Abscisic acid deficiency in the tomato mutant high-pigment 3 leading to increased plastid number and higher fruit lycopene content
Summary
Carotenoids are present in most tissues of higher plants where they play a variety of essential roles. To study the regulation of carotenoid biosynthesis, we have isolated novel mutations in tomato (Solanum lycopersicum) with altered pigmentation of fruit or flowers. Here we describe the isolation and analysis of a tomato mutant, high-pigment 3 (hp3), that accumulates 30% more carotenoids in the mature fruit. Higher concentrations of carotenoids and chlorophyll were also measured in leaves and the pericarp of green fruit. The mutation in hp3 had occurred in the gene for zeaxanthin epoxidase (Zep), which converts zeaxanthin to violaxanthin. Consequently, leaves of the mutant lack violaxanthin and neoxanthin, and flowers contain only minute quantities of these xanthophylls. The concentration in the hp3 mutant of abscisic acid (ABA), which is derived from xanthophylls, is 75% lower than the normal level, making hp3 an ABA-deficient mutant. The plastid compartment size in fruit cells is at least twofold larger in hp3 plants compared with the wild-type. The transcript level in the green fruit of FtsZ, which encodes a tubulin-like protein involved in plastid division, is 60% higher in hp3 than in the wild-type, suggesting that increased plastid division is responsible for this phenomenon. Elevated fruit pigmentation and plastid compartment size were also observed in the ABA-deficient mutants flacca and sitiens. Taken together, these results suggest that ABA deficiency in the tomato mutant hp3 leads to enlargement of the plastid compartment size, probably by increasing plastid division, thus enabling greater biosynthesis and a higher storage capacity of the pigments.
Introduction
Carotenes and their oxygenated derivates, xanthophylls, are 40-carbon lipid-soluble terpenoid molecules. They are present in most tissues of higher plants, where they play a variety of roles in biochemical processes and development. In green tissues, carotenoids exist as integral components of the photosynthetic apparatus. Xanthophylls in the light-harvesting complexes (LHC) protect the plastids against photo-oxidation caused by the formation of reactive singlet-state oxygen (1O2) by dissipating the excess of energy of triplet-state chlorophyll (3Chl*). In many plant species, carotenoids accumulate in reproductive tissues to provide yellow, orange or red colors that attract pollinators and seed distributors. In addition, products of oxidative cleavage of carotenoids (apocarotenoids) are involved in plant development, serve as anti-fungal agents and contribute animal-attracting scents (reviewed by Auldridge et al., 2006; Booker et al., 2004; Simkin et al., 2004). Cleavage of xanthophylls also produces the precursor for the phytohormone abscisic acid (ABA), which regulates a wide range of processes in the plant, including seed development and dormancy, transition from vegetative to the reproductive phase, and responses to environmental stresses, mainly drought and salinity (Finkelstein, 2006; Leung and Giraudat, 1998; Nambara and Marion-Poll, 2003; Shinozaki and Yamaguchi-Shinozaki, 2007).
The carotenoid biosynthesis pathway in plants has been largely worked out (reviewed by Cunningham and Gantt, 1998; DellaPenna and Pogson, 2006; Fraser and Bramley, 2004; Hirschberg, 2001; Romer and Fraser, 2005). The biosynthetic pathway starts with the formation of phytoene from two molecules of geranylgeranyl diphosphate (GGPP) in the central isoprenoid pathway (Figure 1). Four desaturation steps give rise to lycopene; cyclizations at both ends of the lycopene molecule produce α- or β-carotene which undergo hydroxylation at C3 and C3′ to form the xanthophylls lutein and zeaxanthin, respectively. Zeaxanthin epoxidase produces violaxanthin, which is the substrate for neoxathin synthase. As the latter occurs in the light-harvesting complex in the 9-cis configuration, an isomerization step, probably catalyzed by an as yet unknown enzyme, is hypothesized. 9-cis violaxanthin and possibly also 9′-cis-neoxanthin undergo oxidative cleavage to give cis-xanthoxin. In the cytosol, abscisic acid (ABA) is formed from xanthoxin via ABA-aldehyde by two oxidation reactions.
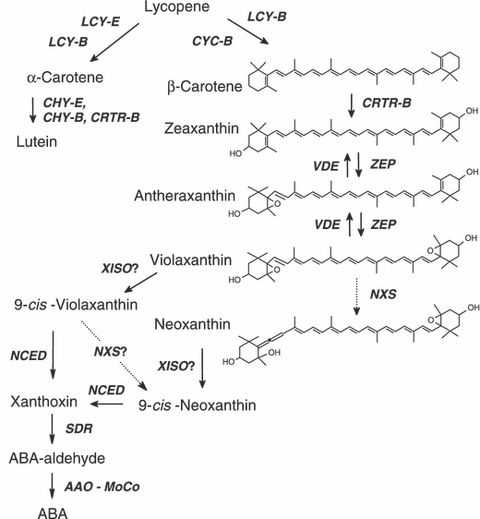
The xanthophyll and ABA biosynthesis pathway.AAO, abscisic aldehyde oxidase; CHY-B and CRTR-B, β-ring hydroxylases; CHY-E, ε-ring hydroxylase; CYC-B, chromoplast-specific lycopene β-cyclase; LCY-B, lycopene β-cyclase; LCY-E, lycopene ε-cyclase; MoCo, molybdenum co-factor; NCED, 9-cis-epoxycarotenoid dioxygenase; NXS, neoxanthin synthase; SDR, short chain dehydrogenase; VDE, violaxanthin de-epoxidase; XISO, xanthophyll isomerase (predicted); ZEP, zeaxanthin epoxidase.
To study the regulation of carotenoid biosynthesis, we have analyzed the genetic mechanisms that control the accumulation of lycopene in tomato (Solanum lycopersicum) fruit during ripening. It is well documented that accumulation of lycopene in fruit is correlated with differential expression of genes encoding biosynthetic enzymes during the ‘breaker’ stage of fruit development (reviewed by Alba et al., 2004; Fraser and Bramley, 2004; Giovannoni, 2004; Hirschberg, 2001; Romer and Fraser, 2005). Expression of genes for all enzymes upstream of lycopene is up-regulated at the ‘breaker’ stage, but the genes for lycopene β-cyclase (Lcy-b) and lycopene ε-cyclase (Lcy-e) are not transcribed. Concomitantly, at this stage, the chloroplasts in the mature green fruit develop into chromoplasts, which are adapted to produce and store large amount of crystalline lycopene (Camara et al., 1995; Price et al., 1995; Pyke and Howells, 2002), carotenoid-associated polypeptides are synthesized (Leitner-Dagan et al., 2006a,b; Simkin et al., 2007) and chlorophyll is degraded (Cheung et al., 1993; Efrati et al., 2005; Hortensteiner, 2006).
