Role of a GDSL lipase-like protein as sinapine esterase in Brassicaceae
Summary
The seeds of most members of the Brassicaceae accumulate high amounts of sinapine (sinapoylcholine) that is rapidly hydrolyzed during early stages of seed germination. One of three isoforms of sinapine esterase activity (BnSCE3) has been isolated from Brassica napus seedlings and subjected to trypsin digestion and spectrometric sequencing. The peptide sequences were used to isolate BnSCE3 cDNA, which was shown to contain an open reading frame of 1170 bp encoding a protein of 389 amino acids, including a leader peptide of 25 amino acids. Sequence comparison identified the protein as the recently cloned BnLIP2, i.e. a GDSL lipase-like protein, which displays high sequence identity to a large number of corresponding plant proteins, including four related Arabidopsis lipases. The enzymes belong to the SGNH protein family, which use a catalytic triad of Ser-Asp-His, with serine as the nucleophile of the GDSL motif. The corresponding B. napus and Arabidopsis genes were heterologously expressed in Nicotiana benthamiana leaves and proved to confer sinapine esterase activity. In addition to sinapine esterase activity, the native B. napus protein (BnSCE3/BnLIP2) showed broad substrate specificity towards various other choline esters, including phosphatidylcholine. This exceptionally broad substrate specificity, which is common to a large number of other GDSL lipases in plants, hampers their functional analysis. However, the data presented here indicate a role for the GDSL lipase-like BnSCE3/BnLIP2 as a sinapine esterase in members of the Brassicaceae, catalyzing hydrolysis of sinapine during seed germination, leading, via 1-O-sinapoyl-β-glucose, to sinapoyl-l-malate in the seedlings.
Introduction
Germinating seeds of members of the Brassicaceae family express high activity of an esterase that catalyzes hydrolysis of the major seed phenylpropanoid component sinapine (sinapoylcholine) to liberate sinapate and choline (Nurmann and Strack, 1979; Strack et al., 1980; Tzagoloff, 1962). Based on the substrate specificity the enzyme has been classified as a ‘sinapine esterase’ (SCE; EC 3.1.1.49). Sinapate released by in vivo SCE activity is re-esterified in the young seedlings, via 1-O-sinapoyl-β-glucose, with l-malate to form the UV-shielding compound sinapoyl-l-malate, which accumulates in epidermal tissues (Landry et al., 1995; Sheahan, 1996). The free choline feeds into phosphatidylcholine biosynthesis (Strack, 1981).
Formation of sinapoyl-l-malate is catalyzed by the enzymes UDP-glucose:sinapate glucosyltransferase (SGT; EC 2.4.1.120) and 1-O-sinapoyl-β-glucose:l-malate sinapoyltransferase (SMT; EC 2.3.1.92). The same mechanism has been described for sinapine formation in developing seeds involving the action of SGT and 1-O-sinapoyl-β-glucose:choline sinapoyltransferase (SCT; EC 2.3.1.91). Recently, a detailed study on molecular regulation during sinapate ester metabolism was performed in Brassica napus (Milkowski et al., 2004).
Initial studies on molecular evolution of the enzymes involved in sinapate ester metabolism in the Brassicaceae family indicated that SMT (Lehfeldt et al., 2000) and SCT (Milkowski et al., 2004; Shirley et al., 2001) belong to a new class of serine carboxypeptidase-like (SCPL) proteins (Milkowski and Strack, 2004) originally defined by isobutyroyl transferases from Lycopersicon penellii (Li and Steffens, 2000). It has been speculated that SCE might also share the characteristics of SCPL proteins (Fraser et al., 2005), but differing from the acyltransferases in retaining its hydrolytic activity. This assumption could not be verified in the present study.
Here we describe separation of three protein fractions exhibiting SCE activity from germinating seeds of B. napus, i.e. BnSCE1, BnSCE2 and BnSCE3. The protein with the highest activity (BnSCE3) was isolated and used for peptide sequencing followed by cDNA cloning. The deduced amino acid sequence of the full-length clone identified BnSCE3 as the recently cloned BnLIP2 (GenBank accession no. AY870270; Ling et al., 2006), a member of the large protein family of GDSL lipases (Akoh et al., 2004). However, its lipase activity and role in plant metabolism have not yet been experimentally proven. In our hands, BnSCE3/BnLIP2 showed, in addition to activity with sinapine and other hydroxycinnamate choline esters, a broad substrate specificity towards various other phenolic choline esters, and, surprisingly, also towards phosphatidylcholine. Our data suggest that the GDSL-like protein previously described as BnLIP2 has either gained a specific role as sinapine esterase during evolution of plant secondary metabolism in Brassicaceae, or accepts sinapine and other phenolic choline esters due to its inherent broad substrate specificity.
Results and discussion
Purification of BnSCE
Protein extracts from 2-day-old Brassica napus seedlings express high ‘sinapine esterase’ activity (Milkowski et al., 2004). To purify the ‘sinapine esterase’, 18 g of ammonium sulphate-precipitated protein from these seedlings was subjected to a combination of chromatographic separation steps, including adsorption, ion-exchange and size-exclusion techniques (Table 1). Figure 1 shows a typical elution profile from Q-Sepharose, by which three protein fractions with esterase activity towards sinapine were separated, i.e. BnSCE1, BnSCE2 and BnSCE3. The protein fraction with the highest enzymatic activity, BnSCE3, was purified to near homogeneity with a 2300-fold enrichment and a yield of 5% of the extracted enzyme activity. Final chromatography on Mono-Q of the BnSCE3 fraction yielded 400 μg protein, from which aliquots were resolved into two bands on SDS–PAGE (Figure 2). The molecular mass of the major band was estimated to be about 44 kDa. Gel filtration of this protein on a Superose 12 HR 10/30 column gave a mass value of 47 kDa, indicating that the enzyme has a monomeric structure. The very faint second band in lane 8 has been tentatively ascribed to a glycosylated isoform of the major one.
| Purification step | Total protein (mg) | Total activity (nkat) | Specificity (nkat/mg) | Enrichment (fold) | Yield (%) |
|---|---|---|---|---|---|
| BnSCE1/BnSCE2/BnSCE3 | |||||
| Ammonium sulphate | 18 000 | 1080 | 0.06 | 1 | 100 |
| Phenylsepharose | 8400 | 773 | 0.092 | 1.5 | 72 |
| Q-Sepharose | |||||
| BnSCE1 | 2610 | 117 | 0.045 | 0.75 | 11 |
| BnSCE2 | 823 | 123 | 0.15 | 2.5 | 11 |
| BnSCE3 | 656 | 366 | 0.56 | 9.3 | 34 |
| BnSCE3 | |||||
| Sephadex G-75 | 91 | 295 | 3.24 | 54 | 27 |
| Q-Sepharose | 30 | 182 | 6.1 | 102 | 17 |
| Phenylsepharose | 3.0 | 103 | 34 | 566 | 9.5 |
| Superdex G-75 | 1.8 | 86 | 48 | 800 | 8.0 |
| Mono-Q | 0.40 | 55 | 138 | 2300 | 5.1 |
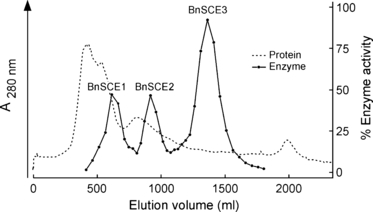
Ion-exchange chromatography of BnSCE proteins on Q-Sepharose resulting in separation of three sinapine esterase activities: BnSCE1, BnSCE2 and BnSCE3.
