Degradation of the cyclin-dependent kinase inhibitor KRP1 is regulated by two different ubiquitin E3 ligases
Summary
In animals and fungi, a group of proteins called the cyclin-dependent kinase inhibitors play a key role in cell cycle regulation. However, comparatively little is known about the role of these proteins in plant cell cycle regulation. To gain insight into the mechanisms by which the plant cell cycle is regulated, we studied the cyclin-dependent kinase inhibitor KRP1 in Arabidopsis. KRP1 interacts with the CDKA;1/CYCD2;1 complex in planta and functions in the G1–S transition of the cell cycle. Furthermore, we show that KRP1 is a likely target of the ubiquitin/proteasome pathway. Two different ubiquitin protein ligases, SCFSKP2 and the RING protein RKP, contribute to its degradation. These results suggest that SCFSKP2b and RPK play an important role in the cell cycle through regulating KRP1 protein turnover.
Introduction
The cell cycle consists of a series of events that ultimately lead to the formation of two daughter cells. In eukaryotes, the fundamental mechanisms of cell cycle regulation are highly conserved. The cycle is divided into four phases, G1, S, G2 and M, and has two major checkpoints to control cell cycle progression: the G1–S transition and the G2–M transition. Cell cycle progression is controlled by the activities of cyclin-dependent kinase (CDK)/cyclin complexes. Different combinations of CDKs and cyclins regulate passage from one phase of the cycle to the next (De Veylder et al., 2003; Dewitte and Murray, 2003). The activities of CDK/cyclin complexes can be regulated by CDK inhibitors (CKIs), which function as negative regulators of CDK activity (Sherr and Roberts, 1999). CKIs have been identified in yeast, mammals and plants (De Clercq and Inze, 2006). In mammals, there are seven CKIs, which are classified into two families: the INK4 family and the Cip/Kip family (Nakayama and Nakayama, 1998; Vidal and Koff, 2000). Plants do not appear to have INK4-type CKIs, but proteins related to the Cip/Kip family have been identified in Arabidopsis, tobacco, maize and tomato (Bisbis et al., 2006; Coelho et al., 2005; De Veylder et al., 2001; Jasinski et al., 2002; Wang et al., 1997). The Arabidopsis genome encodes seven proteins related to the mammalian CKI p27Kip1, known as Kip-related proteins (KRPs) or interactors/inhibitors of Cdc2 kinase (ICKs; De Veylder et al., 2001; Vandepoele et al., 2002; Zhou et al., 2002a,b). The only sequence similarity between KRPs and p27Kip1 is in the conserved CDK-binding/inhibitory domain (De Veylder et al., 2001; Wang et al., 1997). Recently, an additional CKI was identified in Arabidopsis called SIAMESE (SIM; Churchman et al., 2006). The SIM protein includes a cyclin binding domain and a domain also found in the KRP proteins. Genetic studies indicate that SIM has a role in the control of endoreduplication.
Recent studies have begun to shed light on the function of plant CKIs in cell cycle regulation and growth. Ectopic expression of KRP1, KRP2, KRP4 and KRP6 confirm that these proteins function as inhibitors of the cell cycle, resulting in dwarfed plants with reduced cell number and organ size (Bemis and Torii, 2007; De Veylder et al., 2001; Wang et al., 2000; Zhou et al., 2003). Studies of transcriptional regulation have shown that KRP1 transcription is increased by low temperature and abscisic acid (ABA), whereas KRP2 transcription is downregulated by auxin during lateral root initiation (Himanen et al., 2002; Wang et al., 2000). The expression patterns of KRPs during the cell cycle were characterized using synchronized Arabidopsis cultured cells (Menges and Murray, 2002; Menges et al., 2005). These data show that there are three main patterns of transcriptional regulation of KRP genes. KRP1 is highly expressed in non-dividing cells and is strongly downregulated during the G1 phase in cell cycle re-entry. KRP1 shows a further clear peak of expression at the G2–M transition, although this is threefold lower than the expression in non-dividing cells. KRP2 is highly expressed in non-dividing cells, and is unique in showing a peak of expression only during the G1 phase as cells re-enter the cell cycle. In contrast, KRP3, KRP4, KRP5, KRP6 and KRP7 are not highly expressed in non-dividing cells, but are upregulated or peak during the S and early G2 phases. These results implicate KRP1 and KRP2 as primary candidates for controlling activation of division by non-dividing cells. Among the plant CKIs, KRP1 and KRP2 are known to be degraded by the 26S proteasome (Jakoby et al., 2006; Verkest et al., 2005a). Interestingly, degradation of KRP2 requires its phosphorylation by the CDKB1;1 complex (Verkest et al., 2005b). However, the detailed mechanisms of KRP1 and KRP2 degradation remain to be elucidated.
In eukaryotes, ubiquitin-mediated protein degradation plays a critical role in the cell cycle by destroying many important cell cycle regulators (Hershko, 2005). Conjugation of ubiquitin to a substrate requires the sequential action of three enzymes: ubiquitin-activating enzyme (E1), ubiquitin-conjugating enzyme (E2) and ubiquitin-protein ligase (E3). The E3 enzymes are responsible for the specificity of the pathway, and several classes of E3s have been implicated in cell cycle regulation including the SCF (SKP1-cullin-F-box protein) and RING domain ubiquitin ligases. An SCF complex is composed of four subunits: RBX1, CUL1, SKP1 and an F-box protein. The CUL1 subunit functions as a scaffold to bind RBX1 and the SKP1-F-box protein subcomplex. The F-box protein subunit provides specificity for SCFs and binds substrates (Moon et al., 2004; Petroski and Deshaies, 2005). In yeast and mammals, the role of SCFs in cell cycle regulation has been extensively investigated. SCFs are responsible for the degradation of cyclins, CKIs, transcription factor E2F-1 and many other cell cycle regulators (Hershko, 2005). A well-known example is the regulation of p27Kip1 degradation by SCFSKP2 in mammals. SCFSKP2 targets p27Kip1 for degradation to trigger the G1–S transition of the cell cycle (Sutterlüty et al., 1999;Tsvetkov et al., 1999).
