsis root cells (Haas et al., 2007). In addition, several recent studies have also demonstrated that the TGNs and PVCs/MVBs may serve as early endosomes and late endosomes, respectively, because the internalized endosomal marker FM4-64 reached the SCAMP-positive TGNs prior to the VSR-labeled PVCs en route to the tonoplasts in tobacco BY-2 cells (Dettmer et al., 2006; Lam et al., 2007a,b; Tse et al., 2004).
In this study, we showed that, when transiently expressed in pairs together in Arabidopsis protoplasts, the fluorescent reporters of Rha1/AtRabF2a, Ara6/AtRabF1 and Ara7/AtRabF2b were all largely co-localized with the PVC marker AtVSR in both untreated or wortmannin-treated BY-2 and Arabidopsis cells (5, 6), but were separated from the early endosomal marker YFP-SCAMPI (Figure S6). These results demonstrated that VSRs and the three plant Rab5 homologs were localized to the same PVCs/MVBs, and that the PVCs/MVBs and late endosome are the same compartments in both BY-2 and Arabidopsis cultured cells. In addition, in wortmannin-treated cells, the GFP-tagged Ara6 or Ara7 were mainly found to localize to the limiting membranes of the enlarged PVCs, which may represent the activated GTPase forms of Ara6 or Ara7 for membrane association in the presence of wortmannin, as compared with their inactive cytosolic form.
Conclusions and perspectives
Several conclusions can be made from this study. First, all seven XFP-AtVSR fusions were localized to the same PVC populations in both tobacco BY-2 and Arabidopsis cultured cells, which may reflect the subcellular localization of endogenous AtVSR proteins. Second, vacuolar transport of lytic enzymes and storage proteins, as represented by aleurain-GFP and 2S albumin-GFP, respectively, is likely to be mediated by the same PVC populations in tobacco BY-2 and Arabidopsis cultured cells. Third, the VSR-positive PVCs/MVBs may also serve as the Rha1/Ara6/Ara7-positive late endosomal compartments in Arabidopsis cultured cells. Fourth, the distinct signals between the lumenal aleurain-GFP or 2S albumin-GFP and the membrane mRFP-AtVSR in the wortmannin-induced vacuolated PVCs/MVBs may reflect the in vivo cargo–receptor relationship in PVCs of plant cells. Last, the relatively constant fluorescent signals of aleurain-GFP or 2S albumin-GFP inside the vacuolated PVCs indicated a possible wortmannin-induced homotypic fusion of PVCs/MVBs in BY-2 and Arabidopsis cells. However, as no visible protein storage vacuole or protein body has been identified in vegetative or suspension cultured cells, it would be interesting to find out in a future study if other non-VSR cargo proteins, such as the XFP-AFVY and barley lectin-GFP, would reach the same VSR-positive PVCs when they are expressed in BY-2 or Arabidopsis protoplasts. Future studies can be carried out using either the 35S constitutive promoter, or the VSR promoters, in transgenic Arabidopsis plants to address the nature of VSR proteins and PVCs in plants, by asking the following questions. What are the distinct functions or specific cargo proteins for the seven VSR proteins in Arabidopsis? Will the seven VSR proteins localize to the same PVCs/MVBs in developing or germinating Arabidopsis seeds? What are the molecular mechanisms and biological consequences of wortmannin-induced vacuolation of PVCs/MVBs in plant cells? What are the molecular mechanisms of PVC/MVB biogenesis? Our current studies are addressing some of these questions.
Experimental procedures
The general methods for the construction of recombinant plasmids, characterization of cloned inserts and maintenance of Nicotiana tabacum BY-2 culture cells have been described previously (Jiang and Rogers, 1998; Tse et al., 2004).
Plasmid construction
All transient expression constructs used in this study were derived from the plasmids with a PUC19, PBR322 or PBI221 backbone. All fluorescent fusion constructs contain the cauliflower mosaic virus (CaMV) 35S promoter and the nopaline synthase (NOS) terminator (Figure S1). In order to generate various transient expression constructs that contain the signal peptide (SP) sequences from the barley proaleurain linked to GFP (Tse et al., 2004) and the TMD/CT sequences of individual AtVSR (GFP-AtVSR1-7), corresponding GFP-AtVSR fragments in the binary vectors (Miao et al., 2006) were cut out and ligated into PBI221 with BamHI/SacI sites (Miao et al., 2006). To use other fluorescent proteins (XFP) for comparison, monomeric red fluorescent proteins (mRFPs) were also amplified via polymerase chain reaction from plasmid Ara6-mRFP (Campbell et al., 2002; Ueda et al., 2001) using two oligonucleotides: SP-mRFP-forward (5′-GGGGATCCATGGCCCACGCCCGCGTCCTCCTCCTGGCGCTCGCCGTCCTGGCCACGGCCGCCGTCGCCGTCGCCGCCTCCTCCGAGGACGTCATCAAGGAG-3′) and mRFP-reverse (5′-GGGGAATTCGGCGCCGGTGGAGTGGCGGCCCTCGGC-3′). The corresponding transient expression mRFP-AtVSR fusion constructs were generated by the replacement of GFP with mRFP in PBI221-derived GFP-AtVSR1–GFP-AtVSR7 using the BamHI/EcoRI sites. For the construction of the 2S albumin-GFP construct (Figure S1), the full-length coding region of the Arabidopsis 2S albumin cDNA was amplified via PCR using the cDNA clone (RAFL05-14-B09), obtained from RIKEN (http://www.riken.jp/engn), as a template, and the amplified DNA fragment was fused at the N terminal of GFP, and resulted in the 2S albumin-GFP construct. All constructs were checked by both restrictions mapping and DNA sequencing.