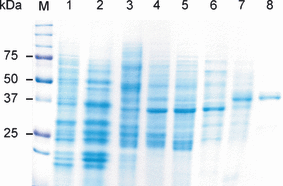
SDS-PAGE analysis of the BnSCE purification steps from two-day-old B. napus seedlings. M, Precision Plus Protein™ Standards Dual Color, BIO-RAD; lane 1, crude protein extract; lane 2, protein solution after Phenylsepharose FF 50/20; lane 3, Q-Sepharose 50/20; lane 4, Sephadex G-75 50/60; lane 5, Q-Sepharose 16/10; lane 6, Phenylsuperose 5/5; lane 7, Superdex G-75 16/60; lane 8, Mono-Q.
cDNA cloning and sequence analysis
To obtain sequence information for the purified BnSCE3, the major protein band in lane 8 of the SDS–PAGE (Figure 2) was excised from the Coomassie-stained gel and subjected to trypsin digestion and mass spectrometric sequencing. Sequences of five peptides were determined, and were used to derive degenerate primers for RT-PCR with RNA from B. napus seedlings. This approach led to the amplification of a 471 bp cDNA carrying the 3′ portion of an open reading frame. The 5′ cDNA sequence was amplified by 5′ RACE using poly(A+) RNA extracted from 2-day-old seedlings and a set of gene-specific primers (R1, R2, R3, R4; see Experimental procedures for sequences). PCR using one of these gene-specific primers (R2) led to the amplification of a 1037 bp cDNA fragment that had a 57 bp sequence overlap with the 471 bp 3′ cDNA fragment and a putative in-frame translation initiation codon near the 5′ end. The full-length BnSCE3 cDNA was isolated from seedling RNA by RT-PCR using specific primers derived from the 5′ RACE fragment and the 471 bp partial sequence. The resulting cDNA was shown to contain an open reading frame that consists of 1170 bp and encodes a protein of 389 amino acid residues. The derived amino acid sequence carried all sub-sequences determined by peptide sequencing of the purified BnSCE3 protein (Figure 3). Sequence-based prediction of the molecular mass of the mature enzyme revealed a value of 40.46 kDa, and the isoelectric point (pI) was 4.69. The calculated mass differs by about 4–7 kDa from the estimated values from SDS–PAGE and gel filtration. This indicates that the native enzyme is a glycoprotein, which is in accordance with the potential N-glycosylation sites identified in the amino acid sequence (see below).

Multiple alignment of deduced amino acid sequence of BnLIP2/BnSCE3 with four putative Arabidopsis GDSL lipases (At1g28640, At1g28650, At1g28650, At1g28670). The N-terminal leader peptide (first 25 amino acids) of BnSCE3/BnLIP2 is double underlined. Fully conserved residues are shaded in blue. Four conserved blocks in the SGNH-hydrolases family are boxed by black lines (I, II, III, V; Akoh et al., 2004; Ling et al., 2006). Amino acid residues forming the catalytic triad (Ser, Asp, His) in the consensus sequences of blocks I and V are marked by red triangles. One of the Gly residues in block II and Asn in block V potentially may also act as catalytic residues. The GDSL motif (blue framed box) and four other sub-sequences (black framed boxes) were identified by mass spectrometric sequencing after trypsin digestion. Putative N-glycosylation sides are underlined.
Sequence comparison by tblastn (http://www.ncbi.nlm.nih.gov/BLAST/) identified the BnSCE3 protein as the previously submitted GDSL lipase-like BnLIP2 (accession no. AY871275), based on a sequence identity of 97%. The cDNA was recently cloned from germinating seeds of B. napus, named BnLIP2 and mistakenly published as BnLIP1 (Ling et al., 2006), a homologue of the realBnLIP1 (Ling et al., 2006b). The database entry describes BnLIP2 as a putative lipase, but this is based exclusively on sequence identities without any experimental evidence.
The protein domain architecture derived from the deduced amino acid sequence of B. napus BnSCE3/BnLIP2 was examined by computer-assisted protein family analysis using the database Pfam (http://www.sanger.ac.uk/Software/Pfam/). The results showed that the sequence contains a conserved GDSL lipase family domain spanning residues 35–361. A GDSL/I motif was detected between amino acids 39 and 42 (Figure 3).
Inspection of the deduced BnSCE3/BnLIP2 amino acid sequence using the sorting server TargetP (Emanuelsson et al., 2000) predicts that the N-terminus of the protein contains a signal sequence of 25 residues, which routes the protein to the secretory pathway with a probability of 0.983 (>0.800 = strongest prediction; Figure 3). This is in accordance with a probable localization of the assumed natural substrate sinapine to vacuoles of germinating seeds. In the amino acid sequence, five potential N-glycosylation sites, NX(S/T), were identified (Figure 3).
GDSL lipases constitute a subclass of lipolytic enzymes characterized by a distinct GDSL sequence motif that is different from the GXSXG motif found in many lipases (Akoh et al., 2004). Within the GDSL subclass, BnSCE3/BnLIP2 belongs to the subgroup of SGNH hydrolases that are characterized by the presence of the four strictly conserved residues Ser, Gly, Asn and His in four conserved peptide blocks (Ling et al., 2006). The active centre of these enzymes is formed by a catalytic triad consisting of the seryl residue from the GDSL motif, which is part of conserved peptide block I, and aspartyl and histidyl residues that are both located in conserved peptide block V. This implies that, in BnSCE3/BnLIP2, the catalytic triad is most probably composed of Ser41, Asp345 and His348, i.e. Ser16-Asp320-His323 of the mature protein (Figure 3).
Role of GDSL lipases in plant metabolism
The sequence identity of BnSCE3 with BnLIP2 was the first indication that the ‘sinapine esterase’ is related to lipases instead of being a member of the serine carboxypeptidase-like (SCPL) protein family as previously speculated based on expression analysis of the Arabidopsis SCPL gene family (Fraser et al., 2005). Given the broad substrate specificity of GDSL lipases, which may be due to the remarkable flexibility of the active site of the protein (Akoh et al., 2004), we conclude that members of this enzyme family may have been recruited during evolution for functions in plant secondary metabolism such as sinapine hydrolysis.
