RcsB-BglJ activates the Escherichia coli leuO gene, encoding an H-NS antagonist and pleiotropic regulator of virulence determinants
Summary
The LysR-type transcription factor LeuO is involved in regulation of pathogenicity determinants and stress responses in Enterobacteriaceae, and acts as antagonist of the global repressor H-NS. Expression of the leuO gene is repressed by H-NS, and it is upregulated in stationary phase and under amino acid starvation conditions. Here, we show that the heterodimer of the FixJ/NarL-type transcription regulators RcsB and BglJ strongly activates expression of leuO and that RcsB-BglJ regulates additional loci. Activation of leuO by RcsB-BglJ is independent of the Rcs phosphorelay system. RcsB-BglJ binds to the leuO promoter region and activates one of two leuO promoters mapped in vivo. Moreover, LeuO antagonizes activation of leuO by RcsB-BglJ and acts as negative autoregulator in vivo and in vitro. Further, the H-NS paralogue StpA causes repression of leuO in addition to H-NS. Together, our data suggest a complex arrangement of regulatory elements and they indicate a feedback control mechanism of leuO expression.
Introduction
LeuO is a LysR-type transcription factor and a master regulator of multiple loci including genes related to stress response and pathogenicity of Enterobacteriaceae. LeuO has been studied mostly in Escherichia coli and Salmonella enterica, but also in other bacteria including Yersinia enterocolitica, and Vibrio cholerae (Moorthy and Watnick, 2005; Rodriguez-Morales et al., 2006; Lawrenz and Miller, 2007). Among other targets, LeuO acts as a transcriptional activator of the bgl (β-glucoside) operon (Ueguchi et al., 1998), of outer membrane porins ompS1 and ompS2 (Flores-Valdez et al., 2003; Fernandez-Mora et al., 2004; Rodriguez-Morales et al., 2006), the yjjQ-bglJ operon (Stratmann et al., 2008), and the CRISPR-associated cas operon (Hernandez-Lucas et al., 2008; Westra et al., 2010; Medina-Aparicio et al., 2011), while LeuO represses transcription of the acid stress regulator CadC (Shi and Bennett, 1995) and the small RNA dsrA (Repoila and Gottesman, 2001). Many of the target genes regulated by LeuO are repressed by the pleiotropic H-NS (heat-stable nucleoid structuring) protein, and thus LeuO emerged as important H-NS antagonist and global regulator (Hernandez-Lucas et al., 2008; Shimada et al., 2009; 2011). H-NS is an abundant nucleoid-associated protein (NAP) forming extended complexes with DNA that are important in nucleoid structuring and that confer transcriptional repression of multiple loci (Dillon and Dorman, 2010; Liu et al., 2010; Wang et al., 2011). Activation of H-NS repressed loci by LeuO is presumably achieved by competition of LeuO with H-NS for DNA binding (De la Cruz et al., 2007; Shimada et al., 2011). It has also been shown that LeuO when binding in between an H-NS bound fragment and a promoter can delimit repression by H-NS (Chen et al., 2003; Chen and Wu, 2005).
Remarkably, expression of the leuO gene is itself repressed by H-NS under standard laboratory growth conditions (Klauck et al., 1997; Chen et al., 2001). As LeuO is an H-NS antagonist with pleiotropic function, activation of leuO expression should occur in response to specific environmental cues. Indeed, moderately increased expression of leuO has been detected upon amino acid starvation and in the stationary growth phase (Fang et al., 2000; Majumder et al., 2001; Shimada et al., 2011). The promoter and regulatory region of leuO gene is highly AT-rich (Haughn et al., 1986), typical of H-NS repressed loci (Navarre et al., 2007), and H-NS and LeuO binding sites have been mapped to this region (Chen et al., 2001; 2003; 2005; Chen and Wu, 2005).
The leuO gene is located in between the leu and ilvIH operons encoding enzymes for branched-chain amino acid synthesis. This leu-leuO-ilvIH gene cluster is a paradigm for the role of transcription-induced local changes of DNA supercoiling in promoter regulation. Initially, the leu-500 promoter mutant of S. enterica serovar Typhimurium that carries a point mutation rendering the promoter of the leu operon inactive was found to be suppressed in topA (topoisomerase I) mutants (Graf and Burns, 1973; Margolin et al., 1985). It was then shown that the mutant leu-500 promoter is supercoiling sensitive and responds to transcription induced local changes of DNA supercoiling as tested with plasmids (Pruss and Drlica, 1985; Richardson et al., 1988; Lilley and Higgins, 1991; Chen et al., 1992; 1994; Tan et al., 1994). Further it was shown using plasmids carrying the natural context of the leu-leuO-ilvIH gene cluster that activation of the leu-500 promoter (and also the wild-type leu promoter) depends on transcription of the divergent ilvIH promoter and the leuO gene both in S. Typhimurium and in E. coli. This suggested that topological coupling operates over 1.9 kb in the chromosome (Wu et al., 1995; Fang and Wu, 1998a,b). Taken together with the finding that expression of leuO and ilvIH in the late stationary phase correlated, these findings lead to the proposal of a supercoiling relay mechanism where activation of ilvIH results in activation of leuO and finally the leu promoter (Fang and Wu, 1998a,b; Wu and Fang, 2003). The reported effect of leuO on leu operon expression is 2- to 4-fold (Hertzberg et al., 1980; Fang and Wu, 1998a; El Hanafi and Bossi, 2000). This positive effect of leuO on the leu operon may be caused by local topological coupling of transcription (Fang and Wu, 1998a; Wu and Fang, 2003). Furthermore, the capability of LeuO to delimit H-NS spreading was taking as indication that LeuO acts as a positive autoregulator in the natural context (Chen and Wu, 2005).
Independent of this interesting case of regulation of the leu-leuO-ilvIH gene cluster by DNA topology some ambiguity about the importance of LeuO in regulation of the divergent leu operon originates in the nomenclature. Initially, leuO was used to describe a mutation mapping in cis to the leu operon that rendered its expression constitutive, assuming that regulation involves an operator (Calvo et al., 1969; Hertzberg et al., 1980). Later regulation of the leu operon by attenuation was shown (Wessler and Calvo, 1981) and the designation ‘leuO’ was then used for a gene of unknown function located next to the leu operon (Henikoff et al., 1988).
RcsB and BglJ are transcription factors of the FixJ/NarL family, characterized by a conserved LuxR-type helix–turn–helix (HTH) motif in the C-terminal DNA binding domain (Henikoff et al., 1990; Gao et al., 2007). RcsB is the response regulator of the Rcs (regulation of capsule synthesis) two-component phosphorelay system (TCS), sensing perturbations of the outer membrane and the peptidoglycan layer (Majdalani and Gottesman, 2005). RcsB is a unique bacterial response regulator in that it acts as homodimer but also as heterodimer with RcsA (regulation of capsule synthesis), GadE (regulation of acid stress response) and BglJ (activation of the bgl operon) respectively (Majdalani and Gottesman, 2005; Castanie-Cornet et al., 2010; Venkatesh et al., 2010). These three proteins likewise belong to the FixJ/NarL family of transcription factors (Henikoff et al., 1990; Giel et al., 1996). The activity of the RcsB-RcsB homodimer and the RcsB-RcsA heterodimer depends on phosphorylation of RcsB (Majdalani and Gottesman, 2005). However, the RcsB-BglJ heterodimer activates transcription of the so far only known target locus, the bgl operon, independently of RcsB phosphorylation (Venkatesh et al., 2010). Interestingly, the three genes rcsA, gadE and bglJ, respectively, encoding the three RcsB heterodimerization partners are all repressed by H-NS (Majdalani and Gottesman, 2005; Stratmann et al., 2008; Sayed and Foster, 2009). Furthermore, LeuO counteracts H-NS mediated repression of the yjjQ-bglJ operon which encodes BglJ (Stratmann et al., 2008).
Here we show that the RcsB-BglJ heterodimer strongly activates transcription of leuO and also of other target loci in E. coli. Activation of leuO is independent of phosphorylation of the RcsB response regulator. We also demonstrate that the H-NS paralogue StpA represses leuO partially and that the LeuO protein acts as an autorepressor in vivo and in vitro. Promoter mapping revealed that two H-NS and StpA repressed promoters direct expression of the leuO gene and that one of these promoters is activated by RcsB-BglJ. Further, our data suggest that LeuO and RcsB-BglJ act antagonistically in regulation of leuO.