Transient expression of plant suspension cultured cells
Arabidopsis thaliana cell suspension cultures (ecotype Landsberg erecta) PSB-D and tobacco (N. tabacum) BY-2 cells were subcultured twice a month in MS agar media, or twice a week in liquid MS media. Arabidopsis cells were grown in a 250-ml flask at 27°C in light-protected shakers. BY-2 cells were maintained in MS media as described previously (Miao et al., 2006; Tse et al., 2004). The detailed procedures, critical steps and the cautions of the transient expression studies were recently described (Miao and Jiang, 2007). After electroporation, the transfected protoplasts of tobacco BY-2 and Arabidopsis suspension cultured cells were incubated at 27°C before confocal observation. Protoplasts were observed for fluorescent signals by confocal microscopy at the early stage (i.e. 6–12 h after transformation), middle stage (i.e. 12–18 h after transformation) or late stage (i.e. 18–24 h after transformation).
Drug treatment
Stock solutions of wortmannin (Sigma-Aldrich, http://www.sigmaaldrich.com) at 2.5 mg ml−1 dissolved in dimethyl sulfoxide (DMSO), was used. The drug was diluted in protoplast incubation medium to appropriate working concentrations before incubation with protoplasts. For drug treatment, protoplasts were mixed with drug working solutions at a 1 : 1 ratio to ensure minimal variation. Treated samples were then harvested at the indicated times for the subsequent confocal analysis, as described previously (Miao et al., 2006; Tse et al., 2004).
Confocal immunofluorescence studies
Images of fluorescent signals in intact protoplasts were obtained from protoplasts in incubation medium on a glass slide covered with a cover slip. Confocal fluorescent images were collected using a Bio-Rad Radiance 2100 system (http://www.bio-rad.com). The settings for collecting confocal images within the linear range were described by Jiang and Rogers (1998). Images were processed using Adobe Photoshop software (http://www.adobe.com) as previously described (Jiang and Rogers, 1998). All experiments were repeated at least twice with similar results.
All novel materials described in this publication will be made available in a timely manner for non-commercial research purposes, subject to the requisite permission from any third-party owners of all or parts of the material. Obtaining any of the approriate permissions will be the responsibility of the requestor.
Acknowledgements
We would like to thank the two anonymous reviewers for their critical and constructive comments that led to the significant improvement of this article. We thank Dr J.C. Rogers (Washington State University, USA) for providing the aleurain-GFP and GFP-chitinase constructs, Dr P. Pimpl (University of Heidelberg, Germany) for advice on the transient expression system, Dr H. He (Hong Kong University of Science and Technology, China) for designing the program used to analyze the signal intensity of confocal images, Dr A. Nakano (University of Tokyo, Japan) for sharing the Ara6-mRFP/mRFP-Ara7 constructs and Dr I. Hwang (Pohang University of Science and Technology, Korea) for sharing the mRFP-Rha1 construct. We also thank the RIKEN Genomic Sciences Center (GSC) for providing the full-length cDNA of 2S albumin RAFL05-14-B09 (Seki et al., 1998, 2002). This work was supported by grants from the Research Grants Council of Hong Kong (CUHK4307/03M, CUHK4580/05M, CUHK488707 and CUHK465708), UGC-AoE, CUHK Scheme C, NSF of China (30529001) and the National 863 Program of China (2007AA02Z102) to LJ.
References
Accession numbers: At3g52850 (Arabidopsis AtVSR1), At2g30290 (Arabidopsis AtVSR2), At2g14740 (Arabidopsis AtVSR3), At2g14720 (Arabidopsis AtVSR4), At2g34940 (Arabidopsis AtVSR5), At1g30900 (Arabidopsis AtVSR6), At4g20110 (Arabidopsis AtVSR7), CAA28804 (Hordeum vulgare aleurain), At4g27140/RAFL05-14-B09 (RIKEN, Arabidopsis 2S albumin), At3g54840 (Arabidopsis Ara6), At5g45130 (Arabidopsis Rha1) and At4g19640 (Arabidopsis Ara7).