The amount of lycopene that accumulates in the fruit is determined by the rate of synthesis as well as the storage capacity. Two tomato mutants, high-pigment 1 (hp1; Reynard, 1956) and high-pigment 2 (hp2; Soressi, 1975), have been found to accumulate higher concentrations of lycopene in fruit. Phenotypic characterization revealed exaggerated photoresponses during de-etiolation, higher chlorophyll levels in immature fruit, and increased plastid compartment size in leaf and fruit cells (Cookson et al., 2003; Kendrick et al., 1994; Kerckhoffs et al., 1997; Peters et al., 1998). It was determined (Lieberman et al., 2004; Liu et al., 2004) that hp1 encodes a UV-DAMAGED DNA-BINDING PROTEIN 1 (DDB1) homolog, which, in Arabidopsis, has been demonstrated to interact with DE-ETIOLATED 1 (DET1) to regulate expression of many genes in response to light (Schroeder et al., 2002), and hp2 is mutated in the tomato ortholog of Det1 in Arabidopsis (Mustilli et al., 1999). Both mutations presumably impair regulatory functions that control plastid development.
Here we describe the isolation and analysis of a novel tomato mutant that exhibits higher concentrations of carotenoids and chlorophylls in leaves and fruit. The high-pigment 3 (hp3) mutation was found to occur in the gene for zeaxanthin epoxidase. Our results suggest that the reduced level of abscisic acid (ABA) enhances fruit lycopene content by increasing the total size of plastid compartment in cells.
Results
Isolation and phenotypic characterization of the high pigment 3 mutant
To facilitate the genetic analysis of carotenoid biosynthesis, we have established a collection of novel mutations in tomato (Solanum lycopersicum cv. M82) that affect pigmentation of flowers and fruit. Seeds were treated with ethyl-methane sulfonate (EMS) or fast neutron bombardment (Menda et al., 2004). Over 200 000 M2 plants were visually screened for changes in the characteristic pigmentation of flowers or fruit. More than 100 such mutant lines were identified. Total carotenoid level and pigment composition were determined by HPLC in leaves, flowers and fruit of each mutant. The final collection comprised 64 single-gene color mutations that were clustered into 24 groups, based on carotenoid composition in the various organs. Twelve lines were found by crosses to be allelic with four known carotenoid mutants: two white-flower alleles (wf), four yellow-flesh (r), five tangerine (t) and one new old-gold (ogc) allele.
The EMS-induced mutant line e1472 exhibited fruit with an intensified red color, relatively greener leaves and beige flowers (Figure 2). The total carotenoid concentrations in the leaves and red fruit of the mutant were approximately 40 and 30% higher than in the wild-type, respectively (Table 1). Elevated carotenoid content was observed at all stages of fruit development (Figure 2b). Based on this phenotype, we named the mutation high-pigment 3 (hp3). The carotenoid composition in the leaves of hp3 was altered (Table 1). Dark-adapted leaves of hp3 lacked violaxanthin and neoxanthin, two abundant xanthophylls that are integral components of the light-harvesting complexes (LHC) in higher plants. Instead, they contained a little more lutein, a substantial amount of zeaxanthin and a small amount of antheraxanthin. Normally, zeaxanthin appears in measurable amounts in leaves only after exposure to light, which activates de-epoxidation of violaxanthin through the ‘xanthophyll cycle’. Flowers of hp3 had a reduced level of carotenoids due to the elimination of violaxanthin and neoxanthin, the main xanthophylls that accumulate in tomato flowers (Table 1 and Figure 2a). Another mutant line, e3452, which was identified by altered flower pigmentation, resembled hp3 in accumulating a high level of zeaxanthin, but exhibited a weaker phenotype (Table 1). This mutant was found by genetic crosses to be allelic to hp3. Hence, we designated line e1472 as hp3-1 and line e3452 as hp3-2.
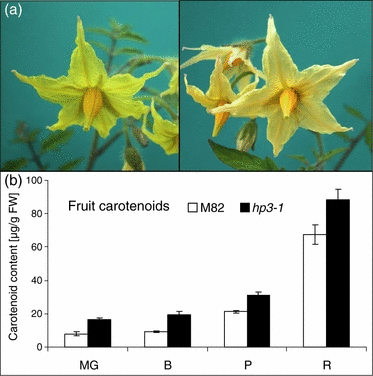
Phenotypic characteristics of the mutant high-pigment 3 (hp3).(a) Flowers of wild-type (cv. M82; left) and high-pigment 3-1 (e1472).(b) Total carotenoid content in fruit of hp3-1 and M82 at various developmental stages: MG, mature green; B, breaker; P, pink; R, mature red. Data are means ± SE (n = 22, except for stage R for which n = 8). Numbers correspond to μg carotenoids g−1 FW.
| Chlorophyll a + b | Neoxanthin | Violaxanthin | Antheraxanthin | Lutein | Zeaxanthin | β-Carotene | Lycopene | Phytoene + phytofluene | Others | Total carotenoids | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fruit | |||||||||||
| Wild-type | 0.9 ± 0.2 | 0 | 4.3 ± 1 | 56.8 ± 4 | 5.5 ± 0.4 | 0.3 | 67.8 ± 5.7 | ||||
| hp3-1 | 1.7 ± 0.2 | 5.9 ± 0.6 | 5.2 ± 0.4 | 69.0 ± 7.2 | 6.0 ± 0.4 | 0.5 | 88.3 ± 6.5 | ||||
| hp3-2 | 1.7 ± 1.1 | 0.9 ± 0.4 | 6 ± 2.4 | 54.9 ± 4 | 7.9 ± 1.7 | 0.5 | 71.9 ± 8.4 | ||||
| Petals | |||||||||||
| Wild-type | 517.2 ± 25.5 | 216.4 ± 11.8 | 1.5 ± 0.6 | 2.4 ± 0.8 | 0 | 1.4 ± 0.4 | 738.9 ± 37.5 | ||||
| hp3-1 | 10.6 ± 6.1 | 0.9 ± 0.3 | 20.0 ± 3.6 | 2.4 ± 0.4 | 175.9 ± 13.7 | 0 | 209.8 ± 17.8 | ||||
| hp3-2 | 305.9 ± 7.3 | 96.7 ± 5.5 | 70.5 ± 4.8 | 4.4 ± 0.3 | 46.2 ± 1.5 | 0 | 523.7 ± 19.5 | ||||
| Leaves | |||||||||||
| Wild-type | 1453 ± 41 | 18.7 ± 4.5 | 25.7 ± 2.8 | 3.5 ± 0.8 | 75.6 ± 8 | 4.2 ± 1.1 | 30.3 ± 3.7 | 0.1 | 158.1 ± 17.5 | ||
| hp3-1 | 2151 ± 68 | 0.5 ± 0.4 | 5.6 ± 1.2 | 81.9 ± 6.9 | 93.7 ± 7.3 | 38.7 ± 4.9 | 0.1 | 220.5 ± 20.2 | |||
| hp3-2 | 1944 ± 135 | 13.6 ± 0.7 | 19.6 ± 3.2 | 7.1 ± 1.2 | 67.5 ± 3.4 | 15.9 ± 1.2 | 33.4 ± 1.7 | 0.6 | 157.6 ± 10 | ||
- Data shown are means (μg g−1 FW) ± SE (n = 6, except for fruit total carotenoids for which n = 22).