The Arabidopsis genome encodes two F-box proteins (called SKP2a and SKP2b) that are related to mammalian SKP2 (del Pozo et al., 2002). SKP2a appears to recruit the phosphorylated form of the transcription factor E2Fc for degradation. Whether SKP2b also regulates E2Fc degradation is unknown. In addition to E2Fc, another cell cycle regulator that may be an SCF substrate is CYCD3;1. This cyclin is unstable and its degradation depends on the 26S proteasome (Planchais et al., 2004). In transgenic plants with reduced levels of RBX1, CYCD3;1 accumulates indicating that SCF is involved in its degradation. However, the F-box protein component of this SCF has not been identified (Lechner et al., 2002; Liu et al., 2004).
To investigate the post-translational regulation of plant CKIs, we focused on KRP1. Our data demonstrate that KRP1 interacts with the CDKA;1/CYCD2;1 complex in planta. Furthermore, we show that KRP1 degradation is dependent on SCFSKP2b and the RING protein RKP. These results provide new insight into the mechanisms by which the plant cell cycle is regulated by protein degradation.
Results
KRP1 expression
Previous studies by RNA blot and RT-PCR have shown that KRP1 is expressed in roots, stems, leaves, flowers, inflorescences and actively dividing cultured cells (De Veylder et al., 2001; Lui et al., 2000; Wang et al., 1998). In addition, KRP1 expression was examined in leaves and in the shoot apex by in situ hybridization (Ormenese et al., 2004). KRP1 RNA was detected in endoreduplicating tissues of leaves, but not in dividing cells of the shoot apical meristem. To further characterize KRP1 expression, we generated Arabidopsis transgenic lines in which the bacterial β-glucuronidase reporter gene (GUS) was placed adjacent to the KRP1 promoter. Over 10 independent transgenic lines were analyzed, and all lines exhibited similar GUS expression patterns. In young seedlings, GUS staining was first observed in cotyledons (Figure 1a). With longer incubation times, staining became apparent in roots, hypocotyls and emerging leaves (Figure 1b). In older seedlings, GUS staining was equivalent in cotyledons and rosette leaves (Figure 1c). In the flower, GUS staining was observed in the sepals, anthers and mature pollen (Figure 1d,e). GUS staining was also detected in siliques, with peak expression at the base (Figure 1f,g). These results show that KRP1 is expressed in various tissues and organs throughout plant development. An examination of publicly available expression data confirms this view (http://jsp.weigelworld.org/expviz/expviz.jsp; Schmid et al., 2005). KRP1 transcript is detected in all tissues examined, but is most abundant in cotyledons and leaves (Table S1).
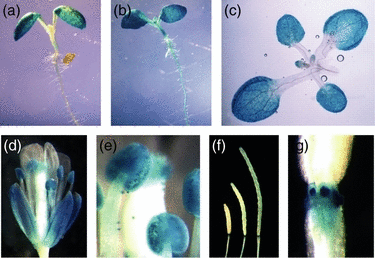
KRP1 is broadly expressed.A 2062-bp KRP1 promoter was fused to GUS, and GUS expression was examined by GUS staining of transgenic plants carrying a KRP1:GUS transgene. (a) and (b) 7-day-old light-grown seedling stained for 4 (a) or 24 h (b). (c) Rosette leaves of a 10-day-old light-grown seedling stained for 4 h. (d) Mature flower. (e) A closer look at the anthers and mature pollens shown in (d). (f) Siliques. (g) A closer look at the base of siliques shown in (f).
KRP1 overexpression inhibits auxin-mediated pericycle cell division during lateral root initiation
To understand the role of KRP1 in plant growth and development, we investigated the effects of KRP1 loss of function and gain of function on plant growth and development. A krp1 line with a T-DNA insertion in the third intron was identified in the SALK collection, and RT-PCR results indicate that the mutation prevents formation of a functional KRP1 protein (data not shown; Alonso et al., 2003). Despite the fact that the krp1 mutant was grown in a wide variety of growth conditions, we were not able to detect any changes in phenotype compared with the wild-type line (data not shown). To determine the effects of KRP1 overexpression, we generated Arabidopsis transgenic plants that express a c-Myc epitope tagged KRP1 under the control of the Cauliflower mosaic virus (CaMV) 35S promoter. More than 30 independent lines exhibited similar phenotypes. Plants had serrated rosette leaves, reduced apical dominance, reduced fertility and a reduced numbers of lateral roots. This phenotype is very similar to that conferred by overexpression of KRP1 (Wang et al., 2000), indicating that Myc-KRP1 is a functional protein in planta. A 35S:Myc-KRP1 line that has a weak phenotype and carries a single T-DNA insertion was chosen for further analysis. Interestingly, KRP1 overexpressors were temperature sensitive. If grown at 18°C, plants were more robust and exhibited increased fertility (data not shown).
An interesting and previously uncharacterized aspect of the KRP1-overexpression phenotype is a severe defect in lateral root formation. The 35S:Myc-KRP1 line exhibited only a slight decrease in primary root growth, but lateral root formation was dramatically inhibited (Figure 2a,b). The density of emerged lateral roots was reduced by 41% and 96% in the hemizygous and homozygous 35S:Myc-KRP1 plants, respectively, suggesting that the effect of KRP1 on lateral root formation is dose dependent. This was confirmed by determining the level of Myc-KRP1 in these lines by protein blot (Figure 2e).
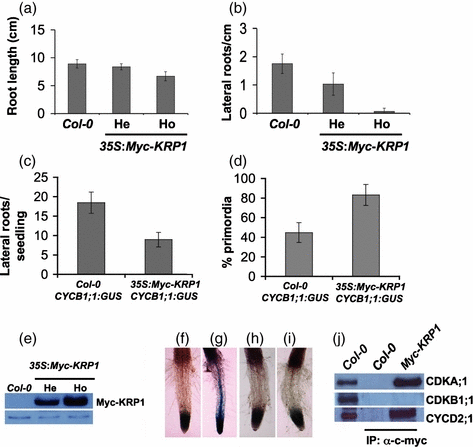
KRP1 overexpression inhibits auxin-mediated pericycle cell division.(a) Primary root length of 2-week-old light-grown plants. He, hemizygous; Ho, homozygous. (b) Number of emerged lateral roots in 2-week-old light-grown plants. (c) Total lateral roots/seedling (primordia + emerged) visualized using the CYCB1;1:GUS marker. (d) Percentage lateral root primordia from (c). (e) Immunoblot analysis of Myc-KRP1 levels with an α-c-myc antibody. Protein extracts were prepared from 2-week-old light-grown plants. An unknown protein recognized by the α-c-myc antibody was used as a loading control. (f) Root of a CYCB1;1:GUS seedling grown on an ATS + 10 μmN-1-naphthylphthalamic acid (NPA) plate for 72 h. (g) CYCB1;1:GUS seedling transferred to an ATS + 10 μm 1-naphthaleneacetic acid (NAA) plate for 12 h after initial growth on NPA. (h) Root of a 35S:Myc-KRP1 (homozygous) seedling carrying a CYCB1;1:GUS transgene grown on an ATS + 10 μm NPA plate for 72 h. (i) GUS expression in the root of a 35S:Myc-KRP1 (homozygous) seedling transferred to an ATS + 10 μm NAA plate for 12 h after initial growth on NPA. (f–i) All seedlings were stained for GUS. (j) KRP1 interacts with CDKA;1 and CYCD2;1 in planta. Protein extracts from wild-type (Col) and 35S:Myc-KRP1 seedlings were immunoprecipitated with an α-c-myc antibody. Immunoblot analyses of protein extracts from wild-type (Col) and α-c-myc immunoprecipitates were performed with α-CDKA;1, α-CDKB1;1 and α-CYCD2;1 antibodies. Asterisk indicates an unknown protein recognized by the α-CYCD2;1 antibody.
