NAC transcription factors NST1 and NST3 regulate pod shattering in a partially redundant manner by promoting secondary wall formation after the establishment of tissue identity
Summary
Three distinct pattern elements of the silique are thought to contribute to its dehiscence: a separation layer, cells with a secondary wall adjacent to the separation layer, and a valve endocarp layer with secondary wall. However, the role of the secondary wall has not been proven, and the factors that regulate its formation in siliques remain to be characterized. We show here that secondary wall formation in siliques is necessary for dehiscence, and that two plant-specific transcription factors, NAC SECONDARY WALL THICKENING PROMOTING FACTOR 1 and 3 (NST1 and NST3), regulate its formation in siliques of Arabidopsis. The promoters of the NST1 and NST3 genes were active in the valve endocarp layer and in cells surrounding vascular vessels in the replum, and NST1 promoter activity only was faintly detectable at valve margins. In nst1 mutants, specific loss of secondary walls was evident at valve margins, while nst1 nst3 double mutants lacked secondary walls in all parts of the siliques, with the exception of vascular vessels. These siliques were similarly indehiscent. The promoters of two tissue-identity genes, INDEHISCENT (IND) and SHATTERPROOF2 (SHP2), were as active in the nst1 nst3 mutant as in the wild-type. Moreover, the ectopic secondary wall formation that occurs in the fruitfull (ful) mutant was absent in the ful nst1 double mutant. We propose that secondary walls in valve margins are required for dehiscence, and that NST1 and NST3 regulate their formation in siliques in a partially redundant manner after the establishment of tissue identity.
Introduction
Many plant species, including those of the Brassica family such as oilseed rape and Arabidopsis, disperse their seeds by a mechanism known as ‘pod shattering’. This mechanism allows plants to disperse their seeds effectively. However, as much as 50% of the potential commercial yield is lost because some seeds have already dispersed before the harvest begins (MacLeod, 1981; Spence et al., 1996). Each silique is composed of two symmetrical valves, which are separated by a septum with a replum at each end. The secondary walls develop in the endodermis of the valve, which is known as the endocarp b layer (enb), and at a lignified layer in the valve margin, as well as in vascular vessels and their surrounding cells (Spence et al., 1996). Pod shattering in Arabidopsis is proposed to occur as follows: as the silique matures and dries, the inner cell layers of each valve, in which no secondary wall has been synthesized, shrink quickly, but the valve margins and the enb layer do not shrink because of the presence of secondary walls. The resulting tension allows the silique to spring open after secreted hydrolytic enzymes have enabled a cell–cell separation event to occur at the separation layer in the valve margin (Spence et al., 1996). Although this hypothesis is well known, no genetic evidence has been reported, and factors regulating secondary wall formation in the silique remain to be characterized. On the other hand, several factors that control cell identity within siliques have been characterized (Dinneny et al., 2005; Ferrandiz et al., 2000; Liljegren et al., 2000, 2004; Rajani and Sundaresan, 2001; Roeder et al., 2003). The bHLH transcription factor ALCATRAZ (ALC) controls differentiation of the separation layer in the valve margin. Loss of the ALC gene disrupts normal development of the separation layer and induces the development of ectopic lignified cells at the innermost layer of the valve margin (Rajani and Sundaresan, 2001). These ectopic lignified cells form an unruptured ‘bridge’ between the enb layer and the lignified cells of the replum, resulting in indehiscent siliques (Rajani and Sundaresan, 2001). A gene for the bHLH transcription factor INDEHISCENT (IND) and the MADS box genes SHATTERPROOF1 (SHP1) and SHP2 regulate differentiation of the valve margin (Liljegren et al., 2000, 2004). Loss of the IND gene or simultaneous loss of both SHP1 and SHP2 induce loss of the valve margin and result in indehiscent siliques (Liljegren et al., 2000, 2004). FRUITFULL (FUL) is a MADS box gene that is necessary for differentiation of the fruit valves and acts as a negative regulator of IND, SHP1, SHP2 and ALC (Liljegren et al., 2004). Disruption of FUL induces ectopic expression of IND, SHP1 and SHP2, which confers valve margin identity on the cells in the valve, resulting in short siliques and ectopic lignification of valves (Liljegren et al., 2004). Furthermore, FUL is considered to play a role in specifying the identity of the enb layer, because loss of FUL activity in the ind alc shp1 shp2 quadruple mutant induces complete loss of the lignified enb layer (Liljegren et al., 2004). However, the ful mutant still has a lignified enb layer, suggesting that all these genes are involved in establishment of the enb layer or that FUL is the primary gene for specifying the identity of enb layer and the ectopic activity of IND, SHPs and ALC induces lignification of the enb layer in the ful mutant (Liljegren et al., 2004).
We have shown previously that three plant-specific transcription factors, namely NAC SECONDARY WALL THICKENING PROMOTING FACTOR 1, 2 and 3 (NST1–3) (designated SND1 by Zhong et al., 2006), are responsible for secondary wall formation and act in a partially redundant manner (Mitsuda et al., 2005, 2007). NST1 and NST2 regulate secondary wall formation in the anther endothecium (Mitsuda et al., 2005), and NST1 and NST3 act in the inflorescence stem and hypocotyl (Mitsuda et al., 2007). However, the factors that regulate secondary wall formation in siliques remain uncharacterized despite their agronomic importance. In this study, we focused on the role of NST transcription factors in secondary wall formation in siliques. Gene-disruption analysis revealed that secondary wall formation in siliques is required for dehiscence and is regulated redundantly by NST1 and NST3. Together with previous studies, our data suggest that NST transcription factors might be key regulators of secondary wall formation in all plant tissues with the exception of vascular vessels.