The pleiotropic nature of the mutation is evident from additional phenotypic changes. Field-grown hp3-1 plants had a wilted appearance at midday, their overall biomass was reduced by 20%, and the average fruit size and total fruit yield per plant were 56–68% and 50–68%, respectively, lower than in the wild-type (Table 2). Greenhouse-grown hp3-1 plants did not show any of these phenomena (data not shown).
| Experiment | Line | Plant weight (kg) | Total fruit weight (kg) | Average fruit weight (g) |
|---|---|---|---|---|
| 2004 (winter) | Wild-type | 1.03 ± 0.06 | 5.39 ± 0.53 | 67.90 ± 3.13 |
| hp3-1 | 0.83 ± 0.09 | 2.72 ± 0.21 | 38.70 ± 1.52 | |
| 2005 (summer) | Wild-type | 1.89 ± 0.20 | 9.37 ± 0.90 | 60.47 ± 2.18 |
| hp3-1 | 1.18 ± 0.14 | 4.91 ± 0.30 | 41.39 ± 1.72 | |
| 2006 (winter) | Wild-type | 0.89 ± 0.06 | 5.63 ± 0.29 | 74.80 ± 1.47 |
| hp3-1 | 0.83 ± 0.07 | 3.86 ± 0.37 | 43.14 ± 2.78 |
- Plant weight refers to total vegetative parts after harvesting the fruit. Field-grown plants at the stage of 80% red fruit were measured. Data shown are means ± SE (n = 10, except for average fruit weight for which n = 20).
The genetic and molecular nature of hp3
The carotenoid composition in hp3 plants suggested a possible obstruction in the biosynthetic pathway of carotenoids at the step of epoxidation of zeaxanthin. The enzyme zeaxanthin epoxidase (ZEP) catalyzes the conversion of zeaxanthin to violaxanthin through the intermediate antheraxanthin (Marin et al., 1996; Figure 1).
We have previously mapped the gene Zep to bin K on chromosome 2, in an overlapping region between IL 2-5 and IL 2-6 (Liu et al., 2003). The Zep gene was found to be tightly linked to the RFLP marker TG 167.
Mutant hp3-1 was crossed with the red-fruited wild species S. pimpinellifolium. F2 plants derived from this cross segregated with a ratio of 3:1 for wild-type to hp3 phenotypes, indicating that hp3 is a monogenic, recessive mutation. Complete co-segregation of the hp3 phenotype and homozygous TG 167 was detected in 28 F2 plants as determined by DNA blot hybridization of genomic DNA (Figure S1). This result suggests that hp3 is encoded by Zep.
Analysis by quantitative RT-PCR in flowers of hp3-1 and hp3-2 did not identify any alteration in the mRNA levels of Zep in these mutants compared to wild-type (data not shown). Separation by agarose gel electrophoresis of PCR products from the entire cDNA showed a full-length sequence of Zep in both alleles (data not shown). However, sequence analysis of Zep cDNA from hp3-1 and hp3-2 revealed mutations in the coding region that lead to amino acid substitutions in the ZEP polypeptide (Figure 3 and Supplementary material). A transition of G to A, leading to substitution of the glutamic acid at position 142 by lysine (E142K), was detected in hp3-1, and an A to G transition that replaces the glutamic acid by lysine at position 150 (E150K) was identified in hp3-2 (Figure 3). Interestingly, the two mutations occurred in highly conserved residues in close proximity in the polypeptide, and in both cases the negatively charged amino acid glutamic acid was replaced by positively charged lysine.
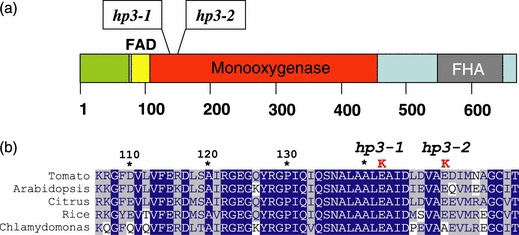
Mutations in zeaxanthin epoxidase of hp3 mutants.(a) Structure of ZEP. FAD, flavin adenine dinucleotide binding domain; FHA, forkhead-associated domain.(b) Alignment of amino acid sequences of ZEP from various plants in the region where mutations were found in the tomato hp3-1(E142K) and hp3-2 (E150K) alleles.
Reduced abscisic acid levels in hp3
The xanthophyll violaxanthin is a precursor in synthesis of the plant hormone abscisic acid (ABA; Figure 1; reviewed by Nambara and Marion-Poll, 2005). Mutations inhibiting zeaxanthin epoxidation in Arabidopsis and tobacco have been reported to be ABA-deficient (Barrero et al., 2005; Marin et al., 1996). Indeed, we observed that hp3-1 plants in the field were more sensitive to water stress conditions and tended to wilt more quickly than the wild-type. Plants of hp3-2 behaved like the wild-type, most probably because of the relatively small reduction in violaxanthin and neoxanthin in leaves (Table 1).
Water loss in detached leaflets was measured in greenhouse-grown, well-watered plants of hp3-1 and wild-type (M82). The faster weight loss in hp3-1 leaflets (Figure 4) indicates reduced stomatal closure, which is a characteristic of ABA deficiency.
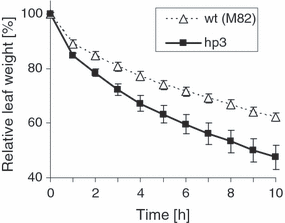
Water loss in detached leaves from M82 (wild-type) and hp3-1 plants. Leaflets were detached, weighed and placed on filter paper on the bench at room temperature. Data shown are means ± SE (n = 3).
The ABA concentration in hp3-1 and in the wild-type (M82) was measured in leaves and fruit at several developmental stages (Table 3). A significant decrease in ABA levels was detected in all tested tissues of field-grown hp3-1 plants compared to the wild-type. These results indicate that the mutation in Zep also impairs the pathway leading to ABA. In greenhouse-grown plants, the ABA level was low and similar in both hp3-1 and wild-type.
| Tissue | Stage | ABA | |
|---|---|---|---|
| Wild-type | hp3-1 | ||
| Leaves (greenhouse) | 11.4 ± 1.6 | 9.6 ± 1.3 | |
| Leaves (field) | 46.3 ± 6.2 | 14.9 ± 5.7 | |
| Fruit (field) | Mature green | 49.1 ± 6.7 | 14.6 ± 2.6 |
| Pink | 14.3 ± 2.7 | 1.9 ± 0.5 | |
| Mature red | 40.1 ± 4.7 | 9.9 ± 1.2 | |
- The concentration of ABA (pmol g−1 FW) was measured in leaves from plants grown in the greenhouse (well-watered) or in the field, and in fruit from field-grown plants. Data shown are means ± SE (n = 5).
