Virus-induced gene silencing in tomato fruit
Summary
Virus-induced gene silencing (VIGS) is a powerful tool for the study of gene function in plants. Here we report that either by syringe-infiltrating the tobacco rattle virus (TRV)-vector into the surface, stem or carpopodium of a tomato fruit attached to the plant or by vacuum-infiltrating into a tomato fruit detached from the plant, TRV can efficiently spread and replicate in the tomato fruit. Although VIGS can be performed in tomato fruit by all of the means mentioned above, the most effective method is to inject the TRV-vector into the carpopodium of young fruit attached to the plant about 10 days after pollination. Several reporter genes related to ethylene responses and fruit ripening, including LeCTR1 and LeEILs genes, were also successfully silenced by this method during fruit development. In addition, we found that the silencing of the LeEIN2 gene results in the suppression of tomato fruit ripening. The results of our study indicate that the application of VIGS techniques by the described methods can be successfully applied to tomato fruit and is a valuable tool for studying functions of the relevant genes during fruit developing.
Introduction
A wide range of genes expressed during fruit ripening has been isolated using classical differential display and DNA microarrays (Aharoni and O'Connell, 2002; Moore et al., 2002; Zegzouti et al., 1999). With the sequencing of expressed sequence tags, the number of ripening-related genes has increased rapidly and there is a pressing need for the large-scale functional identification of genes. Agrobacterium-mediated genetic transformation consisting of downregulation or over-expression has so far been widely used to generate transgenic plants that have allowed us to understand the functions of genes involved in specific ripening pathways (Giovannoni, 2004; Hamilton et al., 1995; Klee, 1993; Moore et al., 2002) or in the biosynthesis or action of the plant hormone ethylene (Giovannoni, 2001; Hamilton et al., 1990; Klee, 1993; Luo et al., 1995). However, the traditional way of studying the functions of various genes has been limited by the complex procedures, laborious screening and relatively poor efficiency of these methods. Moreover, some transgenic manipulations are either lethal or greatly affect the morphology of the plant.
Virus-induced gene silencing (VIGS) is an attractive reverse-genetics tool for the study of gene function in plants (Dinesh-Kumar et al., 2003; Fu et al., 2005; Robertson, 2004). When the virus infects a plant tissue and spreads systemically throughout the tissue, the endogenous gene transcripts, which are homologous to the insert fragment in the viral vector (VIGS-vector), are degraded by post-transcriptional gene silencing (PTGS) (Baulcombe, 2004). PTGS is the normal defense mechanism of plants, which results in the specific degradation of a population of homologous transgenes encoding RNA or virus RNA (Waterhouse et al., 2001). VIGS has been widely used for functional gene characterization in Nicotiana benthamiana (Liu et al., 2004; Ratcliff et al., 2001), Arabidopsis (Turnage et al., 2002), tomato (Liu et al., 2002), barley (Holzberg et al., 2002), pepper (Chung et al., 2004), potato (Brigneti et al., 2004; Faivre-Rampant et al., 2004), legume (Constantin et al., 2004), cassava (Fofana et al., 2004) and petunia (Chen et al., 2004b). In comparison with the stable transformation of plants, the use of virus vectors (VIGS) has the advantage that the time from cloning the gene of interest to phenotype analysis is relatively short. In addition, VIGS allows the analysis of genes possessing a lethal phenotype in T-DNA-tagged knockout mutants (Baulcombe, 1999; Burch-Smith et al., 2004). Many VIGS vectors have been described for studying gene function in plants, such as tobacco rattle virus (TRV) (Ratcliff et al., 2001), potato virus X (Faivre-Rampant et al., 2004) and barley stripe mosaic virus (Holzberg et al., 2002). Within these vectors, TRV is able to spread more vigorously throughout the entire plant, including the meristem tissue, and the overall symptoms of infection are mild compared with other viruses (Ratcliff et al., 2001). The technique of VIGS has been successfully applied in studies within plant organs including leaf (Liu et al., 2002), tuber (Faivre-Rampant et al., 2004), root (Ryu et al., 2004; Valentine et al., 2004) and, more recently, flower (Chen et al., 2004b; Liu et al., 2004). However, little information is available to date as to whether the VIGS technique may be successfully applied to fruit.
In recent studies we have established a highly efficient TRV-based VIGS system in tomato fruit. In the present study, we demonstrate that the VIGS technique can be also successfully applied to identity gene functions in tomato fruit, and is a powerful new tool for corresponding studies of fruit ripening.