Protein sequences related to BnSCE3/BnLIP2 were found in Arabidopsis thaliana, Oryza sativa, Triticum aestivum and Zea mays, with identities of up to 82%. A multiple alignment of the deduced amino acid sequences of BnSCE3/BnLIP2 and four putative Arabidopsis GDSL lipases is shown in Figure 3. These putative Arabidopsis GDSL lipases display the highest sequence identities to BnSCE3/BnLIP2, with scores of 672 for At1g28670, 655 for At1g28640, 642 for At1g28650 and 634 for At1g28660, and E-values of zero. All four Arabidopsis homologues are arranged in tandem repeats on chromosome 1.
The GDSL lipase subclass includes bacterial and a large number of plant proteins that have little sequence identity to true lipases (Akoh et al., 2004). Their function in seed germination and plant development remains unknown, and functional analysis is hampered by their exceptional broad substrate specificity (Ling et al., 2006).
Transcript accumulation and heterologous expression of BnSCE3/BnLIP2 and its Arabidopsis homologues
Upon measurement of SCE activity during B. napus seed germination, we found that the maximum value was reached in seedlings 2 days after sowing (Milkowski et al., 2004), correlating well with a strong decrease in the content of sinapine as one of the assumed BnSCE substrates. This developmental regulation of enzyme activity is roughly reflected by the BnSCE3/BnLIP2 transcript abundance, which showed a sharp induction 2 days after sowing. Figure 4 shows results from RT-PCR analyses of BnSCE3/BnLIP2 expression during B. napus seedling development, together with a modified diagram of SCE activity and sinapine degradation based on Milkowski et al. (2004). A high level of transcript was maintained until 6 days after sowing, followed by a decrease up to 12 days of seedling development. The decline in enzyme activity was found to be more rapid during these developmental stages, indicating that additional post-transcriptional regulation of BnSCE3/BnLIP2 activity may occur. Moreover, the enzyme could be involved in other as yet unknown metabolic pathways in seedlings (Ling et al., 2006), especially in the light of its expression in all mature B. napus tissues, including root, stem, leaf, bud, flower and pod (Ling et al., 2006). In germinating seeds, BnSCE3/BnLIP2 may be involved in oil body mobilization. It has been shown, for example, that phospholipase A activity is associated with oil bodies in cotyledons of Helianthus annuus (Gupta and Bhatla, 2007) and Cucumis sativus (May et al., 1998).
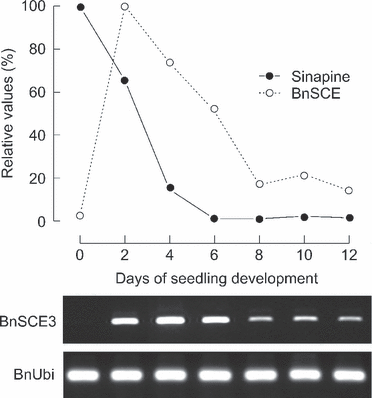
RT-PCR analysis of BnLIP2/BnSCE3 expression along with a modified diagram after Milkowski et al. (2004) showing enzyme activities (100 = 511 pkat mg−1 protein) and amount of sinapine (100 = ca. 135 nmol individuum−1) in B. napus seeds and developing seedlings. Expression of the ubiquitin gene is given as control. Staining was achieved with ethidium bromide.
For comparison, the transcript accumulation of the four Arabidopsis BnSCE3/BnLIP2 homologues encoding putative GDSL lipases was analyzed (Figure 5). During early seedling development, characterized by rapid sinapine degradation (Lorenzen et al., 1996), we found high transcript levels for At1g28650, At1g28660 and At1g28670 in seedlings up to 3 days old. For the putative lipase gene At1g28640, weak transcript abundance was restricted to the very early stage of germination, i.e. 1 day after sowing, indicating that this gene may not be involved in sinapine hydrolysis. Only At1g28670 was transcribed in seedlings as well as in adult leaves, in which sinapine is not detectable as a possible substrate. Thus, At1g28670 and BnSCE3/BnLIP2 may encode GDSL lipase-like proteins that are involved in lipid metabolism (Ling et al., 2006) or other functions of the mature plant as discussed by Akoh et al. (2004).
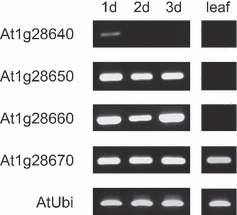
RT-PCR analysis of expression of four putative Arabidopsis GDSL lipases (At1g28640, At1g28650, At1g28660, At1g28670) in early seedling stages and leaf samples. Expression of the ubiquitin gene is given as control. Tissues analyzed were developing seedlings 1–3 days after sowing (1d, 2d, 3d) and adult leaves (leaf). Staining was achieved with ethidium bromide.
To provide experimental proof that the cloned cDNA from B. napus and the four putative Arabidopsis homologues encode enzymes that exhibit the expected sinapine esterase activity, cDNA fragments encoding the mature proteins were fused to the strong rbcs promoter and cloned into the binary vector pBINPLUS. Transiently transformed leaves of Nicotiana benthamiana (Kapila et al., 1997) were subjected to protein extraction, and the presence of sinapine esterase activity was proven by incubating the protein with sinapine as substrate. Enzyme activities were found in extracts from N. benthamiana leaves expressing BnSCE3/BnLIP2 as demonstrated in Figure 6. The Arabidopsis proteins At1g28650, At1g28660 and At1g28670, expressed in these leaves, also showed esterase activity, with specific activities (nkat mg−1 protein) of 2.1, 27.1 and 9.0, respectively. No SCE activity was detected for At1g28640.
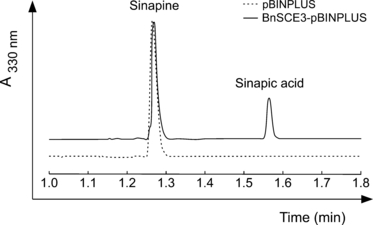
Traces of HPLC analyses of sinapine esterase assays run with protein extracts of Nicotiana benthamiana leaves harboring the empty vector pBINPLUS and the expression cassette BnSCE3-pBINPLUS.
