ROXY1 and ROXY2, two Arabidopsis glutaredoxin genes, are required for anther development
Summary
Glutaredoxins (GRXs) are small oxidoreductases that are involved in various cellular processes and play a crucial role in responses to oxidative stress. Three GRX subgroups exist in plants, and GRXs with active sites of the CPYC and CGFS types are common to pro- and eukaryotes. In contrast, GRXs with the CC type motif have so far only been identified in land plants. Here, we report that the two CC-type GRXs ROXY1 and ROXY2 together control anther development in Arabidopsis thaliana. Single roxy1 and roxy2 mutants are fertile and produce normal anthers. However, roxy1 roxy2 double mutants are sterile and do not produce pollen. Strikingly, abaxial and adaxial anther lobe differentiation are differently affected, with early lobe differentiation being defective in the adaxial lobes, whereas later steps during pollen mother cell differentiation are disrupted in the abaxial lobes. Expression studies show that ROXY1 and ROXY2 are expressed with overlapping patterns during anther development. Lack of ROXY1 and ROXY2 function affects a large number of anther genes at the transcriptional level. Genetic and RT-PCR data imply that ROXY1/2 function downstream of the early-acting anther gene SPOROCYTELESS/NOZZLE and upstream of DYSFUNCTIONAL TAPETUM1, controlling tapetum development. Mutagenesis of a conserved glutathione-binding glycine in the ROXY1 protein indicates that CC-type GRXs need to interact with glutathione to catalyze essential biosynthetic reactions. Analysis of these two novel anther genes indicates that redox regulation, as well as participating in plant stress defense mechanisms, might play a major role in the control of male gametogenesis.
Introduction
Glutaredoxins (GRXs) are small, ubiquitous oxidoreductases that mediate the reversible reduction of intracellular disulfide bonds. GRXs reduce disulfides using conserved cysteines located in active site motifs, and depend on glutathione (GSH) for reduction of the oxidized form (reviewed by Buchanan and Balmer, 2005; Fernandes and Holmgren, 2004). These oxidoreductases are involved in a large variety of cellular processes, and play a major role in defense against oxidative stress.
The Arabidopsis thaliana GRX family comprises 31 members, which fall into three subgroups (Rouhier et al., 2006). The GRXs of the CPYC and CGFS subgroups occur ubiquitously, whereas GRXs of the CC-type subgroup have so far only been identified in land plants (Lemaire, 2004). Comparison of GRX subgroup composition in evolutionarily informative plant species revealed that the CC type, but not the other GRX groups, expanded during the evolution of land plants (Xing et al., 2006). The existence of a disproportionately large subgroup of CC-type GRXs in angiosperms raises the question as to whether their functions might have been integrated into crucial processes controlling higher plant development.
The recent isolation of the first plant CC-type GRX mutant from Arabidopsis, roxy1, provides strong evidence for this notion, as the phenotype of this mutant reveals a function for the GRX in flower development. roxy1 mutants initiate fewer petals than wild-type plants, and later petal morphogenesis is often aberrant (Xing et al., 2005). Here, we show that ROXY1, together with its closest homolog, ROXY2, are required for anther development.
The Arabidopsis anther is a bilaterally symmetrical four-lobed structure that produces the pollen. Each lobe develops from successive divisions of sub-epidermal archesporial cells formed in the anther primordium that give rise to three morphologically distinct cell layers. The endothecium, middle layer and tapetum surround the pollen mother cells (PMCs) that will undergo meiosis and thereby form the haploid microspores (Sanders et al., 1999). The tapetum is a source for nutrients and plays an indispensable role during further microspore maturation.
To date, only a few early anther genes have been identified by mutant analysis. In sporocyteless/nozzle (spl/nzz) mutants, sporogenous cell formation is impaired and bam1 bam2 mutants produce extra PMCs at the expense of normal anther somatic cells. This indicates that the SPL/NZZ gene promotes sporogenous cell formation, and BAM1/2 promote somatic cell formation (Hord et al., 2006; Schiefthaler et al., 1999; Yang et al., 1999). Other mutants, such as excess microsporocytes1/extra sporogenous cells (ems1/exs), tapetum determinant1 (tpd1) and somatic embryogenesis1/2 (serk1/2), completely lack the tapetum layer but instead produce excess PMCs that fail to complete meiosis, showing that EMS1/EXS, TPD1 and SERK1/2 function in the control of tapetum identity (Albrecht et al., 2005; Canales et al., 2002; Colcombet et al., 2005; Yang et al., 2003; Zhao et al., 2002). Genetic data indicate that EMS1/EXS and TPD1 act in the same pathway (Ma, 2005; Yang et al., 2003) and function upstream of DYSFUNCTIONAL TAPETUM1 (DYT1), a positive regulator of tapetum differentiation that is required for pollen formation (Zhang et al., 2006).
Loss of ROXY1 and ROXY2 functions results in defects in sporogenous cell formation in adaxial anther lobes, whereas later stages such as PMC and tapetum differentiation are affected in abaxial lobes. These data indicate that, in addition to functioning in stress responses, the activities of glutaredoxins are also required for normal development of male reproductive plant organs and gametophytes.
Results
Mutant isolation and characterization
Intrigued by the finding that the roxy1 mutant exhibits an aberrant petal phenotype, and forms on average 2.5 rather than four petals, we analyzed the function of its closest homolog, At5g14070 (Xing et al., 2006), which was named ROXY2 (GenBank accession EU332351). The two CC-type GRX products are 71% identical and share a CCMC active site motif.
Two ROXY2 mutant lines were characterized (Figure 1a). The roxy2-1 line, carrying a T-DNA insertion localized 123 bp upstream of the putative ATG start codon, was obtained from the SALK T-DNA collection (Col-0 ecotype). The roxy2-2 mutant, derived from the RIKEN collection (Nössen ecotype), carries a Ds insertion in the sole exon of ROXY2. Neither of these alleles causes any obvious phenotype in single mutants. Therefore, roxy1 roxy2 double mutants were constructed to reveal redundant functions. As RT-PCR experiments detected ROXY2 transcripts in the roxy2-1 allele, further analyses were conducted using the roxy2-2 allele, which represents a knockout mutant (Figure 1c). All three of the previously described alleles of roxy1 are in the Col-0 background (Xing et al., 2005). In order to avoid complications caused by crossing two ecotypes, two additional null mutants of roxy1 in the Nössen background, roxy1-4 and roxy1-5, were obtained from RIKEN (Figure 1b,c). These roxy1 mutants have a slightly weaker petal phenotype compared with the Col-0 alleles, and develop 3.0 petals on average (Table S1). No other obvious phenotypes outside the floral context were observed.