Results
Transcription of leuO is activated by RcsB-BglJ
The heterodimer RcsB-BglJ activates the bgl operon, its only known regulatory target to date. To identify novel target loci of RcsB-BglJ, we performed a DNA microarray analysis. For this we expressed BglJ from low-copy vector pKETS1 in E. coli strain T75 carrying a deletion of the yjjP-yjjQ-bglJ locus (termed ΔyjjPQ-bglJ in the following, Table 1) and compared expression levels to T75 harbouring empty vector pKESK22 as control. The microarray data revealed that leuO was one of the genes most strongly affected by BglJ expression, showing a 45-fold upregulation (Tables 2 and S1). Therefore, we repeated the microarray analysis and overexpressed BglJ in ΔleuO strain T177 to exclude indirect targets. Loci which were significantly (> 4-fold, P < 0.05) activated by BglJ include the known target bgl, and as novel targets chiA (periplamic endochitinase), the RhsA, RhsB and RhsC elements (encoding hydrophilic proteins with repetitive sequence elements), setA (sugar efflux system), as well as several genes of predicted function in the inner or outer membrane (Tables 2 and S1). In addition, microarray analysis in ΔrcsB strain T175 revealed that activation of leuO by BglJ and of all other target genes was completely dependent on RcsB (Tables 2 and S1). This suggests that heterodimerization of BglJ and RcsB is essential for activation of gene expression. BglJ caused downregulation of only one locus, csrB, encoding the CsrA regulating RNA CsrB. We further found that some of the known targets of LeuO were activated by RcsB-BglJ in the leuO+ strain T75 but not in its ΔleuO derivative (T177) which was analysed in parallel (Tables 2, S1 and S2). These LeuO targets include the CRISPR-associated casA gene, elfA (encoding a fimbrial-like adhesion protein) as well as the RhsE cluster. However, most of the LeuO regulated loci (see Table S2 for LeuO microarray results) were not affected by BglJ expression. Interestingly, only two loci, bgl and chiA, are activated by RcsB-BglJ as well as by LeuO independently of each other (Table 2).
| Strain | Relevant genotype | Constructiona/reference |
|---|---|---|
| MG1655 | K12 wild-type strain (CGSC #6300) | Guyer et al. (1981) |
| BW30270 | MG1655 rph+ | CGSC #7925 |
| S3974 | BW30270 ilvG+ | Venkatesh et al. (2010) |
| S4197 | S3974 ΔlacZ-Y217 | Venkatesh et al. (2010) |
| S159 | M182 stpA::TetR | Zhang et al. (1996) |
| S1734 | yjjQ/bglJ-Y6::miniTn10-cat (= bglJC) | Madhusudan et al. (2005) |
| S3010 | CSH50 ΔlacZ-Y217 Δbgl-AC11 ΔhnsKD4-Kan | Nagarajavel et al. (2007) |
| S3754 | MG1655 ΔhnsKD4-Kan | × T4GT7 (S3010) |
| T21 | S4197 ΔrcsBFRT (NC_000913: 2314199–2314846) | × PCR S819/S820 (pKD3) × pCP20 |
| T23 | S4197 ΔyjjPQ-bglJFRT (NC_000913: 4600115–4602857) | × PCR S676/S783 (pKD3) × pCP20 |
| T28 | S4197 attB::(SpecR PleuO lacZ) | × pKES200 |
| T30 | S4197 attB::(SpecR PleuO lacZ) ΔrcsBFRT | T21/pLDR8 × pKES200 |
| T32 | S4197 attB::(SpecR PleuO lacZ) ΔyjjPQ-bglJFRT | T23/pLDR8 × pKES200 |
| T70 | S3974 ΔyjjPQ-bglJKD3-Cm | Venkatesh et al. (2010) |
| T71 | S4197 ΔleuOFRT (NC_000913: 84368–85312) | Venkatesh et al. (2010) |
| T73 | S3974 ΔrcsBFRT | × PCR S819/S820 (pKD3) × pCP20 |
| T75 | S3974 ΔyjjPQ-bglJFRT | T69 × pCP20 |
| T77 | S3974 ΔleuOFRT | × PCR T209/T210 (pKD3) × pCP20 |
| T87 | S4197 attB::(SpecR PleuO lacZ) ΔleuOFRT | T71/pLDR8 × pKES200 |
| T175 | S3974 ΔrcsBFRTΔyjjPQ-bglJFRT | T73 × T4GT7 (T70) × pCP20 |
| T177 | S3974 ΔleuOFRTΔyjjPQ-bglJFRT | T77 × T4GT7 (T70) × pCP20 |
| T208 | S3974 ΔhnsKD4-kan | × T4GT7 (S3754) |
| T221 | S3974 ΔhnsFRT (NC_000913: 1292145-1291735) | × T4GT7 (S3754) × pCP20 |
| T288 | S4197 attB::(SpecR PleuO lacZ) ΔyjjPQ-bglJFRTΔhnsFRT | T32 × T4GT7 (S3754) × pCP20 |
| T290 | S3974 ΔyjjPQ-bglJFRTΔhnsFRT | T75 × T4GT7 (S3754) × pCP20 |
| T292 | S4197 attB::(SpecR PleuO lacZ) ΔleuOFRTΔhnsFRT | T87 × T4GT7 (S3754) × pCP20 |
| T308 | S4197 attB::(SpecR PleuO lacZ) ΔleuOFRTΔyjjPQ-bglJFRT | T87 × T4GT7 (T70)) × pCP20 |
| T316 | S4197 attB::(SpecR PleuO lacZ) ΔleuOFRTΔhnsFRTΔyjjPQ-bglJFRT | T292 × T4GT7 (T70) × pCP20 |
| T324 | S4197attB::(SpecR PleuLABCD lacZ) | × pKETS3 |
| T328 | S4197 attB::(SpecR PleuLABCD lacZ) ΔleuOFRT | T71/pLDR8 × pKETS3 |
| T352 | S4197 attB::(SpecR PleuOlacZ) ΔyjjPQ-bglJFRTΔleuOFRTΔhnsFRT stpA::TetR | T316 × T4GT7(S159) |
| T447 | S3974 ΔhnsFRT stpA::TetR | T221 × T4GT7 (S159) |
| T453 | S3974 ΔrcsCKD3-Cm | × PCR T331/T332 (pKD3) |
| T538 | S4197 attB::(SpecR PleuO lacZ) ΔrcsCFRT (NC_000913: 2315049–2317930) | T28 × T4GT7 (T453) × pCP20 |
| T570 | S4197 attB::(SpecR PleuO lacZ) bglJC | T28 × T4GT7 (S1734) |
| T572 | S4197 attB::(SpecR PleuO lacZ) ΔrcsBFRT bglJC | T30 × T4GT7 (S1734) |
| T574 | S4197 attB::(SpecR PleuO lacZ) ΔrcsCFRT bglJC | T538 × T4GT7 (S1734) |
| T862 | S4197 attB::(SpecR PleuO lacZ) ΔleuOFRT bglJC | T87 × T4GT7 (S1734) |
| T1032 | S4197 ΔleuOFRT bglJC | T71 × T4GT7 (S1734) |
| T1048 | S3974 ΔyjjPQ-bglJFRTΔhnsFRT stpA::TetR | T290 × T4GT7 (S159) |
| T1075 | S4197 attB::(SpecR PleuO RcsB-BglJmutlacZ) ΔleuOFRT bglJC | T1032/pLDR8 × pKETS21 |
| T1106 | S4197 attB::(SpecR PleuO RcsB-BglJmutlacZ) ΔleuOFRT bglJCΔhnsKD4-Kan | T1075 × T4GT7 (T208) |
| T1108 | S4197 attB::(SpecR PleuO RcsB-BglJmutlacZ) ΔleuOFRT bglJCΔhnsKan stpA::TetR | T1106 × T4GT7 (S159) |
- a. Transduction was performed using bacteriophage T4GT7 (Wilson et al., 1979) grown on donor strains given in brackets. Transduction of alleles into recipient strains was verified by selection on LB plates supplemented with suitable antibiotics and by PCR (primers as listed in Table S4). Gene deletion was performed using the λ red-gam recombinase system, as described (Datsenko and Wanner, 2000). PCR fragments obtained with the indicated primers (Table S4) and plasmids pKD3 or pKD4 (Table S3) as templates were transformed into strains harbouring plasmid pKD46. Resistance cassettes were excised using the flp recombinase system and plasmid pCP20. Integration of plasmids into λ attB site was performed as described (Diederich et al., 1992; Dole et al., 2002) by transformation of origin-less re-circularized lacZ reporter plasmids into strains carrying the integrase-expressing plasmid pLDR8. Correct integration was verified by PCR (for oligonucleotides see Table S4). Two independent clones of each strain were used in the expression analyses.