Carotenoid levels in fruit of the ABA mutants flacca and sitiens
To examine the relationship between ABA level and fruit carotenoid levels, we measured total carotenoid concentration in fruit of the recognized ABA-deficient tomato mutants flacca (flc) and sitiens (sit) and in their parental isogenic line Rheinlands Ruhm (RR; LA 0535). As can be seen in Figure 5, red fruit of the mutants contained an approximately 35% higher concentration of carotenoids than the wild-type. The relative abundance of the various carotenoids (Table 5) indicates that this increase is due to the accumulation of lycopene. Similarly to hp3-1, the difference between these mutants and the wild-type was already evident at the ‘mature green’ stage of fruit development, but to a lower extent. In contrast to hp3, the average size of the fruit was not reduced in these mutants (data not shown). Reliable data on total fruit yield have not been obtained.
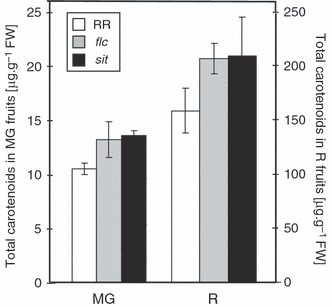
Total carotenoid content in fruit of the ABA mutants flacca (flc) and sitiens (sit) and their parental wild-type (RR).Numbers correspond to μg carotenoids g−1 FW. MG, mature green; R, mature red. Data shown are means ± SE (n = 8).
| Wild-type (M82) | hp3-1 | Wild-type (RR) | flc | sit | |
|---|---|---|---|---|---|
| Plastids per cell (n = 60) | 19.7 ± 0.78 | 38.0 ± 1.25 | 19.5 ± 0.7 | 26.6 ± 0.8 | 31.0 ± 1.5 |
| Plastid area (μm2; n = 100) | 2.7 ± 0.11 | 3.6 ± 0.13 | ND | ND | ND |
| Cell area (μm2; n = 210) | 10 523 ± 407 | 9978 ± 649 | ND | ND | ND |
| Ratio of area plastid per cell | 0.005 ± 0.00021 | 0.0137 ± 0.00025 | ND | ND | ND |
- Plastid compartment size in mature green fruit of mutants hp3-1, flc and sit, and in their corresponding isogenic wild-type lines M82 and RR. Cells from layers 4–6 from the pericarp of fruit at the mature green (MG) stage were analyzed. Three to six sections from two fruit were analyzed. Data shown are means ± SE. ND, not determined.
Expression of carotenoid biosynthesis genes in hp3 and wild-type fruit
Carotenoid biosynthesis is regulated mainly at the level of gene expression of biosynthetic enzymes (reviewed by DellaPenna and Pogson, 2006; Hirschberg, 2001; Romer and Fraser, 2005; Sandmann et al., 2006). To test whether increased pigmentation in hp3 fruit can be attributed to enhanced expression of the rate-limiting enzymes in the carotenoid pathway, we used quantitative RT-PCR to measure the mRNA levels of Psy1 (phytoene synthase 1), Psy2 (phytoene synthase 2) Dxs (1-deoxy-d-xylulose-5-phosphate synthase) and Ggps (geranylgeranyl diphosphate synthase) during fruit development in hp3-1 and its isogenic wild-type line M82 (Figure 6). At all developmental stages except ripe fruit, the mRNA level for the chromoplast-specific gene Psy1 was lower in the mutant. Similar results were observed for Psy2, which is constitutively expressed in chloroplasts, and for Dxs. The Ggps mRNA level was significantly higher in hp3-1 only at the late stages of ripening, and not when most of the carotenoid biosynthesis takes place (stages ‘breaker’ and ‘pink’). These results imply that expression of carotenoid biosynthesis genes is not responsible for the intensified pigmentation in hp3 fruit.
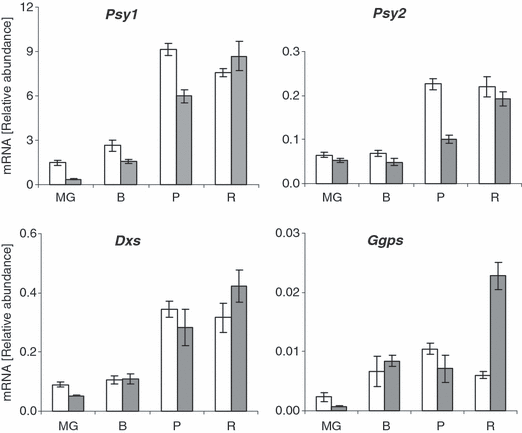
Expression of carotenoid biosynthesis genes during fruit development in hp3-1 and wild-type.Psy1, phytoene synthase 1; Psy2, phytoene synthase 2; Dxs, 1-deoxy-d-xylulose-5-phosphate synthase; Ggps, geranylgeranyl diphosphate synthase. The steady-state level of mRNA was determined by quantitative RT-PCR. RNA was extracted from four developmental stages: MG, mature green; B, breaker; P, pink; R, mature red. White bars are M82 (wild-type); gray bars are hp3. Data shown are means ± SE (n = 5).
Plastids in the fruit of hp3 and other ABA-deficient mutants
The carotenoid content in the cell is a combination of biosynthesis rate, storage capacity and turnover. In fruit of the tomato mutant high-pigment 1 (hp1), an increase in plastid compartment size has been observed (Cookson et al., 2003). We examined the plastids in pericarp cells of hp3-1 and wild-type fruit. Cell size, plastid number per cell, and plastid size were determined microscopically. The number of plastids per cell at the ‘mature green’ stage was doubled in hp3-1 compared with wild-type, and they were 30% larger (Table 4). A similar increase in plastid number per cell was found at the ‘breaker’ and ‘pink’ stages (data not shown). As the size of cells was similar in hp3-1 and wild-type, it is estimated that total volume of the plastid compartment in hp3-1 pericarp tissue is approximately threefold larger than in the wild-type. In addition, we found increased stacking of granal thylakoids in chloroplasts of hp3-1 at the ‘mature green’ stage (Figure 7). However, the appearance of chromoplasts at the ‘pink’ stage was similar to that in the wild-type (data not shown).
| Lutein | Zeaxanthin | β-Carotene | Lycopene | Phytoene + phytofluene | Others | |
|---|---|---|---|---|---|---|
| Wild-type (RR) | 1.9 ± 0.2 | 2.6 ± 0.6 | 1.3 ± 0.2 | 142.1 ± 2.5 | 7.8 ± 1.3 | 0.1 ± .01 |
| flc | 1.1 ± 0.2 | 1.8 ± 0.2 | 1.8 ± 0.7 | 181.8 ± 2.4 | 15 ± 3.4 | 1.4 ± 0.1 |
| sit | 0.8 ± 0.1 | 2.1 ± 0.5 | 3.4 ± 1.6 | 182.8 ± 3 | 14.1 ± 3.5 | 2 ± 0.2 |
- Data shown are means (μg g−1 FW) ± SE (n = 3).
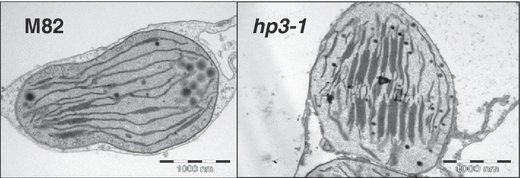
Ultra-structure of chloroplasts in hp3-1 and wild-type.Characteristic electron micrographs of chloroplasts from ‘mature green’ fruit of M82 (wild-type) and hp3-1 showing enhanced stacking of granal thylakoids in the mutant.
