Chlamydomonas chloroplasts can use short dispersed repeats and multiple pathways to repair a double-strand break in the genome
Summary
Certain group I introns insert into intronless DNA via an endonuclease that creates a double-strand break (DSB). There are two models for intron homing in phage: synthesis-dependent strand annealing (SDSA) and double-strand break repair (DSBR). The Cr.psbA4 intron homes efficiently from a plasmid into the chloroplast psbA gene in Chlamydomonas, but little is known about the mechanism. Analysis of co-transformants selected using a spectinomycin-resistant 16S gene (16Sspec) provided evidence for both pathways. We also examined the consequences of the donor DNA having only one-sided or no homology with the psbA gene. When there was no homology with the donor DNA, deletions of up to 5 kb involving direct repeats that flank the psbA gene were obtained. Remarkably, repeats as short as 15 bp were used for this repair, which is consistent with the single-strand annealing (SSA) pathway. When the donor had one-sided homology, the DSB in most co-transformants was repaired using two DNAs, the donor and the 16Sspec plasmid, which, coincidentally, contained a region that is repeated upstream of psbA. DSB repair using two separate DNAs provides further evidence for the SDSA pathway. These data show that the chloroplast can repair a DSB using short dispersed repeats located proximally, distally, or even on separate molecules relative to the DSB. They also provide a rationale for the extensive repertoire of repeated sequences in this genome.
Introduction
Double-strand breaks (DSBs) in DNA can lead to loss of genetic information, instability, or even cell death. DSBs may be caused by exogenous agents such as ionizing radiation, or by endogenous stressors such as reactive oxygen species, endonucleases or topoisomerases (Bleuyard et al., 2006). Eukaryotic plant cells have three genomes (nuclear, mitochondrial and chloroplastic) to maintain, but chloroplast DNA (cpDNA) may be subject to the most genotoxic stress, because of its close proximity to oxygen-producing photosynthetic membranes (Lindbeck and Rose, 1990; Rose, 1979). A recent study of spontaneous mutagenesis in Chlamydomonas suggested that oxidative damage plays an important role in nucleotide substitution patterns (GuhaMajumdar and Sears, 2005).
Despite the implications, little is known about DNA repair in chloroplasts, including repair of DSBs. It is known, however, that chloroplasts must have efficient repair machinery (GuhaMajumdar and Sears, 2005), and are capable of photoreactivation and dark repair of UV damage (Selby and Sancar, 2006; Small and Greiman, 1977), oligonucleotide-directed repair (Kmiec et al., 2001), and gene conversion (Khakhlova and Bock, 2006; Lemieux et al., 1988).
Recent efforts toward understanding DSB repair in plant nuclear DNA have resulted in significant progress, particularly in somatic cells of Arabidopsis (reviewed by Bleuyard et al., 2006; Puchta, 2005). An instrumental part of many studies has been the utilization of I-SceI, a rare-cutting endonuclease derived from a group I intron (Puchta, 2005). As in yeast and mammals, repair of a nuclear DSB in plants occurs by one of two general mechanisms, homologous recombination (HR) or non-homologous end joining (NHEJ). Repair by NHEJ – which does not require homologous sequences, but is promoted by microhomology – is more frequent and more error-prone. Repair of DSBs by HR can be very accurate, but gene conversion may occur if the intact and broken DNAs are not identical. This is the case for the example in Figure 1, where one allele has a mobile intron that is also the cause of the DSB; after recombination, both alleles contain the intron.
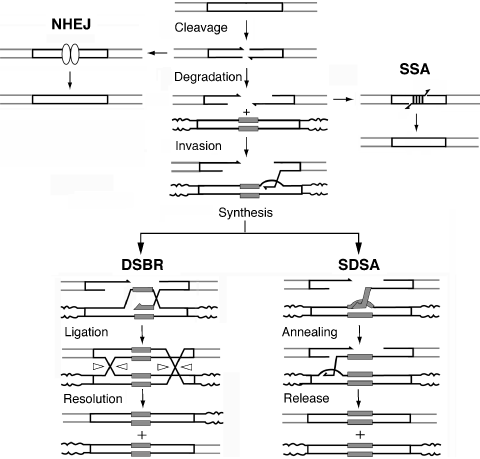
Pathways for repair of a DSB by intron homing and alternative mechanisms.Intron homing involves the two central pathways, DSBR and SDSA. DNA cleavage is followed by degradation of 5′ ends to generate 3′ single-stranded tails, which have limited degradation. Invasion of homologous DNA occurs with both pathways, but only DSBR forms Holliday junctions, whose resolution can result in crossing over (as shown). In NHEJ, the free ends are re-ligated, but often not before some damage to the ends. SSA can occur when homologous sequences on either side of the break become single-stranded; the sequences anneal, and the non-homologous flaps are removed by an endonuclease.
Two distinct gene-conversion mechanisms are shown in Figure 1: classical double-strand break repair (DSBR; Szostak et al., 1983) and synthesis-dependent strand annealing (SDSA). In both mechanisms, single-stranded 3′ tails formed by a 5′ exonuclease invade the intact allele and prime DNA synthesis. However, in DSBR, Holliday junctions form and are subsequently resolved to produce crossovers in approximately 50% of the repair events (Pâques and Haber, 1999). SDSA does not result in crossovers.
An alternative HR mechanism for DSB repair that results in DNA loss is single-strand annealing (SSA) between direct repeats (Figure 1). In SSA, the 3′ single-stranded tails anneal to each other, and the non-homologous DNA (or ‘flaps’) is removed before repair DNA synthesis (Pâques and Haber, 1999). In plants, SSA is more efficient than the gene-conversion mechanisms (Puchta, 2005). In yeast and mammalian cells, SSA can occur between direct repeats as short as 29 bp, although 300–400 bp of homology is much more efficient (Perez et al., 2005; Sugawara et al., 2000).
Less is known about the consequences and repair of a DSB in organellar genomes. Homing of group I introns is well known in fungal and Chlamydomonas mitochondria (reviewed in Belfort et al., 2002), suggesting that they have the capacity for DSBR or SDSA. Also, NHEJ and HR activity have been reported for mammalian mitochondria in vitro (Lakshmipathy and Campbell, 1999; Thyagarajan et al., 1996), suggesting that mitochondria may use both general repair mechanisms.