| Number | Loci of known function | |
|---|---|---|
| Total | 39 | |
| RcsB-dependent | 38 | leuO, rhsA, rhsB, rhsC, setA, sfsB, osmB, btuB, blc |
| LeuO-dependent | 5 | cas, rhsE, elfA |
| Co-activated by LeuO and BglJ | 2 | bgl, chiA |
| Loci of putative function in inner/outer membrane | 13 | |
| Loci of unknown function | 11 | |
| RcsB-independent | 1 | csrB |
To study the regulatory effect of RcsB-BglJ on transcription of leuO, we constructed a leuO promoter lacZ reporter fusion and integrated it into the chromosomal phage λ attachment site attB of isogenic derivatives of ΔlacZ strain S4197 (Table 1). The lacZ fusion encompasses the complete 659 bp intergenic region between the leu operon and leuO (Fig. 1A). In the wild-type strain (T28) this leuO promoter lacZ fusion directed only 3 Miller units of β-galactosidase activity (Fig. 1B). Low expression is in agreement with repression of leuO by H-NS (Chen et al., 2001). However, when we expressed BglJ from plasmid pKETS1 the expression level increased to 338 Miller units (Fig. 1B). Note that in this strain the chromosomal copy of bglJ is repressed by H-NS (Stratmann et al., 2008). Similar results were obtained in the ΔyjjPQ-bglJΔleuO double mutant T308. In this strain background, BglJ caused an increase in expression from 3 units to 406 units (Fig. 1B). Accordingly, the expression analyses demonstrate a more than 100-fold activation of leuO by BglJ, confirming our microarray data.
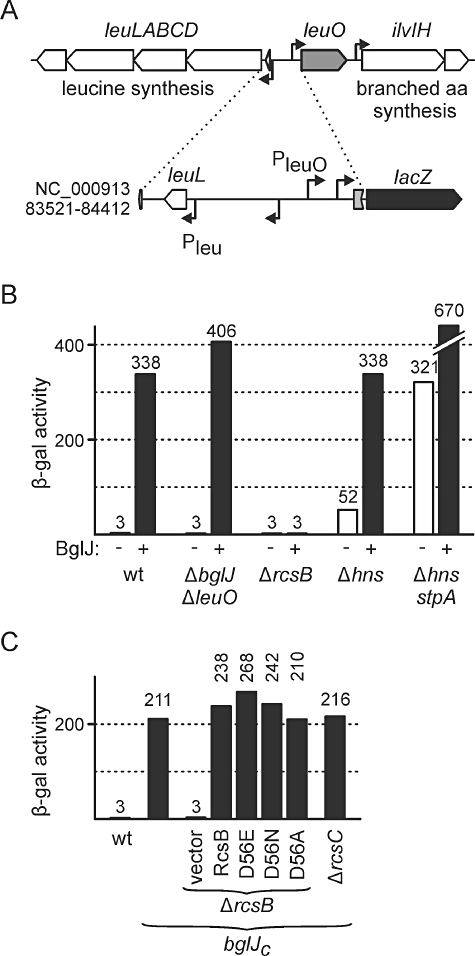
Transcription of leuO is activated by RcsB-BglJ independently of the Rcs phosphorelay and repressed by H-NS/StpA. A. Schematic representation of the chromosomal leu operon-leuO locus and a leuO promoter lacZ fusion integrated at the phage λattB site. B. The expression level directed by the leuO promoter lacZ fusion was determined in wild-type strain T28, ΔyjjPQ-bglJΔleuO strain T308, ΔrcsB strain T30, Δhns strain T288 and Δhns stpA strain T352. BglJ was expressed from plasmid pKETS1 (+, black bars). Empty vector pKESK22 served as control (−, white bars). Cultures for β-galactosidase assays were grown in LB medium to an OD600 of 0.5 supplemented with 1 mM IPTG and 25 µg ml−1 of kanamycin. C. Expression levels directed by the leuO promoter lacZ fusion in strains carrying a miniTn10 transposon insertion in yjjQ, which leads to constitutive expression of downstream bglJ (bglJC). β-Galactosidase activity was determined in strains bglJC T570, bglJCΔrcsB T572 and bglJCΔrcsC T574. Wild-type RcsB and RcsB mutants D56E, D56N and D56A were provided in trans using plasmids pKETS6, pKETS7, pKETS8 and pKES235, respectively, as indicated. Empty cloning vector pKESK22 served as control (vector).
leuO is activated by an RcsB-BglJ heterodimer independently of the Rcs phosphorelay
BglJ requires RcsB as dimerization partner to activate transcription of the bgl operon (Venkatesh et al., 2010), and our microarray data suggested that BglJ requires RcsB for activation of leuO and other target genes. We therefore tested the expression of the leuO promoter lacZ fusion in the ΔrcsB mutant T30. Indeed, in the ΔrcsB mutant expression of BglJ from plasmid pKETS1 did not cause activation of the leuO promoter lacZ fusion and only background expression levels of 3 units were detected (Fig. 1B). This shows that both proteins, RcsB and BglJ, are required for activation of leuO transcription. Previously, we have shown that activation of the bgl operon by RcsB-BglJ is independent of phosphorylation of RcsB by the Rcs two-component phosphorelay system (Venkatesh et al., 2010). We therefore tested whether activation of leuO by RcsB-BglJ is also independent of phosphorylation of RcsB. For this analysis, we used a ΔrcsB mutant carrying a miniTn10 transposon insertion causing constitutive expression of bglJ (bglJC, strain T572). In this bglJCΔrcsB mutant the leuO promoter lacZ fusion was not expressed (3 units), while in the rcsB wild-type background constitutive expression of BglJ from allele bglJC resulted in an expression level of 211 units (Fig. 1C). We then complemented the ΔrcsB mutant with low-copy plasmids encoding wild-type RcsB (pKETS6), or mutants RcsB-D56E (pKETS7), RcsB-D56N (pKETS8) and RcsB-D56A (pKES235) respectively. Mutation of the presumptive phosphorylation site D56 mimics the active phosphorylated form of RcsB (D56E) or the non-phosphorylated form of RcsB (D56A and D56N) (Scharf, 2010). Complementation with wild-type RcsB restored activation of the leuO promoter lacZ fusion by BglJ, with an increase of the expression level to 238 units. Similarly, we determined 267 units for complementation with RcsB-D56E, 242 units for RcsB-D56N, and 210 units for RcsB-D56A (Fig. 1C). These data demonstrate that there is no significant difference between wild-type RcsB, and the D56 mutants, suggesting that activation of leuO by RcsB-BglJ is independent of the phosphorylation status of RcsB at D56. This conclusion is further supported by the result that activation of the leuO promoter by RcsB-BglJ was not dependent on RcsC, the upstream sensor kinase of the Rcs phosphorelay. In the ΔrcsC bglJC mutant (T574) the expression level remained as high (216 units) as in the bglJC background (211 units, Fig. 1C).
Transcription of leuO is repressed by H-NS and StpA
Previous studies have shown that transcription of leuO is repressed by the nucleoid-associated protein H-NS. To elucidate the role of the H-NS paralogue StpA for regulation of leuO we determined the activity of the leuO promoter lacZ fusion in Δhns mutant T316 and Δhns stpA double mutant T352. In the Δhns mutant the expression level increased to 52 Miller units (Fig. 1B). Intriguingly, in the Δhns stpA double mutant expression was even sixfold higher and reached 321 units (Fig. 1B). Our results confirm that transcription of leuO is repressed by H-NS, and further show that StpA contributes to this repression. Indeed, the binding profile of H-NS and StpA to the leuO locus is very similar suggesting that both proteins bind and repress simultaneously (Uyar et al., 2009). Activity of the leuO promoter lacZ fusion was even twofold higher (669 units) when we additionally expressed BglJ from plasmid pKETS1 in the Δhns stpA double mutant (Fig. 1B). However, we cannot rule out that this additional increase of expression is due to pleiotropic effects, as expression of BglJ in the Δhns stpA double mutant led to severe growth deficiencies (data not shown).