To learn more about how KRP1 functions in lateral root formation, we introduced a CYCB1;1:GUS transgene into 35S:Myc-KRP1 plants by crossing. CYCB1;1, a mitotic cyclin, is expressed in late G2 and M phase, and is therefore a marker for cell cycle progression from the G2 to the M phase. Two experiments were performed with these plants. In the first we used the activity of the CYCB1:1:GUS gene as a marker for initiation of lateral root primordia. When both lateral root primordial and emerged lateral roots were counted, overexpression of Myc-KRP1 resulted in a decrease in the total number of lateral roots (Figure 2c). However, a much larger fraction of these lateral roots did not emerge in the overexpression lines compared with the wild type (Figure 2d). These results indicate that increased levels of KRP1 inhibit both formation of lateral root primordia and continued growth of primordia once they are formed.
We then used the CYCB1:1:GUS transgene to examine the effect of KRP1 overexpression on auxin-mediated pericycle cell division. Seedlings were grown using lateral root induction conditions developed by Himanen et al. (2002). Seedlings were first treated with the auxin transport inhibitor N-1-naphthylphthalamic acid (NPA). This compound prevents pericycle cell division, and all pericycle cells remain in the G1 phase. Subsequently, seedlings were treated with the auxin 1-naphthaleneacetic acid (NAA) to activate pericycle cells, causing them to pass the G1–S and G2–M transitions and to undergo cell division. After NPA treatment, wild-type and 35S:Myc-KRP1 seedlings did not exhibit GUS staining in the pericycle (Figure 2f). As expected, treatment of wild-type seedlings with NAA produced significant GUS staining in the pericycle, showing that these cells have passed through the G2–M transition. In contrast, no staining was observed in the 35S:Myc-KRP1 plants (Figure 2f), indicating that KRP1 overexpression inhibits auxin-mediated pericycle cell division during lateral root initiation.
KRP1 interacts with CDKA;1 and CYCD2;1 in planta
A crucial step towards understanding the role of KRP1 in cell cycle regulation is to identify its CDK/cyclin complex targets. Analyses using the yeast two-hybrid system have shown that KRP1 interacts with CDKA;1 and D-type cyclins (CYCD1;1, CYCD2;1 and CYCD3;1), but that KRP1 does not interact with CDKB1;1, CYCA2;2 and B-type mitotic cyclins (CYCB1;1 and CYCB2;1; De Veylder et al., 2001; Wang et al., 1998). However, these interactions have not been confirmed in the plant. Because we have transgenic plants overexpressing Myc-KRP1 as well as antibodies to CDKA;1, CDKB1;1 and CYCD2;1, we tested for these interactions by immunoprecipitation. Protein extracts prepared from wild-type and 35S:Myc-KRP1 seedlings were immunoprecipitated with an α-c-myc antibody. Immunoblot analyses were performed with α-CDKA;1, α-CDKB1;1 and α-CYCD2;1 antibodies. As shown in Figure 2g, CDKA;1 and CYCD2;1, but not CDKB1;1, co-immunoprecipitates with Myc-KRP1. These results indicate that KRP1 interacts with CDKA;1 and CYCD2;1 in planta.
KRP1 is an unstable protein and its degradation depends on the 26S proteasome
KRP1 is related to mammalian CKI p27Kip1, a protein that is degraded through the action of two ubiquitin ligases called SCFSKP2 and KPC1 (Carrano et al., 1999; Kamura et al., 2004b; Sutterlüty et al., 1999; Tsvetkov et al., 1999). Although KRP1 has been shown to be degraded by the 26S proteasome (Jakoby et al., 2006), the detailed mechanism of KRP1 degradation has not yet been described. To study KRP1 protein stability in planta, we generated Arabidopsis transgenic plants that express KRP1–GUS fusion protein under the control of the KRP1 promoter. In 10-day-old KRP1:GUS plants stained for 4 h, GUS staining was observed in the cotyledons and leaves (Figure 1c). To assist in comparison, this image is also shown in Figure 3. In contrast, no GUS staining was observed in KRP1:KRP1–GUS seedlings that were incubated in staining solution for 24 h (Figure 3b). As GUS is a stable protein, these results indicate that KRP1 destabilizes GUS, suggesting that KRP1 is an unstable protein that is quickly degraded in planta (Jefferson et al., 1987).
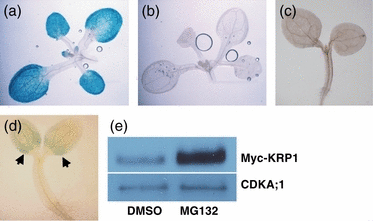
KRP1 degradation depends on the 26S proteasome.(a) GUS staining of a 10-day-old light-grown KRP1:GUS seedling stained for 4 h. (b) GUS staining of a 10-day-old light-grown KRP1:KRP1–GUS seedling stained for 24 h. (c) GUS staining of 4-day-old light-grown KRP1: KRP1–GUS seedlings treated with DMSO for 8 h. (d) GUS staining of 4-day-old light-grown KRP1:KRP1–GUS seedlings treated with 50 μm MG132 for 8 h. MG132 is a 26S proteasome inhibitor. DMSO was used as a control. Arrows indicate GUS staining. (e) Immunoblot analysis of protein extracts from 35S:Myc-KRP1 (homozygous) seedlings treated with DMSO and 50 μm MG132 for 12 h, respectively, with α-c-myc and α-CDKA;1 antibodies. CDKA;1, a stable protein, was used as a loading control.