Chloroplasts in Chlamydomonas spp. have mobile group I introns (reviewed in Sears, 1998), indicating they are able to repair a DSB by HR and gene conversion. Homing of the Cr.LSU intron into an ectopic target (23S cDNA) occurred when the target was in an inverted orientation relative to the donor gene (Dürrenberger et al., 1996). Crossover products were not detected, suggestive of the SDSA pathway, thus raising the possibility that the chloroplast is incapable of DSBR. When the ectopic target was in the same orientation as the donor, a deletion occurred, consistent with repair by SSA (Dürrenberger et al., 1996).
Odom et al. (2001) used a different approach to demonstrate homing of the Cr.psbA4 intron (psbA intron 4) of C. reinhardtii; the donor (+ intron) DNA was located on a plasmid that was delivered into a strain (IL) with an intronless psbA gene (psbAintronless; Johanningmeier and Heiss, 1993). There was essentially 100% co-conversion of the downstream exon to 3-(3,4-dichlorophenyl)-1,1-dimethylurea resistance (DCMUR), which enabled semi-quantitative assessment of homing efficiency. The Cr.psbA4 intron-encoded endonuclease I-CreII (Kim et al., 2005) recognizes a 30 bp sequence in psbAintronless DNA that spans the intron 4 insertion site. I-CreII also has its own promoter, and can sustain homing without the rest of the psbA gene (Odom et al., 2001). In this study, we wished to determine which pathway(s) the chloroplast uses for intron homing and DSB repair by introducing into the IL strain donor (I-CreII) DNA with limiting, one-sided or no homology with the psbA target. This was accomplished by co-transforming the constructs with a 16S rRNA gene (rrn) that confers resistance to spectinomycin (16Sspec). The 16Sspec marker (Harris et al., 1989) does not yield copious numbers of chloroplast transformants, but those that are obtained have almost always taken up and expressed I-CreII (Odom et al., 2001). Although use of homology-limited constructs decreased the chloroplast transformation frequency further, we were able to discern repair events in 50 such co-transformants. This analysis reveals the remarkable ability of chloroplasts to repair their genome by recombination.
Results
DSB repair by Cr.psbA4 intron homing
To examine the consequences of limiting homology between donor and target DNAs, we used a plasmid-to-organelle homing assay: a plasmid containing the Cr.psbA4 intron plus flanking exon sequences (Figure 2) was introduced into the IL strain using biolistics. Insertion of the intron into psbAintronless from these plasmids (pKS4 and pBX4 in Figure 2) is dependent on I-CreII (Kim et al., 2005; Odom et al., 2001), probably because of the short exon 4. The DCMUR marker in exon 5, which consists of a single nucleotide substitution 34 bp from the intron, allows homing efficiency to be estimated directly from the number of DCMUR transformants (Odom et al., 2001). Also, as DCMU inhibits electron transport through photosystem II, growth of the transformants on minimal medium + DCMU requires accurate homing.
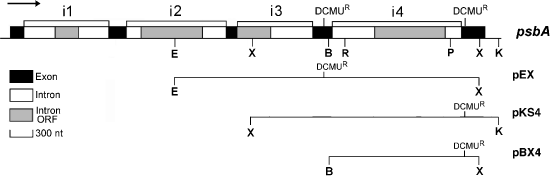
Maps of the psbA gene and plasmids used in this study.The gene has five exons and four introns; the 4th intron (i4), Cr.psbA4, and its flanking exons are most relevant here. The location of mutations conferring resistance to 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMUR) are indicated on the psbA map, and on the plasmids pEX, pKS4 and pBX4. The ORF in i4 encodes the I-CreII endonuclease. The restriction sites are: B, BstEII; E, EcoO109I; K, KpnI; P, PstI; R, EcoRI.
It was shown previously that efficient homing of Cr.psbA4 occurred with pBX4, which has 50 bp of exon 4 and 269 bp of exon 5 (Figure 2). To examine the effect of limiting homology, new constructs with exon 4 shortened as indicated in Table 1 were tested in the homing assay; longer (263–269 bp) and shorter (100 bp) lengths of exon 5 were also compared. The data (Table 1) indicate that, while homing of Cr.psbA4 occurred with as little as 10 bp of exon 4, the efficiency decreased considerably when exon 4 was <50 bp (and exon 5 was >260 bp). Table 1 also shows that the relative decrease in homing efficiency precipitated by the shortening of exon 4 from 50 to 41 bp is not as drastic with the shorter (100 bp) exon 5 (a drop of 57 to 43 versus 3500 to 56), but that is because the homing efficiency with the 100 bp exon 5 was already so low. We attempted to use a DCMUR marker in exon 4 (Figure 2), located 101 bp from the intron, to examine further the effect of shortening exon 5, but the number of DCMUR transformants obtained with pEX (Figure 2) was very low (<10), suggesting that co-conversion of exon 4 is quite limited in length (<101 bp). We do not know how far the conversion of exon 5 extends, but it must be at least 35 bp to convert the DCMU4 marker. Co-conversion of a marker in a flanking exon requires exonucleolytic degradation of that exon’s 3′ tail until the recipient DNA is removed; then the marker can be copied from the donor DNA. Thus, this result suggests that degradation of the 3′ tail, at least for exon 4, is very limited.
| Donor DNA | Exon 4a (bp) | Exon 5 (bp) | DCMUR transformantsc |
|---|---|---|---|
| E450–E5269b | 50 | 269 | ∼5000 |
| E450–E5263 | 50 | 263 | ∼3500 |
| E441–E5264 | 41 | 264 | 56 |
| E410–E5264 | 10 | 264 | 2 |
| I4−11–E5264 | −11 | 264 | 0 |
| E450–E5100 | 50 | 100 | 57 |
| E441–E5100 | 41 | 100 | 43 |
| E432–E5100 | 32 | 100 | 11 |
| E422–E5100 | 22 | 100 | 8 |
| E411–E5100 | 11 | 100 | 0 |
| I4−11–E5100 | −11 | 100 | 0 |
- aThe −11 constructs lack exon 4 and 11 bp of the intron.
- bThis construct is plasmid pBX4 (Figure 2).
- cThe DCMUR marker is in exon 5, and shows 100% co-conversion with homing. The data are the means of two experiments.
Phage intron homing has been proposed to occur by both DSBR and SDSA (Figure 1), with the latter being more prevalent (Mueller et al., 1996a). To determine which mechanism is preferred in the chloroplast, we looked for evidence of crossover products using PCR. The DSBR model invokes the formation of Holliday junctions (Figure 1), which are randomly resolved to produce either crossover or non-crossover products in a 1:1 ratio (Szostak et al., 1983). With SDSA, however, crossing over is not an inherent part of the mechanism, and such products should be highly under-represented.