Results and discussion
Infecting tomato fruit with the TRV
Current VIGS methods, including Agro drench and leaf infiltration, are typically used to investigate gene function in young seedlings or during seedling development (Faivre-Rampant et al., 2004; Liu et al., 2002).
To test whether the TRV-vector can directly infect the tomato fruit, a mixture of Agrobacterium cultures containing pTRV1 and pTRV2 constructs (TRV) in a 1:1 ratio was syringe-infiltrated into the stem (Figure 1a), the carpopodium (Figure 1b) and the surface (Figure 1c) of a fruit attached to the plant and was vacuum-infiltrated into a fruit detached from the plant. Ten days after infiltration, total RNA (sampled far from the injection/infiltration point) was prepared from the fruit and the first-strand cDNA was generated. This cDNA was used as a template for RT-PCR with TRV-RNA2-specific primers. The TRV-RNA2-specific PCR product was detected only in the TRV-infiltrated fruit by all of the four methods listed above, and was absent in the control fruit (no infiltration) (Figure 1d top row). These results were consistent with the results of PCR–Southern blots with probe derived from TRV-RNA2 (Figure 1d bottom row). The identity of the PCR products was further confirmed by sequencing and the resulting sequence was consistent with the fragment of RNA2 included in the plasmid pYL156 (GenBank accession number AF406991, data not shown). All these results clearly demonstrate that recombinant TRV can efficiently spread and replicate in the tomato fruit, suggesting that TRV-based vector could be used to establish the VIGS system in tomato fruit.

Recombinant TRV infects tomato fruit. Agrobacterium cultures transformed with TRV alone were mixed in a 1:1 ratio and infiltrated on to the stem (a), carpopodium (b) and fruit surface (c) of tomato fruit attached to the plant using a 1 ml needle-less syringe. RT-PCR (top row) and PCR–Southern blot (bottom row) analysis of TRV-RNA2 accumulation in tomato fruit infiltrated with TRV (d). Total RNA, isolated from the attached tomato fruit infiltrated with TRV through the stem (lane 3), carpopodium (lane 4) and fruit surface (lane 5) and from the vacuum-infiltrated detached fruit with TRV (lane 6), was used to generate first-strand cDNA. This first-strand cDNA was used in a PCR reaction using TRV-RNA2-specific primers. Lane 1 represents the control in which RT reaction mix with the pTRV2 plasmid was used as a template in reaction. Lane 2 represents the negative control in which RT reaction mix with the cDNA from no-infiltration tomato fruit was used as a template in reaction. PCR blots were hybridized with a probe derived from RNA2 (bases 917 ± 1343). Arrows indicate the infiltrated site.
Silencing of the LeACS2 gene in tomato fruit detached from the plant by vacuum infiltration with TRV-LeACS2
To test whether the TRV-vector can be used to silence a gene expressed in tomato fruit, we targeted the LeACS2 gene in the ethylene biosynthesis pathway. Ethylene plays an important role in the regulation of tomato fruit ripening (Alexander et al., 2002b; Fu and Li, 2002; Jiang and Fu, 2000; Schuch et al., 1989). It has been proposed that the LeACS2 gene encodes a key speed-restricting enzyme of ethylene synthesis in tomato fruit (Alexander and Grierson, 2002a; Picton et al., 1993) and antisense suppression of the LeACS2 gene in transgenic tomato fruit results in strong inhibition of ethylene production and fruit ripening (Alexander and Grierson, 2002a; Luo et al., 2000). Therefore, the LeACS2 gene is an ideal choice as a reporter gene for the successful application of VIGS in tomato fruit.
In order to silence the LeACS2 gene in tomato fruit, the vector of pTRV2-LeACS2 was generated by inserting a 707-bp fragment of the LeACS2 gene into pTRV2. A mixture of Agrobacterium cultures containing pTRV1 and pTRV2-LeACS2 constructs (TRV-LeACS2) in a 1:1 ratio was vacuum-infiltrated into fruit detached from the plant (mature green, i.e. the LeACS2 gene starts expressing at this stage). About 10 days after infiltration, the TRV-LeACS2 infiltrated fruit developed a yellow color phenotype similar to LeACS2 antisense tomato fruit and remained yellow for at least 1 month. Meanwhile, the control fruit infiltrated with TRV alone completely turned red like the fruit of wild type (Figure 2a).