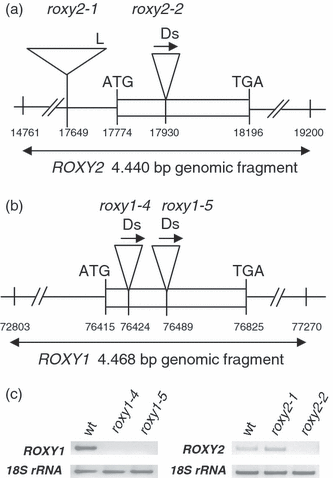
Isolation of roxy2 and roxy1 mutants.(a) Structures of roxy2 alleles in Col-0 (roxy2-1) and Nössen (roxy2-2) backgrounds. Numbering of the coordinates is based on the BAC clone MUA 22 containing the ROXY2 gene.(b) Structures of two roxy1 alleles in the Nössen background. Numbering of coordinates is based on the BAC clone F1C9 containing the ROXY1 gene.(c) Comparison of ROXY1 and ROXY2 expression in inflorescences from wild-type and mutant lines by RT-PCR. As a control, 18S rRNA was amplified.L, left T-DNA border; Ds, transposon; arrows indicate the Ds orientation.
roxy1roxy2 double mutants display defects in fertility
Strikingly, seven of 108 F2 plants derived from a cross between the two null mutants roxy1-5 and roxy2-2 were sterile. Genotyping confirmed that this phenotype was restricted to double mutants that, in addition to producing a reduced number of petals, also failed to elongate their siliques and did not set seed (Figure 2b). Additionally, anthers were smaller and did not produce pollen (Figure 2d,e). In wild-type flowers, anthers are grouped around the carpel at the time of dehiscence and deliver their pollen to the stigma (Figure 2c). In roxy1-5 roxy2-2 flowers, anthers are arbitrarily positioned and often do not face the carpel (Figure 2d).
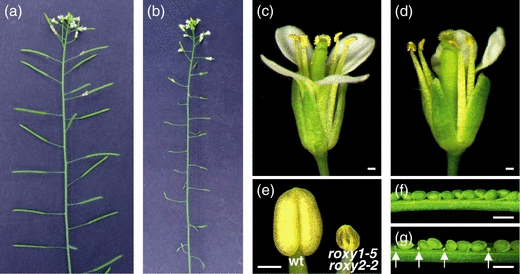
The roxy1 roxy2 phenotype.(a) Wild-type inflorescence with normal seed set in siliques.(b) roxy1-5 roxy2-2 inflorescence with small siliques lacking seeds.(c) Wild-type flower showing dehisced anthers with pollen grains. Stigmatic papillae are topped with pollen grains.(d) roxy1-5 roxy2-2 flower showing anthers without pollen grains.(e) Normal wild-type anther just before dehiscence (left) and a roxy1-5 roxy2-2 anther (right) at an equivalent stage.(f) Dissected silique from a wild-type cross, showing normally developed ovules 8 days after pollination.(g) A roxy1-5 roxy2-2 silique dissected 8 days after pollination with wild-type pollen. Aborted ovules are indicated by arrows.Bars = 200 μm.
Failure to set seed is not solely due to lack of pollen production, because pollination of roxy1-5 roxy2-2 pistils using wild-type pollen still yielded 21.7% aborted ovules (Table 1 and Figure 2g). In wild-type control crosses, only 2.4% of the ovules failed to develop into seeds. Crosses performed with wild-type pollen applied to the carpels of roxy2-2 single mutants resulted in wild-type-like siliques (Table 1 and Figure 2f). These findings indicate that ROXY1 and ROXY2 also participate – although to a lesser degree than observed for anthers – in controlling female organ development.
| Male parent | Female parent | Percentage of aborted ovulesa | No. of counted ovulesa |
|---|---|---|---|
| Wild-type | roxy1-5 roxy2-2 | 21.7 | 735 |
| Wild-type | roxy2-2 | 2.4 | 642 |
| Wild-type | Wild-type | 2.4 | 707 |
- aTen siliques were analyzed.
An identical sterility phenotype was observed for roxy1-4 roxy2-2 double mutants. Furthermore, a 4440 bp ROXY2 genomic fragment (Figure 1a) rescues the sterility phenotype of the double mutant (Figure S1). Given these observations, we conclude that the fertility defects in the double mutant are due to loss of the ROXY1 and ROXY2 functions.
Comparison of ROXY1 and ROXY2 expression patterns
In situ hybridization experiments were performed to compare ROXY1 and ROXY2 expression patterns in floral tissues. ROXY1 is expressed in distinctive areas of the inflorescence apical meristem where new floral buds will be initiated. Later, strong expression is detectable in young floral organ primordia, and expression levels decrease when floral organs start to differentiate (Figure 3a; Xing et al., 2005). In contrast, ROXY2 RNA is weakly expressed throughout the whole inflorescence apical meristem and in young buds (Figure 3e). After the onset of floral organ differentiation, when archesporial cell divisions give rise to the sporogenous cells at anther stage 3 (Sanders et al., 1999), overlapping expression of ROXY1 and ROXY2 is detectable in the four corners of young anthers (Figure 3b,f). During further differentiation, at anther stages 4 and 5, ROXY1 and ROXY2 are mainly expressed in PMCs formed in the center of each anther lobe and in the somatic cell layers embracing the sporogenous cells. No signals are detectable in the anther epidermis (Figure 3c,g). At stage 6, shortly before meiosis, ROXY1 and ROXY2 are both expressed in PMCs and in the tapetum (Figure 3d,h). After completion of meiosis, signals become restricted to the tapetum and are absent from maturing microspores (data not shown). The observation that ROXY1 and ROXY2 expression patterns coincide during early premeiotic to meiotic anther stages in sporogenous and parietal cells is in accordance with their redundant function during anther development as revealed by genetic studies.
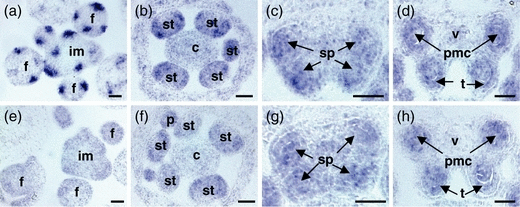
In situ hybridization analysis of ROXY1 and ROXY2 expression in wild-type plants.Cross-sections through wild-type inflorescences and flowers at various stages were analyzed for ROXY1 (a–d) and ROXY2 (e–h) expression.(a, e) Cross-sections through inflorescence apices, showing that ROXY1 is strongly expressed in sepal primordia, whereas ROXY2 is weakly expressed throughout the floral meristem.(b, f) ROXY1 and ROXY2 expression overlap in young anthers at anther stage 3 in the four sectors where sporogenous and somatic cells are formed by division of archesporial cells. ROXY2 is also weakly detectable in petal primordia.(c, g) At anther stage 4, ROXY1 and ROXY2 are expressed in all four lobes of anthers, in both sporogenous cells and somatic cell layers (with the exception of the epidermis).(d, h) Before meiosis, at anther stage 6, expression of both genes is mainly confined to PMCs and tapetum.c, carpel; f, floral bud; im, inflorescence meristem; p, petal; pmc, pollen mother cell; sp, sporogenous cells; st, stamen; t, tapetum; v, vascular bundle. Bars = 25 μm.