Results
Expression of NST1 and NST3 during silique development
In previous studies, we have shown that the promoters of the NST1 and NST3 genes are very active in the inter-fascicular fibers of inflorescence stems, in cells that are differentiating into vascular vessels, in the secondary xylem of the root hypocotyl, and, in the case of NST1, in the anther endothecium (Mitsuda et al., 2005, 2007). To address whether these genes play significant roles in siliques, we examined cross-sections of siliques of plants harboring ProNST1:GUS and ProNST3:GUS constructs (Figure 1) (Mitsuda et al., 2005, 2007). The promoter activities of NST1 and NST3 were detectable in the vascular cells of the replum or enb layer, respectively, from stage 16 (Roeder and Yanofsky, 2006; Smyth et al., 1990), at which time siliques begin to elongate (Figure 1a,c). At early stage 17, promoter activities were evident in both the replum and the enb layer, even though the secondary wall had not been formed (Figure 1e–h). These promoter activities were observed at the same locations until silique maturity (Figure 1i,k). In the case of NST1, some promoter activity was also detected at the valve margin where lignified secondary walls develop (Figure 1m). RT-PCR analysis showed that expression of NST1 was rapidly induced after stage 16, when the silique began to elongate. NST1 expression reached its maximum level at early stage 17, before secondary walls became evident, and then decreased (Figure S1). This expression pattern is consistent with the results of the promoter–reporter experiment described above (Figure 1). Expression of IRREGULAR XYLEM 3 (IRX3), a gene encoding cellulose synthase, was strongly correlated with that of NST1. These data suggest the functional involvement of NST1 and NST3 in secondary wall formation in siliques. On the other hand, expression of IND, which confers the tissue identity of valve margins, reached a maximum at an early stage of maturation, and decreased before expression of NST1 was strongly induced. Expression of ABSCISIC ACID INSENSITIVE 3 (ABI3), a marker gene for seed maturation, gradually increased at late stages of development, indicating that the RNA of late stages was not degraded.
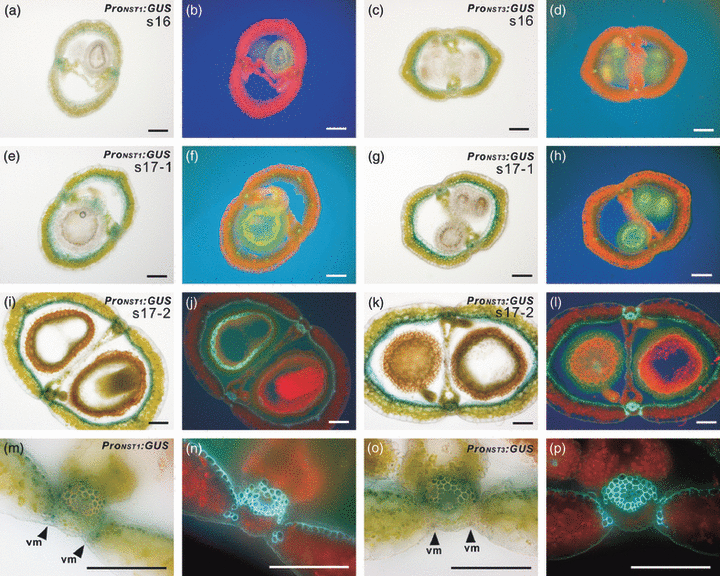
Activities of the promoters of the NST1 and NST3 genes in siliques.(a–p) Cross-sections of siliques of Arabidopsis harboring ProNST1:GUS (a, e, i, m) and ProNST3:GUS (c, g, k, o). The same sections were viewed under UV illumination (b, d, f, h, j, l, n, p). The region around the valve margin is shown at higher magnification in (m–p). vm, valve margin. The NST1 promoter had weak activity at the valve margin but the NST3 promoter did not. Scale bars = 100 μm.
Disruption of NST1 and NST3 results in loss of secondary walls in siliques and indehiscent siliques
To examine the roles of NST1 and NST3 in secondary wall formation in siliques, we examined homozygous ‘knockout’ lines of the NST1 and NST3 genes nst1-1 (SALK_120377), nst1-2 (SALK_149993), nst3-1 (SALK_149909) and nst3-2 (SALK_131657), previously described by Mitsuda et al. (2005, 2007). We also examined the newly identified NST1 gene knockout line, SM_3_28212, referred to as nst1-3 (Figure S2a). The observed phenotype of all three independent NST1 knockout lines was the same, and the phenotype of nst1-2 mutant is therefore described as representative of all three lines unless otherwise stated. Compared to wild-type, the secondary walls in the part of the enb layer close to valve margins in the nst1-2 mutant formed later, and secondary walls were absent at the valve margins (Figure 2c,d and Figure S3c,d). Even though both NST3 single knockout lines had a normal phenotype, nst1-1 nst3-1 double knockout plants lacked all secondary walls in siliques except those of vascular vessels (Figure 2e,f and Figure S3e,f), as for the inflorescence stem and hypocotyl, as described previously (Mitsuda et al., 2007). The loss of secondary walls resulted in naturally indehiscent siliques, even in NST1 single knockout lines, which lacked secondary walls only at the valve margins (Figure 2g). The siliques of nst1-2 and nst1-1 nst3-1 mutants could be opened along the valve margin by rubbing between the fingers. The degree of indehiscence appeared to be similar between the nst1-2 and nst1-1 nst3-1 mutants. By contrast, siliques of shp1 shp2 mutant could not be opened easily, probably because valve margins were absent. These observations suggested that secondary wall formation in valve margins is required for silique dehiscence, and that NST1 and NST3 regulate secondary wall formation in siliques in a partially redundant manner.