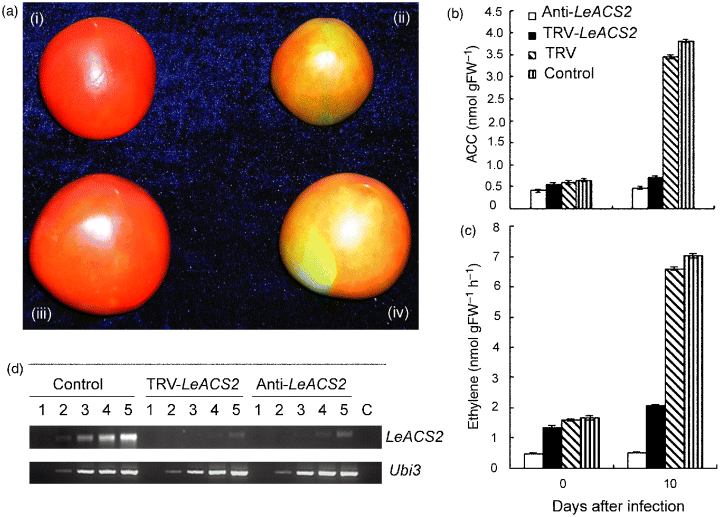
Silencing of the LeACS2 gene in tomato fruit detached from the plant by vacuum infiltration. No infiltration (a i) and antisense-LeACS2 tomato fruit (a ii) were used as the control. The detached tomato fruit in mature green were vacuum infiltrated with Agrobacterium transformed with TRV alone (a iii), and with TRV carrying a fragment of LeACS2 (TRV-LeACS2) (a iv). Silencing of the LeACS2 gene led to the decrease of the ACC content (b) and of ethylene production (c). Mean ± SE (n = 3). Columns with the same letter are not significantly different (P < 0.05), according to Tukey's multiple range. RT-PCR analysis shows the effect of VIGS on LeACS2 transcription in the silenced tomato fruit (d). Total RNA was isolated from silenced and control fruit. The abundance of LeACS2 transcripts was evaluated using 21, 24, 27, 30 and 35 cycles of RT-PCR with LeACS2-specific primers. Ubi3 was used as the internal control. Lane C represents the control in which the reverse transcriptase-free RT reaction mix was used as a template in the reaction (35 cycles).
As shown in Figure 2(b,c), the content of 1-aminocyclopropane-1-carboxylic acid (ACC, the precursor compound of ethylene biosynthesis) and the rate of ethylene production in the LeACS2-silenced fruit was significantly lower than those observed in the control fruit (infiltrated with TRV alone). About 65% of the vacuum-infiltrated fruit (total 100 pieces) were discarded because of decay, but symptoms of the gene silenced were observed in all the fruit (35 pieces) during infiltration.
To confirm the LeACS2 suppression at the molecular level, we performed semiquantitative RT-PCR. The primers that anneal to the outside region of the LeACS2 gene targeted for silencing were used. As shown in Figure 2(d), the LeACS2 message in the TRV-LeACS2-infiltrated fruit was reduced by more than 73% when compared with the control fruit (infiltrated with TRV alone). In contrast, the level of Ubi3 gene (encoding for ubiquitin) mRNA was similar in LeACS2-silenced and control fruit and served as an internal control for RNA quality and RT-PCR amplification.
From these data, we conclude that the LeACS2 gene in the tomato fruit was effectively silenced by vacuum infiltration with Agrobacterium cultures containing pTRV1 and pTRV2-LeACS2 constructs. However, because of not being in contact with the plant, fruit detached from the plant are no longer able to obtain their nutrients from it, and thus they easily rot after infiltration, maybe more in the case of young fruit.
Silencing the LeCTR1 gene in tomato fruit attached to the plant by syringe injection with TRV-LeCTR1
In order to overcome the limitations of the vacuum infiltration method, we also tried to introduce the VIGS system in tomato fruit attached to the plant. It has been demonstrated that the LeCTR1 is a negative regulator of the ethylene signaling pathway in tomato (Leclercq et al., 2002; Zegzouti et al., 1999). The high expression level of the LeCTR1 gene during ripening may indeed prevent ripening (Leclercq et al., 2002). From these data, we deduced that the suppression of the LeCTR1 gene would promote the ripening of tomato fruit. We thus determined that the LeCTR1 gene could be also used as an effective reporter gene for application of VIGS in tomato fruit attached to the plant.