Substrate specificity of native BnSCE3/BnLIP2 and inhibition of enzymatic activity
To analyze the substrate specificity of BnSCE3/BnLIP2, the enzyme was assayed with 16 potential phenolic substrates (Table 2) and also with some lipids, e.g. phosphatidylcholine (Figure 7). The phenolic substrates were chosen to allow systematic comparison of the influence of ring substituents and side-chain structures, as well as the nature of the ester-bound moieties. The results summarized in Table 2 show that, among the hydroxycinnamate esters, the enzyme prefers sinapine as substrate, followed by cinnamoylcholine for BnSCE3 and feruloylcholine for BnSCE1 and BnSCE2. Shortening of the side chain of the phenylpropanoid substrates drastically reduced the hydrolytic activity. Compounds with a C1 instead of a C3 side chain, e.g. syringoylcholine, were found to be almost inactive as substrates. In contrast, elimination of the C3 side-chain double bond, as in 3(3,4-dimethoxyphenyl)-propionylcholine and 3-phenylpropionylcholine, revealed much higher relative specific activity compared with that towards sinapine. Thus, 3-phenylpropionylcholine was hydrolyzed with 50-fold higher activity compared to the related structure cinnamoylcholine. Replacing the choline moiety by ethylamine led to a strong decrease in enzyme activity. These results indicate that specificity is directed towards the phenyl-C3 part of the phenolic substrate and the conjugating moiety, predominantly choline. To resolve some of the contradictions in the substrate specificities listed in Table 2, the kinetic constants of BnSCE3/BnLIP2 for 3-phenylpropionylcholine in comparison with sinapine and cinnamoylcholine were determined (Table 3). The data show that the high conversion rate of the enzyme with the phenylpropionyl conjugate corresponds to a Vmax value that is 40 times higher than those obtained with the two other substrates. It should be noticed, however, that there is no difference in the catalytic efficiencies with sinapine and 3-phenylpropionylcholine, as the affinity of the protein (Km) towards sinapine is 40 times higher than that for 3-phenylpropionylcholine.

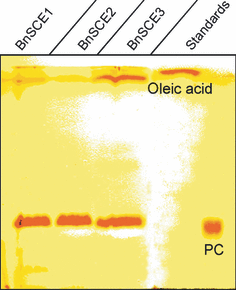
Thin layer chromatography (TLC) in hexan/diethyl ether/acetic acid (85:15:1) of phosphatidylcholine (PC) assays with protein extracts of BnSCE1 (184 μg), BnSCE2 (95 μg) and the purified BnSCE3 (1 μg). The applied standards were phosphadidylcholine (1,2-dioleoyl-PC) and oleic acid.
| Substrate | K m (μm) | V max (nkat mg−1) | V max /K m (nkat mg−1 μm−1) | Efficiency (%) |
|---|---|---|---|---|
| Sinapine (sinapoylcholine) | 2.6 ± 0.9 | 66 ± 3.3 | 25.4 | 100 |
| Cinnamoylcholine | 11 ± 0.9 | 59 ± 1.4 | 5.4 | 21 |
| 3-Phenylpropionylcholine | 101 ± 14 | 2525 ± 124 | 25.0 | 98 |
- Data were obtained and evaluated using an enzyme kinetics tool (SigmaPlot; Systat Software, Inc.).
Interestingly, two other non-choline sinapoyl conjugates, i.e. 1-O-sinapoyl-β-glucose and sinapoyl-l-malate, major phenolic components of the sinapate ester pathway in the seedlings (Milkowski et al., 2004), were not accepted as substrates by BnSCE3/BnLIP2. This excludes a function of the enzyme as non-specific general esterase, and more specifically also as a cholinesterase (EC 3.1.1.8). The latter was also excluded for Raphanus sativus SCE activity (Nurmann and Strack, 1979), which did, however, hydrolyze sinapoylglucose. Our previous assumption that this activity may be caused by a contaminating hydrolase activity has thus been corroborated.
The results listed in Table 2 indicate that the presence of choline moieties is a basic structural feature recognized by BnSCE3/BnLIP2. Given the homology of this enzyme with lipases, we tested phosphatidylcholine as potential substrate in comparison with some neutral lipids, e.g. a digalactosyldiacylglycerol and a triacylglycerol. Thin-layer chromatography of the reaction mixtures showed that all three BnSCE proteins were able to hydrolyze phosphatidylcholine (1,2-dioleoyl-PC), as confirmed by the appearance of oleic acid as a reaction product (Figure 7). The neutral lipids tested were not accepted (data not shown). This result indicates a phospholipase A-like catalysis activity (Wang, 2001) for BnSCE3/BnLIP2.
Due to the sequence identity with GDSL lipases, the enzyme most probably uses a catalytic triad of Ser16-Asp320-His323 of the mature protein, with the seryl side chain as the nucleophile. To prove the involvement of a catalytic seryl residue in sinapine hydrolysis, the enzyme was subjected to treatment with phenylmethylsulfonylfluoride (PMSF), a potent inhibitor that phosphorylates the seryl residues of enzymes; this treatment has previously been applied to lipolytic GDSL proteins (e.g. Teissére et al., 1995). Treatment of BnSCE3 with 1, 10 and 50 mm PMSF led to strong decreases in sinapine esterase activity, with 50% inhibition at about 8 mm PMSF (Figure 8). Thus, Ser16 of the mature protein of the GDSL motif may be part of the catalytic triad in BnSCE3/BnLIP2.
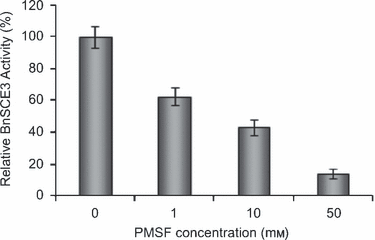
Inhibition of BnSCE3/BnLIP2 enzyme activity by the serine inhibitor PMSF; mean relative activity of three assays ± SD (n = 3); 100% = 232 nkat mg−1 protein.
As the enzyme was active towards phosphatidylcholine (Figure 7), we addressed the question of whether this activity may be due to the broad substrate specificity of a phospholipase (Akoh et al., 2004; Huang, 1993; Wang, 2001), or an extended function of a GDSL lipase as a ‘sinapine esterase’ in Brassicaceae. Therefore, we tested possible sinapine hydrolysis using commercially available phospholipase A2 from cobra venom (Sigma-Aldrich, München, Germany) and protein preparations from GDSL lipase-rich cotyledons of H. annuus and C. sativus, according to the methods described byGupta and Bhatla (2007) and May et al. (1998), respectively. Phospholipase A2 did not accept sinapine as a possible substrate (results not shown), and, although the protein preparations showed hydrolytic activity towards sinapine, this was less than 1% of that determined for B. napus (Milkowski et al., 2004). The specific function of BnSCE3/BnLIP2 as ‘sinapine esterase’ is further supported by results from an earlier study indicating a correlation between the occurrence of sinapine and esterase activity in members of the Brassicaceae (Strack et al., 1980).
In more applied studies focusing on the potential utilization of B. napus not only as oilseed crop but also as valuable protein supply in human nutrition, we have expressedBnSCE3/BnLIP2 in developing seeds of transgenic lines. This may suppress the sinapine accumulation that compromises the use of the seed meal as human food supplement (Ismail et al., 1981). In these studies, Arabidopsis plants were transformed with Agrobacterium tumefaciens harboring a BnSCE3/BnLIP2 expression plasmid under the control of the seed-specific napin promoter. Our results show that sinapine accumulation in some of the transgenic lines was drastically reduced, as shown in Figure 9 for a line that showed a residual amount of sinapine that was less than 5% of that of control plants. In HPLC analyses of the transgenic seed extracts, sinapine could scarcely be detected, and there is no accumulation of another sinapate ester compensating for the strong suppression of sinapine accumulation. This result strongly supports the evidence presented in this paper that the GDSL lipase-like BnSCE3/BnLIP2 in B. napus is involved in sinapine hydrolysis.