We designed downstream primers to distinguish between crossover and non-crossover products by taking advantage of the fact that crossing over integrates the whole plasmid (assuming it is circular), as shown in Figure 3(a), whereas non-crossover recombination does not. Oligos 248 and 528 (Figure 3a) are complementary to the vector portion of pKS4, and should be specific for the crossover product, whereas oligo 476 is complementary to the intergenic region near 5S rrn, and was used to detect the non-crossover product (Figure 3a,b). The same upstream primer, specific for exon 3 of the genomic psbA gene (oligo 176), was used, thus excluding amplification of any residual or contaminating plasmid. In this experiment, pKS4 was co-transformed with 16Sspec into the IL strain, and selection was performed on spectinomycin/TAP plates; under these conditions, nearly all spectinomycin-resistant transformants have the intron (Odom et al., 2001). Although selecting for 16Sspec gives 100-fold fewer transformants than selecting for the DCMUR marker in exon 5, this protocol was used to minimize selective pressure at the psbA locus that might affect the product ratio. Figure 3(b) shows the PCR results for five of the 15 co-transformants analyzed in this experiment; all of the clones contained the intron, as revealed by the 176-476 primer pair, and two showed evidence of crossing over, as indicated by the product obtained with the 176-248 primer pair (lanes 2 and 4). The other 10 transformants gave only the 176-476 product, indicative of no crossing over. Similar results were obtained when oligo 528 (Figure 3a) was substituted for 248 (data not shown). Also, sequencing of the PCR products confirmed that the vector sequence of pKS4 is fused to the psbA region as indicated in Figure 3(a). In theory, the crossover product could have also been detected using the 176-476 primer pair, but the PCR product is too large (>10 kb) to be amplified under these conditions. In another experiment, 18 spectinomycin-resistant co-transformants were recovered and analyzed by PCR; all contained the intron and non-crossover product, but only three gave the crossover product (data not shown). Thus, approximately 15% of the transformants contained the crossover product expected for the DSBR mechanism, but none of them contained only the crossover product.
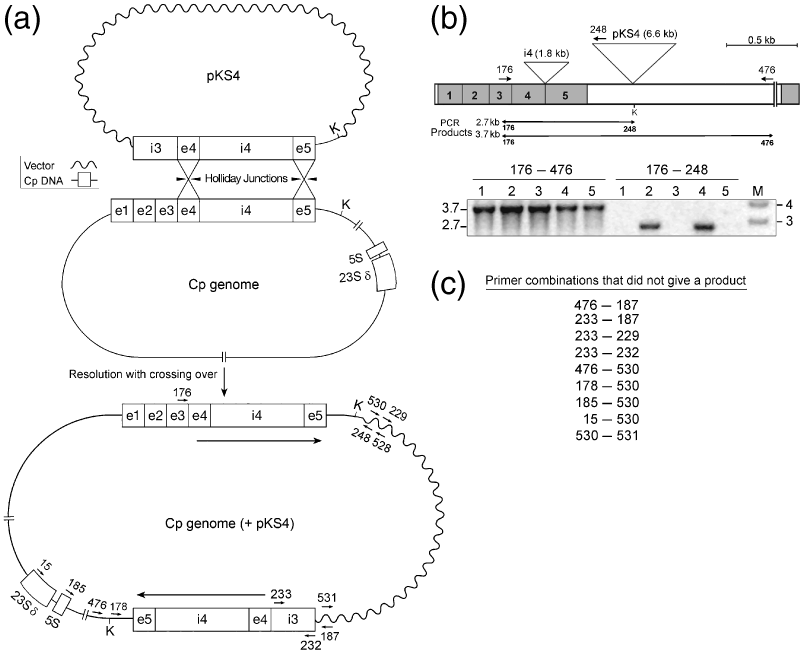
Detection of crossover recombinants associated with Cr.psbA4 homing by PCR.(a) Intermediate and product DNAs from crossover recombination occurring during homing of intron Cr.psbA4 (i4). The cpDNA insert in the intron donor plasmid, pKS4 (Figure 2), is labeled, as is the relevant part of the chloroplast genome of the IL strain (i.e. the psbA gene and partial downstream rRNA genes, (5S and 23S δ). In the intermediate (top diagram), psbA is shown after the intron has been copied in, but before the Holliday junctions have been resolved. The lower diagram is the crossover product, which is the chloroplast genome with pKS4 integrated immediately downstream of psbA. The locations of primers used for PCR are indicated. The components are not drawn to scale.(b) PCR analysis of transformants that had integrated the intron from pKS4 by homing. (Top) Diagram of the psbAintronless gene and intergenic region in IL, and primers for PCR. The insertion sites for the 1.8 kb intron (i4) and pKS4 (6.6 kb) sequences are indicated, and the drawing is to scale. Primer 248 anneals to the vector portion (pBluescript SK+) of pKS4, whereas 176 and 476 anneal to the chloroplast genome as indicated. The expected PCR products are shown below the map. K is the KpnI site at the end of the cpDNA insert in pKS4. The gel panels show the PCR products obtained for five of the co-transformants (lanes 1–5) from a pKS4 and 16Sspec shooting. The transformants were selected on spectinomycin, and total DNA was used for PCR with the indicated primer pairs (176-476 and 176-248). Two DNA size markers (lane M) are indicated to the right (in kb). The image from the ethidium fluorescence was reversed, yielding a black-on-white image.(c) List of primer combinations that did not give a PCR product using DNA from the same transformants shown in (b). The locations of the primers are shown in the diagram of the crossover product DNA in (a). All of these primers worked when tested in control reactions with either pKS4 or cpDNA from IL.
We were concerned about the possible instability of the crossover product affecting the results, especially as the clones with the crossover product are heteroplasmic and contain non-crossover DNA in greater relative amounts (based on the PCR signal). As shown in Figure 3(a), the crossover product should also contain a direct repeat comprising psbA exon 4, intron 4, exon 5 and 3′ flanking sequence (to the Kpn I site, approximately 3 kb total) that are separated by approximately 3 kb of vector sequence. Recombination between the repeats could remove the vector sequence as well as one copy of the repeat. We attempted to verify the presence of the downstream copy in these clones, but it appears to be deleted based on PCR using primers that either flank the region (Figure 3a,c) or lie completely within it (e.g. oligos 232 and 233 in intron 3). The deletion does not result from a simple recombination between the direct repeats, however, as at least one end of the vector sequence is present, and the deletion seems to extend well into the inverted repeat (IR) region containing the rRNA genes. Despite a number of attempts (Figure 3c), we were unable to obtain a PCR product spanning the deletion, so we do not know how it occurred. However, these data are consistent with the notion that the crossover product is unstable. Therefore, it is likely that we are underestimating the prevalence of the DSBR pathway.