RcsB-BglJ binds to regulatory region of leuO
Next we assessed whether activation of the leuO promoter by RcsB-BglJ is mediated by binding to the leuO regulatory region. However, so far we were not successful in purifying active BglJ protein. Therefore, we searched the leuO regulatory sequence for possible RcsB-BglJ binding sites by comparing it with the only known RcsB-BglJ binding site located at the bgl locus (Fig. 2A) (Venkatesh et al., 2010). In addition, we compared the sequence to the consensus sequence of the RcsB-RcsA heterodimer (Wehland and Bernhard, 2000). Candidate sequence motifs were mutated, and the effect of the mutations on activation of the leuO promoter lacZ fusion by BglJ was tested (Fig. 2A). Mutation of the best candidate sequence motif (mapping at NC_00913 position 84239 to 84252) completely abrogated activation by BglJ (Fig. 2A). In the bglJC strain expressing BglJ constitutively, the expression level dropped from 184 units obtained with the wild-type leuO promoter lacZ fusion (strain T862) to 4 units of the RcsB-BglJ site mutant in strain T1075 (Fig. 2B). As a control, we confirmed that another RcsB-BglJ target (the bgl operon) remained activate in all tested strains (data not shown). Importantly, the mutation of the RcsB-BglJ site did not affect expression in the Δhns stpA mutant T1107. In the absence of H-NS and StpA the leuO promoter lacZ fusion with the mutant RcsB-BglJ site was expressed at high levels (222 units). Taken together, these data suggest that RcsB-BglJ activates leuO transcription by binding to the mapped site.
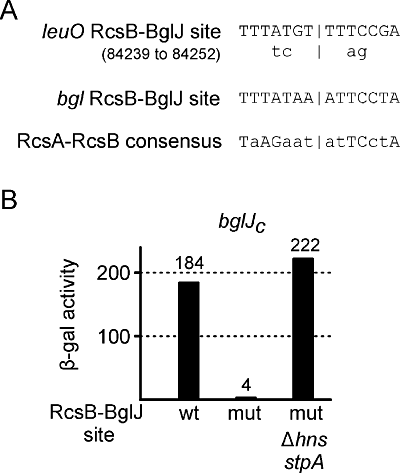
Mapping of RcsB-BglJ binding site. A. The leuO promoter region was searched for sites showing high similarity to the RcsB-BglJ binding site mapped at the bgl promoter (Venkatesh et al., 2010) and to RcsA-RcsB consensus binding site (Wehland and Bernhard, 2000). Point mutations were introduced into the putative binding sites located at position 84239–84252 by combined chain reaction (positions according to the genome sequence NC_000913), as indicated. B. Mutation of the RcsB-BglJ binding site abrogates activation of the leuO promoter lacZ fusion by RcsB-BglJ. Activities directed by the wild-type leuO promoter lacZ fusion in bglJCΔleuO strain T862, and by the RcsB-BglJ site mutant leuO promoter in bglJCΔleuO strain T1075 and in the Δhns stpA derivative (strain T1107), respectively, were determined in cultures grown in LB to an OD600 of 0.5.
RcsB-BglJ activates one of two H-NS-StpA repressed leuO promoters in vivo
To map in vivo transcription start sites in the intergenic region between leuO and the leu operon, we performed 5′ RACE analyses. We isolated RNA from ΔyjjPQ-bglJ strain T75, from Δhns stpA mutant T447, and from Δhns stpAΔyjjPQ-bglJ mutant T1048. In the Δhns stpA mutants, we expected the leuO promoter to be active. In addition, BglJ should be present in the Δhns stpA (chromosomal bglJ derepressed) but not in the Δhns stpAΔyjjPQ-bglJ mutant. For 5′ RACE analysis, half of each RNA sample was treated with tobacco acid pyrophosphatase (TAP) to distinguish between primary 5′ mRNA ends (transcription start sites) and processed 5′ mRNA ends. Then 5′ RACE products were analysed on agarose gels, the fragments of TAP treated samples were cloned and at least 4 clones each were sequenced to map the transcription start sites (Fig. 3).
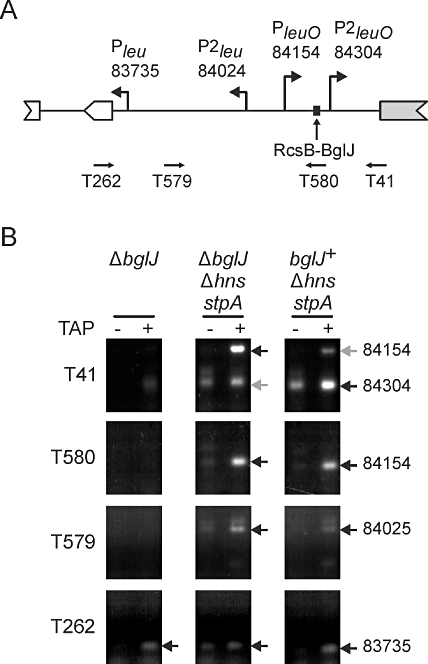
RcsB-BglJ activates one of two H-NS/StpA-repressed leuO promoters in vivo. A. Schematic summary of 5′ RACE mapping of promoters in the leu-leuO intergenic region. Indicated are the primers used for 5′ RACE, the mapped transcription starts (given as coordinates of sequence reference file NC_000913), and the RcsB-BglJ binding site. B. Gel electrophoresis of 5′ RACE PCR products. For 5′ RACE analysis, total RNA was extracted from strains ΔyjjPQ-bglJ (ΔbglJ) T75, Δhns stpAΔyjjPQ-bglJ T1048 and Δhns stpA (bglJ+) T447. To map primary 5′ ends of transcripts, half of each RNA sample was treated with tobacco acid pyrophosphatase (+ TAP). Then RNA oligo T268 was ligated to RNA 5′ ends of TAP treated and untreated samples, and the RNA was used for first-strand cDNA synthesis. For PCR amplification of leu-leuO specific cDNA, oligonucleotides T262, T579, T580 and T41, respectively, were used in combination with adapter-specific DNA oligonucleotide T265. TAP-dependent 5′ RACE products were cloned into pUC12 and at least four clones each were sequenced. The position of the most 5′ nucleotide neighbouring the RACE adapter was taken as transcription start site (arrows). Two transcription starts oriented towards the leu operon were mapped to NC000_913 positions 83 735 (Pleu) and 84 024 (P2leu) using primers T262 and T579, respectively, confirming the previously published transcription start mapped to position 83 735 (Wessler and Calvo, 1981). Two transcription start sites oriented towards leuO, were mapped to positions 84 155 (PleuO) and 84 304 (P2leuO) using primers T580 and T41 respectively. In strain Δhns stpA (BglJ present, derepressed in absence of H-NS), P2leuO at 84304 is more prominent than in strain Δhns stpA ΔyjjPQ-bglJ (no BglJ, grey arrow). P2leu, PleuO and P2leuO were only detected in the Δhns stpA mutants strains. Experiments were carried out twice and typical 2% agarose gel images are shown.
Two transcription start sites of the leuO gene were detected when 5′ RACE was performed with primers T580 and T41 (Fig. 3A). One of these transcription start sites mapped to position 84 155 and corresponds to the leuO promoter previously mapped in S. enterica (Fang and Wu, 1998a). This promoter is designated PleuO. The second novel transcription start of leuO, P2leuO, was mapped to position 84 304 using primer T41 (sequence shown in Fig. S1). Both transcription starts were only detectable in the Δhns stpA mutants indicative of repression by H-NS and StpA. Moreover, the TAP-dependent band corresponding to the newly identified transcription start site at position 84 304 was much more prominent in the Δhns stpA strain, in which chromosomal bglJ is de-repressed (i.e. in the presence of BglJ), than in the Δhns stpAΔyjjPQ-bglJ mutant (absence of BglJ). These results show that expression of leuO is directed by two H-NS and StpA repressed promoters (PleuO and P2leuO) and they suggest that RcsB-BglJ activates transcription from promoter P2leuO at position 84 304. Correspondingly, the centre of the RcsB-BglJ binding site maps at −58.5 bp relative to this transcription start (Fig. 3B). Further, transcription initiation at P2leuO was detected by 5′ RACE in a wild-type strain in which BglJ was overexpressed from a plasmid (data not shown).
In parallel, we performed 5′ RACE analyses using primers specific for leu operon transcripts (Fig. 3). In all three strain backgrounds, we detected a band that corresponded to the Pleu promoter with the transcription start site at pos. 83 735, which was mapped previously (Fig. 3A) (Wessler and Calvo, 1981). An additional transcription start of the leu operon was mapped to position 84 024 using PCR primer T579 (Fig. 3A). This transcription start site was only detectable in the Δhns stpA mutants, indicative of repression of this newly identified promoter P2leu by H-NS and/or StpA.