To determine whether the 26S proteasome is involved in KRP1 degradation, we examined the effect of MG132, a proteasome inhibitor, on KRP1–GUS and Myc-KRP1 protein stability. Unlike the DMSO-treated seedlings, GUS staining was observed in the cotyledons of MG132-treated KRP1:KRP1–GUS seedlings (Figure 3c,d). Similarly, MG132 treatment resulted in increased Myc-KRP1 levels in 35S:Myc-KRP1 seedlings (Figure 3e). CDKA;1 was used as a loading control. MG132 did not affect CDKA;1 protein levels (because this protein is stable). The stabilization of both KRP1–GUS and Myc-KRP1 by MG132 indicates that KRP1 degradation depends on the 26S proteasome.
The AXR1-dependent RUB conjugation pathway regulates KRP1 degradation
Like ubiquitin, RUB1 (related to ubiquitin-1, also called NEDD8) is a post-translational modifier that regulates diverse cellular processes. At present the best-characterized RUB1 targets are the cullin proteins, including the CUL1 subunit of SCFs (Parry and Estelle, 2004; Petroski and Deshaies, 2005). In Arabidopsis, RUB1 conjugation to CUL1 requires the AXR1-ECR1 heterodimer (RUB-activating enzyme E1), RCE1 (RUB-conjugating enzyme E2) and RBX1 (RUB-protein ligase E3). RUB conjugation modulates the activity of many, perhaps all, CUL1-based SCFs (Parry and Estelle, 2004). Our previous data demonstrated that the ubiquitin-proteasome pathway regulates KRP1 degradation. To determine if the RUB conjugation pathway is required for this degradation, we examined KRP1–GUS and Myc-KRP1 protein stability in the axr1-3 mutant, which has an impaired RUB conjugation pathway (del Pozo et al., 1998).
We introduced the KRP1:KRP1–GUS transgene into axr1-3 plants by crossing. The results shown in Figure 4a,b indicate that the axr1-3 mutation acts to stabilize KRP1–GUS in both the cotyledon and hypocotyl. Homozygous KRP1:KRP1–GUS plants are very similar to wild type in appearance, whereas axr1-3 plants exhibit a pleiotropic phenotype that includes reduced stature and decreased apical dominance (Figure 4c,d). Interestingly, the introduction of the KRP1:KRP1–GUS transgene into the axr1-3 background enhances this mutant phenotype. The effects of the transgene were variable, but 34% (29/85) had a severe phenotype as illustrated in Figure 4c,d.

The AXR1-dependent RUB conjugation pathway regulates KRP1 degradation.(a) GUS staining of a 4-day-old light-grown KRP1:KRP1–GUS seedling. (b) GUS staining of a 4-day-old light-grown axr1-3 seedling carrying a KRP1:KRP1–GUS transgene. (c) Seven-week-old plants. From left to right, wild-type (Col-0), axr1-3, axr1-3 KRP1:KRP1–GUS, and KRP1:KRP1–GUS. Bar = 2 cm. (d) Inflorescences of 7-week-old mature plants shown in (c). Bar = 1 cm. (e) Immunoblot analysis of Myc-KRP1 with an α-c-myc antibody. Protein extracts were prepared from 2-week-old light-grown plants. An unknown protein recognized by the α-c-myc antibody was used as a loading control (bottom). Lane 1, Col-0; lane 2, 35S:Myc-KRP1 (hemizygous); lane 3, axr1-3 35S:Myc-KRP1 (hemizygous); lane 4, axr1-3.
We also introduced the 35S:Myc-KRP1 transgene into axr1-3 plants by crossing. As shown in Figure 4e, the axr1-3 mutation stabilized Myc-KRP1. These data indicate that the AXR1-dependent RUB conjugation pathway regulates KRP1 degradation.
KRP1 degradation is dependent on SCFSKP2b
The RUB conjugation pathway is probably required for the function of all cullin-based E3 ubiquitin ligases, including SCF, CUL3-BTB and CUL4-DDB E3s (Bernhardt et al., 2006; Chen et al., 2006; Parry and Estelle, 2004). To determine if an SCF might be involved in KRP1 degradation, we introduced the KRP1:KRP1–GUS and 35S:Myc-KRP1 transgenes into the axr6-3 mutant by crossing. The axr6-3 mutant contains a recessive and temperature-sensitive mutation of CUL1 that has been shown to stabilize SCF substrates (Quint et al., 2005). As shown in Figure 5a,b,d, the axr6-3 mutant stabilized both KRP1–GUS and Myc-KRP1, exhibiting GUS staining in the cotyledon and an increased Myc-KRP1 protein level. Interestingly, overexpression of either KRP1–GUS or Myc-KRP1 enhances the axr6-3 growth defects. Plants exhibited a dramatically decreased height and were sterile (5, 6). These results indicate that CUL1 is required for KRP1 degradation.