Histological analysis of roxy1roxy2 anthers
Wild-type and roxy1-5 roxy2-2 anther development was compared by analyzing semi-thin cross-sections. Morphological differences between the double mutant and wild-type become discernable from anther stage 3 onwards, at which time primary parietal cells and primary sporogenous cells are formed in all four lobes in the wild-type. However, sporogenous cell formation fails to occur on the adaxial side of roxy1 roxy2 anthers (Figure 4a,f). Further divisions of the primary parietal and sporogenous cells occur only in the abaxial portions of double mutant anthers at stage 5, and give rise to the three cell layers of the anther wall and the PMCs, respectively (Figure 4b,g). In the adaxial lobes, no further divisions occur, and thus no differentiation into PMCs and somatic cell layers is observed (Figure 4g). From stage 6 onwards, PMCs in the abaxial lobes adopt an aberrant shape (Figure 4c,h). Loss of ROXY1 and ROXY2 functions leads to a reduction in PMC callose formation, such that PMCs stick together and degenerate (Figure 4k,l). The tapetum then grows hypertrophically (Figure 4d,i) and finally degrades, leading to the formation of small, empty anther locules (Figure 4j,e).
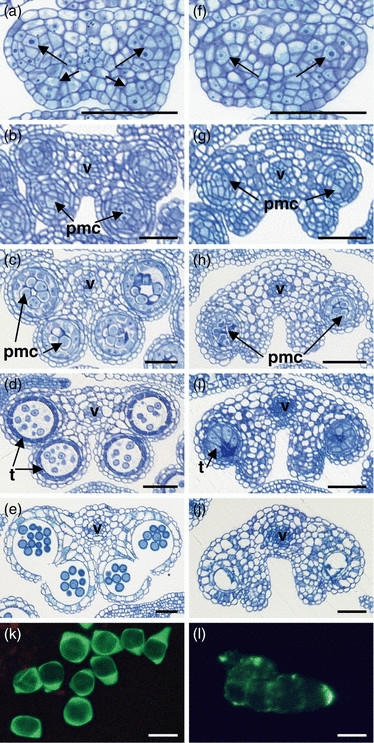
Comparison of wild-type and roxy1 roxy2 anther development.Semi-thin cross-sections through wild-type (a–e) and roxy1 roxy2 (f–j) anthers were stained with toluidine blue.(a) Anther stage 3. Anthers have four lobes containing sporogenous cells (arrows).(b) Anther stage 5. The typical four-lobed anther morphology is established, and PMCs have formed in the center of each lobe.(c) Anther stage 6. PMCs have separated and are ready to undergo meiosis.(d) Anther stage 8. Microspores are released from the tetrads. Strong staining of the tapetum indicates its degeneration.(e) Anther stage 13. Anthers are dehisced. Pollen sacs contain mature pollen grains, while the tapetum and middle layer have disappeared completely.(f) Anther stage 3. Sporogenous cells have formed in abaxial anther lobes (arrows), but not in adaxial ones.(g) Anther stage 5. Anther size is slightly reduced compared to wild-type. Two abaxial lobes are formed by somatic cells surrounding PMCs. However, no sporogenous cells are generated in the adaxial lobes.(h) Anther stage 6. Abaxial PMCs are irregularly shaped and start to degenerate. The typical pollen sac wall is not formed in the adaxial lobes.(i) Anther stage 8. PMCs have degenerated. The expanded tapetum occupies the anther lobe space.(j) Anther stage 13. The tapetum is degraded and no pollen grains are detectable in the locules. A residual middle layer still exists.(k) Wild-type PMCs separate at anther stages 6–7, and thick callose walls form (visualized by aniline blue staining).(l) A group of PMCs from a roxy1-5 roxy2-2 anther at stage 6. These PMCs have less callose in their walls, and therefore, unlike wild-type PMCs, remain clumped together. Bars = 50 μm in a–j; 20 μm in k,l.
The differential effects of ROXY1 and ROXY2 on abaxial and adaxial anther lobe development were further investigated by in situ hybridization experiments using a SPL/NZZ probe. SPL/NZZ is one of the earliest-acting anther genes and is expressed in archesporial and sporogenous cells as well as later in PMCs and tapetum (Schiefthaler et al., 1999; Yang et al., 1999). SPL/NZZ expression is detectable at anther stage 2, when archesporial cells are formed in the four corners of early wild-type and double mutant anthers (Figure 5a,e). When sporogenous cells are initiated at stage 3, expression persists in the four wild-type anther lobes (Figure 5b–d). However, in roxy1 roxy2 double mutants, after formation of sporogenous cells in abaxial lobes at stage 3, adaxial mutant lobes are largely devoid of SPL/NZZ expression (Figure 5f–h). When tetrads are formed after stage 7, less SPL/NZZ expression is detectable in the hypertrophically growing tapetum, which is in accordance with the abnormal tapetum phenotype (Figure 5d,h). These data show that the earliest defect detectable in the double mutant specifically disrupts archesporial cell differentiation in the adaxial lobes of anthers. Later defects in the abaxial lobes are due to failure of further PMC differentiation, and could therefore also perturb meiosis.
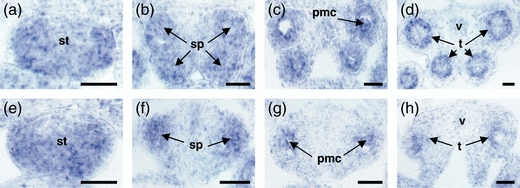
In situ hybridization analysis of SPL/NZZ expression in wild-type and roxy1-5 roxy2-2 anthers.Cross-sections through early anther stages were prepared from wild-type (a–d) and roxy1-5 roxy2-2 (e–h) flowers.(a, e) Anther stage 2. SPL/NZZ expression is detectable in the four corners of anthers, including archesporial cells, but absent from the epidermis.(b, f) Anther stage 4. SPL/NZZ RNA is localized in sporogenous cells. In wild-type, the sporogenous cells in all four corners of the anther show strong expression of SPL/NZZ (b), whereas, in roxy1 roxy2, strong SPL/NZZ expression is restricted to the two abaxial lobes (f).(c, g) Anther stage 6. SPL/NZZ is expressed within the tapetum and PMCs of all four wild-type lobes (c). In contrast, strong SPL/NZZ signal is only detectable in the two abaxial locules of roxy1 roxy2 anthers (g).(d, h) Anther stage 7. SPL/NZZ expression is mainly confined to the tapetum in wild-type anthers (d). A weak signal is detectable in the two abaxial locules of roxy1-5 roxy2-2 anthers (h).pmc, pollen mother cell; sp, sporogenous cell; st, stamen; t, tapetum; v, vascular bundle. Bars = 20 μm.