Transcription of leuO is negatively autoregulated
Previous studies suggested that LeuO might act as an activator of its own transcription by antagonizing H-NS-mediated repression (Chen et al., 2003; Chen and Wu, 2005). Therefore, we also tested the autoregulatory effect of LeuO in vivo. Firstly, we expressed LeuO from low-copy plasmid pKEDR13 and measured activity of the leuO promoter lacZ fusion in the ΔleuOΔyjjPQ-bglJ double mutant T308. In this strain the leuO promoter lacZ fusion is repressed by H-NS and StpA, while BglJ is absent. In the presence of plasmidic LeuO, expression of the leuO promoter lacZ fusion increased slightly from 3 units to 5 units (Fig. 4A), demonstrating a marginal approximately twofold upregulation by LeuO. Secondly, we measured expression of the leuO promoter lacZ fusion in Δhns mutant T316 and in Δhns stpA double mutant T352, respectively, in which the leuO promoter is partially or fully derepressed. When plasmidic LeuO was provided in trans the expression level dropped from 52 units (Δhns) to 13 units (Δhns + LeuO, Fig. 4A). Even more strikingly, in the Δhns stpA double mutant expression also decreased to 13 units as compared with 321 units (Fig. 4A). Thirdly, to investigate whether LeuO can downregulate its expression in the presence of RcsB-BglJ, we performed the experiments using bglJC strain T570. Here, the leuO promoter lacZ fusion directed an expression level of 211 Miller units in absence of LeuO but only of 35 units when LeuO was expressed in trans (Fig. 4A). This sixfold downregulation of leuO promoter activity by LeuO in the presence of RcsB-BglJ suggests that LeuO counteracts activation of P2leuO by RcsB-BglJ.
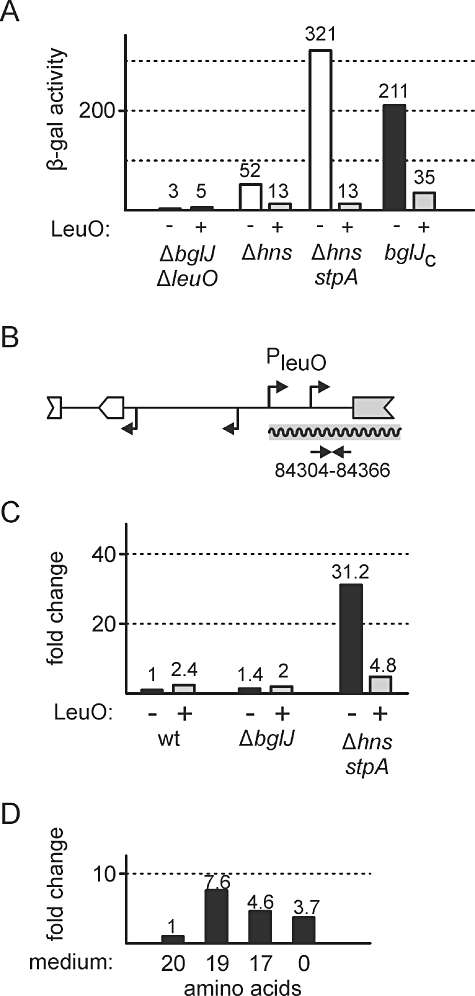
Transcription of leuO is negatively autoregulated. A. Expression of the leuO promoter lacZ fusion (as shown in Fig. 1A) is repressed by LeuO. Expression levels were determined in strains ΔyjjPQ-bglJΔleuO T308, ΔleuOΔhns T316, Δhns stpA T352 and bglJC T570 in the absence (−, white bars) and presence (+, grey bars) of LeuO. LeuO was expressed from plasmid pKEDR13 (+), empty vector pKESK22 served as control (−). Cultures were grown to OD600 of 0.5 in presence of 1 mM IPTG and 25 µg ml−1 kanamycin. B. Schematic of the chromosomal leu-leuO intergenic region and primers used for RT-qPCR analysis of leuO transcription. The RT-qPCR primers T351 and T352 are specific for amplification of a 63 base-pair fragment of the non-translated leuO mRNA leader (NC_00913 coordinates indicated) which is not present on LeuO expression vector pKETS5. C. RT-qPCR analysis of chromosomal leuO expression. RNA was isolated from cultures of wild-type strain S3974, ΔyjjPQ-bglJ strain T75 and Δhns stpA strain T447, all harbouring either control vector pKESK22 (−) or LeuO expression vector pKETS5 (+). For first-strand cDNA synthesis, random hexameric DNA oligonucleotides were used. Quantitative PCR was performed using serial dilutions of cDNA and primers T351 and T352. Ct values were normalized to rpoD expression determined with primers T247 and T248. Expression level is given as fold-change compared with the wild-type control (S3974 harbouring pKESK22). Cultures were inoculated in LB supplemented with 25 µg ml−1 kanamycin to an OD600 of 0.1 for exponential growth. After 30 min, IPTG was added to a final concentration of 1 mM and cultures were grown for additional 60 min. D. RT-qPCR analysis of chromosomal leuO expression in response to amino acid starvation. RNA was isolated from wild-type strain S3974 harbouring empty vector pKESK22 grown in M9 minimal medium containing 1% glucose, 25 µg ml−1 kanamycin and either 20 amino acids (20), 19 amino acids (19, without leucine), 17 amino acids (17, without leucine, isoleucine and valine), or no amino acids (0). Growth of cultures, RNA isolation, cDNA synthesis and RT-qPCR were carried out as described in (C). Expression level of leuO is given as fold-change compared with samples grown in presence of 20 amino acids.
The above data suggest that LeuO is an autoregulator that may have a moderate positive autoregulatory effect, but that strongly represses leuO transcription in the absence of H-NS and StpA and that counteracts activation of leuO by RcsB-BglJ. The moderate positive autoregulation is in agreement with previous data (Fang and Wu, 1998a; Chen et al., 2003). These authors further demonstrated that LeuO can hinder spreading of an H-NS repressing complex and assumed that binding of LeuO to the leu-leuO region results in positive regulation in a process that involves supercoiling-dependent transcriptional coupling in the chromosomal context (Chen and Wu, 2005). However, autoregulation of leuO so far was not tested in hns and hns stpA mutants. Therefore, we additionally analysed autoregulation of leuO in its natural chromosomal context by RT-qPCR (reverse transcription followed by quantitative PCR) to test whether autorepression of leuO by LeuO was an artefact based on the leuO promoter lacZ fusion. To this end, we used plasmid pKETS5 for ectopic expression of LeuO. This plasmid encompasses the leuO coding region from position −20 (relative to the ATG, NC_00913: 84348 to 85332) and thus allows quantification of transcription of chromosomal leuO using primers that are specific for the non-translated leader of the native leuO mRNA (Fig. 4B, NC_00913: 84304 to 84366).
For RT-qPCR analysis of the native leuO locus, we isolated RNA from the wild-type strain S3974, the ΔyjjPQ-bglJ mutant T75, and from Δhns stpA mutant T447, all harbouring either plasmid pKETS5 (+ LeuO) or empty vector pKESK22 (-LeuO). The lowest level of chromosomal leuO leader mRNA was measured in the wild-type strain in the absence of LeuO, as expected (Fig. 4C). Upon expression of plasmidic LeuO in trans, we again observed a moderate twofold increase of leuO expression (Fig. 4C). Rather similar results were obtained in the ΔyjjPQ-bglJ mutant (Fig. 4C). In comparison, expression of the chromosomally encoded leuO mRNA was elevated approximately 31-fold in the Δhns stpA mutant, confirming derepression of leuO in absence of H-NS and StpA. However, when LeuO was expressed in trans in the Δhns stpA background, transcription of chromosomal leuO decreased to a level only fivefold higher than that obtained in the wild-type strain. This demonstrates strong repression of leuO transcription by LeuO in the Δhns stpA mutant within the chromosomal context.