Consequences of transforming the chloroplast with constructs containing I-CreII but lacking sufficient homology for homing: repair by the SSA pathway
To examine repair of the DSB by means other than intron homing, constructs containing I-CreII but with only one-sided (exon 5) or no (lacking both exons) homology with the target (Figure 4a) were co-transformed into IL with the 16Sspec plasmid. The homology-limited constructs decreased the chloroplast transformation frequency approximately threefold compared to transformation with the 16Sspec marker alone; nonetheless, we obtained and analyzed a total of 50 spectinomycin-resistant transformants. None of these transformants grew on minimal medium, and their growth on acetate medium was inhibited by moderately bright light (40 μE m−2 sec−1), suggesting that the psbA gene, at least, had been damaged. DNA analysis showed that most of the transformants obtained using the one-sided homology constructs had a similar, albeit complex, DNA organization, and this will be discussed below.
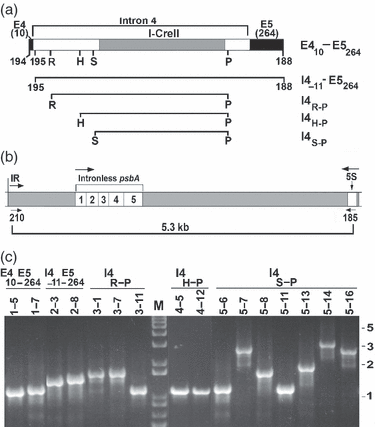
Consequences of the donor DNA lacking sufficient homology for homing: repair by SSA.(a) Diagram of constructs that were co-transformed into IL with 16Sspec. The top diagram is the longest construct, E410–E5264, which has 10 bp of exon 4, the intron, and 264 bp of exon 5. The I4−11–E5264 construct lacks exon 4 and 11 bp of the intron. All the constructs contain an intact ORF (endonuclease). The numbers at the ends of the diagrams refer to the primers used to amplify the DNA. H, HincII; P, PstI; R, EcoRI; S, SspI.(b) Diagram of the psbA region in the recipient IL strain, and the location of PCR primers. IR (inverted repeat) indicates one end of the large inverted repeat that contains the psbA and rRNA genes. The primers and the expected PCR product in IL are indicated below the map.(c) PCR analysis of 16 co-transformants using the 210-185 primer pair. The construct that was co-transformed into the cells along with the 16Sspec gene is indicated above the lanes, together with the strain numbers. The transformants were selected with spectinomycin, and total DNA was used for PCR. Lane M of the agarose gel contained DNA size markers, and the sizes (kb) of selected fragments are indicated to the right.
However, the DNA structure in most of the transformants bombarded with the exon-lacking constructs is relatively straightforward. Figure 4(a) shows the constructs that were co-transformed into the cells; although the longest construct, E410–E5264, does have 10 and 264 bp, respectively, of exon homology with psbA, none of these DNAs really have enough homology for homing. Figure 4(c) shows the analysis of 16 spectinomycin-resistant transformants by PCR using primers that flank the psbA gene. A product smaller than expected for the recipient strain (5.3 kb, not shown) was obtained with each transformant, and the products range from 1 to 3 kb in size. It is also noteworthy that only a single major PCR product was obtained with all of these strains, suggesting that both copies of the IR are similar in this region.
All of the PCR products in Figure 4(c), plus additional ones from other transformants, were sequenced, and the results are summarized in Table 2. All of these strains have a deletion that is apparently due to recombination between directly repeated sequences that flank the psbA gene (see also Figure 5), as indicated by the fact that the deletions include one of the two repeats and the DNA between them. This repair pattern is characteristic of the SSA pathway, and is not usually associated with the DSBR and SDSA pathways (Figure 1). The data also show that deletions as large as 4.9 kb were obtained, and that direct repeats as short as 15 bp were used for repair. Analysis of the sequences of the repeats involved (Table 2) indicate that some are closely related, e.g. repeat 7 is a truncated form of repeat 6, whereas some others share partial sequence identity. Also, some of the repeats (e.g. repeat 4) contain smaller, internal repeats. On the other hand, the data do not reveal a conserved sequence, or a base composition bias in the repeats. There does seem to be a clear preference, however, for longer repeats, based on the fact that there are far more short (<20 bp) direct repeats flanking psbA than the relatively long repeats used in many of these repair events (not shown).
| Transformant | Donor DNAa | Deletion size (kb) | Repeat size (bp) | Direct repeat sequence (and copies involved in the deletion) |
|---|---|---|---|---|
| 1–5 | E410–E5264 | 4.3 | 49 | GTATGTAAACCCCTTCGGGCAACTAAAGTTTATCGCAGTATATAAATAT (10a and 10c) |
| 1–7 | E410–E5264 | 4.2 | 54 | AGGACGTCCCCTTCGGGTAAATAAATTTTAGTGGCAGTGGTACCACCACTGCCT (2a and 2b) |
| 2–3 | I4−11–E5264 | 3.9 | 31 | AGTATATAAATATAGAATGTTTACATACTCC (5a and 5b) |
| 2–8 | I4−11–E5264 | 3.9 | 41 | GAAGGAGGACGCCAGTGGCAGTGGTACCGCCACTGCCTGCT (9a and 9b) |
| 3–1, 3–7 | I4R-P | 3.7 | 20 | CCCGAAGGGGAAGGAGGACG (8a and 8b) |
| 3–8 | I4R-P | 4.3 | 20 | CCCGAAGGGGAAGGAGGACG (8a and 8c) |
| 3–10 | I4R-P | 5.0 | 15 | TAGGCAGTTGGCAGG (3a and 3e) |
| 3–11 | I4R-P | 4.2 | 49 | GTATGTAAACCCCTTCGGGCAACTAAAGTTTATCGCAGTATATAAATAT (10a and 10c) |
| 4–5 | I4H-P | 4.2 | 49 | GTATGTAAACCCCTTCGGGCAACTAAAGTTTATCGCAGTATATAAATAT (10a and 10c) |
| 4–6, 4–13 | I4H-P | 2.8 | 62 | TAAGTTTACTTGCCCAATATTTATATTAGGACGTCCCCTTCGGGTAAATAAATTTTAGTGGC (1a and 1b) |
| 4–12 | I4H-P | 4.2 | 24 | GGCAGTTGGCAGGCAACTGCCACTG (11a and 11f) |
| 5–6 | I4S-P | 4.2 | 54 | AGGACGTCCCCTTCGGGTAAATAAATTTTAGTGGCAGTGGTACCACCACTGCCT (2a and 2b) |
| 5–7, 5–16 | I4S-P | 2.8 | 62 | TAAGTTTACTTGCCCAATATTTATATTAGGACGTCCCCTTCGGGTAAATAAATTTTAGTGGC (1a and 1b) |
| 5–8 | I4S-P | 3.8 | 27 | GTTTACATACTCCTAAGTTTACTTGCC (6a and 6b) |
| 5–11 | I4S-P | 4.2 | 49 | GTATGTAAACCCCTTCGGGCAACTAAAGTTTATCGCAGTATATAAATAT (10a and 10c) |
| 5–13 | I4S-P | 3.6 | 21 | ATACTCCTAAGTTTACTTGCC (7a and 7b) |
| 5–14 | I4S-P | 2.4 | 29 | AAGGGGACGTCCCGAAGGGGAAGGGGAAG (4a and 4b) |
- aThe endonuclease-encoding donor DNAs are shown in Figure 4(a).