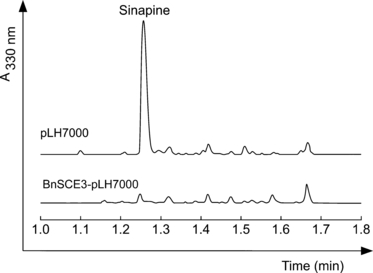
Traces of HPLC analyses of methanolic seed extracts from transformed Arabidopsis plants harboring the empty vector pLH7000 or the expression cassette BnSCE3-pLH7000. The seeds expressing the BnSCE3 gene reveal a strong suppression of sinapine formation.
This result indicates that members of the GDSL lipase family have gained new functions in plant phenylpropanoid metabolism. Together with a UDP-glucose:sinapate glucosyltransferase (SGT) and two sinapoylglucose-dependent sinapoyltransferases (SMT, SCT), characterized as SCPL-like acyltransferases (Milkowski and Strack, 2004; Milkowski et al., 2004; Stehle et al., 2006) that catalyze the formation of sinapoyl-l-malate (Lehfeldt et al., 2000) in the seedling cotyledons and sinapine (Shirley et al., 2001) in the seeds, these enzymes form the sinapate ester pathway in members of the Brassicaceae.
Further studies are required to determine the nature of the interactions of BnSCE3/BnLIP2 with choline esters, and the conformational changes due to substrate binding (Akoh et al., 2004) according to the induced fit mechanism (Koshland, 1958). Elucidating the structures of the protein–ligand complexes, e.g. with hydroxycinnamoyl- and phenylpropionylcholines as well as phosphatidylcholine, will lead to an understanding of the factors that contribute to the plasticity and extraordinarily broad substrate specificity of GDSL lipases.
Experimental procedures
Plant material and growth conditions
Seeds of winter oilseed rape (Brassica napus L. var. napus cv. Express) were obtained from the Norddeutsche Pflanzenzucht (http://www.norddeutschepflanzenzucht.de). For enzyme purification, seeds were germinated for 2 days under greenhouse conditions at 12–18°C on moist filter paper in closed plastic boxes. Seedlings were grown for up to 12 days in the greenhouse under a 16 h light regimen. After harvest at various developmental stages, germinating seeds and seedlings were immediately frozen and stored at −80°C. Arabidopsis plants (Arabidopsis thaliana L. Heynh., wild-type Columbia) were grown in growth chambers on soil under a 16 h light regimen at 24°C. Transient heterologous expression of BnSCE3 was performed in host plants of Nicotiana benthamiana L. grown under greenhouse conditions as described for B. napus.
Purification of BnSCE
All protein manipulations were performed at 4°C. Protein concentrations were determined according to the method described by Bradford (1976), using bovine serum albumin as standard. Purification steps were performed using an ÄKTAexplorer (Amersham Biosciences, http://www5.amershambiosciences.com/). The chromatographic columns were purchased from GE Healthcare Life Sciences (http://www.gehealthcare.com). The buffers used were buffer A, 0.1 m Tris–HCl, pH 7.5; buffer B, 0.02 m Tris–HCl, pH 7.5; buffer C, 0.02 m Tris–HCl, pH 7.5, 1 m ammonium sulphate; buffer D, 0.02 m Tris–HCl, pH 7.5, 1 m NaCl. Buffers were supplemented with 10% glycerol, 0.5 mm EDTA and 2 mm 2-mercaptoethanol. Purification of BnSCE3 involved eight chromatographic steps.
Step 1. Three kilograms of 2-day-old seedlings were ground to a fine powder in a mortar with liquid nitrogen. Buffer A was added, and the homogenate treated with an Ultra-Turrax homogenizer (Janke & Kunkel GmbH & Co. KG-IKA-Labortechnik (http://www.ika.net) and centrifuged at 27 485 g for 10 min. To the supernatant, 1% protamine sulfate (PS) solution was added to a final concentration of 0.05%, stirred for 15 min and centrifuged at 27 485 g for 10 min. The collected protein was precipitated at 80% ammonium sulphate saturation and collected by centrifugation at 27 485 g for 15 min.
Step 2. The resulting pellet was dissolved in buffer C, and the enzyme solution was applied in five runs to a phenyl Sepharose FF 50/20 column equilibrated with buffer C. Unbound protein was washed off with the same buffer. Bound protein was eluted with a 300 ml gradient from 0 to 70% buffer B in C, followed by 400 ml at 70% buffer B in C with a flow rate of 5 ml min−1. Fractions (10 ml) were collected, and those containing enzyme activities were pooled, precipitated with ammonium sulphate at 80% saturation, and re-dissolved in buffer B.
Step 3. After desalting using a Sephadex G-25 column the protein solution was applied in 2 runs to a Q-Sepharose FF 50/20 column equilibrated with buffer B. Unbound protein was washed off using the same buffer. Bound protein was eluted by applying a 200-ml gradient from buffer 0 to 15% buffer D in B, followed by 1600 ml to 35% buffer D in B and 200 ml of 100% buffer D at a flow rate of 8 ml min−1. Fractions (10 ml) were collected. Enzyme activity was detected in three fractions: BnSCE1 eluted between 500 and 700 ml, BnSCE2 between 800 and 950 ml, and BnSCE3 between 1200 and 1320 ml. These fractions were pooled separately, and the protein was precipitated with ammonium sulphate at 80% saturation and re-dissolved in buffer B.
Step 4. Purification was continued using fraction BnSCE3, which was applied to a Sephadex G-75 50/60 column equilibrated with buffer B. Bound protein was eluted with the same buffer at a flow rate of 5 ml min−1 and 10 ml fractions were collected. The esterase activity eluted between 350 and 450 ml. Active fractions were pooled and concentrated by ultrafiltration through Centriprep-10 (Millipore; http://www.millipore.com).
Step 5. The protein was applied to a HiLoad 16/10 Q-Sepharose HP anion-exchange column and eluted with a 100-ml gradient from 0 to 20% buffer D in B, followed by a 250-ml gradient from 20 to 40% buffer D in B at a flow rate of 3 ml min−1 (5 ml fractions). The active fractions that eluted between 175 and 230 ml were pooled, concentrated by ultrafiltration through Centripep YM-10 Centrifugal Filter Units (Millipore) and re-dissolved in buffer B.
Step 6. Buffer B was replaced by buffer C and the enzyme solution was then applied to a phenylsepharose HR 5/5 column that had been equilibrated with buffer C. Elution was achieved with a 60 ml linear gradient from buffer C to 100% buffer B at a flow rate of 0.5 ml min−1. Fractions containing enzyme activities were pooled and concentrated by ultrafiltration through Centripep YM-10 Centrifugal Filter Units (Millipore).