Previous studies showed that leuO expression is elevated in response to starvation for branched-chain amino acids (Fang et al., 2000; Majumder et al., 2001). Therefore, we additionally tested by RT-qPCR whether leuO expression changes in minimal medium supplemented with different sets of amino acids. We isolated RNA from wild-type strain S3974 grown in M9 minimal glucose medium containing either all 20 amino acids (20 aa), all amino acids but leucine (19 aa), all amino acids but the three branched-chain amino acids (17 aa), or no amino acids (0 aa). Note that strain S3974 is ilvG+ and thus valine resistant (Salmon et al., 2006). Compared with medium containing all 20 amino acids (Fig. 4C, 20), we measured the highest level of 7.6-fold elevated expression in medium lacking leucine (19 aa), a 4.6-fold elevated expression in medium lacking all three branched-chain amino acids (17 aa), and a 3.7-fold elevated expression in medium containing no amino acids (0). These results confirm that branched-chain amino acid starvation leads to higher expression of leuO. Finally, analysis of a leu promoter lacZ reporter fusion in a wild-type strain and a ΔleuO mutant confirmed that the LeuO protein has a moderate (2.5-fold) effect on expression of the divergent leu operon (Fig. S4).
In vitro mapping of promoters in the leuO-leu operon intergenic region and analysis of their regulation by LeuO
Our data suggest that LeuO acts predominantly as an autorepressor. Furthermore, we mapped two promoters directing expression of leuO of which the newly identified P2leuO promoter is activated by RcsB-BglJ. In addition, a second promoter P2leu directing expression of the leu operon was mapped (Fig. S1). To further characterize the leuO-leu intergenic region and autoregulation of leuO we mapped in vitro RNA polymerase binding sites by KMnO4 footprinting in the absence and presence of LeuO protein. In addition we performed in vitro transcription assays, and we re-mapped the LeuO binding sites by DNase I footprinting (for a summary of the results see Fig. 5).
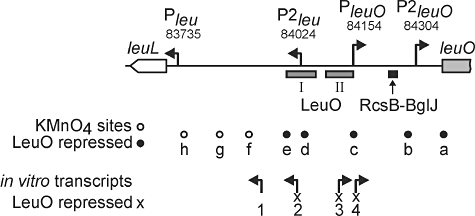
In vitro mapping of promoters in the leu-leuO intergenic region and analysis of their regulation by LeuO. Schematic summary of regulatory features mapped to the 659 bp leu-leuO intergenic region. Four transcription start sites were mapped in vivo by 5′ RACE termed Pleu, P2leu, PleuO and P2leuO, positions are given as NC_000913 coordinates (Fig. 3). The RcsB-BglJ binding site is drawn as black rectangle (Fig. 2). In addition, LeuO binding sites were mapped in vitro by DNase I footprinting assays and are represented as grey rectangles termed LeuO I and II (see Fig. S2 for details). To detect the formation of putative open complexes, KMnO4 footprinting experiments were performed in presence and in absence of LeuO protein (see Fig. S2 for details). Eight KMnO4-sensitive sites (unfilled/filled circles) were detected (termed ‘a’ to ‘h’). Formation of sites a to e is repressed by LeuO (filled circles). In vitro transcription in presence and in absence of LeuO protein was performed to roughly map start sites and orientation of transcripts (see Fig. S3 for details). Four transcripts were detected (arrows). Transcription starts 1 and 2 are oriented towards the leu operon, transcription starts 3 and 4 are oriented towards leuO. Transcripts 2, 3 and 4 are repressed by LeuO (marked with ‘x’, see Fig. S3 for details).
For DNase I and KMnO4 footprinting the leuO-leu operon intergenic region was sub-cloned so that the whole region was covered by overlapping fragments (Fig. S2A). DNase I footprinting using LeuOHis6 protein revealed two LeuO binding sites, LeuO I and LeuO II (Fig. S2B and Fig. 5). The LeuO binding sites correspond to sites previously mapped (Chen et al., 2003; Chen and Wu, 2005). The binding sites overlap with the P2leu and PleuO promoters (Figs 5 and S1). The footprinting analysis also showed that LeuO prevents binding of RNA polymerase (Fig. S2B). No additional LeuO binding site was detected by DNase I footprinting and by gel retardation assays (data not shown).
KMnO4 footprinting in the absence and presence of LeuO and sigma70-RNA polymerase revealed the presence of several KMnO4 sensitive sites at which RNA polymerase may form open complexes (labelled ‘a’ to ‘h’ in Figs 5 and S2). The map positions of three of these KMnO4 sensitive sites correspond well with the mapped promoter of the leuO gene (PleuO, site c), the divergent leu operon (Pleu, site h), and the second leu operon promoter (P2leu, site d). However, KMnO4 sensitive site b maps 20 bp upstream of the transcription start site of P2leuO. Further, one site was mapped to the coding region of leuO (site a), and three sites were mapped between the two leu operon promoters (sites e, f and g). Interestingly, formation of KMnO4 sensitive sites ‘a’ to ‘e’ was inhibited when LeuO was added to the reactions (Figs 5 and S2). Suppression of formation of these putative open complexes by LeuO supports the finding that LeuO acts as an autorepressor. Intriguingly, formation of site b, mapping next to the RcsB-BglJ activated P2leuO promoter was repressed by LeuO when the fragment included the LeuO I and LeuO II binding sites but not when a shorter fragment was probed (Fig. S2F, compare fragments 4 and 6).
To determine whether transcription is initiated at the mapped open complexes and to corroborate repression by LeuO, we additionally performed in vitro transcription assays in the absence and presence of LeuO protein. To this end, we used a set of four DNA fragments of the leuO-leu region as templates (fragments 1 to 4, Fig. S3) that allowed to simultaneously monitor transcription oriented towards the leu operon and towards leuO (see Fig. S3 for details). Four major RNA transcripts were detected in the in vitro transcription assays performed in the absence of LeuO protein but only one transcript was detected in the presence of LeuO protein (Fig. S3). Two transcripts were oriented towards the leu operon (transcripts 1 and 2, Figs 5 and S3) and two start sites directed transcription towards leuO (transcripts 3 and 4, Figs 5 and S3). In vitro transcripts 2, 3 and 4 were repressed in the presence of LeuO protein. The starts sites of these transcripts and the LeuO binding sites overlap and the result suggests that binding of LeuO represses transcription. Roughly mapped transcripts 2 and 4 correspond to P2leu and PleuO while the presence of transcripts 1 and 3 suggests that additional promoters may exist in this region. Interestingly, no in vitro transcript corresponding to transcription initiation at the P2leuO promoter was detected. This may indicate that P2leuO is RcsB-BglJ dependent. Taken together the in vitro data suggest that the leuO-leu intergenic region represents a complex regulatory promoter region and they support autorepression of leuO.
Discussion
In this report we present molecular details of transcriptional regulation of leuO encoding the pleiotropic regulator LeuO. We show that transcription of leuO is strongly activated by the RcsB-BglJ heterodimer independently of signalling by the Rcs two-component phosphorelay. Microarray data suggest that RcsB-BglJ in addition activates the expression of multiple other loci. We further show that StpA, the H-NS paralogue, can cause repression of leuO in an hns mutant. Moreover, our data suggest that LeuO acts predominantly as a negative autoregulator, with a moderate positive effect on transcription of leuO and the divergent leu operon. Regulation of leuO by RcsB-BglJ and LeuO as shown here and activation of yjjQ-bglJ by LeuO (Stratmann et al., 2008) indicates a feedback control mechanism of two global transcriptional regulators that may ensure turn on of their expression in response to specific environmental signals.
Previously it was shown that expression of leuO is moderately upregulated in response to starvation for branched-chain amino acids and in the stationary phase (Fang et al., 2000; Majumder et al., 2001; Shimada et al., 2011). Further, it was proposed that LeuO acts as a positive regulator of the leu operon and leuO gene with activation based on transcription induced changes in DNA supercoiling and delimiting of H-NS spreading by LeuO (Fang and Wu, 1998b; Chen et al., 2003; Chen and Wu, 2005). Our data confirm a moderate (two- to threefold) positive effect of LeuO on expression of the leu operon and the leuO gene, respectively, as well as a moderate upregulation in response to the availability of branched-chain amino acids. However, our further data suggest that LeuO acts predominantly as an autorepressor on the leuO promoter PleuO and the newly identified promoters P2leuO and P2leu (for a model see Fig. 6). PleuO corresponds to a promoter previously mapped in S. enterica while the P2leuO promoter is activated by RcsB-BglJ. Both of these promoters are repressed by LeuO, and LeuO acts antagonistically to activation by RcsB-BglJ (Fig. 6). Repression of PleuO by LeuO is presumably direct as the two LeuO binding sites mapped by DNase I footprinting are located next to this promoter and one of the sites overlaps with the core sequence of PleuO. Repression of the second P2leuO promoter also requires the LeuO I and II binding sites and it is open whether repression might be caused by formation of a more extended LeuO-DNA complex. LeuO is a member of the LysR-type family of transcription factors which bind as tetramers and affect the DNA structure by bending (Maddocks and Oyston, 2008). LysR-type transcription factors are characterized by an N-terminal HTH DNA binding domain and a central domain for binding of small co-inducer molecules that can alter protein activity (Maddocks and Oyston, 2008). However, thus far, no co-inducer altering activity of the LysR-type transcription factor LeuO has been identified.