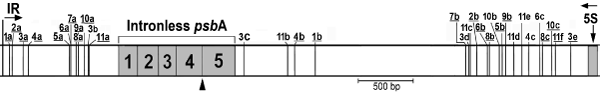
Map of direct repeats that flank the psbA gene and were used for repair.The repeats were numbered from left to right (1–11), and each copy was given a lower-case letter (e.g. 11a–11f). The repeat copies that were used in at least one transformant are underlined. The arrowhead indicates the DSB site. IR, inverted repeat.
A map-based analysis of the full complement of these repeats relative to the cleavage site is shown in Figure 5. In this map, the repeats are numbered from left to right (1-11), and their respective copies labeled alphabetically using lower-case letters (e.g. repeats 11a–11f). Only sequences that were used at least once for repair were put on the map, and the underlined copies represent those actually used in a repair event. One thing that stands out from this analysis is the asymmetry of the deletions with respect to the DSB; in general, much more DNA was lost on the 3′ side of the break compared to the 5′ side. This is due, in part, to the relatively large (approximately 1.3 kb) region downstream of psbA (bounded by repeats 1b and 7b) that was not used for repair. Computational analysis of repeated sequences using Reputer (Kurtz et al., 2001) revealed a paucity of repeats ≥15 bp in this 1.3 kb region, and none longer than 17 bp. It is also interesting to note that this region corresponds closely to a cold spot for recombination (Newman et al., 1992). Putting aside repeat length, the utilization of repeats for this repair process seems to be relatively stochastic; however, repeat 10 may be an exception. In all four of the deletions involving repeat 10, copy 10b, which is closer to the DSB on the 3′ side, was bypassed in favor of copy 10c (Figure 5 and Table 2). Although our sample size is limited, this could indicate that some repeat copies are better substrates for SSA than others, and that factors in addition to proximity to the DSB may play a role.
Repair of the DSB induced by one-sided homology constructs: tripartite repair
Although several of the clones from the transformations with the one-sided homology constructs had a simple deletion as discussed above (Figure 4c, leftmost lanes), the majority (12 of 17) did not, giving no product with primers 210 and 185 (Figure 4b) under standard conditions (not shown). However, increasing the extension time in PCR yielded a faint product of approximately 9 kb (not shown), which is approximately 4 kb larger than the recipient strain's DNA and suggestive of a large insertion. We reasoned that repair of the 3′ fragment of the DSB could have occurred by recombination with the exon 5 sequence (Figure 4a), and PCR using primers 214 (which anneals to exon 5, Figure 6b) and 185 verified that this was correct (data not shown). Further analysis of the integrated DNA by PCR using a number of different primers (Figure 6b) indicated that essentially the whole I4−10–E5264 plasmid had been incorporated (Figure 6b), and that this structure was similar in all 12 transformants. Figure 6(a) shows representative PCR reactions for six of the transformant clones (numbers 1–6 in both panels) using two sets of primers, 208-230 and 187-116, respectively. The 208-230 primer pair was useful in establishing how the 5′ side of the break was repaired, whereas the 187-116 primer pair shows that the inserted intron is still attached to the plasmid vector. As primer 208 anneals to a gene in the single-copy region of the genome (ChlN), the 208-230 pair probes one copy of the IR. PCR using an upstream primer (224) that anneals to a gene in the other single-copy region (ycf12) indicated that both copies of the IR were similar (not shown).
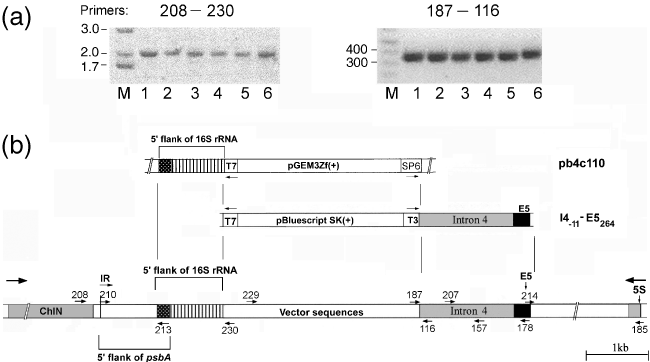
Tripartite DNA structure in co-transformants bombarded with a one-sided homology construct.(a) Representative PCR reactions for six of the co-transformants. The primer pair is indicated above each gel, and the locations of primers are shown in (b). The sizes of the markers (M) for the 208-230 gel are in kb; those for the 187-116 gel are in bp.(b) Map and origins of the DNA in the tripartite transformants. The map, with PCR primers, is the lower diagram, and above it are relevant portions of the two donor plasmids, pb4c110 and I4−11–E5264. The DNA marked with vertical lines occurs upstream of the 16S gene (in pb4c110). The hatched DNA (173 bp) is found upstream of both 16S and psbA. The vector region is a mixture of the two vector sequences. pb4c110 has more cpDNA than is shown, including all of the 16Sspec gene and part of the 23S gene. IR, inverted repeat; ChlN, gene for chlorophyll synthesis.