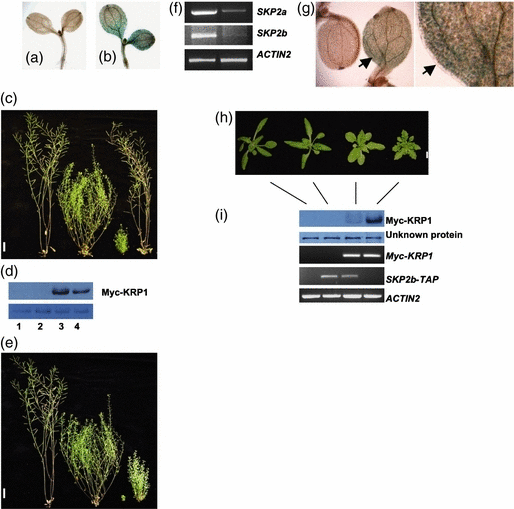
KRP1 degradation is dependent on SCFSKP2b.(a) GUS staining of a 6-day-old light-grown KRP1:KRP1–GUS seedling. (b) GUS staining of a 6-day-old light-grown axr6-3 seedling carrying a KRP1:KRP1–GUS transgene. (c) Eleven-week-old mature plants grown at 18°C. From left to right, wild-type (Col-0), axr6-3, axr6-3 KRP1:KRP1–GUS and KRP1:KRP1–GUS. Scale bar = 2 cm. (d) Immunoblot analysis of Myc-KRP1 with an α-c-myc antibody. Protein extracts were prepared from 40-day-old plants grown at 18°C. An unknown protein recognized by the α-c-myc antibody was used as a loading control (lower panel). Lane 1, wild-type (Col-0); lane 2, axr6-3; lane 3, axr6-3 35S:Myc-KRP1 (hemizygous); lane 4, 35S:Myc-KRP1 (hemizygous). (e) Eleven-week-old mature plants grown at 18°C. From left to right, wild-type (Col-0), axr6-3, axr6-3 35S:Myc-KRP1 (homozygous) and 35S:Myc-KRP1 (homozygous). Scale bar = 2 cm. (f) RT-PCR analysis of SKP2a, SKP2b and ACTIN2 expression in wild-type (Col-0) and SKP2-RNAi line (RNAi-29). Total RNAs were extracted from 7-day-old light-grown seedlings. PCRs were performed for 25 cycles (ACTIN2) and 40 cycles (SKP2a and SKP2b). (g) Left panel: GUS staining of 5-day-old light-grown KRP1:KRP1–GUS seedlings in the wild-type (Col-0) and SKP2-RNAi line (RNAi-29) (arrow). Right panel: close-up view of the SKP2-RNAi KRP1:KRP1–GUS seedling. (h) Three-week-old plants. From left to right: Col-0, 35S:SKP2b-TAP, 35S:SKP2b-TAP 35S:Myc-KRP1 (hemizygous), 35S:Myc-KRP1 (hemizygous). Scale bar = 0.5 cm. (i) Myc-KRP1 and Myc-KRP1 levels in plants shown in (h). Top two panels show Myc-KRP levels measured with an α-c-myc antibody. Protein extracts were prepared from 3-week-old plants. An unknown protein recognized by the α-c-myc antibody was used as a loading control. Bottom three panels show Myc-KRP1, SKP2b-TAP and ACTIN2 levels measured by RT-PCR. Total RNA was extracted from 3-week-old plants. The c-myc epitope and tandem affinity purification (TAP) tag transcripts were amplified to show Myc-KRP1 and SKP2b-TAP expression, respectively. PCRs were performed for 25 cycles.
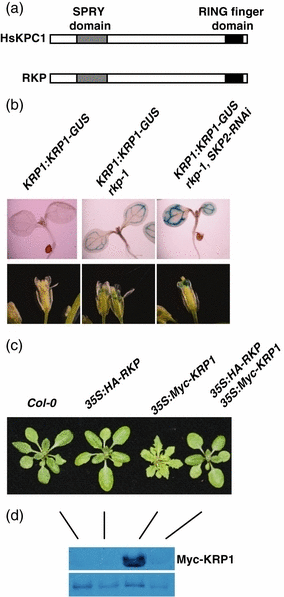
KRP1 degradation is dependent on the RING protein RKP.(a) Structure of human KPC1 (HsKPC1) and Arabidopsis RKP showing the SPRY and RING domains (not drawn to scale). (b) Seven-day-old seedlings or mature flowers stained for 24 h. (c) Three-week-old plants. (d) Immunoblot analysis of Myc-KRP1 levels. Protein extracts prepared from 4-week-old plants.
The involvement of CUL1 in KRP1 degradation reveals that an SCF mediates KRP1 protein turnover. Among the SCF subunits, the F-box protein recognizes and binds substrates. In mammals, the F-box protein SKP2 binds CKI p27Kip1 (Sutterlüty et al., 1999; Tsvetkov et al., 1999) and the transcription factor E2F-1 (Marti et al., 1999), as well as other cell cycle regulators (Nakayama and Nakayama, 2005), targeting them for degradation. Arabidopsis has two SKP2-related F-box proteins SKP2a and SKP2b that are 83% identical at the amino acid sequence level. It has been shown that SKP2a binds the transcription factor E2Fc and appears to mediate its degradation (del Pozo et al., 2002, 2006). Because of the sequence and functional relationship between KRP1 and p27Kip1, we decided to investigate the possibility that Arabidopsis SKP2 is involved in KRP1 degradation.
To test this possibility, we examined KRP1–GUS protein stability in the SKP2a and SKP2b T-DNA insertion mutants. We identified SKP2a and SKP2b T-DNA insertion mutants in the GABI-KAT and SALK collection (Alonso et al., 2003; Rosso et al., 2003; see Figure S1a). Neither skp2a-1 and skp2b-1 nor a skp2a-1 skp2b-1 double mutant exhibited an obvious phenotype. In addition, none of these mutants stabilized KRP1–GUS (data not shown). To understand the molecular nature of the skp2a-1 and skp2b-1 mutants, we examined SKP2a and SKP2b expression by RT-PCR in the skp2a-1 skp2b-1 double mutant. The full-length transcripts of SKP2a and SKP2b could not be detected. However, truncated transcripts were detected at a high level for SKP2a and at a low level for SKP2b (see Figure S1c). Therefore, truncated SKP2a and SKP2b proteins could be produced. If these truncated proteins exist, SKP2a and SKP2b will have the F-box domain, as well as four and two leucine-rich repeats (LRRs), respectively (see Figure S1b). Thus, it is possible that truncated SKP2a and SKP2b could form a functional SCF complex that targets substrates for degradation.
As an alternative, we examined KRP1–GUS protein stability in SKP2 RNA interference (RNAi) transgenic plants with reduced levels of both SKP2a and SKP2b. We worked with two independent lines and obtained similar results. SKP2-RNAi transgenic lines did not exhibit any obvious defects in growth and development (data not shown). Here, we show results for the line RNAi-29. Compared with wild type, the expression of both SKP2a and SKP2b was strongly decreased in this line (Figure 5f). The KRP1:KRP1–GUS transgene was introduced into SKP2-RNAi transgenic plants by crossing, and F1 plants were examined for GUS expression. GUS staining was observed in the cotyledon of RNAi-29 seedlings (Figure 5g). Therefore, SKP2-RNAi transgenic plants stabilize KRP1–GUS, indicating that SKP2a and/or SKP2b are involved in KRP1 degradation.