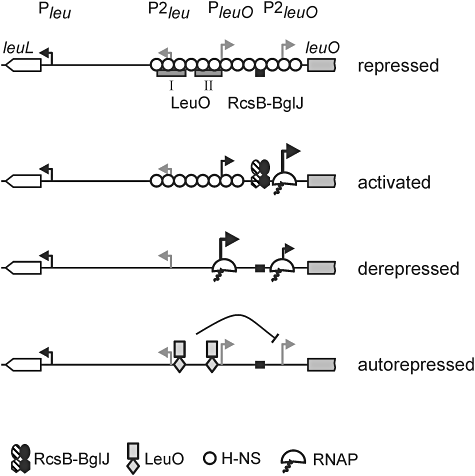
Model of transcriptional regulation of leuO. Under standard laboratory growth conditions transcription is repressed by H-NS and/or StpA (repressed). Transcription can be activated by binding of RcsB-BglJ upstream of P2leuO (activated). It is open how RcsB-BglJ interferes with repression by H-NS/StpA and whether RcsB-BglJ acts as class I regulator and recruits RNA polymerase to P2leuO. In absence of H-NS, StpA and RcsB-BglJ, RNA polymerase can access both PleuO and P2leuO and initiate transcription (derepressed). In this case PleuO is favoured over P2leuO. In case of high LeuO concentration (e.g. by overexpression) LeuO may bind to the two central binding sites and repress transcription from promoter P2leu and PleuO that overlap with LeuO binding sites (autorepressed). In addition, it represses transcription from the more distal P2leuO.
The RcsB-BglJ heterodimer activates a newly characterized promoter P2leuO apparently by binding to a site located directly upstream of the core sequence of P2leuO. This indicates that RcsB-BglJ acts as a class I transcriptional activator. Activation of leuO and bgl is independent of phosphorylation of the Rcs two-component system response regulator RcsB, and our microarray data suggest that regulation by BglJ in general depends on RcsB. The data further suggest that RcsB-BglJ acts as a global transcriptional regulator with a distinct set of targets, many of which putatively relate to membrane and surface functions. Curiously, just two RcsB-BglJ target loci are co-regulated by LeuO. These are the H-NS repressed bgl and chiA loci, encoding enzymes for utilization of aromatic β-glucosides and a periplasmic endochitinase respectively.
Activation of leuO by RcsB-BglJ and negative autoregulation of leuO (shown here) as well as activation of the yjjQ-bglJ operon by LeuO (Stratmann et al., 2008) constitutes a regulatory feedback loop that connects the two global regulators, LeuO and RcsB-BglJ. Remarkably, RcsB-BglJ has only rather mild effects on the expression of some LeuO target loci, while many LeuO targets are not at all affected by RcsB-BglJ. This indicates that a negative feedback mechanism exists which operates in addition to the mutual positive control of leuO and bglJ expression. Such a feedback may ensure tightly controlled turn on of LeuO and/or BglJ in response to specific environmental signals.
Experimental procedures
Bacterial strains, plasmids and media
Escherichia coli K12 strains used in this study are listed in Table 1. Cultures were grown in LB or M9 medium supplemented with 50 µg ml−1 ampicillin, 15 µg ml−1 chloramphenicol, 25 µg ml−1 kanamycin, 25 µg ml−1 spectinomycin, and 12 µg ml−1 tetracycline, where indicated. Construction of strains by gene replacement, transduction and integration of lacZ reporter fusions followed published protocols (Wilson et al., 1979; Diederich et al., 1992; Datsenko and Wanner, 2000; Ausubel et al., 2005). Standard molecular techniques and cloning work were carried out according to published protocols (Ausubel et al., 2005). Plasmids used for this study are summarized in Table S3; sequences of oligonucleotides are given in Table S4.
Site-specific mutagenesis by combined chain reaction (CCR)
Combined chain reaction (CCR) to mutate the RcsB-BglJ binding site at the leuO locus was performed as described previously (Bi and Stambrook, 1998; Hames et al., 2005). In brief, 1 µl of forward PCR primer T334 (10 pmol µl−1) and 1 µl of reverse PCR primer S118 (10 pmol µl−1) were used in combination with 4 µl of internal, 5′-phosphorylated mutagenesis primer (10 pmol µl−1, Table S4). Plasmid pKES200 was used as template for the leuO promoter lacZ fusion. A total of 25 µl of dNTP mix (10 mM each), 5 µl of 10× CCR buffer (200 mM Tris-HCl (pH 8.5), 30 mM MgCl2, 500 mM KCl, 5 mM NAD+), 2 µl of HighFidelity EnzymeMix (Fermentas), 3 µl of Ampligase (Epicentre), 2 µl of BSA (10 mg ml−1) and H2O were added to a final volume of 50 µl. The PCR cycler program was 5 min 95°C, 35× (30 s 95°C, 30 s 55°C, 2 min 65°C), 5 min 65°C, hold at 4°C. CCR fragments were excised from agarose gels and used for cloning.
β-Galactosidase assay
β-Galactosidase assays were performed as described (Miller, 1992). Briefly, cultures were grown overnight in LB medium with antibiotics. Then, 8 ml cultures were inoculated to an optical density at 600 nm (OD600) of 0.05–0.1 and grown to an OD600 of approximately 0.5. IPTG (isopropyl-β-d-thiogalactopyranoside) was added, where indicated, to a final concentration of 1 mM to the overnight and the exponential cultures for induction. The bacteria were harvested, and β-galactosidase activities were determined. The assays were performed at least of three independent cultures. Standard deviations were less than 15%.
RNA isolation
Exponential cultures were inoculated from fresh overnight cultures to an OD600 of 0.1 in LB (with antibiotics for transformants). In case of induction, IPTG was added to a final concentration of 1 mM after 30 min of growth. After additional 60 min the bacteria were harvested using RNAprotect (Qiagen, Hilden, Germany) and used for RNA isolation using the RNeasy MiniKit system (Qiagen, Hilden, Germany). In brief, 1 ml of each culture (OD600 between 0.5 and 0.6) was used and processed according to the manufacturer's instructions including an on-column DNaseI treatment. RNA quality was assayed by denaturing urea-PAGE and by measuring the ratio of absorption at 260/280 nm. RNA concentration was determined by measuring UV light absorption at 260 nm.
cDNA synthesis
RNA was isolated as described above. For first strand cDNA synthesis, 1 µg of RNA was reverse transcribed using the SuperScript III First Strand Synthesis Kit (Invitrogen, Karlsruhe, Germany) according to the manufacturer's instructions and random hexameric oligonucleotides as primers. In brief, RNA was mixed with primers and dNTPs, denatured by heating to 65°C and then kept on ice. For the RT reaction, 200 U of SuperScript III reverse transcriptase and 40 U of RNaseOUT were used. The final reaction volume was 20 µl. Samples were first incubated at 25°C for 10 min, then at 50°C for 60 min, then at 85°C for 5 min and put on ice. RNase H (Fermentas, St. Leon-Rot, Germany) was added and samples were incubated for 20 min at 37°C. cDNA was stored at −20°C.
Microarray
To determine putative target genes of BglJ and LeuO, ΔyjjPQ-bglJ strain T75, ΔyjjPQ-bglJΔrcsB strain T175 and ΔyjjPQ-bglJΔleuO strain T177 (Table 1) were transformed with either pKETS1 (BglJ) or pKESK22 (control, Table S3). Strain T75 was additionally transformed with plasmid pKEDR13 (LeuO). From fresh overnight cultures, cultures were inoculated in 15 ml LB supplemented with kanamycin to an OD600 of 0.1 and grown to an OD600 of approximately 0.15. At this point IPTG was added to the medium to a final concentration of 1 mM. After 60 min, cells were harvested for RNA isolation. RNA isolation was performed as described above. Hybridization to Affymetrix GeneChip E. coli Genome 2.0 microarrays was carried out according to the manufacturer's instructions. Microarrays were scanned using an Affymetrix GeneChip Scanner 3000 7G. Data were processed using Affymetrix apt-probeset-summarize software version 1.10 and RMA algorithm. Samples were normalized using the standard normalization probes present on the Affymetrix GeneChip. Differential expression values were calculated as fold change. Microarray data were submitted to the NCBI Gene Expression Omnibus Website (http://www.ncbi.nlm.nih.gov/geo/) under accession number GSE34023.