The plastid numbers in fruit cells at the ‘mature green’ stage of the ABA-deficient mutants flacca and sitiens were increased by 36 and 59%, respectively, compared to the wild-type (cv. RR; Table 4). This result, which is in agreement with data from hp3-1, strongly supports linkage between ABA deficiency and overpigmentation in the tomato fruit.
The number of plastids per cell is determined by the rate of plastid division relative to cell division. To verify that the higher number of plastids in hp3-1 is caused by an increased rate of plastid division, we measured the transcript levels of the genes FtsZ and MinD, which encode structural components of the plastid division apparatus. Expression of FtsZ in stages ‘green’ and ‘mature green’ was 60 and 17% higher, respectively, in hp3 compared with the wild-type (Figure 8). However, no significant difference in the mRNA levels of MinD was found in the mutant.
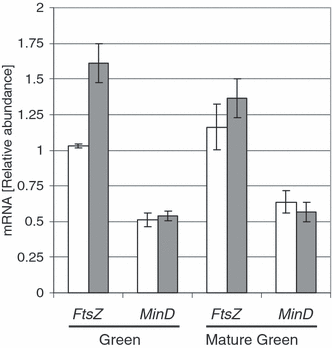
Expression of FtsZ and MinD in developing fruit of hp3-1 and wild-type.The steady-state level of mRNA was determined by quantitative RT-PCR. RNA was extracted from two developmental stages: ‘green’ and ‘mature green’. Data shown are means ± SE (n = 4).
Ethylene production during fruit ripening in hp3-1
Reduced ABA levels may lead to greater production of ethylene, and therefore it is possible that the increase in plastid number and higher pigmentation in hp3 could be mediated by the latter hormone. Ethylene production during fruit development was approximately 40% higher in hp3-1 during the ‘breaker’ to ‘pink’ stages of ripening, but similar to M82 in the ‘green’ and ‘mature green’ stages and lower at the ‘mature red’ stage (Figure 9). As higher pigmentation is seen throughout the fruit development process (Figure 2), these results exclude the possibility that the phenotype is caused by increased ethylene.
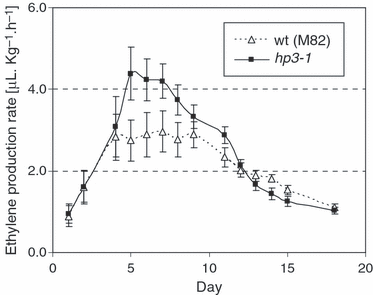
Ethylene production rate in ripening fruit of hp3-1 and wild-type.Ethylene production was measured in detached fruit (n = 10) stored at room temperature. Day 1, ‘mature green’; days 2–5, ‘breaker’; days 6–9, ‘pink’; days 10–19, ‘mature red’.
Discussion
The molecular nature of high-pigment 3
Three lines of evidence support the conclusion that mutations in zeaxanthin epoxidase, which converts zeaxanthin to trans-violaxanthin, are responsible for the phenotype of high-pigment 3.
- 1
Reduction in the activity of zeaxanthin epoxidase provides a straightforward explanation for the altered carotenoid profile observed in hp3 plants.
- 2
A complete genetic linkage exists between the gene Zep and the locus hp3.
- 3
Two independent mutations were detected in the Zep gene in two alleles of hp3.
The gene Zep was initially cloned from Nicotiana plumbaginifolia (locus aba2) and Arabidopsis (aba1; Marin et al., 1996; Xiong et al., 2002). The amino acid sequence of ZEP is 69% identical (86% similar) in all higher plants sequenced so far, and 53% identical (67% similar) between plants and the alga Chlamydomonas rheinhardtii (accession no. AAO48941). The amino acid sequence of ZEP in S. lycopersicum cultivar M82 matches the previously published sequences from S. lycopersicum Mill. (accession no. CAB06084; Burbidge et al., 1997) and cultivar Zhongshu (accession no. EF581828). It contains a typical plastid transit peptide in its N-terminus, an FAD-binding domain and a lipocalin motif, which also exists in violaxanthin de-epoxidase (Hieber et al., 2000). The mutations in hp3-1 and hp3-2 occurred in a highly conserved region in the polypeptide (Figure 4 and Supplementary material). Substitution of the negatively charged glutamic acid by the positively charged lysine in position 142 drastically reduced enzyme activity. However, residual activity in hp3-1 plants is evident from the occurrence of small amounts of antheraxanthin in leaves and of violaxanthin and neoxanthin in petals. One explanation is that the E142K mutation is ‘leaky’, and thus enables some residual ZEP activity. An alternative possibility is the existence of an additional zeaxanthin epoxidase in tomato. It has been previously suggested, based on Southern hybridization, that the tomato genome may contain an additional sequence similar to Zep (Thompson et al., 2000). Moreover, a low-stringency Northern hybridization with cDNA of Zep apparently detected an additional signal in root mRNA (Thompson et al., 2000). We have previously noted only a single Zep sequence in tomato (Liu et al., 2003), and as all available sequence databases of tomato, potato and pepper, including numerous ESTs, show only a single Zep in each of these species, we have ruled out the existence of another zeaxanthin epoxidase similar to Zep.
Substitutions of conserved amino acids in ZEP that impair its activity have been reported in Arabidopsis and in C. rheinhardtii (Baroli et al., 2003; Barrero et al., 2005; see Supplementary material). Interestingly, a substitution mutation similar to that in hp3-1 occurred in ZEP from a viviparous mutant of rice (Oryza sativa, japonica cultivar group), although it was claimed to be generated by an endogenous retrotransposon Tos17 insertion (Agrawal et al., 2001). A E134A substitution in the rice mutant is apparent from comparison the published sequence of the mutant gene (accession no. BAB39765) to ZEP from wild-type plants of the japonica (accession no. BAF14840 and EAZ30898) or indica (accession no. CAH67090) cultivar groups (Supplementary material). The effect of mutation E150K on ZEP activity was smaller, making hp3-2 a leaky mutation. The homologous residue of E150 in ZEP from Chlamydomonas is alanine, indicating that this residue is not highly conserved.
Increased fruit carotenoid levels are caused by ABA deficiency
Mutations in Zep explain the absence of violaxanthin and neoxanthin in leaves and the marked reduction of these xanthophylls in flowers. However, the increase in total fruit carotenoids in hp3, mainly lycopene, is intriguing because lycopene is an intermediate in the carotenoid biosynthesis pathway, three steps upstream of the block in zeaxanthin epoxidation (Figure 1). Therefore, a metabolic block at zeaxanthin epoxidation does not explain the phenotype of hp3.