To gain further evidence for a role for Arabidopsis SKP2 in KRP1 degradation, we examined the effect of SKP2 overexpression on KRP1 degradation in planta. We generated Arabidopsis transgenic plants that express a TAP (tandem affinity purification) tagged SKP2a or SKP2b under the control of the CaMV 35S promoter. We introduced the 35S:SKP2a-TAP and 35S:SKP2b-TAP transgenes into 35S:Myc-KRP1 plants by crossing. A 35S:SKP2a-TAP line did not appear to alter the effects of Myc-KRP1 overexpression (data not shown). Interestingly, two independent 35S:SKP2b-TAP lines suppressed the effects of Myc-KRP1 overexpression. Here, we show results for line 5. An obvious phenotype of KRP1 overexpressers is serrated rosette leaves. Plants that overexpress both SKP2b-TAP and Myc-KRP1 did not exhibit serrated rosette leaves (Figure 5h). The loss of serrated leaf phenotype was associated with a decreased Myc-KRP1 protein level. Three-week-old 35S:SKP2b-TAP 35S:Myc-KRP1 plants had much less Myc-KRP1 than 35S:Myc-KRP1 plants (Figure 5i). We also examined Myc-KRP1 transcript levels in both lines and found them to be similar (Figure 5i), confirming that decreased Myc-KRP1 protein levels are caused by increased degradation. Taken together, our data indicate that KRP1 degradation is dependent on an SCF complex that consists of CUL1 and SKP2b.
The RING protein RKP also contributes to KRP1 degradation
Recent studies in mammalian cells indicate that at least two different E3s contribute to p27Kip1 degradation: SCFSkp2 and a RING-type E3 called KPC1 (Kamura et al., 2004b; Kotoshiba et al., 2005). Because KRP1 is related to p27Kip1, a similar KPC-dependent protein degradation mechanism might exist in Arabidopsis to regulate KRP1 protein turnover. Interestingly, there is a KPC1-related RING finger protein called At2g22010 in Arabidopsis. Like mammalian KPC1, At2g22010 has a RING finger domain in the C-terminus and a SPRY domain with an unknown function near the N-terminus (Stone et al., 2005; Figure 6a). The two proteins are approximately 25% identical, and this conservation extends along the entire length of the proteins. Based on this relationship we have named this protein RKP (related to KPC1). Microarray experiments indicate that RKP RNA is present throughout plant development, with particularly high levels of expression in the embryo and senescing leaves (Schmid et al., 2005).
To determine if RKP functions in KRP1 degradation, we first examined the effect of loss of RKP on KRP1–GUS levels. A RKP T-DNA insertion mutant was identified in the SAIL collection (Sessions et al., 2002). The rkp-1 mutant contains a T-DNA insertion in exon 2 of RKP that prevents the formation of full-length RKP RNA. As any protein encoded by the truncated transcript would not include the RING domain, rkp-1 is likely to be a null mutant. When the KRP1:KRP1–GUS transgene was crossed into the rkp-1 line we found higher levels of GUS staining in mutant cotyledons and flowers, indicating that RKP participates in KRP1 degradation (Figure 6b). To further investigate the role of RKP in KRP1 degradation, we examined the effect of RKP overexpression on KRP1 protein turnover in planta. We introduced a 35S:HA-RKP construct into the 35S:Myc-KRP1 line exhibiting the serrated leaf phenotype typical of high KRP1 levels (Figure 6c). In the T1 generation, 92% (34/37) of kanamycin- and hygromycin-resistant 35S:HA-RKP 35S:Myc-KRP1 plants did not exhibit serrated rosette leaves. We examined Myc-KRP1 protein levels in six independent lines that lost the serrated rosette leaf phenotype by immunoblot analysis using an α-c-myc antibody. All six lines had much lower Myc-KRP1 protein levels than the 35S:Myc-KRP1 plants (data not shown). Here we show results for line 6. Figure 6c shows that RKP overexpression suppressed the serrated rosette leaf phenotype of 35S:Myc-KRP1 plants. In addition, this suppression was associated with reduced levels of Myc-KRP1 (Figure 6d).
Despite the clear role of RKP in KRP1 degradation, the rkp1 mutant did not exhibit any clear defects in growth and development. As our results indicate that both RKP and SKP2 participate in KRP1 degradation, we crossed the SKP2:RNAi transgene into rpk-1 plants. The results in Figure 6b show that KRP1–GUS levels are higher in this line than either parental line, confirming that both SKP2 and RPK contribute to KRP1 degradation, probably independently. However, again no defects in plant growth and development were observed.
Discussion
In recent years, the ubiquitin-proteasome pathway has been implicated in many aspects of cellular regulation and development in plants, particularly hormone signaling. Here, we provide clear evidence for an important role for two different ubiquitin protein ligases in cell cycle regulation. We show that both SCFSKP2b and the RING protein RKP mediate the degradation of KRP1. In addition, the absence of a clear growth defect in mutant lines deficient in both E3s suggests that additional mechanisms regulate KRP1 levels.
KRP1 interacts with the CDKA;1/CYCD2;1 complex
In eukaryotes, cell cycle progression is controlled by the activities of CDK/cyclin complexes. In Arabidopsis, there are five CDKs with known direct roles in the cell cycle, and at least 31 cyclins of the three main classes of A, B and D types (Menges et al., 2005; Vandepoele et al., 2002). In previous studies, KRP1 was shown to bind CDKA;1 and three D-type cyclins (CYCD1;1, CYCD2;1 and CYCD3;1) in yeast, but not with CDKB1;1 (De Veylder et al., 2003; Jakoby et al., 2006; Wang et al., 1998; Zhou et al., 2003). Furthermore, overexpression of CDKA;1 and CYCD2;1 in Arabidopsis whole plants and trichomes, respectively, suppress the effects of KRP1 overexpression (Schnittger et al., 2003; Zhou et al., 2003).We have extended these results by showing that KRP1 forms a complex with CDKA;1 and CYCD2;1 in vivo, but not with CDKB1;1.
A number of lines of evidence suggest that CDKA;1/CYCD2;1 has a critical role in the G1–S transition (Healy et al., 2001; Planchais et al., 2004). The in vivo interactions between KRP1 and CDKA;1/CYCD2;1 strongly suggest that KRP1 functions to regulate the G1–S transition, consistent with genetic studies in which KRP1 overexpression was shown to inhibit cell division and endoreduplication ( Wang et al., 2000; Zhou et al., 2002a,b).
In addition to the CDKA;1/CYCD2;1 complex, KRP1 may have other targets. A recent study reported that besides its role in the G1–S transition, KRP1 also functions in the G2–M transition to regulate mitosis entry (Weinl et al., 2005). However, the CDK/cyclin complex target of KRP1 at the G2–M transition is unknown. It will be important to identify other CDK/cyclin complex targets of KRP1 in the future to further define the role of KRP1 in cell cycle regulation.