The conserved glycine in the putative GSH binding site is crucial for ROXY1 function
Various GRXs from prokaryotes to humans are known to utilize the tripeptide glutathione (GSH) to catalyze redox-dependent functions. In the GRX catalytic cycle, GSH binds to oxidized glutaredoxin and reduces it, making the enzyme available for further reduction reactions (Fernandes and Holmgren, 2004). In addition to a conserved active site motif, a GSH binding site sequence with one conserved glycine and a few less well conserved amino acids has been described for GRXs (Fladvad et al., 2005; Lundberg et al., 2001; Sun et al., 1998). Amino acid alignment of GRXs from Escherichia coli and various plant and mammalian species revealed that this conserved glycine also exists in ROXY1 (G110) and ROXY2 (Figure 6a). To test its functional significance for a plant GRX, G110 in ROXY1 was mutated into an alanine (G110A). In addition, a conserved proline (P100) located in a conserved GRX hydrophobic surface area (Lundberg et al., 2001) ten amino acids upstream of G110 was mutated (P100A, Figure 6a). The two mutated versions of ROXY1 were expressed under the control of a 3.6 kb ROXY1 promoter fragment that has been shown to confer endogenous ROXY1 expression (Xing et al., 2005) in roxy1-5 plants. A wild-type version of ROXY1 served as a control. The petal phenotype of roxy1-5 mutants was rescued in all 40 pROXY1::ROXY1 T1 plants (Figure 6b,c and Figure S2). In contrast, none of the 52 pROXY1::ROXY1m(G110A) plants produced any wild-type flowers (Figure 6d and Figure S2). The single conserved glycine is thus crucial for rescue of the roxy1 petal phenotype, and ROXY1 and ROXY2 probably bind GSH and use it to regenerate their reductive capacity during flower development. Mutagenesis of the conserved proline (P100A) did not prevent full complementation of 36 investigated transgenic roxy1-5 mutants (Figure 6e and Figure S2), indicating that the hydrophobic proline is dispensable for ROXY1 function.
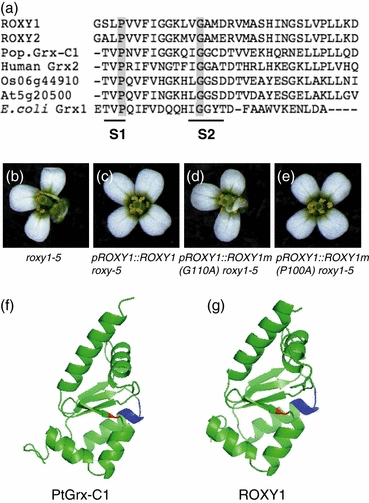
G110 in the putative GSH binding site is crucial for ROXY1 function.(a) Alignment of C-terminal regions of ROXY1, ROXY2, poplar PtGrx-C1 (AAV73806), the human Grx2 (AAK72499), a rice GRX (Os06g44910), a CPYC GRX from Arabidopsis (At5g20500) and Grx1 from E. coli (P68688). S1 and S2 indicate a hydrophobic surface area and the GSH binding site, respectively. The positions of conserved proline and glycine residues are indicated by gray boxes; in ROXY1, these amino acids are P100 and G110, respectively.(b) A typical roxy1-5 flower.(c) Flower from a T1roxy1-5 plant expressing the pROXY1::ROXY1 transgene, showing a wild-type petal phenotype.(d) Flower from a T1roxy1-5 plant expressing the pROXY1::ROXY1m(G110A) transgene. Replacement of the conserved Gly110 by Ala prevents rescue of the mutant petal phenotype.(e) Flower from a roxy1-5 T1 plant harboring the pROXY1::ROXY1m(P100A) transgene. Substitution of the conserved Pro100 by Ala has no effect on the ability of ROXY1 to rescue the petal phenotype.(f) Ribbon representation of poplar Grx-C1. Four central β-sheets are surrounded by five α-helices. The position of the conserved glycine in the GSH binding site is indicated in red, the CGYC active site is blue.(g) Ribbon representation of ROXY1 obtained by homologous modeling using poplar Grx-C1 as a template (PDB entry 1Z7R). The conserved glycine (G110, red) and the CCMC active site (blue) are located on the same β-sheet and α-helix, respectively, as in poplar Grx-C1.PyMOL (DeLano Scientific, Palo Alto, CA, USA; http://www.pymol.org) was used to generate Figure 6(f, g).
Structural characterization of the first plant glutaredoxin protein from poplar, the reduced Grx-C1 containing a CGYC active site (Feng et al., 2006), enabled us to conduct homology modeling using Grx-C1 as a template (Figure 6f). ROXY1 and Grx-C1 share 43% sequence identity. Modeling predicts that the ROXY1 core structure is highly similar to that of Grx-C1, and shows a typical thioredoxin-fold composed of five α-helices and four β-strands (Figure 6g). This overall structure may be shared between various plant GRXs (Feng et al., 2006). The conserved G110 and the CCMC active site of ROXY1 are localized on the β4-sheet and α2-helix, respectively, and both regions are localized on the protein surface, as are the corresponding amino acids in the poplar Grx-C1. Homology modeling is thus consistent with the results of mutagenesis, and suggests that ROXY1 could bind to GSH.
Expression of a large number of genes is deregulated in roxy1 roxy2 double mutants
To explore the pathways that are affected by loss of ROXY1 and ROXY2 activity during anther development, microarray experiments were conducted. Affymetrix 24K GeneChips were used to compare the transcriptomes of young roxy1-5 roxy2-2 and wild-type inflorescences from anther stages 1–7. The expression level of 386 genes is downregulated in the double mutant, and upregulation was observed for only 64 genes (Figure 7a and Tables S3 and S4). The largest affected group of genes belongs to the category ‘other and unknown functions’ (186 genes downregulated and 22 genes upregulated). Loss of ROXY1/2 activity severely disturbs transcription of genes in the category ‘metabolic function’, with 60 genes downregulated and 22 genes upregulated. Further large groups of downregulated genes belong to the categories ‘transcription regulation’ (39 genes) and ‘signal transduction’ (21 genes), for which five genes and one gene, respectively, were upregulated. Loss of ROXY1/2 activity caused downregulation of 23 redox-related genes and did not increase the transcript level of any member of this category.