Step 7. The concentrated protein was loaded at a flow rate of 1 ml min−1 onto a HiLoad 16/60 Superdex G-75 column equilibrated with buffer B. The collected fractions were assayed for BnSCE3 activity. Active fractions were concentrated by ultrafiltration through Centricon 10 Centrifugal Filter Units (Millipore).
Step 8. The enzyme was applied to a Mono-Q HR 5/5 column that had been equilibrated with buffer B, and eluted with a 60-ml gradient from 0 to 30% buffer D in B at a flow rate of 1 ml min−1. Active fractions were concentrated, the buffer was replaced by 0.01 m aqueous ammonium sulphate, and the protein was lyophilized in 100 μg portions.
Assay for sinapine esterase activity
For standard assay of sinapine esterase activity, enzyme solutions were incubated for 10 min at 30°C in 100 mm Tricine buffer, pH 8.5, containing 250 μm sinapine thiocyanate isolated from B. napus seeds, and various chemically synthesized phenolic choline esters (see below) in a total volume of 200 μl. Reactions were terminated by the addition of 10 μl trifluoroacetate. After centrifugation, at 20 817 g for 2 min, enzyme activities were determined by liquid chromatography using an ACQUITY UPLC (Waters; http://www.waters.com). Aliquots of the reaction mixtures (2–5 μl) were injected onto an ACQUITY UPLC C18 (1.7 μm) column (50 mm × 2.1 mm internal diameter). The liberated phenolic moieties were analyzed by a 2-min elution from phosphoric acid (1.5% aqueous solution) with an acetonitrile linear gradient from 0 to 30% buffer D in B at a flow rate of 1 ml min−1. The column eluates were monitored through max-plot UV spectroscopy by an ACQUITY uPLC Photodiode Array (PDA) Detector (Waters). Identification and quantification were achieved using sinapate and the phenolics used in choline ester syntheses as standards.
Determination of molecular mass
The molecular mass of native BnSCE3 was determined by FPLC on a Superose 12 HR 10/30 gel filtration column calibrated using a set of reference proteins including bovine serum albumin (67 kDa), chymotrypsinogen (25 kDa), ovalbumin (43 kDa) and ribonuclease A (13.7 kDa) from a gel filtration calibration kit (LMW, Amersham) (for chromatographic parameters, see step 7 of the protein purification protocol). The molecular mass of denatured BnSCE3 was determined by 10% w/v linear gradient SDS–PAGE (Laemmli, 1970) in comparison with a Bio-Rad molecular weight protein mixture (Precision Plus Protein Standard Dual Color; Bio-Rad Laboratories, http://www.bio-rad.com/). Proteins were visualized using Coomassie brilliant blue.
Mass spectrometric sequencing
The protein band on SDS–PAGE corresponding to BnSCE3 was excised, washed with water, reduced, carboxyamidomethylated and digested with trypsin, and the resulting peptides were extracted and purified according to standard protocols (e.g. Shevchenko et al., 1996). A QTOF II mass spectrometer (Micromass; http://www.micromass.com) equipped with a nanospray ion source and gold-coated capillaries (Protana; http://www.protana.com) was used for electrospray MS/MS of peptides. For collision-induced dissociation (CID) experiments, multiple charged parent ions were selectively transmitted from the quadrupole mass analyzer into the collision cell (collision energy 25–35 eV for optimal fragmentation). The resulting daughter ions were then separated using the orthogonal TOF mass analyzer. These MS/MS spectra were enhanced (Max. Ent. 3; Micromass) and used for manual de novo peptide sequencing using the PepSeq program (Micromass). The isomeric amino acids leucine and isoleucine could not be distinguished by the low-energy MS/MS techniques used.
RNA isolation
Extraction of total RNA from B. napus and Arabidopsis was performed by selective adsorption onto a silica matrix using a kit for plant RNA purification (Qiagen, http://www.qiagen.com/). Total RNA from 2-day-old B. napus seedlings was used to enrich poly(A+) RNA by selective binding to oligo(dT) Oligotex beads (Qiagen). Purification of total RNA from germinating seeds, seedlings and leaves of Arabidopsis was done as described previously (Vicient and Delseny, 1999).
Isolation of BnSCE3 cDNA, sequence analyses and alignment
Three peptide sequences derived from MS sequencing of purified BnSCE3 were used (bold underlined) to derive degenerated primers (NYADYYNSLYR: 5′-AAYTAYGCYGAYTAYTACAAC-3′, 5′-AAYTAYGCRGAYTAYTACAAC-3′, 5′-AAYTAYGCYGAYTAYTACAAT-3′, 5′-AAYTAYGCRGAYTAYTACAAT-3′, 5′-AAYTAYGCYGAYTAYTATAAC-3′, 5′-AAYTAYGCRGAYTAYTATAAC-3′, 5′-AAYTAYGCYGAYTAYTATAAT-3′, 5′-AAYTAYGCRGAYTAYTATAAT-3′; GGNDYNYPFFEDK: 5′-GGYAAYGAYTAYAAYTACC-3′, 5′-GGRAAYGAYTAYAAYTACC-3′, 5′-GGYAAYGAYTAYAAYTATCC-3′, 5′-GGRAAYGAYTAYAAYTATCC-3′; LYQEPTKYGFK: 5′-TAYCARGARCCRACRAARTACGG-3′, 5′-TAYCARGARCCYACYAARTACGG-3′, 5′-TAYCARGARCCRACYAARTACGG-3′, 5′-TAYCARGARCCYACRAARTACGG-3′). The resulting oligonucleotides were used in reverse transcription reactions as forward primers, combined with a customized poly(dT) reverse primer and RNA extracted from B. napus seedlings 1 or 2 days after germination. RT-PCR was performed using the Omniscript reverse transcription kit (Qiagen) according to the manufacturer’s protocol. Amplified cDNA fragments were subcloned into plasmid pGEM-T Easy (Promega, http://www.promega.com/) and subjected to sequence analysis. A 5′ incomplete 471 bp cDNA fragment with sequence similarity to putative lipases was used to derive gene-specific reverse primers (R2, 5′-GCCATCTTCTGGTGTGCGGCTTC-3′; R3, 5′-CCGTTGAGTATGCCATGAGCCATCTTCTG-3′; R4, 5′-GCAGCGTCAAGGCAGGACCAGTTG-3′) to amplify a full-length cDNA by 5′ RACE PCR from RNA from B. napus seedlings at 2 days of germination using the BD Smart Race cDNA amplification kit (BD Clontech, http://www.clontech.com/). Nested PCR was performed using 5′ nested reverse primer R1 (5′-CTCCAACTCCACAACAAGCAGCCAAAG-3′) and a 50-fold dilution of cDNA obtained from the first PCR run as template. The resulting products were cloned into pGEM-T Easy (Promega) and sequenced. The full-length cDNA was PCR-amplified using 5′ RACE-ready cDNA from B. napus seedlings at 2 days after germination as template and the primer pair BnSCEall_F (5′-ATGGCTTCTTCACTAAAGAAGCTTATC-3′) and BnSCEall_R (5′-TCAACTACCGAAAGAAGATTCGTTATC-3′). Sequence analysis and alignments of DNA and proteins were performed using the software package Clone Manager (Sci-Ed software; http://www.scied.com).