To introduce VIGS in tomato fruit attached to the plant, a 1346-bp fragment of LeCTR1 was inserted into pTRV2 (pTRV2-LeCTR1). A mixture of Agrobacterium cultures containing pTRV2-LeCTR1 and pTRV1 constructs (TRV-LeCTR1) was syringe injected into the fruit (about 2 weeks after pollination) through the peel or injected into the carpopodium of young fruit about 10 days after pollination.
As shown in Figure 3, in the surface-infiltrated fruit, the infiltrated sector turned red, while the other sectors remained green in the 10 days after TRV-LeCTR1 infiltration (Figure 3a bottom). In the respective control fruit the infiltrated sector remained green (Figure 3a top). In the carpopodium-infiltrated fruit, a strong ripening phenotype (red color) was observed on the upper sector near the carpopodium of the TRV-LeCTR1-infiltrated fruit (Figure 3b right), whereas the respective control tomato fruit (infiltrated with TRV alone) remained green in the 3–4 weeks after infiltration (Figure 3b left). Colorwise, the TRV-LeCTR1-infiltrated fruit were half red and half green (Figure 3d,f), whereas non-infiltrated fruit had a uniform pink color on 100% of their surface (Figure 3c). Interestingly, we observed that the red color, like a signal, could diffuse from the upper to the lower sector on the surface during the development of the TRV-LeCTR1 fruit (Figure 3d–f). About 75% of the TRV-LeCTR1-infiltrated fruit (40 pieces) exhibited the promotively ripening phenotype.
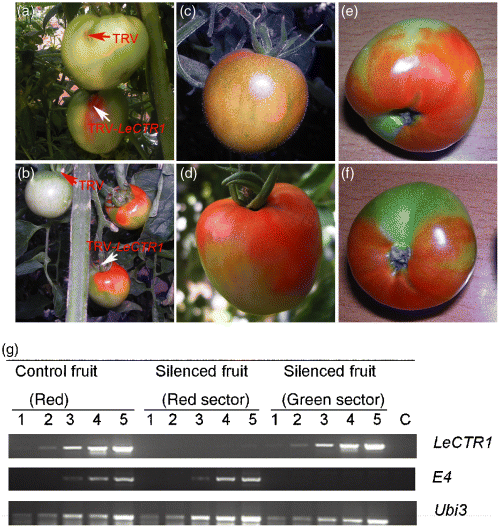
TRV-mediated VIGS of the LeCTR1 gene in tomato fruit attached to the plant. Tomato fruit attached to the plant were infiltrated with Agrobacterium containing TRV alone (a top), (b left) or TRV carrying a fragment of LeCTR1 (a bottom), (b right) from the fruit surface (a) or from the carpopodium (b). No-infiltration tomato fruit were used as the control (c). Phenotypes of LeCTR1-silenced tomato fruit infiltrated from the carpopodium (d–f). Photographs of the tomato fruit were taken 2–4 weeks after infiltration, respectively. RT-PCR analysis of the LeCTR1 and E4 genes in the LeCTR-silenced tomato fruit (g). The first-strand cDNA was generated from total RNA isolated from the LeCTR1-silenced and the control fruit (red) and was used in a PCR reaction using LeCTR1 and E4 gene-specific primers. Lanes 1–5 correspond to products from PCR cycle numbers 21, 24, 27, 30 and 35. Lane C represents the control in which the RT reaction mix without reverse transcriptase was used as a template (35 cycles). Arrows indicate the infiltrated site.
To confirm VIGS of LeCTR1 at the molecular level, semiquantitative RT-PCR analysis was performed using LeCTR1-specific primers. For the LeCTR1-silenced fruit, the LeCTR1 mRNA levels in the red tissue was only 3% of that of the control fruit (entirely red), however, LeCTR1 mRNA levels in the green tissue was similar to that of control fruit (entirely red) (Figure 3g).