Ectopic expression of KRP1 inhibits pericycle activation during lateral root initiation
Lateral root formation is an example of post-embryonic de novo organogenesis. In Arabidopsis, lateral roots are derived from pericycle cells adjacent to the xylem poles (Casimiro et al., 2003; Himanen et al., 2002). Pericycle activation appears to involve changes in the level of a protein related to KRP1, called KRP2 (Himanen et al., 2002). The KRP2 gene is expressed in pericycle cells and downregulated in conditions that induce lateral root formation. Based on these results it was proposed that KRP2 controls the G1–S transition of pericycle cells during pericycle activation.
The KRP1 gene is also expressed in roots and is downregulated during lateral root formation (Himanen et al., 2002). We demonstrate that KRP1 overexpression inhibits auxin-mediated pericycle cell division, resulting in a dramatic decrease in the number of lateral roots. These results suggest that KRP1 may also have a role in regulating pericycle cell activation. However, the lack of a clear effect of the krp1 mutation on lateral root development precludes a definitive conclusion. In any case, it is clear that KRP1 is not the only factor regulating this process.
SCFSKP2b mediates KRP1 degradation
Previous studies demonstrated that KRP1 is an unstable protein that is degraded by the 26S proteasome (Jakoby et al., 2006; Zhou et al., 2003). We have extended this work using a KRP1–GUS fusion protein. Our results demonstrate that the AXR1-RUB conjugation pathway is required for KRP1 degradation. Because the only known substrates for RUB conjugation are the cullin proteins, these results imply that KRP1 degradation requires a cullin-based E3, such as an SCF. Indeed we show that CUL1 is required for KRP1 degradation, indicating that an SCF regulates KRP1 degradation.
In mammals p27Kip1 is degraded by SCFSkp2. In Arabidopsis, there are two proteins related to Skp2: SKP2a and SKP2b. Despite the fact that KRP1 is only related to p27Kip1 through the cyclin- and CDK-binding/inhibitory domain, our results indicate that SKP2b is involved in KRP1 degradation. SKP2-RNAi lines with strongly decreased levels of both SKP2a and SKP2b stabilize KRP1–GUS. Furthermore, we have shown that SKP2b overexpression promotes KRP1 degradation in planta. Surprisingly, overexpression of the closely related SKP2a protein did not suppress the effects of Myc-KRP1 overexpression.Whether this is caused by a difference in the function of SKP2a and SKP2b, or by an artifact related to the specific 35S:SKP2a line, is unknown. Thus, our results suggest that SKP2b targets KRP1 for degradation, but do not exclude the possibility that SKP2a has a similar function. It will be important to examine the biochemical interactions between KRP1 and SKP2b in the future. Taken together, our data suggest that an SCF complex that is composed of CUL1 and SKP2b mediates KRP1 degradation.
KRP1 degradation is also regulated by the RING protein RKP
Previous studies have identified two domains on KRP1 that contribute to its instability, suggesting that there may be two independent mechanisms of KRP degradation (Jakoby et al., 2006). In mammalian cells, p27Kip1 degradation is mediated by both SCFSkp2 and a RING protein called KPC1 (Kamura et al., 2004b). Our genetic studies suggest that RKP, an Arabidopsis protein related to KPC1, also contributes to KRP1 degradation. The rkp-1 mutant stabilizes KRP1–GUS. In addition, RKP overexpression promotes KRP1 degradation in planta. The observation that KRP1–GUS is significantly more stable in rkp 35S:SKP2-RNAi plants than either the rkp or 35S:SKP2-RNAi lines suggest that the two E3s function independently. Furthermore, both pathways appear to be active in the same tissue. In mammals, SCFSkp2-dependent degradation of p27Kip1 occurs in the nucleus, whereas KPC functions in the cytoplasm (Kamura et al., 2004a). Jakoby et al. (2006) have shown that KRP1 localizes primarily to the nucleus, but is found in different subnuclear domains. It will be interesting to determine if RKP and SCFSKP2b mediate KRP1 degradation in distinct cellular compartments.
Despite the clear involvement of SCFSKP2 and RKP in KRP1 degradation, plants deficient in either or both of these E3s do not display any growth defects. This is surprising, as relatively modest changes in KRP1 levels exert an effect on development. For example, the KRP1:KRP1–GUS transgene acts to enhance the phenotype of the axr1-3 and axr6-3 mutants. Our failure to detect a phenotype in SKP2-RNAi and rkp lines may be caused by residual SCFSKP2 activity in the RNAi lines and redundancy between SCFSKP2 and RPK. Alternatively, it is possible that Arabidopsis has an additional mechanism of KRP1 degradation, perhaps via another E3 enzyme.
Experimental procedures
Plant materials and growth conditions
Arabidopsis thaliana plants were grown under 24-h light conditions at 22°C, or 18°C when necessary. All mutants and transgenic lines were in the Columbia ecotype. The skp2a-1 mutant (GABI-Kat 293D12), a T-DNA insertion in At1g21410, was acquired from GABI-KAT; Rosso et al., 2003). The skp2b-1 mutant (SALK_028396), a T-DNA insertion in At1g77000, was acquired from the Arabidopsis Biological Research Center (ABRC, http://www.arabidopsis.org;Alonso et al., 2003). The rkp mutant (SAIL_3_E3), a T-DNA insertion in At2g22010, was acquired from the ABRC (Sessions et al., 2002). All T-DNA mutants were confirmed by PCR and sequencing. Seeds were surface sterilized in a 30% bleach and 0.04% Triton X-100 solution, and were cold treated for 2–3 days at 4°C to synchronize germination. Seeds were grown on ATS medium [1% sucrose, 5 mm KNO3, 2.5 mm KH2PO4 (pH 5.6), 2 mm MgSO4, 2 mm Ca(NO3)2, 50 μm CuSO4, 1 μM ZnSO4, 0.2 μm NaMoO4, 10 μm NaCl and 0.01 μM CoCl2) with or without 0.8% agar. N-1-Naphthylphthalamic acid (NPA), 1-naphthaleneacetic acid (NAA), herbicide basta (AgroEvo) and antibiotics were added to autoclaved ATS medium, when necessary.