Semi-quantitative transcript analysis of BnSCE and Arabidopsis homologues
Reverse transcription reactions were performed with 1 μg of total RNA using the Omniscript reverse transcription kit (Qiagen) according to the manufacturer’s protocol. Fifty-fold dilutions of cDNA were used for each PCR amplification performed using Platinum PCR SuperMix High Fidelity (Invitrogen, http://www.invitrogen.com/) and primer pair LIP2RT_F 5′-CAGTTGGATGTAGCGCCGCGTATC-3′ and LIP2RT_R 5′-CTTCTGGTGTGCGGCTTCGGTTATATG-3′ for amplification of BnSCE3/BnLIP2, and primer pairs NM631spez_F 5′-TGTTAACCACCTTCCTCAATCC-3′, NM631spez_R 5′-GTGAGATACGCGGGATAGCAT-3′; NM632spez_F 5′- AACGTGAGCTTCAATCAAGG-3′, NM632spez_R 5′-GATATGCCGTGGAACATCCTATTG-3′; NM633spez_F 5′-GATGTCGGCGCTTTACATCTATC-3′, NM633spez_R 5′-GATGATCAAATCCTGTAGTTTGGTTTC-3′ and NM634spez_F 5′-CCTAACTTATGCGCCTCGTC-3′, NM634spez_R 5′-CGTTGAGCAATGGGTAACAAC-3′ for amplification of the putative Arabidopsis lipases. Control experiments were performed using primer pairs recognizing ubiquitin expression: UbiRT_F 5′-ACTTCACTTGGTGCTCAGGC-3′ and UbiRT_R 5′-CCTTGACGTTGTCAATGGTG-3′ for Arabidopsis and UbiRT_F 5′-AAGCCAAGATCCAGGACAAAG-3′ and UbiRT_R 5′-CGAGCCAAAGCCATCAAAGAC-3′ for B. napus. The RT-PCR runs consisted of 25–40 cycles, depending on the linear range of PCR amplification for the individual genes. Each cycle included incubations at 94°C for 15 sec, 55°C for 30 sec and 72°C for 42 sec. An additional cycle at 72°C for 10 min was run after the last cycle to allow trimming of incomplete polymerizations. The gels were stained with ethidium bromide.
Heterologous expression of BnSCE3 and Arabidopsis homologues in Nicotiana benthamiana leaves
For heterologous expression, cDNAs were fused to the strong promoter of the Rubisco gene encoding the small subunit (rbcs) from Asteraceous chrysanthemum (Outchkourov et al., 2003) by sub-cloning into plasmid pImpact1.1 (Plant Research International; http://www.pri.wur.nl/uk). Expression cassettes consisting of the rbcs promoter, coding sequence and rbcs transcription terminator were then transferred to the binary vector pBINPLUS (Plant Research International), and the resulting recombinant vectors were transformed into Agrobacterium tumefaciens strain GV2260 (McBride and Summerfelt, 1990).
The BnSCE coding sequence was PCR-amplified from cloned cDNA using primers BNSCErub_F (5′-ACTGGGATCCGAAAATGGCTTCTTCACTAAAGAAGCTTATC-3′) and BNSCErub_R (5′-GCAGCGGCCGCTCAACTACCGAAAGAAGATTCGTTATC-3′) designed to introduce an upstream BamHI restriction site and a downstream NotI site (underlined) into the PCR product. The BnSCE coding sequence was inserted as a BamHI–NotI fragment into plasmid pImpact1.1, from which the resulting expression cassette was isolated by AscI–PacI restriction and inserted into similarly cleaved pBINPLUS.
Coding sequences of the Arabidopsis genes At1g28640, At1g28650, At1g28660 and At1g28670 were PCR-amplified from seedling cDNAs using gene-specific primers (given below) designed to introduce an upstream recognition site for endonuclease XbaI and a downstream NotI site (At1g28640: 640rub_F (5′-ACTGTCTAGAGAAAATGGCTTCTTCACTGGAGAAGCTTATTTC-3′) and 640rub_R (5′-GCAGCGGCCGCTTAACTGCTGAAAGAATACTCTTTATCCACTGATTC-3′), At1g28650: 650rub_F (5′-ACTGTCTAGAGAAAATGACGACGACTCTCCTCATGG-3′) and 650rub_R (5′-GCAGCGGCCGCTTATCCACTGAAATCATAAGAGCCAAGG-3′), At1g28660: 660rub_F (5 ′-ACTGTCTAGAGAAAATGGCTTCTTCACTGAAGAAGCTTATC-3′) and 660rub_R (5′-GCAGCGGCCGCTTATGTATCCACTGTACCAGAGCCAAG-3′) At1g28670: 670rub_F (5′-ACTGTCTAGAGAAAATGGCTTCTTCACTGAAGAAGCTTATCTC-3′) and 670rub_R (5′-GCAGCGGCCGCTTATGTATCCACTGTACCAGAGCCAAGG-3′). Coding sequences were inserted as XbaI–NotI fragments into pImpact1.1, and expression cassettes were transferred to the binary vector pBINPLUS as described above. PCR was performed using Platinum PCR SuperMix High Fidelity (Invitrogen). For Nicotiana benthamiana infiltration, recombinant agrobacteria were grown overnight in 5 ml YEB medium supplemented with 25 mg ml−1 rifampicin, 50 mg ml−1 kanamycin and 50 mg ml−1 carbenicillin at 28°C with gentle shaking. After addition of 20 μm acetosyringone, 10 mm glucose and 10 mm MES, pH 5.6, cultures were grown for another 24 h. Bacteria were then pelleted at 2755 g for 8 min and resuspended in a mixture of 1 ml YEB, 2 ml 2x infiltration medium (100 g l−1 saccharose, 3.6 g l−1 glucose and 8.6 g l−1 MS medium, pH 5.6) and 2 ml water to an OD600 of 1. After addition of 0.2 μm acetosyringon, the resuspended agrobacteria were used to infiltrate the lower part of Nicotiana benthamiana leaves using a 1 ml syringe (Roth; http://www.carl-roth.de). As a negative control, agrobacteria harboring the empty vector pBINPLUS were used. Transformed plants were cultivated for 5 days under greenhouse conditions. For protein extraction, 400 mg of inoculated leaf sectors were cut, and homogenized in 2.5 ml extraction buffer (0.1 m Tricine, pH 8.5). Cell debris was pelleted by centrifugation at 20 817 g for 5 min, and the cleared supernatant was desalted using a PD-10 column (Sephadex G25 matrix; GE Healthcare Life Sciences). The soluble proteins were concentrated using a Vivaspin concentrator (VivaScience AG; http://www.vivascience.de).