The phenotype of the LeCTR1-silenced fruit showed that the suppression of the LeCTR1 gene promotes the ripening of tomato fruit, which once more demonstrated that LeCTR1 acts as a negative regulator in the ethylene signaling pathway. Interestingly, we found that the carpopodium-infiltrated silenced tomato fruit caused a more severe phenotype compared with that from surface-infiltrated fruit. For example, the silencing signal (red color) could move rapidly and freely, like a cloud, from the upper to the lower sector of carpopodium-infiltrated fruit (Figure 3d,f). However, for the surface-infiltrated silenced tomato fruit, red color occupied only a small sector around the infiltrated site (Figure 3a bottom). In addition, carpopodium-infiltration avoids scarring the fruit surface. Consequently, we decided to apply carpopodium infiltration for VIGS in tomato fruit.
Silencing the LeEILs genes in tomato fruit attached to the plant by syringe injection with TRV-LeEILs
The LeEILs genes as a family act as positive regulators of the ethylene signaling pathway in tomato (Tieman et al., 2001). Reduced expression of these genes in transgenic tomato resulted in an ethylene-insensitive phenotype for all responses including leaf epinasty, flower abscission and senescence, and fruit ripening (Chen et al., 2004a; Tieman et al., 2001). To further confirm that the infiltration from carpopodium is suitable for carrying VIGS in tomato fruit attached to the plant, we used the LeEILs genes as indicators of gene silencing.
In order to silence the LeEILs gene family in tomato fruit, a 478-bp conserved fragment of LeEILs was cloned into pTRV2 (pTRV2-LeEILs). A mixture of Agrobacterium cultures containing pTRV2-LeEILs and pTRV1 constructs (TRV-LeEILs) was syringe infiltrated into the carpopodium of young fruit about 10 days after pollination. As shown in Figure 4, 5–6 weeks after carpopodium infiltration, the TRV-LeEILs-infiltrated fruit acquired a half red and half green sector (Figure 4b), while the control fruit turned red and became softer (Figure 4a). Upon cutting the fruit we found that a large part of the LeEILs-silenced fruit pulp had remained green and seedless (Figure 4d right), whereas the control fruit pulp had softened and contained many seeds (Figure 4d left). Interestingly, the border between the red and the green part was very clear in the LeEILs-silenced fruit, while at the same time we observed that the green color can diffuse like a cloud from the upper to the lower sector of the LeEILs-silenced fruit (Figure 4c). The control fruit (infiltration with TRV alone) however, had a 100% red-and-pink-colored surface (Figure 4a). About 80% of the TRV-LeEILs-infiltrated fruit (20 pieces) exhibited the ripening suppression phenotype. Meanwhile, for the LeEILs-silenced fruit, the LeEILs mRNA levels in the green tissue was only 10% of that of the TRV-infiltrated control fruit (entirely green), however, LeEILs mRNA levels in the red tissue was similar to that in control (Figure 4e). The results of LeEILs-infiltrated fruit further proved that infiltration of the virus vector into the carpopodium of young fruit is an effective method for introducing VIGS in tomato fruit.
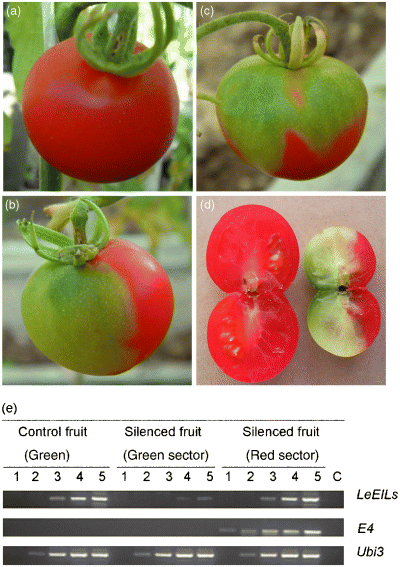
TRV-mediated VIGS of the LeEILs gene in tomato fruit attached to the plant. Tomato fruit attached to the plant were infiltrated with Agrobacterium containing TRV alone (a), or TRV carrying a fragment of LeEILs (b–d) from the carpopodium. Photographs of the tomato fruit were taken 5–6 weeks after infiltration, respectively. RT-PCR analysis of the LeEILs and E4 genes in the LeEILs-silenced tomato fruit (e). The total RNA, isolated from the LeEILs-silenced and control fruit, were used to generated the first-strand cDNA, the first-strand cDNA was used in a PCR reaction using LeEILs and E4 gene-specific primers. Lanes 1–5 correspond to products from PCR cycle numbers 21, 24, 27, 30 and 35. Lane C represents the control in which the RT reaction mix without reverse transcriptase was used as a template (35 cycles).