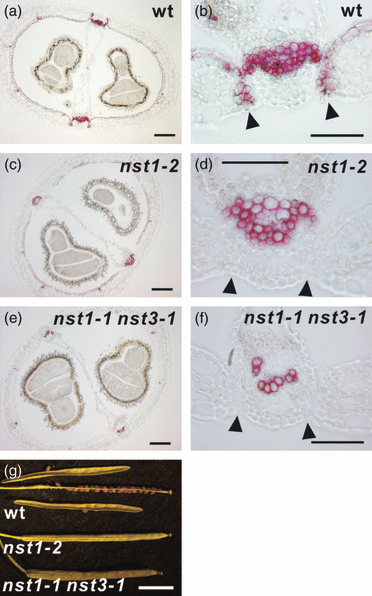
Siliques of nst mutants plants lack secondary walls.(a–f) Cross-sections of siliques of wild-type (a, b), nst1-2 (c, d) and nst1-1 nst3-1 (e, f) plants after staining with phloroglucinol. Regions around the valve margins are shown at higher magnification in (b), (d) and (f). Arrowheads indicate valve margins.(g) Appearance of siliques of wild-type, nst1-2 and nst1-1 nst3-1 plants. Wild-type siliques dehisced easily, but those of nst1-2 and nst1-1 nst3-1 did not. Scale bars = 100 μm (a–f) or 5 mm (g).
Complementation analysis of the nst1 nst3 mutants
To confirm that the phenotype observed in nst mutants was due to the loss of functional NST genes, we introduced a genomic fragment that included either the NST1 or the NST3 gene into the nst1-1 nst3-1 double mutant. The genomic fragment containing NST1 or NST3 reversed the defective phenotype of the enb layer and the region surrounding the vascular vessels in the replum of the nst1-1 nst3-1 double mutant (Figure S3g–j). However, the defect in secondary wall formation at the valve margin of the nst1 nst3 mutant was not completely reversed, and siliques remained indehiscent when the NST1 genomic fragment (which included 6.5 kbp of the 5′ upstream and 1 kbp of the 3′ downstream region of NST1) was introduced (Figure S3g,h). It is possible that the genomic fragment of NST1 used in this study did not contain all the cis element(s) necessary for sufficient expression of NST1 in the valve margin. The NST3 genomic fragment did not restore a normal phenotype at the valve margin (Figure S3i,j). The phenotype of resultant plant (nst1-1 nst3-1 NST3g) was the same as that of NST1 single knockout plants. These results are consistent with those from the phenotypic analyses of knockout plants and the promoter–reporter experiment, in which only weak NST1 promoter activity was detected in the valve margins and NST3 promoter activity was undetectable (Figure 1m,o).
RNAi and expression of a chimeric repressor for NST1 result in a phenotype similar to that of NST1-disrupted plants
To support our hypothesis that the phenotype of nst1 mutants was due to loss of NST1 activity, we attempted to mimic the phenotype of NST1 knockout lines by RNAi. Although the introduced RNAi construct directed against NST1 did not completely suppress expression of endogenous NST1 (Figure S4a), all examined transgenic plants (six lines) had extremely reduced secondary walls at the valve margin and showed reduced dehiscence, as seen for NST1 knockout lines (Figure S4b,c). Furthermore, expression of NST3 was unaffected by RNAi directed against NST1 (data not shown). Thus, the RNAi construct successfully and specifically suppressed the expression of NST1 and mimicked the phenotype of nst1 mutants.
We also attempted to mimic the indehiscent phenotype of siliques using the novel gene-silencing technology known as Chimeric Repressor Silencing Technology (CRES-T) (Hiratsu et al., 2003). An NST1 chimeric repressor, in which NST1 had been fused to the SRDX repression domain, was expressed under the control of the CaMV 35S promoter. Although the resultant 35S:NST1SRDX plants had only a few siliques, as most flowers had indehiscent anthers (Mitsuda et al., 2005), secondary walls of valve margins were absent in the siliques that did develop (Figure S4e). However, secondary wall formation was not suppressed at sites other than the valve margin. The NST1SRDX gene driven by the CaMV 35S promoter may not have been able to overcome the gene redundancy of NST1 and NST3 in tissues other than the valve margin. Alternatively, the effect of the chimeric NST1 repressor in the elongated siliques of 35S:NST1SRDX plants may be weak because most flowers of 35S:NST1SRDX plants do not bear siliques due to indehiscent anthers (Mitsuda et al., 2005). Next we expressed NST1SRDX under the control of the NST1 promoter or the NST3 promoter (ProNST1:NST1SRDX and ProNST3:NST1SRDX, respectively). The plants expressing ProNST1:NST1SRDX had indehiscent anthers in most flowers, resulting in male sterility. This effect was much stronger than that of 35S:NST1SRDX. Partially elongated siliques were occasionally observed in ProNST1:NST1SRDX plants. Although these siliques showed a severe reduction of secondary walls in the enb layer and valve margin, the suppression was not complete, probably due to the weak effect of the chimeric repressor in such elongated siliques (Figure S4f). In contrast to ProNST1:NST1SRDX plants, anther dehiscence was unaffected in ProNST3:NST1SRDX plants and most flowers bore fully elongated siliques. In these siliques, the secondary walls were completely lost except those in the valve margins and vascular vessels (Figure S4g,h). The resultant siliques were indehiscent, but the degree of indehiscence was not as pronounced as that in the nst1-2 mutant, which lacks secondary walls only in the valve margins.