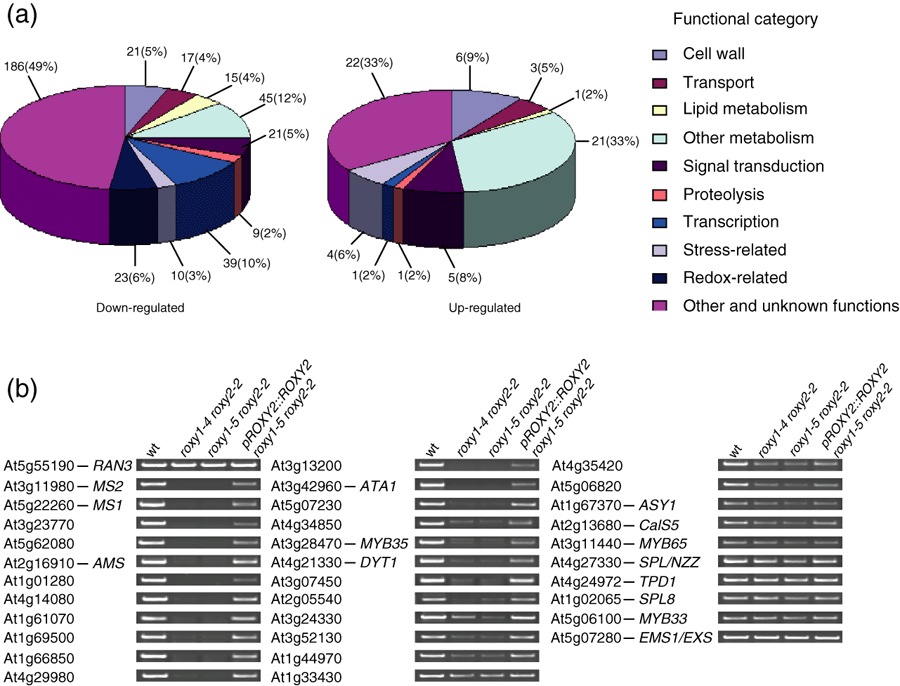
Expression analysis of deregulated genes in roxy1 roxy2 double mutant.(a) Functional classification of the deregulated genes identified by comparing wild-type and roxy1-5 roxy2-2 double mutant young inflorescences using the Affymetrix 24K GeneChip. Lists of downregulated and upregulated genes are provided in Tables S3 and S4, respectively.(b) Representative RT-PCR data for selected genes from the microarray experiment and selected known anther genes that are not present on the Affymetrix 24K GeneChip. RT-PCR was performed for wild-type, roxy1-4 roxy2-2 and roxy1-5 roxy2 mutants, and transgenic plants harboring the pROXY2::ROXY2 transgene in a roxy1-5 roxy2-2 background. Primers used for the PCR are listed in Table S2.
RT-PCR analysis of selected genes from the array experiments confirmed differential expression in young inflorescences from wild-type and roxy1-5 roxy2-2 plants, as well as roxy1-4 roxy2-2 double mutants. Furthermore, it allowed us to determine effects on known anther genes such as DYT1 (Zhang et al., 2006) and SPL8 (Unte et al., 2003) that are not represented on the 24K chip. Genes identified as being downregulated in roxy1-4 roxy2-2 were also downregulated in roxy1-5 roxy2-2 double mutants. This downregulation was at least partially reversed in complemented roxy1-5 roxy2-2 mutants harboring the pROXY2::ROXY2 construct (Figure 7b). Of 33 analyzed genes, including 13 known anther genes, the expression levels of 29 were changed by factors ranging from >2 to >100 (Table 2). A correlation between the degree of expression change and the timing of gene activity during anther development was observed. The most strongly affected genes are MS2 and MS1, which encode two male-sterile proteins (Aarts et al., 1997; Ito and Shinozaki, 2002), for which expression was 164- and 71-fold downregulated, respectively, in the double mutant. Together with AMS, which was 42-fold downregulated, these anther genes act downstream of the tapetum gene DYT1 (Ito and Shinozaki, 2002; Sorensen et al., 2003; Wilson et al., 2001; Zhang et al., 2006). Expression of DYT1 itself, similar to that of the transcription factor MYB35, was reduced over 20-fold. Expression of A9 and A6, which are also known to be required for tapetum differentiation (Hird et al., 1993; Paul et al., 1992), were reduced to a similar extent (29- and 39-fold, respectively). Genes acting during pre-meiotic stages, such as SPL/NZZ and TPD1 (Schiefthaler et al., 1999; Yang et al., 1999, 2003), were only slightly downregulated, about twofold. Similarly, expression of two other early-acting anther genes, MYB33 and MYB65, double mutants of which fail to produce microspores and form a hypertrophic tapetum, is also slightly affected (Millar and Gubler, 2005). The callose synthase gene CalS5 (At2g13680) was downregulated about fourfold, and is known to be expressed in PMCs and mature pollen (Nishikawa et al., 2005), which is compatible with the observed histological defect of reduced callose formation in roxy1-5 roxy2-2 PMCs (Figure 4l). Loss of ROXY1/2 activity does not affect transcription of SPL8, which is known to control early archesporial cell formation (Unte et al., 2003).