In the present report we showed that VIGS of LeEILs genes could inhibit fruit ripening (Figure 4b). These results further support the view that expression of LeEILs genes is necessary for tomato fruit ripening. Our results also suggest that VIGS could co-suppress several members of a gene family and overcome possible functional redundancy by choosing regions that are conserved between genes.
Expressions of ripening-related gene E4 in fruit of the LeCTR1 gene or the LeEIls gene being silenced
Expression of the E4 gene is positively correlated with ripening of tomato fruit (Xu et al., 1996). Using semiquantitative RT-PCR assay, expression of the E4 gene was significantly enhanced in fruit where the LeCTR1 gene was being silenced and correspondingly suppressed in fruit where the LeEIls genes were being silenced (3, 4), These results are consistent with the changes observed in the phenotypes of the fruit and vindicate the suitability of the VIGS method for the functional study of genes involved in the ripening of tomato fruit.
Suppressing fruit ripening by silencing of the LeEIN2 gene
The EIN2 gene has been considered to play an essential role in plant responses to ethylene (Bleecker and Kende, 2000; Guo and Ecker, 2004). To date, the full sequence of the EIN2 gene has been cloned from Arabidopsis (Alonso et al., 1999), rice (Jun et al., 2004) and petunia (Shibuya et al., 2004). A partial sequence of the LeEIN2 gene, which is homologous to the EIN2 gene in other plants, was also reported for tomato (GenBank accession number AY566238); however, the exact function of LeEIN2 in ripening of fruit has not been properly determined.
In order to learn more about the function of the LeEIN2 gene during tomato fruit ripening, we cloned a fragment of the LeEIN2 gene from tomato and constructed the pTRV2-LeEIN2 vector. A mixture of Agrobacterium cultures containing pTRV2-LeEIN2 and pTRV1 constructs (TRV-LeEIN2) was syringe injected into the carpopodium of young tomato fruit about 10 days after pollination. As shown in Figure 5, as the fruit developed and ripened, we observed that the silenced sector of the LeEIN2-silenced fruit remained green and showed no ripening because of the suppression of the LeEIN2 gene, while the other sector turned red (Figure 5b). The control fruit turned red and softened (Figure 5a).
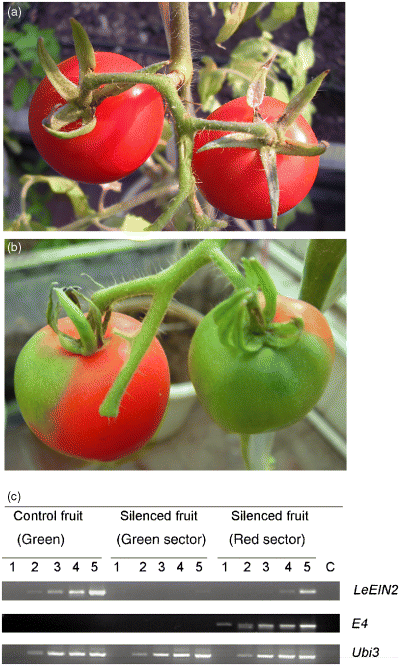
TRV-mediated VIGS of the LeEIN2 gene in tomato fruit attached to the plant. Tomato fruit attached to the plant were infiltrated with Agrobacterium containing TRV alone (a), or TRV carrying a fragment of LeEIN2 (b) from the carpopodium. Silencing of the LeEIN2 gene suppressed fruit ripening (b). Photographs of the tomato fruit were taken 5–6 weeks after infiltration, respectively. RT-PCR analysis of the LeEIN2 and E4 genes in the LeEIN2-silenced tomato fruit (c). The total RNA, isolated from the LeEIN2-silenced and control fruit, were used to generated the first-strand cDNA, the first-strand cDNA was used in a PCR reaction using LeEIN2 and E4 gene-specific primers. Lanes 1–5 correspond to products from PCR cycle numbers 21, 24, 27, 30 and 35. Lane C represents the control in which the RT reaction mix without reverse transcriptase was used as a template (35 cycles).
To confirm VIGS of LeEIN2 at the molecular level, semiquantitative RT-PCR analysis was performed using LeEIN2-specific primers. The PCR primers corresponding to regions of the mRNA of LeEIN2 that was absent from the viral vector were used. In LeEIN2-silenced tomato fruit, the level of LeEIN2 mRNA in green tissue was only 6% of that of the control fruit (entirely green); however, the level of LeEIN2 mRNA in the red tissue was similar to that in the control fruit (entirely green). Moreover, the E4 gene was poorly expressed in the green tissue but highly expressed in the red tissue of the LeEIN2-silenced fruit (Figure 5c). These results indicate that the LeEIN2 gene might play a critical role in regulating tomato fruit ripening.