Expression of genes related to secondary wall formation or cell-separation events in the nst1 nst3 mutant
We used a promoter–reporter experiment to identify genes that are under the control of the NST transcription factors. We examined expression of the IRX3 and CINNAMYL ALCOHOL DEHYDROGENASE-D (CAD-D) genes, which encode cellulose synthase (Taylor et al., 1999) and an enzyme that is involved in lignin biosynthesis (Sibout et al., 2005), respectively. We have previously shown that both promoters had activities in cells with secondary walls in the inflorescence stem and lost them in nst1-1 nst3-1 plants except in cells destined for vascular vessels and their adjacent cells (Mitsuda et al., 2007). In siliques, activities of both promoters were observed in regions where secondary walls normally develop, namely the enb layer of the valve, the valve margins, in cells that are differentiating into vascular vessels, in the cells that surround these vessels in the replum, and, in the case of CAD-D, in cells adjacent to vascular vessels (Figure 3a–d). However, in the nst1-1 nst3-1 background, promoter activities were evident only in cells that were differentiating into vascular vessels, and, in the case of CAD-D, in cells adjacent to vascular vessels (Figure 3e–j). These data suggest that IRX3 and CAD-D function in the formation of secondary walls in siliques under the control of NST transcription factors.
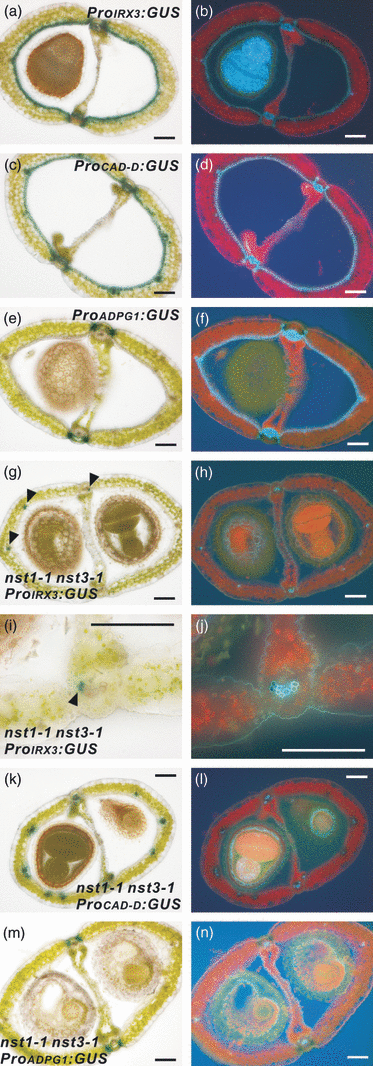
Promoter activities of genes involved in secondary wall formation or cell-separation events in siliques.(a–f) Cross-sections of siliques of ProIRX3:GUS (a), ProCAD-D:GUS (c) and ProADPG1:GUS (e) plants. The same sections were viewed under UV illumination (b, d, f).(g–n) Cross-sections of siliques harboring ProIRX3:GUS (g, i), ProCAD-D:GUS (k) and ProADPG1:GUS (m) in the nst1-1 nst3-1 background. The same sections were viewed under UV illumination (h, j, l, n). Scale bars = 100 μm.
In addition, we examined the promoter activity of the gene encoding endo-polygalacturonase (At3g57510; ADPG1), which is expressed in the dehiscence zone of the silique (Gonzalez-Carranza et al., 2007; Jenkins et al., 1999). This enzyme is thought to separate tissues by degrading cells in the dehiscence zone (Gonzalez-Carranza et al., 2007; Jenkins et al., 1999). Our results showed that ADPG1 promoter activity was detected in the separation layer of the silique in the nst1-1 nst3-1 mutant background, as in the wild-type background (Figure 3e,m). This suggests that the separation layer is properly formed, and the cell-separation event appear to occur without secondary wall formation.
Valve margins are specified properly without the NST genes
To address whether the NST genes are involved in the establishment of valve margin identity, morphological analyses of siliques were performed comparing nst mutants and the shp1 shp2 mutant. As previously described (Liljegren et al., 2000), the boundary between the valve and each replum in the shp1 shp2 mutant was unclear as a result of the absence of valve margins (Figure 4d,h). By contrast, those of nst mutants were clear and well-defined as in wild-type plants (Figure 4a–c,e–g). Further cytological observations revealed that both the cell number and morphology of the valve margin of the nst1-2 or nst1-1 nst3-1 mutant were indistinguishable from that of wild-type plants, with the exception of the lack of secondary walls in the mutant plants (Figure 4e–g). The separation layer observed in nst1-2 and nst1-1 nst3-1 mutants was similar to that of the wild-type (Figure 4e–g). We analyzed the promoter activities of SHP2 and IND using ProSHP2:GUS and ProIND:GUS reporter genes. These promoter activities were evident at valve margins in the wild-type background, and the promoters were also active at the valve margin in the nst1-1 nst3-1 mutant, even though secondary walls failed to develop (Figure 4i–p). This phenotypic and molecular evidence suggests that the identity of the valve margin is specified properly even in the nst1-1 nst3-1 mutant.