Sequencing of these and other PCR products from this region revealed a genomic deletion on the 5′ side of the break that included psbA exons 1–4 and extended 202 bp upstream of the start codon. Replacing this DNA was a novel insertion of 858 bp (indicated by vertical lines in Figure 6b), which was followed by the vector sequence and insert from the I4−10–E5264 plasmid. Analysis of the novel insertion sequence showed that it is identical to a region upstream of the 16Sspec gene in the pb4c110 plasmid (accession no. EF659743), and shows several differences from the published sequence (Schneider et al., 1985). It also became apparent that the same 173 bp DNA flanks both sequences (shown by cross-hatching in Figure 6b). In addition, the 5′ end of the vector portion contained a sequence from the multiple cloning site of pGEM3zf+ (the vector in pb4c110) that is not found in pBluescript SK+ (the vector for I4−10–E5264), confirming that the insertion sequence came from pb4c110 and not from the endogenous rrn gene. It should be noted that most of the approximately 6 kb that was inserted into the break, except for the middle of the vector region, was sequenced in four different transformants, and all gave essentially the same results. There does seem to be some heteroplasmicity, however, in the vector region, based on NdeI digestion of a PCR product obtained using primers 229 and 116 (Figure 6b). Only approximately 50% of the 229-116 product could be cleaved using NdeI for most of these strains (data not shown). The sequence identity between pGEM3zf+ and pBluescript SK+ is approximately 99% for most of their length, but there is an internal region of approximately 300 bp that is unique to pGEM3zf+ and contains the NdeI site.
We propose that the DNA organization in these transformants is the result of recombinational repair of the DSB using DNA from both introduced plasmids, I4−10–E5264 and pb4c110 (Figure 6b). We suggest that repair of the 3′ side of the break involved invasion of I4−10–E5264 by the homologous exon in the chromosome, whereas repair of the 5′ side involved the invasion of pb4c110 by a coincidentally homologous sequence upstream of psbA (Figure 6b). The long regions of vector homology shared by these two plasmids would enable annealing of the new strands for the final stages of repair. As there is cpDNA in pb4c110 (beyond the region shown in Figure 6b) that is not homologous to the I4−10–E5264 plasmid, the unpaired flap(s) was probably removed as in the SSA mechanism (Figure 1). Thus, repair of the DSB in these strains probably involved elements of both the SDSA and SSA mechanisms.
Repair using a distally located sequence
A third type of repair event was discovered in one of the strains transformed with the I4−10–E5264 construct. PCR using primers 210 and 185 (Figure 4) initially indicated that transformant 2-6 had a large (approximately 5 kb) deletion (not shown), but sequencing also revealed an 88 bp insertion between the deletion end points (Figure 7). BLAST searches showed that the insertion, and its immediate flanking sequences (47 and 24 bp, respectively), is identical to a region just (22 bp) downstream of ORF 112, which is located approximately 40 kb from psbA, between the petA and petD genes. The short sequences flanking the 88 bp insertion sequence at ORF 112 are thus repeats that also flank psbA. The 47 bp sequence lies downstream of repeat 4a, whereas the 24 bp sequence is repeat 11f (5, 7). These data suggest that the DSB was repaired by the homologous invasion and copying in of the sequence near ORF 112. This repair event differs from repair by SSA with flanking repeats, because the distal location of the donor DNA would seem to require a mechanism such as SDSA that involves strand invasion.
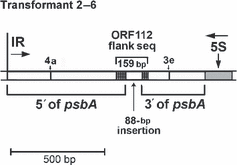
DNA structure in transformant 2–6. This co-transformant was bombarded with I4−11–E5264 . It has a large deletion and a small (88 bp) insertion, as indicated. Flanking the 88 bp insertion were two short sequences (47 and 24 bp, respectively) that also flank an identical 88 bp sequence near ORF 112; the whole region is 159 bp. The locations of repeats 4a and 3e are indicated for reference (Figure 5).
Discussion
DSB repair by HR and intron homing
A DSB is the most severe type of DNA damage. In the nucleus, DSBs are repaired either by NHEJ or HR, and in plants NHEJ predominates. HR actually includes three distinct pathways, SSA, DSBR and SDSA. The primary DSB repair mechanisms used by organelles have not been identified in any systematic way. In this report, we used the mobile group I intron, Cr.psbA4, and its endonuclease, I-CreII, to examine the consequences and repair of a DSB in the chloroplast psbA gene. The results, which are based mainly on molecular analysis of chloroplast transformants, suggest that the chloroplast can use all three HR pathways to repair a DSB. Consistent with this finding is the discovery of a recA-like gene in C. reinhardtii whose product (REC1) is targeted to the chloroplast (Nakazato et al., 2003); this protein could catalyze strand invasion in the SDSA and DSBR pathways. In contrast, no evidence of NHEJ was obtained, and while our sample size is too small to conclude with certainty that the chloroplast does not perform NHEJ, this result is consistent with other observations. For example, there has been no report of a Ku homologue, which is a conserved NHEJ protein (Bowater and Doherty, 2006), that is targeted to chloroplasts, and we could not find a good candidate in the latest version of the C. reinhardtii genome (Joint Genome Institute, version 3). Also, we have not seen any published reports that describe non-homologous recombination by normal plastids.
Previous work, involving homing of the endogenous Cr.LSU intron into an ectopic target, provided evidence for the SDSA pathway, because crossover products were not detected (Dürrenberger et al., 1996). Using a plasmid-to-organelle homing assay, we detected crossover products in approximately 15% of the transformants that had acquired the intron by homing, thus providing evidence of DSBR. Interestingly, these strains also contained the non-crossover product, which, coupled with the low (<1:1) ratio of crossover to non-crossover products, suggests that both mechanisms may operate, but that SDSA may predominate. However, we also present evidence that the crossover product is not completely stable, and this instability could have reduced the observed frequency of crossover products. In bacteriophage T4 homing, the crossover product was also unstable, but a kinetic analysis indicated that crossing over occurred only approximately 18% of the time in that system (Mueller et al., 1996a,b). Thus, SDSA is used more frequently than DSBR by homing phage introns in Escherichia coli. The absence of crossover products in the Cr.LSU study (Dürrenberger et al., 1996) may have been because the resulting cpDNA rearrangement was lethal or too unstable to be detected by Southern blotting.
Repair by SSA
Co-transformation with DNA constructs that were able to produce the endonuclease but lack the flanking homologous sequences (i.e. exons) resulted in deletions of the psbA gene. These deletions involved direct repeats that flank psbA, and are characteristic of the SSA repair pathway (reviewed by Pâques and Haber, 1999). Although the data suggest that longer (>30 bp) repeats are preferentially used for SSA, 40% of the documented deletions involved repeats of <30 bp, with the smallest being a mere 15 bp. This is a remarkable result considering that in yeast, which is quite efficient at reconstituting a broken chromosome, repair by the SSA pathway was barely detectable (0.17%) using a 29 bp repeat (Sugawara et al., 2000); use of shorter (<29 bp) repeats was not attempted.