Substrate specificity of the native BnSCE
The incubation mixture was the same as that used in the BnSCE assay. A total of 16 phenolic substrates (structures in Table 2) and some lipids, e.g. phosphatidycholine (Figure 8), were tested as possible substrates. 1-O-sinapoyl-β-glucose and sinapoyl-l-malate were from our collection of sinapate esters isolated from B. napus seedlings. The substrate concentration in the assay mixtures was 0.4 mm. To ensure reaction linearity, the amount of protein varied with regard to the enzyme activity for the various substrates. Each value for enzyme activity represents the mean of three determinations. Activities were related to that determined using sinapine. The kinetic parameters of the enzyme with sinapine, cinnamoylcholine and 3-phenylpropionylcholine as substrates were determined using Michaelis–Menten and Lineweaver–Burk plots (Segel, 1975), using the program SigmaPlot with the corresponding enzyme kinetics tool (Systat Software, Inc.; http://www.systat.de).
Synthesis of phenolic choline esters
The phenolic choline and ethanolamine esters were synthesized according to a slightly modified bromocholine bromide method (Clausen et al., 1983). The phenolic acids (1 mmol) and bromocholine bromide (1.5 mmol) were suspended in 50 ml water. After addition of 2.5 mmol thriethylamine, the mixture was refluxed for 8 h. The solution was then concentrated in a rotary evaporator (BÜCHI Labortechnik; http://www.buechigmbh.de). The synthesized esters were purified from the reaction mixture by preparative HPLC (Waters) using a DELTAPAK C18 column (15 μm, 30 cm × 5 cm internal diameter) with 1% aqueous HOAc (solvent A) and MeCN (solvent B), using various gradients from A to (A + B) at a flow rate of 20 ml min−1. The ester eluates were monitored using a diode array detector, evaporated to dryness and crystallized from acetone as acetate salts, yielding between 40% and 70%. These compounds, as well as sinapate liberated in the enzyme assays, were identified by mass spectrometry (data not shown).
Assay for lipolytic activity
The enzymatic assays for lipolytic activity of the three BnSCEs (BnSCE1, BnSCE2, BnSCE3) were carried out using phosphatidylcholine (1,2-dioleoyl-glycero-3-phosphocholine, 20 μl), a digalactosyldiacylglycerol (90 μl) and a triacylglycerol (20 μl) from the lipid collection of P. Dörmann (Max-Planck Institute, Golm, Germany). The organic phase of the lipid solutions was evaporated and the residue dissolved in diethyl ether (2 ml). Aliquots (10 μl) of the enzyme fractions (BnSCE1, 84 μg; BnSCE2, 95 μg; BnSCE3, 1 μg) were added and complemented with 490 μl lipase buffer containing 40 mm Tris–HCl (pH 7.2), 2.5 mm CaCl2 and 50 mm H3BO3. As a control, water was used instead of the enzymes. The reactions were stirred for 20 min using a vortex. The organic phases were evaporated and the samples reduced to 500 μl. Lipid extraction was performed using 3 ml chloroform/methanol (2:1 v/v). After centrifugation at 1793 g for 4 min, the organic phases were evaporated and the residues resuspended in 40 μl chloroform/methanol (2:1 v/v), and separated by thin-layer chromatography (TLC) on Si250-PA silica gel (Baker; http://www.mallbaker.com): phosphatidylcholine and digalactosyldiacylglycerol with hexane/diethyl ether/acetic acid 85:15:1 (v/v/v); triacylglycerol with chloroform/methanol/ammonium hydroxide 65:35:5 (v/v/v). The compounds were visualized by exposure to iodine vapour.
Inhibition of BnSCE3/BnLIP2 activity
The serine protease inhibitor PMSF (Sigma-Aldrich) was added to the enzyme assay, and the mixture was incubated for 5 min at 30°C. Three inhibitor concentrations were tested (1, 10 and 50 mm). Assays were carried out with 0.5 μg of the purified enzyme. The reactions were stopped by adding 10 μl of trifluoroacetic acid (TFA), and the enzyme activity was determined using the HPLC-based standard sinapine esterase assay.
Expression of BnSCE3/BnLIP2 in Arabidopsis seeds
For expression of BnSCE3/BnLIP2 in Arabidopsis seeds, the cDNA was fused to the seed-specific promoter of the napin gene from B. napus and cloned into plasmid pLH7000 (Hausmann and Töpfer, 1999). The expression cassette, consisting of the nap promoter, coding sequence and nap transcription terminator, was transferred into Agrobacterium tumefaciens strain EHA105 (Hood et al., 1993).
The BnSCE3/BnLIP2 coding sequence was PCR-amplified from cloned cDNA using primers BNSCEnap_F (5′-ACTGCCCGGGGAAAATGGCTTCTTCACTAAAGAAGCTC-3′) and BNSSCEnap_R (5′-GCAGGATCCTCAACTACCGAAAGAAGATTCGTTATC-3′), designed to introduce an upstream SmaI restriction site and a downstream BamHI site (underlined) into the PCR product. PCR was performed using Platinum PCR SuperMix High Fidelity (Invitrogen). The coding sequence was inserted as an SmaI–BamHI fragment into vector pLH7000.
For transformation of Arabidopsis buds, recombinant A. tumefaciens strain EHA105 was grown overnight in 4 ml LB medium supplemented with 25 mg ml−1 rifampicin and 100 mg ml−1 spectinomycin at 28°C with gentle shaking. The inoculated culture (400 ml LB medium) was grown to an OD600 of 0.8. The bacteria were then pelleted at 8000 g for 8 min and resuspended in a mixture of 1 vol 5% sucrose and 200 μl l−1 Silwet L-77 (Lehle Seeds; http://www.lehleseeds.com). The Arabidopsis buds were incubated with the bacterial solution for 2 min. As a control, bacteria harboring the empty vector pLH7000 were used. Transformed plants were cultivated for 2 days in a greenhouse.
Acknowledgements
We thank Jürgen Schmidt (Leibniz Institute of Plant Biochemistry, Halle (Saale), Germany) for analyses of the synthesized phenolic esters, and Peter Dörmann (Max-Planck Institute for Molecular Plant Physiology, Potsdam-Golm, Germany) for help in determination of lipolytic enzyme activity. Our thanks are also due to Thomas Hartmann (Braunschweig University) for critical reading of the manuscript. The seeds of Brassica napus were kindly provided by the Norddeutsche Pflanzenzucht (Holtsee, Germany). This study was supported by the Deutsche Forschungsgemeinschaft (Bonn, Germany).