Carotenoid biosynthesis in fruit and flowers is tightly regulated at the transcriptional level (reviewed by Fraser and Bramley, 2004; Hirschberg, 2001; Romer and Fraser, 2005). As no significant increase was detected in the mRNA levels for genes for the rate-limiting enzymes phytoene synthase, 1-deoxy-d-xylulose-5-phosphate synthase and geranylgeranyl diphosphate synthase (Figure 6), it is unlikely that enhancement of the pathway is due to up-regulation of gene expression, for example by feedback signaling from the altered carotenoid composition. The possibility of another regulatory mechanism that affects the pathway in hp3 post-transcriptionally, for example at the enzyme activity level, cannot be excluded. However, as the total carotenoid concentration in hp3 is already higher during early stages of fruit development, we have ruled out the possibility that it is associated with the up-regulation of carotenogenesis that normally occurs during the ‘breaker’ stage of ripening.
Our results suggest that the high-pigment phenotype is caused by reduction in the level of the phytohormone abscisic acid (ABA). ABA deficiency has been thoroughly studied in mutants in various plant species (reviewed by Taylor et al., 2000). Loss-of-function mutations in the gene Zep in higher plants resulted in phenotypes that are characteristic of ABA deficiency: precocious germination, plant growth retardation and wilted appearance (Aurdan et al., 2001; Barrero et al., 2005; Marin et al., 1996; Thompson et al., 2000; Xiong et al., 2002). Analysis of hp3 plants revealed a significant reduction, but not a complete elimination, of ABA. This ABA deficiency is explained by the reduction in violaxanthin and neoxanthin, which are the precursors for downstream steps in the synthesis of the hormone (Figure 1). We presume that residual activity of the mutated ZEP in hp3-1 and hp3-2 provides sufficient substrate material for the synthesis of ABA at a low level. Indeed, mutant plants grown in greenhouse under unstressed conditions do not show any physiological phenotype, whereas hp3-1 plants in the field are more susceptible to water stress conditions, have overall lower biomass and yield, and their leaves lose water more rapidly (Table 2 and Figure 4). The latter phenomenon is attributed to lack of control of stomata closure, which is mediated by ABA. No vivipary has been observed in hp3, and seed dormancy was similar to that in wild-type plants (data not shown). It is noteworthy that, in grafting experiments of hp3 shoots on M82 roots and M82 shoots on hp3 roots, the phenotypes of the plants were determined by the genotype of the shoot (data not shown), indicating that root ABA has no effect on the leaves and fruit of hp3.
Association of ABA deficiency and elevated carotenoid content in fruit is further reinforced by the characterization of two other ABA-deficiency mutants in tomato, flacca (flc) and sitiens (sit). These mutations, which block ABA biosynthesis at the ABA-specific aldehyde oxidase (sit) or its molybdenum cofactor (flc), have been extensively used in ABA research (reviewed by Taylor et al., 2000). Another mutation has been created by silencing of Zep in transgenic plants (Taylor et al., 2000). All of these lines display a characteristic ABA-deficiency phenotype. Our data indicate that, similarly to hp3, fruit in the mutants flc and sit consistently accumulate a higher concentration of total carotenoids (Figure 5). This result supports the hypothesis that higher fruit pigmentation is connected to the reduction in ABA rather than to alterations in the regulation of carotenoid biosynthesis due to the impairment of zeaxanthin epoxidase.
Higher pigmentation has also been observed in Arabidopsis mutants that are ABA-deficient because of mutations in Zep. A 60% increase in total leaf carotenoids was found in mutant aba1-1, despite the depletion of violaxanthin and neoxanthin (Duckham et al., 1991). The mutant aba1-6 has 50% more carotenoids than the wild-type in high-light-grown plants and 16% more in low-light-grown plants (Kalituho et al., 2007). In this mutant, the chlorophyll level was also elevated by 15–18%. In another study of aba1-6, the total carotenoids per leaf area were 22% higher than the wild-type after exposure to high light, but similar to the wild-type in low light (Niyogi et al., 1998). A smaller increase in total leaf carotenoids was observed in other studies: 12.4% and 11% in aba1-3 and aba1-4, respectively (Hurry et al., 1997), and a 7% increase in aba1-3 (Pogson et al., 1998) and a 9% increase in aba1-4 (Rock and Zeevaart, 1991). Overall, the data from Arabidopsis corroborate the conclusion that reduced ABA is linked to higher pigment concentration. Treatment of hp3 plants with exogenous ABA did not alleviate the hormone deficiency phenotype (data not shown). This unexpected result requires further investigation.
Is ABA directly involved in the high-pigment phenotype? Ethylene is the major hormone that controls fruit ripening, and it enhances the accumulation of lycopene in the climacteric fruit of tomato (Alexander and Grierson, 2002; Barry et al., 2005). Ample evidence in various plants indicates antagonistic interactions between ethylene and ABA, both physiologically and at the level of signaling pathways (Benschop et al., 2007; Hoffmann-Benning and Kende, 1992; Hussain et al., 2000; Saika et al., 2007; Sauter et al., 1995; Sharp, 2002; Smulders and Horton, 1991; Zeevaart, 1983). At the peak of the climacteric ethylene burst, the fruit of mutant hp3 produced approximately 40% more ethylene than the wild-type (M82), but was similar to the wild-type in the green stages and lower than the wild-type at the ‘mature red’ stage (Figure 9). However, the higher fruit ethylene content in hp3 after the ‘breaker’ stage, probably caused by the reduction in ABA, does not explain the increase in carotenoids that occurs earlier in fruit development. In this respect, hp3 is different from the tomato ethylene over-producing mutant epinastic (epi; Wang et al., 2005). Furthermore, higher ethylene during ripening is not accompanied by the typical increase in gene expression that is associated with fruit carotenoid biosynthesis. Therefore, increased ethylene in hp3 may perhaps contribute to the high-pigment phenotype, but does not play a major role in enhancing fruit lycopene accumulation.
ABA affects plastid number
Carotenoid biosynthesis occurs within plastids, and it is in these organelles, when differentiated to chromoplasts, that carotenoids accumulate to a high concentration, usually in flowers and fruit. The plastid number in pericarp cells from hp3 fruit at the ‘mature green’ stage was doubled compared with wild-type (Table 4). The overall compartment size of plastids is estimated to be approximately threefold higher in hp3-1 than in the wild-type. The larger relative volume of plastids in the cells enables a higher rate of synthesis of carotenoids, and raises the storage capacity in chromoplasts. Increased stacking of granal thylakoids (Figure 7), the major site of carotenoid storage and activity in chloroplasts, indicates the increase in carotenoid storage capacity at the sub-organelle level. The fact that both chlorophyll and carotenoid levels are increased in green tissues of hp3, in addition to the higher pigmentation in fruit chromoplasts, further supports the conclusion that the increase in storage capacity in plastids leads to high pigmentation in hp3. As the increase in carotenoid concentration in the fruit of hp3, flacca and sitiens is lower than the increase in plastid compartment size, we speculate that metabolic constraints on the flux of isoprenoids, or a biochemical regulatory mechanism, limit further increase of carotenoids.