We suggested above that the asymmetry of the psbA deletions relative to the DSB is due mainly to the distribution of direct repeats flanking psbA that are of sufficient length for recombination. Some of these deletions, notably 10a–10c and 11a–11f (Table 2), provide additional insights. In the SSA model, the DSB is followed by generation of 3′ single-stranded tails by a 5′ to 3′ exonuclease activity (Figure 1). For the 11a–11f deletion, degradation of the downstream product must proceed for at least 3 kb to expose 11f for annealing, whereas only approximately 1 kb need be removed from the upstream product to expose 11a. If we assume that degradation of both 5′ ends occurs at about the same rate, the 3′ tail that contains 11a must be very stable, as it must wait for 11f to become available for annealing. However, there must be some degradation of the 3′ tails, or co-conversion of flanking exon sequences during intron homing would not occur. The stability of the 3′ tails is also supported by the fact that the DCMUR marker in exon 4 did not permit analysis of Cr.psbA4 homing the way the marker in exon 5 does; the exon 4 substitution is 101 bp from the intron, whereas the exon 5 substitution is only 34 bp away. The implication is that exonucleolytic degradation of the 3′ tail of exon 4 is insufficient to allow conversion of that marker.
It is interesting that a number of photosystem II mutants of C. reinhardtii have been shown to have deletions whose endpoints map to the flanking regions of psbA (Bennoun et al., 1986). Perhaps some of these mutants, many of which were induced using fluorodeoxyuridine, were the result of recombination between direct repeats. The recombination could have been stimulated by a drug-induced DSB, although other mechanisms are possible. The cpDNA around psbA might be a naturally fragile site, with a propensity for chromosomal breaks and recombination.
Tripartite repair of the DSB: further evidence for SDSA
When constructs with one-sided homology were co-transformed into the chloroplast, the majority of the drug-resistant transformants repaired the DSB using two separate donors. The downstream end was repaired by recombination with the exon sequence in I4−10–E5264, whereas the upstream end was repaired using the pb4c110 plasmid, whose real role was to provide the 16Sspec marker. By an unanticipated coincidence, the cpDNA in pb4c110 contains a sequence upstream of 16Sspec that is repeated in the 5′ flank of psbA. This homology could have enabled invasion by the upstream tail once it was exposed by degradation. The strong homology of the vector sequences could have bridged the newly synthesized strands together by SSA, setting up the final stages of repair. The repair of a DSB using two donor molecules has been reported in yeast, and is considered to be strong evidence for the SDSA pathway (Pâques et al., 1998; Silberman and Kupiec, 1994). However, removal of unpaired flap DNA as in SSA would seem to be required because of the DNA in pb4c110 that is not homologous to I4−10–E5264. Hence, these repair events could be considered a combination of the SDSA and SSA pathways.
Repair using a distally located sequence
A third type of repair event was observed in a strain transformed with a one-sided homology construct. In this transformant, the break was repaired using DNA located approximately 40 kb away (near ORF 112) that had homology to the free ends. However, the homologies in the free ends were some distance from the DSB, so a large deletion also occurred (5, 7). Nonetheless, this strain demonstrates that the chloroplast can recruit a homologous sequence that is very distal to the DSB for repair. It is also significant that the homology on the 3′ side of the DSB for this repair event was only 24 bp, thus providing further evidence of the great efficiency of this process. Mechanistically, this repair could have been via the SDSA pathway (with the removal of a DNA flap as needed), and either intra-molecular or inter-molecular; repair by DSBR is also possible.
Implications for hot and cold spots at psbA
Although not principal targets of this study, aspects of these data are pertinent to the hot/cold spots for recombination near the 3′ end of the psbA gene (Newman et al., 1992). The hot spot, which contributes to the Cr.psbA4 homing efficiency, was initially mapped between the DCMU4 marker in exon 5 and the KpnI site downstream of psbA (Figure 2). As homing of pBX4 is nearly as efficient as that of pKS4 (Odom et al., 2001), the hot spot is probably within exon 5, between the DCMUR marker and the XbaI site. These data (Table 1) indicate the limits of the hot spot as the region between bases 100 and 269 of exon 5. This 169 bp region does not contain repeats that are >12 bp long, but near the 3′ end is the sequence CTTCCCT, which is close to a 7-mer, CCTCCCT, that is preferentially found in recombination hot spots in humans (Myers et al., 2005).
This study also identified a 1.3 kb region downstream of psbA that was not used to repair the DSB by SSA. This region, which is deficient in direct repeats of >15 bp that flank psbA, corresponds closely to the recombination cold spot (Newman et al., 1992). The implication is that short direct repeats might stimulate recombination during mating, although the mechanism is not obvious.
Evolutionary implications
The chloroplast of Chlamydomonas has a remarkable capacity for repairing a DSB by multiple pathways. The fact that all three types of repair events were seen for the same construct suggests that these mechanisms could compete for broken DNA. The organelle utilized homology as short as 15 bp and a repertoire of repeats (Jiao et al., 2004; Maul et al., 2002) to rescue the genome. The direct repeats that flank psbA prevented catastrophic DNA loss in the transformants. Thus, the profusion of repeated sequences in this genome was advantageous.
Of course, these repair events, except homing, were mutagenic. This was not unexpected however, because of the inherent selection for genomes with the target site disrupted; otherwise, the repaired genome would be re-cut by the endonuclease. Presumably, there would be less mutagenesis if the restored genome copies were not cleaved again. It may be possible to set up such a system in Chlamydomonas by controlling the endonuclease using a tightly regulated promoter.
An enduring mystery of the chloroplast genome is why it is deficient in horizontally transferred DNA compared to the mitochondrial and nuclear genomes of plants. The answer might not be the same for all plants, but one shared factor could be the possible absence of an effective NHEJ system that could join completely unrelated DNA ends. These data also implicate an idiosyncratic feature of some plant cpDNAs that could promote the loss of horizontally acquired, or otherwise defective DNA, namely a high frequency of direct repeats. In addition to C. reinhardtii, a highly repeated cpDNA structure is found in other Chlamydomonas spp. (Boudreau and Turmel, 1996), green algae such as Pseudendoclonium (Pombert et al., 2005), and angiosperms such as Pelargonium×hortorum (Chumley et al., 2006). Direct repeats can lead to DNA loss via recombination in the presumed absence of a DSB (e.g. Cerutti et al., 1995; Dürrenberger et al., 1996). Hence, even if foreign, non-homologous DNA was able to integrate into the cpDNA of these organisms, it could be subsequently lost by this type of recombination – unless, of course, it possessed functional significance, or had the ability to maintain itself (such as a mobile intron). On the other hand, the cpDNA in species such as Arabidopsis thaliana (Sato et al., 1999) is not rich in repeats, but also lacks horizontally transferred genes. Further research on DNA recombination in plastids may provide a better answer to this and other pressing questions, as well as providing avenues for improving the frequency of chloroplast transformation in other plants (Maliga, 2004).