Transgenic lines
A 2062-bp KRP1 (At2g23430) promoter was amplified from genomic DNA with primers KRP1-PF (5′-GTTCAAGCGAGTGACACATCTC-3′) and KRP1-PR (5′-CTTCGATTTAGGTTACGTGTGCG-3′). The KRP1:GUS plasmid was constructed by cloning the KRP1 promoter to pCB308 containing the Escherichia coli GUS (Xiang et al., 1999). A 602-bp KRP1 full-length cDNA was amplified from a cDNA library with primers KRP1-F (5′-ACGCACACGTCACCTAAATC-3′) and KRP1-R (5′-CTTCACTCTAACTTTACCCATTCG-3′). The KRP1:KRP1–GUS plasmid was constructed by cloning both the KRP1 promoter and the KRP1 full-length cDNA without the stop codon TGA to pCB308. To make a 35S:Myc-KRP1 construct, the KRP1 full-length cDNA without the start codon ATG was fused to the C-terminus of six c-myc epitopes in pGEM7Z. The Myc-KRP1 insert was then cloned to pROK2.
To make a 35S:SKP2-RNAi construct, a 433-bp cDNA from nucleotides 256–688 of SKP2a (At1g21410) was cloned in an opposite orientation to pHANNIBAL (Wesley et al., 2001). The SKP2-RNAi insert was then cloned to pBIN19. To make a 35S:SKP2b-TAP construct, an SKP2b (At1g77000) full-length cDNA without the stop codon TGA was fused to the N-terminus of a TAP (tandem affinity purification) tag (Rigaut et al., 1999). The SKP2b-TAP insert was then cloned to pPILY (Ferrando et al., 2000).
A 3861-bp RKP full-length cDNA without the start codon ATG was amplified with primers RKP-F (5′-CACCTTGGCTGAAGACAGCCTACGG-3′) and RKP-R (5′-GCAACTAACCCGAGCTTCATGTGC-3′), and was cloned to pENTR/D-TOPO using the pENTR directional TOPO cloning kit (Invitrogen, http://www.invitrogen.com). The RKP insert in pENTR/D-TOPO was then cloned to pGWB15 (http://bio2.ipc.shimane-u.ac.jp/pgwbs/index.htm) containing the CaMV 35S promoter and three hemagglutinin epitopes to make a 35S::HA-RKP construct.
All the above constructs in the binary vectors were introduced into Agrobacterium tumefaciens strain GV3101. Plants were transformed by the vacuum infiltration method (Bechtold and Pelletier, 1998). Transgenic plants were selected on ATS plates supplemented with the necessary antibiotics or herbicide. The KRP1:GUS and KRP1:KRP1–GUS transgenic plants are basta resistant. The 35S:Myc-KRP1 and 35S::HA-RKP transgenic plants are kanamycin- and hygromycin-resistant, respectively.
GUS assays
To examine GUS expression, seedlings were incubated in a GUS staining solution at 37°C as described by Oono et al. (1998). GUS-stained seedlings were incubated in 70% ethanol to remove chlorophyll. GUS staining patterns were examined under a Nikon SMZ1500 dissecting microscope (http://www.nikonusa.com).
RT-PCR analysis
Total RNAs were extracted using the TRI reagent (Sigma, http://www.sigmaaldrich.com). The first-strand cDNAs were synthesized from 5 μg total RNAs using oligo(dT)20 primer and SuperScript II RNase H− reverse transcriptase (Invitrogen). PCRs were performed with the following gene-specific primers: SKP2a-F, 5′-CCGCTTCATTTTAGTCATTAAAC-3′; SKP2a-R1, 5′-GGCCGTTTATATATACAACATAAC-3′; SKP2a-R2, 5′-TGATTGCAGTTATTCCCAATAG-3′; SKP2b-F, 5′-CATATTTACTTTTGATCTCGTGG-3′; SKP2b-R1, 5′-CATACTAGAGAGTAGTAGACC-3′; SKP2a-R2, 5′-CGAGTTTAGTCAGGTTAGTA-3′; Myc-F, 5′-GACTCTAGAGGATCCCCAAAGC-3′; Myc-R, 5′-AGCCGAATTCGATGGGGTACCG-3′; TAP-F, 5′-TAGCCGTCTCAGCAGCCAACC-3′; TAP-R, 5′-CTTCCCCGCGGAATTCGCGTC-3′; ACTIN2-F, 5′-GGCTGAGGCTGATGATATTC-3′; ACTIN2-R, 5′-TCTGTGAACGATTCCTGGAC-3′.
Immunoblot analysis and immunoprecipitation
Protein extracts were prepared as described by Gray et al. (1999). For immunoblot analysis, 50 μg protein extracts were mixed with SDS-PAGE sample buffer and were boiled for 5 min. Denatured proteins were separated on a 10% acrylamide SDS gel and were transferred to a nitrocellulose membrane. The membrane was immersed in Tris-buffered saline (pH 7.6) containing 5% non-fat dry milk and 0.1% Tween 20 to block non-specific binding sites. The α-c-myc 9E 10 antibody (Covance Research Products, http://www.crpinc.com) was used at a 1:1000 dilution. The horseradish peroxidase-conjugated goat α-mouse secondary antibody (Sigma) was used at a 1:3000 dilution. Proteins were detected with the enhanced chemiluminescence (ECL) kit (Amersham Pharmacia Biotech, http://www.gelifesciences.com).
For immunoprecipitation, 5 μl α-c-myc 9E 10 antibody was added to 3 mg protein extracts and was incubated for 1–3 h at 4°C. To collect immune complexes, 30 μl of protein A agarose beads (Roche, http://www.roche.com) were added and were incubated for from 3 h to overnight. Immune complexes were washed three times in 1 ml of protein extraction buffer. Finally, agarose beads were resuspended in SDS-PAGE sample buffer. Immunoblot analysis was carried out as described above. The α-CDKA;1 antibody was used at a 1:5000 dilution. The α-CDKB1;1 and α-CYCD2;1 antibodies (Healy et al., 2001) were used at a 1:3000 dilution. The horseradish peroxidase-conjugated goat α-rabbit secondary antibody (Chemicon International, http://www.millipore.com) was used at a 1:2500 dilution.
Acknowledgements
We thank Dirk Inzé for the α-CDKA;1 antibody and CYCB1;1:GUS line, William M. Gray for the axr6-3 mutant, the SALK Institute Genomic Analysis Laboratory, ABRC and GABI-KAT for T-DNA insertion mutants. This work was supported by grants from the National Science Foundation (NSF 2010 MCB-0115870) to ME and the Spanish MEC (BIO2004-01749) to JCdP.