Conclusions
In the present report, we showed that TRV-vector with the target gene could be efficiently infiltrated into tomato fruit detached from the plant by vacuum infiltration, or by syringe injection into the fruit attached to the plant through the fruit peel, the carpopodium or stem. Several genes related to the ripening of tomato fruit have been successfully silenced by this method. Interestingly, we also found that infiltration with the TRV-vector did not transfer from one fruit to another or to other parts of the plant. This characteristic may therefore allow the silencing of different genes in different fruits on the same plant thus permitting the comparison of the function of various genes under identical conditions. The results clearly demonstrate that the application of VIGS holds great potential in studying fruit development and ripening, and can be an alternative to transgenic technology.
Experimental procedures
Plant material and growth conditions
Tomato plants (Lycopersicon esculentum cv. Lichun and cv. Ailsa Craig) were used for the experiments. Seeds were germinated in flats with a soil-less potting mixture. Three-week-old plants were moved to the greenhouse. Greenhouse conditions were kept at 25/20°C and 70% humidity under a 14/10-h light/dark regime.
Plasmid construction
The pTRV1 and pTRV2 VIGS vectors (described in Liu et al., 2002) were kindly offered by Dr Dinesh-Kumar, Yale University.
pTRV2-LeACS2 construction. A 707-bp fragment of the LeACS2 gene corresponding to bases 83–790 of the LeACS2 gene (GenBank accession number X59145) was PCR-amplified from tomato cDNA sources using primers (forward: 5′-CGA GCT CGT CAC CGA TGA CAC GAC-3′ with an XbaI restriction site and reverse: 5′-GCT CTA GAG CGC AAT GAC GGC AGA AT-3′ with an SacI restriction site). The resulting product was cloned into pTRV2 to form pTRV2-LeACS2.
pTRV2-LeCTR1 construction. A 1346-bp fragment of the LeCTR1 gene (GenBank accession number AF096250) gene was PCR-amplified from tomato cDNA using Taq DNA polymerase and the primers (forward: 5′-CGA GCT CGC GAA ATC TAC GCA GCC ACT-3′ and reverse: 5′-GCT CTA GAG CGT CAA TAC ATA CGC TAA CAA-3′). The resulting PCR product was cloned into XbaI–SacI-cut pTRV2.
pTRV2-LeEILs construction. A 478-bp fragment of the LeEILs gene (GenBank accession number AF328786) was PCR-amplified from tomato cDNA sources using primers (forward: 5′-GTG GAG GGA TCG AATG-3′ and reverse: 5′-TAC CGC CGT CAG AACA-3′). The resulting product was cloned into pGEM-T-easy (Promega, Madison, WI, USA) to form pGLeEILs for sequencing and then the LeEILs cDNA fragment was isolated from the pGLeEILs using EcoRI digestion and subcloned into pTRV2 to generate pTRV2-LeEILs.
pTRV2-LeEIN2 construction. A 568-bp fragment of the LeEIN2 gene (GenBank accession number AY566238) was PCR-amplified from tomato cDNA sources using primers (forward: 5′-TGG AAA TGT CCC TGTA-3′ and reverse: 5′-CCC ATC ATC TTG CCTA-3′). The resulting product was cloned into pGEM-T easy (Promega) to form pGLeEIN2 for sequencing and then the LeEIN2 cDNA fragment was isolated from pGLeEIN2 using EcoRI digestion, and subcloned into pTRV2 to generate pTRV2-LeEIN2.
Agro-infiltration and vacuum infiltration
Plant infiltration were performed as described in Ratcliff et al. (2001), with the modification used in tomato fruit. The Agrobacterium strain GV3101 containing pTRV1 or pTRV2 and its derivatives were used for VIGS experiments. The Agrobacterium strain GV3101 containing TRV-VIGS vectors was grown at 28°C in LB medium containing 10 mm MES and 20 mm acetosyringone with appropriate antibiotics. After 24 h, Agrobacterium cells were harvested and resuspended in the Agrobacterium infiltration buffer (10 mm MgCl2, 10 mm MES, pH 5.6, 150 mm acetosyringone) to a final OD 600 of 1.0 (for both pTRV1 or pTRV2 and its derivatives) and shaken for 4–6 h at room temperature before infiltration.