Similarly to hp3, a relationship between plastid compartment size and increased pigmentation has been recognized in the tomato mutants high-pigment 1 (hp1; Cookson et al., 2003) and hp2, where a fivefold increase in the plastosome:genomic DNA ratio, which is correlated with a higher number of plastids, was reported (Yen et al., 1997). The increase in the plastid compartment size in the tomato mutant suffulta (divergens), which is impaired in plastid division, is different to that in mutants hp1, hp2 and hp3. In contrast to the latter, which have more plastids, suffulta cells contain a small number of huge plastids, and the total pigment content during the green stages of fruit development is smaller than the wt. (Forth and Pyke, 2006). This phenomenon suggests that an increase in overall plastid compartment size alone is not sufficient to bring about higher pigment content.
The lesion in hp3 is more specific than the above mutants; as the precise biochemical block has been revealed. Altered carotenoid composition may affect plastid development directly, for instance if carotenoids, or a specific carotenoid species, are components of the mechanism involved in the regulation of plastid division. Alternatively, carotenoids may affect plastids indirectly, for example, through physiological stresses caused by lack of xanthophylls, over-production of zeaxanthin, or reduced ABA. The observation that the plastid number is 50% higher than the wild-type in the ABA-deficient mutants flacca and sitiens (Table 4), strongly suggests that the increase in plastid number is connected to ABA deficiency.
Previously published results from Arabidopsis support the hypothesis that ABA deficiency might lead to an increase in plastid number (Rock et al., 1992). The number of chloroplasts per cell in mutants aba1-1 and aba1-3 was 21% higher than in the wild-type, and was 59% higher in mutant aba1-4. The ratio of grana to chloroplast was 48% higher in aba1-1 and aba1-3, and 64% higher in aba1-4 (Rock et al., 1992), similar to our observation in mutant hp3 (Figure 7). ABA is involved in chloroplast division in Physcomitrella patens, as reduction in ABA, either by inhibition of its biosynthesis or in transgenic plants, caused the appearance of macrochloroplasts in protonemal cells (Aragane et al., 2007).
It has been suggested (Cookson et al., 2003) that, in mutants hp1 and hp2, which are perturbed in photomorphogenic signal transduction (Lieberman et al., 2004; Liu et al., 2004; Schroeder et al., 2002), one of the pleiotropic effects of the mutations is on plastid division. The mechanism of this process utilizes polypeptides similar to those involved in the machinery of cell division in bacteria (reviewed by Glynn et al., 2007). FtsZ is a major component of the constriction ring that is formed during plastid division, and MinD is important for positioning of the ring. The 60% increase in hp3 in expression of the gene FtsZ (Figure 8) implicates the higher plastid division activity in hp3 as the mechanism underlying this phenotype. The mRNA and protein levels of FtsZ were correlated to cell division in tobacco BY2 cells (El-Shami et al., 2002). Up-regulation of FtsZ expression was also observed in petals of Tagetes varieties that accumulate higher concentrations of carotenoids, whereas pale-colored varieties did not over-express this gene (Moehs et al., 2001). It is unclear how expression of FtsZ is negatively regulated by ABA. Either ABA directly suppresses FtsZ transcription or it positively regulates a suppressor of FtsZ. In contrast to FtsZ, the MinD mRNA level was unchanged in hp3. A possible explanation is that expression of MinD is not regulated, and the protein exists constitutively at levels that do not limit the rate of plastid division.
Fruit size is determined by two processes that take place during fruit development: rapid cell division that occurs in the first few weeks post-anthesis, and cell expansion that occurs mainly afterwards (reviewed by Gillaspy et al., 1993; Giovannoni, 2001). A reduced fruit size in hp3 was evident throughout all stages of fruit development. As no significant alteration was found in cell size (Table 4), it is concluded that the reduced fruit size was the consequence of inhibition of cell division rather than of cell expansion. Photosynthesis was not significantly altered in mutant hp3-1, despite the radical alteration in xanthophyll composition in leaves (data not shown). Therefore, the pleiotropic effects of hp3 on reduction of yield and biomass in field-grown plants (Table 2) do not result from inefficient light energy absorption, electron transfer or changes in photosynthesis photo-inhibition.
A number of discoveries in recent years have highlighted the connections between carotenoids and physiological processes other than photosynthesis and pigmentation. The link between ABA and carotenoids revealed in mutant hp3 suggests a regulatory circuit involving a carotenoid derivative that influences carotenoid biosynthesis through regulation of cellular processes of plastid division. As exemplified by the newly reported apocarotenoid hormone that controls plant development (Auldridge et al., 2006), a network of interactions between carotenoids and other metabolic pathways may be mediated by carotenoid-derived hormones.
Carotenoids, particularly lycopene, are highly potent anti-oxidants that are desired in crop plants. Use of the hp3 gene to obtain tomato fruit with more lycopene has limitations due to the deleterious pleiotropic effects of ABA deficiency. This negative aspect could be possibly overcome if the hp3 effects on ABA and plastids are confined to fruit only, in a similar strategy used for silencing the gene hp2 (det1) in tomato (Davuluri et al., 2005).
Experimental procedures
Plant material
Tomato (Solanum lycopersicum) cv. M82 seeds were mutated by the chemical mutagen ethyl methane sulfonate (EMS; Menda et al., 2004; http://zamir.sgn.cornell.edu/mutants/). Visual screening of over 200 000 M2 plants identified more than 100 mutants with altered pigmentation of flowers or fruit. Following carotenoid analysis and crosses between mutants with similar phenotypes, we verified 64 single-gene non-allelic mutations that potentially could interfere with carotenoid biosynthesis. Two mutant lines, e1472 and e3452, designated hp3-1 and hp3-2, respectively, were isolated and further studied in this research. An F2 segregating mapping population was created by crossing hp3-1 with the red-fruited wild species S. pimpinellifolium. Phenotypic characterization was verified over three consecutive years in two fields that vary in climate and season of growth.
ABA-deficient mutants flacca (flc; LA 0673) and sitiens (sit; LA 0574) and their parental isogenic line Rheinlands Ruhm (LA 0535) were obtained from the CM Rick Tomato Genetics Resource Centre (University of California, Davis, USA; http://tgrc.ucdavis.edu/index.html).
Pigment extraction and analysis
Fresh samples from leaf, flower, and fruit of approximately 50, 40, and 200 mg, respectively, were harvested and immediately frozen in liquid nitrogen. Carotenoids were extracted as previously described (Ronen et al., 1999). Carotenoid and chlorophyll quantifications were performed according to protocols described by (Lichtenthaler, 1987). Carotenoid composition was analyzed by HPLC using a photodiode array detector, as previously described (Ronen et al., 1999).
DNA extraction and mapping with DNA markers
A sample of approximately 15 mg from young tomato leaves was used for DNA extraction as described previously (Eshed and Zamir, 1995). To analyze restriction fragment length polymorphisms (RFLPs), genomic DNA was digested with the restriction enzyme XbaI, according to protocols previously described (Tanksley et al., 1992), using the marker TG 167.