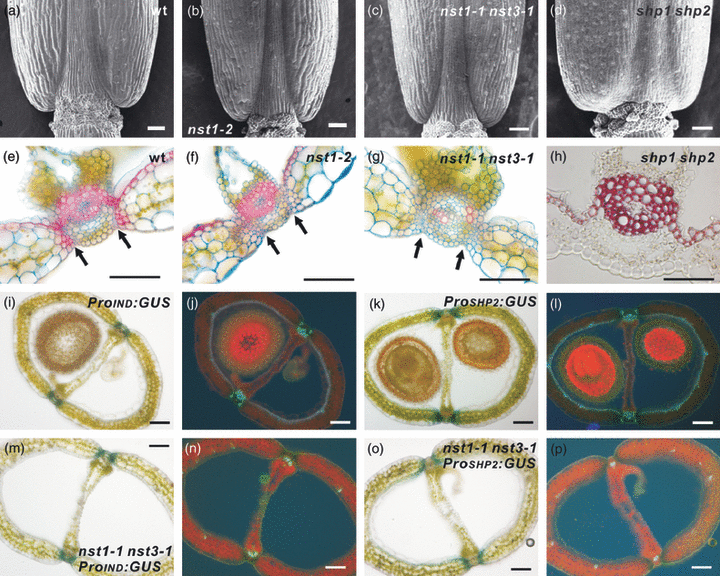
Valve margins are properly specified without NSTs.(a–d) Morphology of basal parts of siliques of wild-type (a), nst1-2 (b), nst1-1 nst3-1 (c) and shp1 shp2 (d) plants, as observed by scanning electron microscopy.(e–g) Cross-sections of siliques of wild-type (e), nst1-2 (f) and nst1-1 nst3-1 (g) plants after staining with Alcian blue and Safranin O. Arrows indicate separation layers.(h) Cross-section of a silique of a shp1 shp2 plant after staining with phloroglucinol.(i–l) Cross-sections of siliques harboring ProIND:GUS (i) and ProSHP2:GUS (k). The same sections were viewed under UV illumination (j, l).(m–p) Cross-sections of siliques harboring ProIND:GUS (m) and ProSHP2:GUS (o) in the nst1-1 nst3-1 background. The same sections were viewed under UV illumination (n, p).Scale bars = 50 μm (a–h) or 100 μm (i–p).
NST1 regulates ectopic secondary wall formation in the ful mutant
Loss of function of the FUL gene (ful) results in very short siliques that are full of seeds (Gu et al., 1998). The absence of FUL induces ectopic expression of IND, SHP1, SHP2 and ALC in the valve. This confers valve margin identity on cells in the valve, with resultant ectopic formation of secondary walls in the valve and loss of stomata formation on the valve surface (Figure 5e,g) (Ferrandiz et al., 2000; Liljegren et al., 2004). To investigate whether the ectopic secondary wall formation was due to the activity of NST1, we prepared a double mutant of FUL and NST1. The ful mutant described by Ferrandiz et al. (2000) and Liljegren et al. (2004) formed ectopic secondary walls in all cells of the three internal valve layers. Although the homozygous T-DNA-tagged ful mutant used in this study (SALK_033647) had very short siliques, similar to the ful mutant described above, only a proportion of the cells in the three internal valve layers was lignified, and secondary walls in the enb layer, as observed in the wild-type and in the ful mutant described previously (Ferrandiz et al., 2000; Liljegren et al., 2004), were not observed (Figure 5a,c,e). These differences might be due to small amounts of normal or aberrant FUL transcripts detected in our ful mutant, in which the T-DNA tag is inserted into the 6th intron (Figure S2b,c). The proteins encoded by these transcripts might partially suppress the ectopic expression of IND, SHP1, SHP2 and ALC, which is necessary for ectopic lignification of the enb layer and valve internal layers in the absence of FUL (Liljegren et al., 2004). Also, the difference in ecotype background of the ful mutants (Ler versus Col) cannot be ruled out as the cause of the phenotypic difference. Nevertheless, most characteristics of ful mutant phenotypes described by Ferrandiz et al. (2000) and Liljegren et al. (2004) were observed in our ful mutant (Figure 5c,e,g). In the ful nst1-1 double mutant, ectopic secondary wall formation was completely suppressed (Figure 5i,k), indicating that ectopic formation of secondary walls in the ful mutant is due to NST1 activity. However, the phenotypes of abnormally short siliques and lack of stomata of the ful mutant were not restored by disruption of NST1, indicating that the formation of short siliques and loss of stomata in the ful mutant is independent of the function of NST1 and the formation of secondary walls (Figure 5a–d). We also generated a ful nst3-1 double mutant and a ful nst1-1 nst3-1 triple mutant. The phenotype of ful nst3-1 mutant was same as that of the ful mutant, and the ful nst1-1 nst3-1 mutant had the same phenotype as the ful nst1-1 mutant (Figure 5 and Figure S5), with an additional loss of secondary walls in cells surrounding vascular vessels of the replum (data not shown). We further analyzed the promoter activities of NST1 and NST3 in the ful background. NST1 promoter activity was weakly detected in cells with ectopic secondary walls and in cells with secondary walls in the replum (Figure 5m). Not all cells with ectopic secondary walls showed GUS activity driven by the NST1 promoter, probably because of the weak activity of the NST1 promoter in the valve margins (Figure 1m). Our results suggest that NST1 is expressed in cells with ectopic secondary walls in the ful mutant. On the other hand, NST3 promoter activity was not detected in cells with ectopic secondary walls (Figure S5l), supporting the idea that NST3 is not expressed in the valve margins.