For tomato fruit infiltration, each Agrobacterium strain containing pTRV1 and pTRV2 or its derivative vectors were mixed in a 1:1 ratio and infiltrated into the carpopodium of tomato fruit attached to the plant in about 10 days after pollination with a 1-ml needle-less syringe. For vacuum infiltration in tomato fruit detached from the plant, we selected the green tomato as experiment material. The vacuum infiltration procedure was as follows: the tomato fruit were harvested at mature green and four fruits for each construct were collected and put into the bacterial suspension and then infiltrated by vacuum at 30 mmHg for 30 sec. After the release of the vacuum, the surface of the fruit was washed with tap water and kept for more than 10 days in a plastic tray at 18°C. Tomato fruit infiltrated with TRV alone was used as the control.
RNA isolation, Southern blot and RT-PCR analysis
To detect the accumulation of virus in tomato fruit infiltrated with TRV, 10 days after infiltration, total RNA was extracted from the sector (far from the infiltrated site) of tomato fruit with TRV and also from the control fruit using the protocol described by Gregory et al. (1988) and treated with RNase-free DNase (Promega). The first-strand cDNA was synthesized using 1 mg of total RNA, random primers and superscript reverse transcriptase (Promega). The RNA2 of TRV was amplified by RT-PCR with the RNA2-specific primers (GenBank accession number AF406991). The primers were as follows: forward: 5′-CGG TCT AGA GGC ACT CAA CTT TAT AAA CC-3′ and reverse: 5′-CGG GGA TCC CTT CAG TTT TCT GTC AAA CC-3′. For PCR–Southern blotting, the PCR products of RNA2 were hybridized with a probe derived from the TRV RNA2 PCR fragment (bases 917 ± 1343) according to the manufacturer's manuals and treated with RNase-free DNase (RQ1; Promega).
To detect the silencing of these reporter genes in tomato fruit, the first-strand cDNA was synthesized using 1 mg of total RNA, oligo (dT) primer and superscript reverse transcriptase (Promega). Semiquantitative RT-PCR was performed as described by Liu et al. (2002). For RT-PCR, primers that anneal outside the region targeted for silencing were used to ensure that only the endogenous gene would be tested. The Ubi3 gene severed as an internal control for RNA quantity in RT-PCR. For a negative control, RT reaction mix without reverse transcriptase was used as a template in the reaction. The intensities of PCR-generated fragments were analyzed and quantified using Gel Doc 2000 and Quantity One Version 4.3 (Bio-Rad, Hercules, CA, USA).
Ethylene measurements
Ethylene production of the tomato fruit was measured at 10 days after infiltration as described in Jiang et al. (1994). The samples (antisense LeACS2 and wild-type tomato fruit as the control) were detached and enclosed in airtight vials and incubated at 25°C for 60 min, after which 1 ml of the headspace was withdrawn. Ethylene concentration in the gas sample was measured by ATI UNICAM 610 series gas chromatograph (Unicam Analytical, Cambridge, UK). Temperatures were as follows: oven/column, 60°C; injector, 120°C; detector, 160°C. The chromatograph was equipped with an alumina column at 100°C and measured using a flame ionization detector at 120°C.
Determination of ACC contents
Determination of ACC contents was performed as described in Jiang et al. (1994). Tomato fruit material was ground in liquid nitrogen and was extracted according to Langebartels et al. (1991). ACC and total ACC following acid hydrolysis (2 n HCl for 3 h at 120°C) were determined as described in Langebartels et al. (1991). The amount of conjugated ACC was calculated by subtracting the amount of ACC from that of the total ACC.
Acknowledgements
We would like to thank Dr S.P. Dinesh-Kumar (Yale University) for offering pTRV1 and pTRV2 vector and Dr Zhengguo Li (Chongqing University of China), Dr Jean-Claude Pech (ENSAT Pole de Biotechnologie Vegetale), and Dr Angelos Kanellis (Laboratory of Pharmacognosy, Department of Pharmaceutical Sciences, Aristotle University of Thessaloniki) for helpful comments on the manuscript. This work was supported by grants (no. 30270934, no. 30371004 and no. 30430940) of the National Nature Science Foundation of China.