Nucleic and amino acid sequence analysis
For analysis of the cDNA sequence of the gene Zep, we amplified four overlapping fragments, each of approximately 700 bp, using the following primers: for fragment 1, 5′-CTTTTCTTGAACTGGGGTTC-3′ (forward) and 5′-CCACAGTAACCTTCTCTGGA-3′ (reverse); for fragment 2, 5′-ATACGTTCACTCCAGCAGTGG-3′ (forward) and 5′-ACCTCTTCCCCATCTAAAGG-3′ (reverse); for fragment 3, 5′-CAGATGTGGGGGGAGGCAAG-3′ (forward) and 5′-TCTGCATCAGTAGCACGCTC-3′ (reverse); for fragment 4, 5′-TGACTTGGGAATGCCTCTGA-3′ (forward) and 5′-AATTGCTCAACGCCTGATGT-3′ (reverse). Pfu DNA polymerase (Promega, http://www.promega.com/) was used for PCR using the following conditions: 95°C for 2 min, followed by 35 cycles of 1 min at 95°C, 1 min at 58°C and 2 min at 72°C. PCR products were separated by electrophoresis in a 1.3% agarose gel, and their sequence was determined using an ABI Prism 377 DNA sequencer (Perkin Elmer; http://www.perkinelmer.com). We used Vector NTI suit software for sequence analysis. The nucleotide sequence of Zep cDNA was deposited in GenBank (accession no. EU004202).
Analysis of ABA
Leaf samples were taken at 9 a.m. from 8-week-old plants grown in the greenhouse or field. Frozen tissue was ground with liquid N2 to powder and extracted with 80% methanol. The extracts were partitioned three times against n-hexanes to eliminate lipids and most of the pigments. The pH was acidified to 2.5 with HCl, and samples were partitioned another three times with dichloromethane. The organic phase was then dried under a constant stream of N2. ABA was dissolved with 80% methanol, and the samples were loaded on HPLC (Ronen et al., 1999). Running conditions were 10% methanol in 1% acetic acid for 10 min, followed by a linear gradient to 100% methanol in 10 min, followed by 100% methanol for 10 min. Fractions with the same retention time as the standard were collected and dried under N2 flow. Quantitative determination of ABA was performed immunologically by enzyme immunoassay using the Phytodetek® ABA test kit (Agdia Inc.; http://www.agdia.com) according to the manufacturer’s instructions.
Measurement of ethylene production
Ethylene was measured as described by Lurie et al. (1996). Each measurement was performed done on five fruits placed in a 0.5 l jar. Ten jars containing fruits at the same stages of ripening were measured for hp3-1 and wild-type. Ethylene production rate was determined for 19 days from the end of ‘green’ stages, through the ‘breaker’ stage, to ripe red fruit.
Measuring mRNA by quantitative RT-PCR
Fruit tissue from the stages ‘mature green’ (MG), ‘breaker’ (B), ‘pink’ (P) and ‘mature red’ (R) were harvested and frozen immediately in liquid nitrogen. The developmental stage of each sample was verified by pigment analysis. Total RNA was isolated from approximately 200 mg tissue, using the Tri-Reagent protocol (Molecular Research Center, http://www.mrcgene.com/). Total RNA (1 mg) was reverse-transcribed with oligo(dT)18 primers as previously described (Ronen et al., 1999). Quantitative PCR reactions, consisting of 4.8 μl cDNA template, 1.2 μl of 3 μm primers, and 6 μl Absolute SYBR Green ROX mix (ABgene; http://www.abgene.com), were carried out using a Rotor-Gene 2000 thermocycler (Corbett Research; http://www.corbettlifescience.com). Thermocycling conditions were 95°C for 2 min, followed by 40 cycles of 95°C for 15 sec, 60°C for 10 sec, 72°C for 15 sec, and fluorescence acquisition for 10 sec. Melting curve analysis and PCR product sequencing verified the reaction specificity. For quantification, calibration curves were run simultaneously with experimental samples, and Ct calculations were performed using Rotor-Gene 5.0 software (Corbett Research). Actin and histone H4 served as internal standards. We used the following primers for PCR amplifications: for Psy1, 5′-AACTTGTTGATGGCCCAAAC-3′ (forward) and 5′-CTGTATCGGACAAAGCACCA-3′ (reverse); for Psy2, 5′-AGTTCTGCTAGTAGATGGCC-3′ (forward) and 5′-GGGCACTAGAGATCTTGCAT-3′ (reverse); for histone H4, 5′-ATGGCCTCAACTTTCATTGG-3′ (forward) and 5′-TCGTTCATGTTGCTTTCAGC-3′ (reverse); for actin, 5′-TTGCTGACCGTATGAGCAAG-3′ (forward) and 5′-GGACAATGGATGGACCAGAC-3′ (reverse); for Ggps, 5′-GTACCTCGCTACCGCTACA-3′ (forward) and 5′-TAATCCCACATTAGGGTTACC-3′ (reverse); for Dxs, 5′-AGCTTCCGGCTGGAAACAAA-3′ (forward) and 5′-CTAGCACAATAGCAGCATCC-3′ (reverse); for FtsZ, 5′-CTGAAGAAGCAGCCGAACAA-3′ (forward) and 5′-ACGCTCATCAACAACAGCAC-3′ (reverse); for MinD, 5′-CTTGCTCCACCAACCTCCAA-3′ (forward) and 5′-GTCTGTATTGGATGTGCAGG-3′ (reverse).
Microscopy
Fresh tomato fruit from the ‘mature green’ (MG), ‘breaker’ (B) and ‘pink’ (P) stages were hand cut. Samples were fixed with 5% glutaraldehyde in 0.1 m cacodylate buffer solution, pH 7.4, overnight at 4°C. After four washes in cacodylate buffer, tissue was post-fixed with cacodylate buffer containing 2% OsO4 and 1.5% potassium ferrycyanide for 2 h at room temperature, dehydrated in a graded ethyl alcohol series followed by propylene oxide, and embedded in Agar 100 resin (Agar Scientific; http://www.agarscientific.com). Polymerization carried out at 60°C for 48 h. For electron microscopy, ultra-thin sections were cut on a LKB III ultratome. Ultra-thin sections were mounted on copper 200 mesh thin bar grids, and stained with uranyl acetate and lead citrate. Images were captured using a Tecnai-12 electron microscope (Philips; http://www.philips.com) equipped with a Megaview II CCD camera and analysis software version 3.0 (Soft Imaging System GmbH).
For light microscopy, semi-thin (3 μm) sections were mounted on slides and stained with 1% methylene blue. Pictures were captured using a digital camera (Nikon Coolpix 950; http://www.nikon.com). The chloroplast number per cell was counted. Chloroplast and cell areas were determined using the analysis software. Six to twelve samples derived from two fruit were used for section preparation.
Acknowledgements
We thank David Kachanovsky for valuable comments on the manuscript and for technical assistance, Ira Ronin for sharing unpublished data, Naomi Feinstein, Yoram Leshem, Dr Naomi Book, Rina Timberg, and Professor Eli Zamski for assistance in microscopy, and Dr Elazar Fallik (Agricultural Research Organization of Israel, Volcani Center, Bet Dagan, Israel) for help in measuring ethylene production. This work was supported by Israel Science Foundation grants numbers 677/01 and 548/05, and by the EU-SOL Project, VI frame program of the European Community. Work in the laboratory of J.H. is carried out under the auspices of the Avron Even-Ari Minerva Center.