RT-qPCR analysis
Quantitative PCR measurements were carried out using gene specific oligonucleotide primers, SYBR Green I and an iQ5 real-time PCR cycler (Bio-Rad) or a C1000 touch thermal cycler with optical reaction module CFX96 (Bio-Rad). RNA isolation and cDNA synthesis were carried out as described above. cDNA derived from 1 µg of total RNA was diluted 1:10 in DEPC-treated water. For one assay, 4 µl of dNTPs (1 mM each), 4 µl of 5× GoTaq buffer (Promega), 6.8 µl of DEPC-treated water, 0.8 µl of DMSO, 0.2 µl of SYBR green (1:1000 in DMSO), 0.2 µl of GoTaq DNA Polymerase (Promega), and 1 µl of each primer (10 pmol µl−1) were used. Two microlitres of diluted cDNA served as template. Assays were pipetted on 96-well PCR plates and sealed with optical quality adhesive film (Bio-Rad). The thermal cycler program was 94°C for 3 min, 40× (94°C for 10 s; 58°C for 30 s; 72°C for 30 s), 72°C for 10 min. A melting curve analysis was carried out starting from 95°C leading to 50°C in steps of 0.5°C. Samples were prepared in triplicate, a pool of cDNA samples of different dilutions served as calibration line for efficiency correction, and the rpoD gene served as reference for data normalization. Data were analysed using the iQ5 Opitcal System Software 2.0 (Bio-Rad) or CFX Manager Software 2.1 (Bio-Rad), applying an efficiency-corrected, normalized expression (ΔΔCt) algorithm.
5′ RACE (rapid amplification of cDNA ends)
5′ RACE analysis was performed as described (Wagner and Vogel, 2005). RNA was isolated as described above. For treatment with tobacco acid pyrophosphatase (TAP, Epicentre Biotechnologies), 12 µg of RNA was brought to a volume of 87.5 µl in water. Ten microlitres of 10× TAP buffer and 0.5 µl (10 U) of RNase inhibitor SUPERaseIn (Ambion) were added. Then, assays were split in half (treated sample and control, 49 µl each) and 1 µl (10 U) of TAP was added to the treated sample. All samples were incubated at 37°C for 30 min. After incubation, 5 µl of RNA adapter oligonucleotide T268 (100 pmol µl−1) and 100 µl of water were added. Enzyme and buffer were removed by phenol-chloroform-isoamyl alcohol (25:24:1) extraction followed by ethanol precipitation of RNA. For ligation of RNA adapter oligonucleotide, RNA was dissolved in 14 µl water, heated to 90°C for 5 min and placed on ice for 5 min. Two microlitres of 10× RNA ligation buffer and 2 µl of DMSO were added. Then 1.8 µl of RNA ligase pre-mixed with 0.2 µl of RNase inhibitor SuperaseIn (Ambion) were added and samples were incubated at 17°C over-night. After incubation, 4 µl of random hexameric DNA oligonucleotides (50 ng µl−1) and 130 µl of water were added for reverse transcription, final volume 150 µl. Enzyme and buffer were removed by phenol-chloroform-isoamyl alcohol extraction followed by ethanol precipitation. RNA pellets were dissolved in 20 µl of water. Ten microlitres of RNA solution was used for first-strand cDNA synthesis as described above. For PCR amplification, PlatinumTaq Polymerase (Invitrogen) was used according to the manufacturer's instructions. Either 1 µl of cDNA or untreated RNA (control), respectively, was used as templates in assays of 25 µl including RNA adapter specific DNA primer T265 and a gene-specific DNA primer as indicated in the results section. PCR products were analysed on 2% agarose gels, isolated and cloned into pUC12. At least 4 clones were sequenced for mapping of the primary transcription start sites.
DNase I footprinting
DNase I footprinting analysis of free DNA and protein–DNA complexes was performed as described (Pul et al., 2007). In brief, the top strand was labelled by EcoRI/PstI digestion of plasmids pKETS13 to pKETS18 (Table S3), and the bottom strand was labelled by HindIII/Ecl136II digestion of the same plasmids. Both strands were separately end-labelled by Klenow polymerase (Promega) incorporation of [α-32P]-dATP. Samples were incubated in the presence of 0.5 mU µl−1 of RNase-free DNase I (Fermentas) for 30 s at 25°C. A total of 50 nM RNAP and 1 µM LeuOHis6 were added, where indicated. Hydrolysis was stopped by addition of 330 mM NaOAc (pH 4.8), 10 mM EDTA and 10 ng µl−1 of glycogen followed by a phenol extraction. For the sequence assignment G- and A-specific chemical cleavage reactions were performed. Cleavage products were separated on denaturing 8% polyacrylamide gels next to sequencing reactions as size standard and visualized by autoradiography.
KMnO4 footprint analysis
KMnO4 footprint analysis was performed as described (Pul et al., 2010). In brief, radioactively labelled DNA fragments were obtained as described above for DNase I footprinting. A total of 40 ng of the labelled DNA fragments in a total volume of 40 µl was incubated for 10 min at 30°C, with 50 nM of RNAP and 1 µM of LeuOHis6, where indicated. Four microlitres of 160 mM KMnO4 was added and the samples were incubated for additional 2 min at 30°C. The reaction was stopped by addition of 4.8 µl of β-mercaptoethanol (14.3 M) and 5.3 µl 500 mM EDTA and the samples were extracted with phenol/chloroform, followed by precipitation with ethanol. The pellets were dissolved with 70 µl of 10% piperidine and incubated for 30 min at 90°C. After lyophylization the pellets were washed twice with 30 µl H2O and lyophylized again. The pellets were then dissolved in 50 µl H2O and precipitated with ethanol. The cleavage products were separated on 10% denaturing polyacrylamide gels and visualized by autoradiography.
In vitro transcription
Transcription assays were performed as described previously (Schnetz and Wang, 1996) with minor modifications. DNA fragments were amplified by PCR using MG1655 single colonies as templates and oligonucleotides T530 to T533 as primers (Table S4). In brief, in vitro transcription was performed at 37°C in transcription buffer (TB; 30 mM Tris-acetate, pH 7.8, 7 mM magnesium acetate, 150 mM potassium glutamate, 1 mM DTT, 100 µg ml−1 BSA, 10% glycerol). DNA (250 ng in 4 µl TB) was mixed with 10 µl TB containing 1 µM LeuOHis6, where indicated. Immediately, 3 µl TB containing 0.2 U RNA polymerase saturated with σ70 (RNAP; Epicentre) were added. After 20 min of incubation to allow binding of RNAP to DNA, run-off transcriptions were started by the addition of 3 µl of a nucleotide/heparin mixture [final concentrations of 200 µM each of ATP, CTP and GTP, 2 µM [32P]UTP (Hartmann Analytic, Germany, 40 Ci mmol−1 final activity)]. Samples were incubated for an additional 10–15 min and 12 µl of RNA Loading Dye (Fermentas; 95% formamide, 0.025% SDS, 0.025% bromphenol blue, 0.025% xylene cyanol FF, 0.025% ethidium bromide, 0.5 mM EDTA) were added to each reaction mixture. Five microlitres of each quenched sample was loaded on a 4% denaturing polyacrylamide gel [4% acrylamide : bisacrylamide (19:1), 7 M urea, 72 mM Tris-borate, pH 8.3, 1.6 mM EDTA]. A sequencing reaction was set up using the Sequenase Version 2.0 DNA Sequencing Kit (Affymetrix, Ohio, USA) according to the manufacturer's instructions and loaded to the gel as size standard. Gels were dried onto Whatman 3MM paper and exposed to a Fuji imaging plate for visualization of radioactivity with a Typhoon scanner (GE Healthcare Lifesciences, Freiburg, Germany).
Acknowledgements
Microarray analysis was supported through the Cologne Center for Genomics (CCG) of the University of Cologne by Peter Frommolt and Peter Nürnberg. We thank Paul Scholz and Selman Öztürk for their contributions to plasmid construction and β-galactosidase assays. Our work was funded through grant Schn 371/10-1 by the Deutsche Forschungsgemeinschaft (DFG).