| AGI accession | Gene symbol | Fold change | Gene annotation |
|---|---|---|---|
| At3g11980 | MS2 | 164.6 ± 2.7 | Male-sterile protein 2 |
| At5g22260 | MS1 | 71.4 ± 0.8 | Male-sterile protein 1 |
| At3g23770 | 49.3 ± 0.2 | β-1,3-glucanase | |
| At5g62080 | 48.8 ± 0.3 | A9 protein precursor-like protein | |
| At2g16910 | AMS | 42.6 ± 0.4 | bHLH transcription factor |
| At1g01280 | 42.0 ± 1.8 | Cytochrome P450 | |
| At4g14080 | 39.3 ± 0.4 | A6 anther-specific protein | |
| At1g61070 | 38.6 ± 0.5 | Unknown protein | |
| At1g69500 | 37.9 ± 0.3 | Cytochrome P450 | |
| At1g66850 | 37.6 ± 0.6 | Lipid transfer protein | |
| At4g29980 | 37.5 ± 0.1 | Hypothetical protein | |
| At3g13200 | 35.7 ± 0.3 | ABC transporter | |
| At3g42960 | ATA1 | 31.6 ± 0.5 | Alcohol dehydrogenase |
| At5g07230 | 29.7 ± 0.2 | A9 protein | |
| At4g34850 | 29.1 ± 0.4 | Chalcone synthase-like protein | |
| At3g28470 | MYB35 | 27.2 ± 0.7 | MYB transcription factor |
| At4g21330 | DYT1 | 22.3 ± 0.2 | bHLH transcription factor |
| At3g07450 | 20.8 ± 0.6 | Putative 5B anther-specific protein | |
| At2g05540 | 11.7 ± 0.2 | Putative glycine protein | |
| At3g24330 | 11.2 ± 0.3 | β-1,3-glucanase | |
| At3g52130 | 9.6 ± 0.4 | 5B-like protein, cysteine-rich protein | |
| At1g44970 | 9.0 ± 0.1 | Peroxidase | |
| At1g33430 | 8.3 ± 0.2 | Elicitor response protein | |
| At4g35420 | 7.6 ± 0.2 | Putative dihydroflavonol-4-reductase | |
| At5g06820 | 7.3 ± 0.2 | Receptor-like protein kinase | |
| At1g67370 | ASY1 | 5.2 ± 0.2 | Meiotic asynaptic mutant 1 |
| At2g13680 | CalS5 | 4.2 ± 0.2 | Callose synthase |
| At3g11440 | MYB65 | 2.1 ± 0.1 | MYB transcription factor |
| At4g27330 | SPL/NZZ | 2.0 ± 0.1 | SPOROCYTELESS/NOZZLE |
| At4g24972 | TPD1 | 1.9 ± 0.1 | TAPETUM DETERMINANT 1 |
| At5g06100 | MYB33 | 1.4 ± 0.1 | MYB transcription factor |
| At1g02065 | SPL8 | 1.1 ± 0.1 | SPL8 transcription factor |
| At5g07280 | EMS1/EXS | 1.1 ± 0.1 | EXCESS MICROSPOROCYTES1/EXTRA SPOROGENOUS CELLS |
- Total RNA for semi-quantitative RT-PCR was isolated from inflorescences harvested from wild-type (Nössen) and roxy1-5 roxy2-2 double mutants. Mean fold changes are indicated, ±standard deviation.
Other genes that are downregulated include genes with an unknown function, such as At1g61070 and At4g29980, and also a β-1,3-glucanase, a cytochrome P450, a lipid transfer protein, an ABC transporter, a chalcone synthase, a peroxidase, an elicitor response protein and a receptor-like protein kinase (Table 2 and Figure 7b). The large variety of genes that are affected at the expression level reveals the highly complex nature of the changes caused by the simultaneous absence of ROXY1 and ROXY2, supporting an important function for ROXY1/2 in anther gene transcription.
Discussion
Within the plant-specific Arabidopsis CC-type GRX subgroup, ROXY1 and ROXY2 show the highest degree of similarity to each other. Single roxy1 mutants display a petal phenotype, whereas single roxy2 mutants do not show any floral phenotype. However, roxy1 roxy2 mutants are sterile and do not produce pollen. In agreement with the observed double mutant phenotypes, ROXY1 and ROXY2 expression domains overlap in anther primordia, PMCs and tapetum. Furthermore, expression of ROXY2 under the control of a ROXY1 promoter fragment can fully rescue the roxy1 mutant phenotype (unpublished data), demonstrating that ROXY2 can substitute for ROXY1 in petal development. These findings show an important redundant function for ROXY1 and ROXY2 in anther development.
ROXY1 and ROXY2 affect abaxial and adaxial anther lobe development differentially
Loss of the ROXY1/2 functions impairs sporogenous cell formation in adaxial lobes, indicating an indispensable early adaxial anther function for ROXY1 and ROXY2. However, in abaxial anther lobes, sporogenous and somatic cell formation proceeds further without requiring ROXY1/2 activity. Therefore, the roxy1 roxy2 double mutant produces only two visible abaxial pollen sacs. Some 3500 Arabidopsis genes may be specifically expressed in the anther and contribute to anther cell differentiation and cell fate (Scott et al., 2004). Several anther mutants have been isolated from Arabidopsis and maize, most of which affect later stages of anther cell differentiation. In the spl/nzz mutant, the four anther lobes are simultaneously affected (Schiefthaler et al., 1999; Yang et al., 1999). In contrast, in spl8 mutants, microsporogenesis does occur occasionally, but with equal probability in all locules within the anther (Unte et al., 2003). Similarly, the maize msca1 mutant also shows defects in archesporial cell division (Chaubal et al., 2003). The differential roxy1 roxy2 anther phenotype could be due to early ROXY1/2 targets being unequally expressed or functioning in anther lobes. Alternatively, other redundantly acting GRXs or redox modulators might exist that function differentially during early anther development.
ROXY1 and ROXY2 contribute to tapetum development
In the abaxial sectors of double mutant anthers, degeneration of PMCs is accompanied by hypertrophic growth of the tapetum, and tapetal cells eventually occupy most of the locule space. A functional tapetum is crucial for pollen development as it provides nutrients and enzymes for the developing PMCs (Scott et al., 2004). Although entry into meiosis can occur in the absence of a normal tapetal cell layer, as observed in gne2 mutants (Sorensen et al., 2002), several tapetum-defective mutants show a male-sterile phenotype. In myb33 myb65 double mutants, PMCs degenerate due to tapetum hypertrophy, which begins early, at the pre-meiotic stage (Millar and Gubler, 2005). The myb33 myb65 phenotype seems to be conditional, as high light intensities and low temperature can restore male fertility (Millar and Gubler, 2005); no such environmental effect was observed in roxy1 roxy2 mutants (data not shown). Other mutants, such as fat tapetum and gne1, also exhibit a hypertrophic tapetum, but, unlike roxy1 roxy2 and myb33 myb65, these mutants also display excessive growth of the middle layer (Sanders et al., 1999; Sorensen et al., 2002). Tapetal hypertrophy in roxy1 roxy2 mutants is probably a direct consequence of the absence of ROXY1 and ROXY2, as both genes are normally expressed in this cell layer. The tapetal effect is accompanied by delayed degeneration of the tapetal cell, a process that is considered to be a programmed cell death event (Papini et al., 1999). These data imply that plant GRXs participate in the modulation of signaling pathways leading to tapetal cell death, which might contribute to the defects observed during further abaxial PMC differentiation.
ROXY1 and ROXY2 probably require interaction with GSH for their function
The mechanistic basis of GRX activity has been intensively studied in E. coli, yeast and mammals, and binding of GSH is known to be required for the action of these enzymes. A GSH binding site has been identified in GRXs, and comprises a conserved glycine, flanked by less well-conserved amino acids (Fladvad et al., 2005; Sun et al., 1998). Substitution of Ala for the conserved G110 in ROXY1 destroys its ability to complement the roxy1 phenotype (Figure 6d), proving that the conserved glycine is also essential for the function of this plant CC-type GRX. Given the sequence similarity between ROXY1 and ROXY2 and their functional redundancy, it seems likely that both proteins act as oxidoreductases and regenerate their reductive capacities via interaction with GSH, mediated by the conserved glycine.