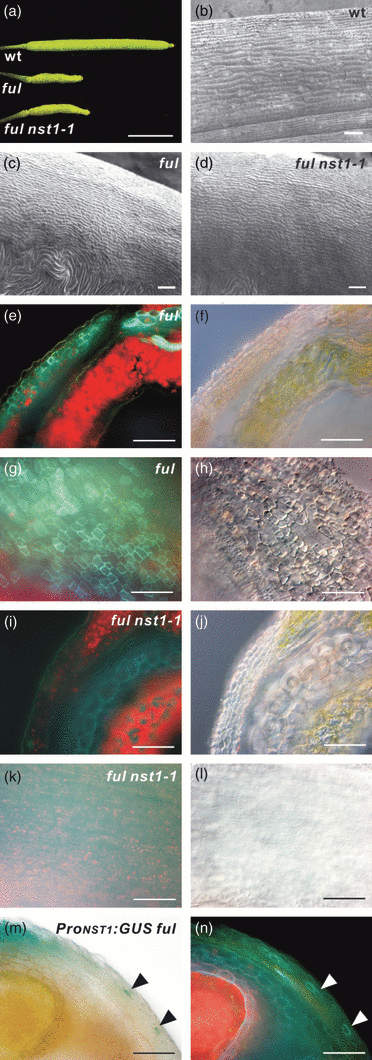
Ectopic secondary wall formation in the ful mutant depends on NST1.(a) Siliques of wild-type, ful and ful nst1-1 plants, as indicated.(b–d) Scanning electron microscopy images of valve surfaces of wild-type (b), ful (c) and ful nst1-1 (d) plants.(e, f, i, j) Cross-sections of ful (e, f) and ful nst1-1 (i, j) plants viewed under UV illumination (e, i) or differential interference contrast (DIC) microscope (f, j).(g, h, k, l) Siliques of ful (g, h) and ful nst1-1 (k, l) plants pressed under a cover slip and viewed under UV illumination (g, k) or DIC microscope (h, l).(m, n) Cross-section of a ProNST1:GUS ful plant viewed under the microscope (m) or UV illumination (n). Arrowheads indicate cells with GUS staining and ectopic secondary walls. Scale bars = 5 mm (a) or 50 μm (b–n).
Discussion
Secondary wall formation is necessary for silique dehiscence
It has long been thought that secondary wall formation in the silique is required for its natural dehiscence, even though there was no genetic evidence to support this. In this study, we found that siliques that lack secondary walls in valve margins are indehiscent. This clearly indicates that secondary wall formation in valve margins is required for silique dehiscence. Interestingly, no difference was observed in the degree of indehiscence between siliques with no secondary walls in valve margins only and those with further loss of secondary walls in the replum and the enb layer. On the other hand, the siliques of the ProNST3:NST1SRDX plants that retained secondary walls in the valve margins but not in the replum and the enb layer had a weaker degree of indehiscence than those lacking secondary walls in valve margins and the enb layer. These data suggest that secondary wall formation in the enb layer is not as important as that in valve margins in Arabidopsis, although the intensities of the indehiscent phenotype of the ProNST3:NST1SRDX plants varied widely because of the various levels of transgene expression. Future research should examine the importance of secondary wall formation in the enb layer in plant species other than Arabidopsis.
NST1 and NST3 regulate secondary wall formation in siliques in a partially redundant manner after the establishment of tissue identity
In this study, we found that three independent NST1-disrupted lines had the same phenotype with respect to loss of secondary walls predominantly at the valve margin, which resulted in naturally indehiscent siliques. Furthermore, RNAi and the chimeric repressor directed against NST1 yielded the same phenotype as that of NST1 knockout plants. A genomic fragment that included the NST1 gene failed to restore a completely normal valve margin. This result was consistent with the observation that the activity of the NST1 promoter was rather weak at the valve margin. It is possible that the genomic fragment used in this study did not include the cis region necessary for restoration of the normal valve margin. The NST3 single knockout plants had no defect and the NST1 and NST3 double knockout plants lacked secondary walls in all tissues except vascular vessels. This observation indicates that the NST1 and NST3 genes function redundantly in the valve endocarp layer and in the cells that surround vascular vessels in the replum. It should be noted that secondary wall formation in the part of enb layer near the valve margins was delayed in the nst1-2 mutant, suggesting a predominant role for NST1 in the formation of secondary walls in this region. At the valve margin, only NST1 appears to be functional.
We found that the promoter activities of IND and SHP2 were maintained at the valve margin even in the nst1-1 nst3-1 double mutant plant. This observation suggests that valve margin identity can be established without NST genes. In addition, expression of IND is induced prior to that of NST1 at an early stage of silique maturation, suggesting that NST genes act after the establishment of tissue identity. We also found that the promoter activity of ADPG1, the gene product of which is involved in the cell-separation event in the separation layer, was also maintained in the nst1-1 nst3-1 mutant. This suggests that the identity of the separation layer is also established without NST genes, and the cell-separation event might occur independently of secondary wall formation. Furthermore, we found that the ectopic formation of secondary walls in valves of the ful mutant, which was mainly induced by the ectopic expression of IND, was suppressed by disruption of NST1, even though the phenotypes of short siliques and no stomata of the ful mutant were not suppressed. Thus, of the various phenotypes of the ful mutant, only that of ectopic secondary wall formation depends on the function of NST1. The phenotypes of short siliques and no stomata of the ful mutants are probably due to the formation of small valve-margin-like cells in the valve. These could result from ectopic conferral of valve margin identity to the valve cells. In other words, NST1 induces secondary wall formation but is not involved in the establishment of valve margin identity. From these data, we conclude that the NST genes act as master regulators of secondary wall formation after the establishment of tissue identity by FUL, SHPs and IND (Figure 6a).