Experimental procedures
Strains and culture conditions
The C. reinhardtii intronless psbA strain, IL (Johanningmeier and Heiss, 1993), was obtained from U. Johanningmeier (Martin Luther University, Halle, Germany), and the wild-type 2137 strain was obtained from G.W. Schmidt (University of Georgia, Athens, USA). Growth was at 24°C under low to moderate light (1–40 μE m−2 sec−1) in Tris-acetate phosphate (TAP) or minimal (no acetate) medium (Harris, 1989). For chloroplast transformation, the IL strain was pre-grown to a density of 1–2 × 106 cells ml−1.
Plasmids
Plasmid pb4c110 contains a 7 kb fragment of cpDNA from the spr-u-1-6-2 mutant (Thompson and Herrin, 1991). Construction of plasmids pKS4, pBX4, I4R-P (RP), I4H-P (HP), and I4S-P (SP) has been described previously (Odom et al., 2001). New truncated exon constructs were produced by PCR of pKS4 using Pfu DNA polymerase (Stratagene; http://www.stratagene.com). The upstream primers, with the exon 4 length indicated in parentheses, were 190 (50 bp), 191(32 bp), 192 (41 bp), 193 (22 bp), 194 (10 bp), and 195 (−11 bp). The downstream primer was either 189 (100 bp of exon 5) or 188 (264 bp of exon 5), but 188 was not paired with primers 191 and 193. The sequences of primers used for PCR are given in Table S1. The products were cloned into SmaI-digested pBluescript SK+, (Stratagene), and selected clones were sequenced. All had the DCMU4 mutation in exon 5 (T→G) as expected (Erickson et al., 1989); the E432–E5100 construct also contained a silent C→T mutation at residue 16 of exon 5 that did not seem to affect its biological activity. The inserts in these plasmids are all in the antisense orientation with respect to T7, except for E450–E5263 and I4−11–E5100, which are in the sense orientation. It should be noted, however, that we have no evidence that insert orientation or vector identity affect intron homing (Odom et al., 2001 and unpublished results).
Plasmid pEX (Figure 2) was created as follows. pEC23 (Herrin and Michaels, 1985) was digested using EcoO109I and EcoRI, and the 2.4 kb fragment was cloned into the same site in pBluescript SK+. The new plasmid, pER, was cut using EcoRI and NotI, and the EcoRI-NotI insert of I4R-P, which includes the PstI site, was added to create pEP. To introduce the dr-u-2 mutation (Erickson et al., 1989), a 264 bp NsiI–BstEII fragment was exchanged with the same fragment from strain CC-1403 generated by PCR using primers 176 and 116 and double digestion. This plasmid (pEPDCMUe4) was converted to pEX by replacing the EcoRI–PstI fragment at the 3′ end of psbA with the nested EcoRI–XbaI fragment of pRX4 (which contains a wild-type exon 5). Sequencing confirmed the dr-u-2 mutation in exon 4.
Chloroplast transformation
Chloroplast transformation of IL utilized bombardment with DNA-coated tungsten particles (Boynton and Gillham, 1993) using a helium apparatus (Bio-Rad He1000; http://www.bio-rad.com/). The bombarded plates were kept overnight in dim light (approximately 2 μE m−2 sec−1). The cell layer was scraped from each plate and re-spread onto two selective plates (either 100 μg ml−1 spectinomycin in TAP, or 3 μm DCMU in minimal medium), which were incubated in light (approximately 20 μE m−2 sec−1) at 24°C. Spectinomycin-resistant transformants appeared in 1–3 weeks, and DCMUR transformants in 2–4 weeks. The clones were transferred to fresh selection plates, and maintained on these plates; the spectinomycin-resistant strains were kept in dim light.
Analysis by PCR and sequencing
Total DNA was isolated from cells grown on selective plates (Dürrenberger et al., 1996). Standard protocols were used for PCR with Taq polymerase, and primer sequences are given in Table S1. All of the primers were tested in control reactions with plasmid or cpDNA to verify their functionality. The standard temperature regimen was: 4 min at 94°C, 24 cycles of 60°C for 1 min, 70°C for 5 min and 94°C for 30 sec, then 1 min at 60°C and 8 min at 70°C. For some reactions, the cycle extension step was increased to 10 min. The PCR products were analyzed on agarose–ethidium bromide gels, and, for sequencing, were purified using Qiagen QIA quick columns (http://www.qiagen.com/). Automated sequencing was performed using the PCR primers and internal primers as necessary.
Chloroplast DNA sequencing
DNA sequencing was performed at the DNA Sequencing Facility of the Institute for Cellular and Molecular Biology (University of Texas, Austin, TX, USA). Table 2 and Figure 5 are based on our own sequences for the psbA flanking regions in IL (accession nos EF659742 and EF659744) where they diverged from published sequences (Erickson et al., 1984; Maul et al., 2002). Most of the differences could be ascribed to polymorphisms, but a 262 bp region between psbA and 5S (949 bp from the psbA stop codon) is not in the published sequence. Hence, we sequenced 700 bp around this site in the 2137 wild-type strain (accession no. EF685150) and in plasmid p322 (accession no. EF685151), whose DNA is from a double mutant (Newman et al., 1992). Both of these contained the 262 bp region. A 1.5 kb region upstream of 16S was sequenced in plasmid pb4c110 (accession no. EF659743), whose DNA is from the spr-u-1-6-2 mutant (Harris et al., 1989). It differs from the published sequence by a few 1 and 2 bp changes and a 23 bp insertion, and has a predicted ORF of 232 amino acids that comprises the two smaller ORFs reported previously (Schneider et al., 1985).
Acknowledgements
We acknowledge the contributions of Mandi Vaughn and Jay Farrington. This research was supported by the Department of Energy (DE-FG03-02ER15352), the R.A. Welch Foundation (F-1164), and an undergraduate research fellowship (to R.D.) from the University of Texas at Austin.