Homologous protein modeling, using the NMR solution structure of Grx-C1 from poplar as a template (Feng et al., 2006), shows that ROXY1 and also ROXY2 (data not shown) most probably form a typical thioredoxin-fold structure, composed of central β-sheets surrounded by α-helices. The conserved glycine and the crucial N-terminal cysteine of the active site motif are both located on the surface of the predicted protein structure, and are thus available for interaction with other molecules. Reduced levels of GSH are known to affect flowering time and root development in Arabidopsis (Cobbett et al., 1998; Ogawa et al., 2004), and this key component of plant antioxidant defenses seems to also have a function in various developmental processes, probably in conjunction with GRXs.
A model for ROXY1/2 function in anther development
Based on our genetic and expression data, we provide a model for ROXY1/2 function during anther development (Figure 8e). The putative transcription factor SPL/NZZ is required for sporogenous anther cell formation. spl/nzz mutant anthers lack endothecium, middle layer, tapetum and meiocytes in all four lobes (Schiefthaler et al., 1999; Yang et al., 1999). As the anther phenotype of the spl-1 roxy1 roxy2 triple mutant resembles that of the spl-1 single mutant (Figure 8a,b), and no strong SPL-1 expression changes were determined in the double mutant, the two GRXs probably act downstream of SPL/NZZ. The tapetum regulator DYT1 was suggested to function downstream of SPL/NZZ (Zhang et al., 2006). Our expression analysis shows that DYT1, as well as its targets MS1, AMS and MYB35, are strongly downregulated in roxy1 roxy2 double mutants. Furthermore, the dyt1 roxy1 roxy2 triple mutant phenotypically resembles the roxy1 roxy2 double mutant (4, 8). Together, these data imply that ROXY1/2 acts upstream of DYT1, regulating tapetum differentiation and thereby probably affecting PMC meiosis and further microspore development. However, we cannot exclude the possibility that ROXY1/2 exert a direct function in PMC differentiation, which is supported by the fact that both genes are expressed in PMCs. Expression profiling studies revealed that a large variety of genes are affected at the transcriptional level in the double mutant. The observed correlation between the onset of anther gene expression and the strength of their downregulation in the double mutant may serve as a starting point to predict the position of more genes in the early anther regulatory pathway and to investigate their functions.
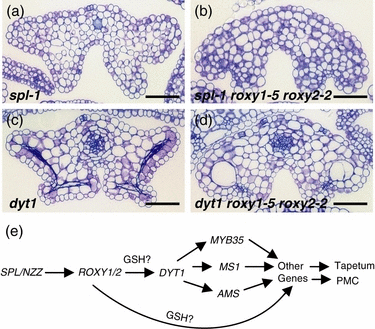
Mutant anther morphology and model for ROXY1/2 function in anther development.(a–d) Cross-sections of mutant anthers at stage 12. (a) spl-1. (b) spl-1 roxy1-5 roxy2-2. (c) dyt1. (d) dyt1 roxy1-5 roxy2-2. (e) Arrows indicate positive genetic regulation between the genes. GSH, glutathione. Bars = 50 μm.
To conclude, the plant-specific glutaredoxins ROXY1 and ROXY2 require a conserved glycine interacting with GSH for modification of target proteins. Environmental stress is known to decrease plant fertility, and gametophyte development is highly sensitive to stress and reactive oxygen species (Ambrus et al., 2006; Sun et al., 2004). Mutagenesis studies have suggested that conserved cysteines in MYB R2R3 proteins mediate their redox-dependent ability to bind to promoter sequences (Heine et al., 2004), and thus these proteins might represent targets for post-translational modification by ROXY1 and ROXY2. Further analysis of these plant glutaredoxins should shed light on the importance of redox control in normal anther development and plant fertility.
Experimental procedures
Growth of plants and isolation of mutants
Plants were grown in the greenhouse at 21–23°C under long-day conditions (16 h light). roxy2-1 and roxy2-2 mutants were isolated from the SIGnAL T-DNA collection (line SALK_057273, Col-0) and from the RIKEN Ds insertions collection (line 13-1723-1, Nössen; Ito et al., 2002; Kuromori et al., 2004), respectively. Homozygous mutants were identified by PCR genotyping. For roxy2-1, gene-specific primers 5′-AGCCGACAAGAAGGATAGATATATCC-3′and 5′-ACGCATCACTCTTCTCTCTCACTGTC-3′ were used. The T-DNA insertion was detected using the left border primer 5′-GCGTGGACCGCTTGCTGCAACT-3′ and the gene-specific reverse primer. Gene-specific primers for roxy2-2 were 5′-AAAGCTAGCAACCATGATGGTCC-3′and 5′-GTTAACATTTATATATTAGTGTG-3′. The Ds primer 5′-TCCGTTCCGTTTTCGTTTTTTAC-3′ was used in combination with the reverse gene-specific primer to detect the insertion. Two new ROXY1 alleles in the Nössen background, roxy1-4 and roxy1-5, were obtained from the RIKEN Ds collection (lines 11-2698-1 and 13-1220-1). The homozygocity of mutants displaying petal abnormalities was confirmed using the ROXY1 primers 5′-CAGAGTTAGACTCAGAGGTGTAGTAGG-3′ and 5′-GAATAGATACGGCGTCAGTTAATACCG-3′. For roxy1-4, the reverse ROXY1 primer was combined with the Ds primer 5′-CCGGATCGTATCGGTTTTCG-3′, and, for roxy1-5, the reverse ROXY1 primer was used together with the Ds primer 5′-TCCGTTCCGTTTTCGTTTTTTAC-3′.
spl-1 (N6586, The European Arabidopsis Stock Centre) and dyt1 (line 15-3398-1, RIKEN) mutants were identified by phenotype screening and genotyping. The gene-specific primer (5′-TGTTCTTCATCAATCTCAGGAGGAGCTTC-3′) and Ds primer (5′-CCGGATCGTATCGGTTTTCG-3′) were used for detecting the Ds insertion in dyt1, while the gene-specific primer pair 5′-TCGAAATGTTACCATTCCTTTGTCTG-3′ and 5′-ATCGAGATTTGGGACTTACGTTGGTG-3′ was used to confirm dyt1 homozygocity.