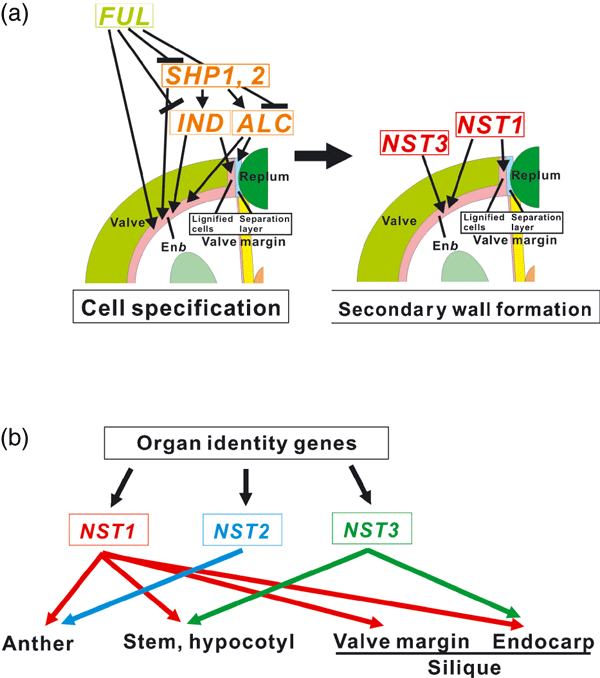
Redundant roles of NST genes in secondary wall formation in plants.(a) Proposed function of NSTs in secondary wall formation of siliques. Acting in a partially redundant manner, NST1 and NST3 promote secondary wall formation after the establishment of tissue identity by SHP1, SHP2, IND, ALC and FUL, at the valve margin, in cells surrounding vascular vessels in the replum, and in the enb layer.(b) Roles of NST genes in the entire plant. NST1 promotes secondary wall formation in the anther, stem, hypocotyls, valve margin, and cells surrounding vascular vessels in the replum and valve endocarp. NST2 functions only in the anther. NST3 functions in the stem, hypocotyl, cells surrounding vascular vessels in the replum and valve endocarp. NST genes function downstream of the tissue-identity genes that determine cell fate.
Control of pod shattering by manipulation of NST1
Manipulation of silique dehiscence would be agronomically useful in plants that have naturally dehiscent siliques, such as oilseed rape, sesame and soybean. In this study, we showed that RNAi and the chimeric repressor directed against NST1 successfully induced indehiscent siliques at high frequency. As shown in Figure 4, there appeared to be no difference in silique morphology between nst mutants and wild-type plants, in which the valve and replum are completely separated, while siliques of the shp1 shp2 double mutant were clearly different from those of the wild-type, with valve and replum coalescing as a result of loss of the valve margin. The seeds of nst mutants could be released from siliques manually by rubbing between the fingers without breaking the silique wall. In an agronomic context, this feature might be preferable to suppression of formation of the valve margin itself, if we could manipulate the NST genes to specify the effect only in the siliques, but not in anthers or stems.
NST transcription factors are key regulators of secondary wall formation in almost all plant tissues
Plants produce thickened secondary walls, composed mainly of lignin and cellulose, beneath their primary cell walls. In contrast to primary cell walls, secondary walls are synthesized in specific tissues, such as fibers, xylem, anthers and siliques. Secondary walls in the xylem of woody plants, and especially in the secondary xylem, provide mechanical strength to support the plant body and to withstand water pressure in vascular vessels, while secondary walls in the anthers and siliques are required for dehiscence, allowing different rates of shrinkage between cell layers with and without secondary walls. In previous studies, we showed that NST1 and NST2 function redundantly to promote secondary wall formation in anthers (Mitsuda et al., 2005), and, moreover, that NST1 and NST3 function redundantly in inter-fascicular fibers and secondary xylem after the establishment of cells destined to form these tissues (Mitsuda et al., 2007). We also showed that all three NSTs can induce ectopic formation of secondary walls in various tissues when they are overexpressed (Mitsuda et al., 2005, 2007), indicating that NSTs are necessary and sufficient for the formation of secondary walls. Given the results of this study, we propose that NSTs are key regulators of secondary wall formation in almost all plant tissues, and that NSTs act in a complex and redundant manner after the establishment of tissue identity (Figure 6b). Downstream genes encoding enzymes involved in the synthesis of lignin and cellulose are also part of the process. For example, IRX3, encoding cellulose synthase, and CAD-D, encoding an enzyme that is involved in lignin synthesis, function downstream of NSTs both in inter-fascicular fibers (Mitsuda et al., 2007) and siliques (this study). This suggests that the molecular mechanism of secondary wall formation might be conserved in the stem and the silique. However, it should be noted that the promoter activity of AtCAD1, which is involved in lignin biosynthesis, is not detected in inter-fascicular fibers of inflorescence stems, but is observed in the replum and enb layer of siliques. AtCAD1 promoter activity is absent in shp1 shp2 mutant (Eudes et al., 2006). This indicates that some genes that are involved in secondary wall formation are differentially expressed between stems and siliques.
Regulation of the formation of secondary walls in mature phloem tissue remains to be fully characterized (Mitsuda et al., 2007). It is unclear how expression of the genes that are involved in biosynthesis of the components of secondary walls, such as lignin and cellulose, is actually regulated. Further studies are required to unravel all the complexities of gene regulation during formation of the secondary wall.
Experimental procedures
Construction of plasmids
Plasmids for expression of ProNST1:GUS, ProNST3:GUS, ProIRX3:GUS, ProCAD-D:GUS, 35S:NST1SRDX, ProNST1:NST1SRDX, ProNST3:NST1SRDX and the genomic fragments of NST1 and NST3 were prepared as described previously (Mitsuda et al., 2005, 2007). 5′ upstream regions of 3140, 3030 and 2297 bp from the sites of initiation of translation of the ADPG1, IND and SHP2 genes, respectively, were used for preparation of ProADPG1:GUS, ProIND:GUS and ProSHP2:GUS. These genes were constructed in modified vectors derived from p35SSRDXG (Mitsuda et al., 2006). Each transgene was transferred to the pBCKH plant expression vector or its derivative (Mitsuda et al., 2006). For RNAi, the region from bp 18–434 of the coding sequence of NST1 was sub-cloned into the pHellsgate8 vector (Wesley et al., 2001).
Plants with disrupted NST1 or FUL
For the mutants of the NST1 gene, we used the T-DNA-tagged lines SALK_120377 (nst1-1) and SALK_149993 (nst1-2) described previously (Mitsuda et al., 2005, 2007), and a transposon-tagged line SM_3_282128 (nst1-3) (Figure S2a). For the ful mutant, we used a T-DNA-tagged line, SALK_033647 (ful) (Figure S2b).
RT-PCR analysis
Total RNA was isolated from rapidly growing siliques using guanidium thiocyanate-phenol-chloroform as described previously (Fukuda et al., 1991). First-strand cDNA, reverse-transcribed from the total RNA, was used as the template for PCR. The full-length and partial fragments of NST1 and FUL were amplified by 26 (Figure S4a) or 30 (Figure S2) cycles of PCR with appropriate primers (Table S1). Quantitative RT-PCR was performed as described previously (Mitsuda et al., 2005).
Conditions for plant growth and transformation
Arabidopsis thaliana (Col-0) plants were used in all experiments. They were grown in soil at 22°C with a 16 h/8 h light/dark photoperiod. For transformation, a T-DNA vector carrying the appropriate construct was introduced into Agrobacterium tumefaciens strain GV3101 by electroporation, and the resultant Agrobacterium was infiltrated into Arabidopsis by the floral dip method (Clough and Bent, 1998).
Light and fluorescence microscopy
For observations of the autofluorescence of lignin, we used a filter with the following specifications: glass:365, dichroic mirror:395 and lone pass 400. Unless otherwise stated, mature siliques (late stage 17) were used to prepare cross-sections. To prepare sections of siliques (70–100 μm), we embedded the tissue in 3% agar and then sectioned it on a vibrating microtome (HM-650V; Microm Inc., http://microm-online.com). To examine the deposition of lignin, we embedded siliques in Paraplast Plus (McCormick Scientific Inc., http://www.mccormickscientific.com) for preparation of 8 μm cross-sections. After removal of paraffin, samples were stained with 2% w/v phloroglucinol in 95% ethanol for 2–5 min, washed in 10 n HCl for 1 min, and mounted in 5 n HCl. To observe the valve margin in more detail, 70 μm sections of siliques were stained using 0.01% Safranin O and 0.1% Alcian blue solutions (Wako Inc., http://www.wako-chem.co.jp). Assays for β-glucosidase (GUS) activity were performed using T1 transgenic plants. Siliques were cut in half horizontally and fixed briefly (10–15 min) in a solution that contained 0.3% formalin, 0.2% MES (pH 5.8) and 0.3 m mannitol before incubation in 100 mm sodium phosphate buffer (pH 7.0) that contained 0.1% Triton X-100, 1 mm 5-bromo-4-chloro-3-indolyl-β-d-glucuronide (X-gluc) and 0.5 mm potassium ferricyanide at 37°C for up to 12 h. Stained siliques were embedded in 3% agar and sectioned. All observations by light and fluorescence microscopy were made with the Axioskop2 Plus system (Carl Zeiss Inc., http://www.zeiss.com/).
Scanning electron microscopy
Mature but still green siliques of 3- to 4-week-old plants were examined with a scanning electron microscope (VE8800; Keyence Inc., http://www.keyence.co.jp), at an accelerating voltage of 1 kV, without any pre-treatment.
Acknowledgements
The authors thank the Salk Institute, the Arabidopsis Biological Resource Center (ABRC) and the European Arabidopsis Stock Centre (NASC) for providing seeds of plants with disrupted NST1, NST3 or FUL genes, Yukie Kimura, Sumiko Takahashi, Manami Watanabe, Yoshimi Sugimoto, Naomi Ujiie, Akiko Kushida-Kuwazawa and Yuko Takiguchi for their skilled technical assistance, and Dr Miho Ikeda, Dr Tomotsugu Koyama and Dr Kyoko Matsui for helpful discussions and advice.