Crosses and mutant phenotype analysis
Various roxy1 alleles were crossed to roxy2-2. Sterile double mutant phenotypes in a F2 population were confirmed by genotyping. Reciprocal crosses were performed to test for effects on male and/or female fertility. Siliques from crosses were harvested and dissected 8 days after pollination to determine the number of aborted ovules under a binocular microscope (Leica MZ-FLIII; http://www.leica-microsystems.com). To generate spl-1 roxy1-5 roxy2-2 and dyt1 roxy1-5 roxy2-2 triple mutants, spl-1 (+/−) and dyt1 (+/−) hemizygotes, respectively, were crossed with roxy1-5 roxy2-2 double mutants, and F2 plants were phenotypically screened and genotyped. For microscopic examination, young floral buds from anther stages 2–13 were processed as described by Sorensen et al. (2002). For analysis of the callose wall, PMCs were stained with 0.1% aniline blue at anther stage 6. Samples were photographed under a Zeiss Axiophot microscope (http://www.zeiss.com/) using a digital camera (JVC KY-F75U; http://www.jvc-victor.co.jp).
Transgenic plant construction
To test whether ROXY2 can complement the roxy1-5 roxy2-2 phenotype, a 4440 bp genomic fragment was amplified by PCR using the primers 5′-GCGGATCCTTTCAGGTAAACATCTCATTGATAGTG-3′ and 5′-ACGGATCCACCTGTTGGCGTCTTTCTGTTCATCATC-3′, and cloned into the binary vector pGSA1252. After sequence verification, the binary vector was transformed into Agrobacterium strain GV3101. Transgenic plants were generated by transformation of roxy2-2−/−roxy1-5−/+ plants.
To generate the ROXY1 promoter construct, a 3.6 kb region located immediately upstream of the ROXY1 start codon was amplified from Col-0 genomic DNA (adding appropriate restriction sites to facilitate cloning), using the primer pair 5′-GCGTAGATCTCAATAGTCGAGGATCATTCGGAGTGC-3′ and 5′-ATGCCCATGGTCTAGATTTGATATCTCTTCTCTTTCTCTTGTTAC-3′. The resulting product was cloned into the binary vector pGSA1252. Point mutations at specific positions in the ROXY1 coding sequence were created using a PCR-based technique as described by Xing et al. (2005). To introduce the P100A and G110A mutations, pairs of mutagenic oligomers were used (5′-AGGGTCTCTCgCGGTCGTCTTCATC-3′ and 5′-ATGAAGACGACCGcGAGAGACCCTG-3′ for P100A, 5′-ACTGGTTGcAGCTATGGACAGAGTCATGG-3′ and 5′-AGCCATGACTCTGTCCATAGCTgCAACCAG-3′ for G110A; mutated bases are indicated in lower case). After sequencing, the wild-type version or the two mutated ROXY1 genes were fused to the 3.6 kb ROXY1 promoter and the constructs were transformed into roxy1-5 mutants. Transgenic T1 plants were tested for complementation of the roxy1 phenotype.
In situ RNA hybridization
In situ hybridization was performed as previously described, using PCR templates containing binding sites for the T3 or T7 RNA polymerase (Zachgo, 2002). The ROXY1 antisense probe was prepared according to the method described by Xing et al. (2005). To avoid cross-hybridization with ROXY1, the ROXY2 antisense probe was prepared using a unique 160 bp fragment from the 5′ end of ROXY2 (5′-CAACCAACTCTCACACAAATTCTC-3′ and 5′-CGGCAATTAACCCTCACTAAAGGGCACCTTACTGTTGTTG-3′) and a unique 220 bp fragment from the 3′ end of ROXY2 (5′-ATCAATGGCTCACTCGTCCC-3′ and 5′-CGGCAATTAACCCTCACTAAAGGGGACAAAGAGCTAAGC-3′) as templates. For SPL/NZZ antisense probe production, a template was used that comprised the whole coding region sequence, and was amplified using the primers 5′- GCGGAATTAACCCTCACTAAAGGGATCAATGGCGACTTCTCTCTTCTTC-3′ and 5′-GCTCGTAATACGACTCACTATAGGGCTTAAAGCTTCAAGGACAAATCAATGG-3′.
Homology modeling
Comparative modeling was performed using the automated Swiss-MODEL server (accessible via the ExPASy web server at http://swissmodel.expasy.org//SWISS-MODEL.html), which uses ProMod for three-dimensional protein modeling. Grx-C1 from poplar (1Z7RA) was used as the model as it shows the highest homology to ROXY1 (43%) among available templates. This allowed modeling of ROXY1 amino acids 21-136.
Isolation of total RNA and semi-quantitative RT-PCR
Total RNA was isolated from young inflorescences comprising apical meristems and young flower buds up to flower stage 10 (as defined by Smyth et al., 1990) obtained from wild-type (Nössen), roxy1-5 roxy2-2 and roxy1-4 roxy2-2 double mutants and T1 plants harboring the pROXY2::ROXY2 transgene in a roxy1-5 roxy2-2 background, using the RNeasy Plant Mini Kit (Qiagen, http://www.qiagen.com/). For semi-quantitative RT-PCR, 4 μg aliquots of total RNA were treated with RNase-free DNase I (Roche; http://www.roche-applied-science.com), and SuperScript™ II reverse transcriptase (Invitrogen, http://www.invitrogen.com/) was used to produce first-strand cDNA according to the manufacturer’s instructions. Aliquots of 5 μl from 25 μl reactions were loaded on an ethidium bromide-stained 1.0% w/v agarose gel. Bands were quantified by scanning gels using the phosphor imager Typhoon 8600 (Amersham Biosciences, http://www5.amershambiosciences.com/), using Image Quant version 5.2 software (Amersham Biosciences). Ran3 (At5g55190) was used for normalization of signal strength and as a reference for expression levels. Three RT-PCR repetitions were conducted for each gene, and primer pairs are listed in Table S2.
Microarray analysis
Three biological samples for total RNA were prepared from wild-type (Nössen) and the roxy1-5 roxy2-2 double mutant, respectively, as described for semi-quantitative RT-PCR. Probe preparation, hybridization to ATH1 Arabidopsis Genome Arrays (Affymetrix, Santa Clara, California; http://www.affymetrix.com) and statistical data analysis were carried out at the Integrated Functional Genomic service unit (University of Münster, Germany; http://ifg-izkf.uni-muenster.de). Data processing was performed using Affymetrix microarray suite 5.0. Only genes with an expression ratio (fold change, down or up) ≥2.0 and P-values <0.01 were considered. Functional classification of deregulated genes was performed manually, based on established and putative functions (TAIR, http://www.arabidopsis.org).
Acknowledgements
We thank Anja Hörold for excellent assistance and Dr Maren Heese for support with homology modeling. We also thank Dr Peter Huijser and Dr Andrea Busch for commenting on the manuscript. S.Z. is grateful to Professor Heinz Saedler for ongoing support and stimulating discussions. This work was supported by a grant from the Deutsche Forschungsgemeinschaft to S.Z. (ZA 259/4-1).




