Spatial resolution of two bacterial cell division proteins: ZapA recruits ZapB to the inner face of the Z-ring
Summary
FtsZ, the essential regulator of bacterial cell division, is a dynamic cytoskeletal protein that forms helices that condense into the Z-ring prior to division. Two small coiled-coil proteins, ZapA and ZapB, are both recruited early to the Z-ring. We show here that ZapB is recruited to the Z-ring by ZapA. A direct interaction between ZapA and ZapB is supported by bacterial two-hybrid and in vitro interaction assays. Using high-resolution 3-D reconstruction microscopy, we find that, surprisingly, ZapB is located inside the Z-ring in virtually all cells investigated. We propose a molecular model in which ZapA increases lateral interactions between FtsZ proto-filaments and ZapB mediates further stabilization of this interaction by cross-linking ZapA molecules bound to adjacent FtsZ proto-filaments. Gene deletion and complementation assays show that ZapB can mitigate cell division and Z-ring assembly defects even in the absence of ZapA, raising the possibility that ZapB stimulates Z-ring assembly by two different mechanisms.
Introduction
Division of rod-shaped bacteria, such as Escherichia coli and Bacillus subtilis, is a highly regulated morphological process that produces two equal-sized daughter cells. It involves the temporal and topological co-ordination of many different factors: following chromosome replication and segregation, the division site is selected at mid-cell and the cell wall constricts before the synthesis of the central multilayered cell envelope, consisting of the inner and outer membranes and peptidoglycan.
A dynamic protein complex, called the divisome, composed of more than 10 different factors, is required for the co-ordination and completion of these biosynthetic processes (Lutkenhaus, 2002; Goehring and Beckwith, 2005; Rothfield et al., 2005; Vicente and Rico, 2006). Central to cell division is the initial formation of a ring-like structure composed of FtsZ – the Z-ring – at the future division site (Bi and Lutkenhaus, 1991). Formation of the Z-ring is co-ordinated with DNA replication and segregation (Errington et al., 2003; Romberg and Levin, 2003; Bernhardt and de Boer, 2005). FtsZ is a highly conserved GTPase with a tubulin signature motif (Erickson and Stoffler, 1996; Addinall and Holland, 2002) that can polymerize into filaments and rings in vitro (Erickson et al., 1996; Lu et al., 2000) and short filaments in vivo (Li et al., 2007).
After the formation of the Z-ring, that initially consists of FtsZ polymers stabilized by FtsA and ZipA, downstream division proteins are recruited in a largely linear hierarchy (FtsZ > FtsA/ZipA > FtsK > FtsQ > FtsL/FtsB > FtsW > FtsI > FtsN) to form the division machinery. The proper recruitment of a protein requires all the upstream division factors (Aarsman et al., 2005; Goehring and Beckwith, 2005). If either FtsA or ZipA are inactivated, the Z-ring can form but the downstream division factors (FtsK, etc.) are not recruited to the divisome (Pichoff and Lutkenhaus, 2002). If FtsA and ZipA are inactivated simultaneously, the Z-ring does not form at all (Pichoff and Lutkenhaus, 2002).
The assembly of the Z-ring at mid-cell is the first known event in bacterial cytokinesis. Interestingly, although the Z-ring assembles early in the cell cycle, constriction is not initiated until late in the cell cycle (Aarsman et al., 2005). This delay suggests that Z-ring constriction is subject to regulation and is consistent with observations from both E. coli and B. subtilis that show Z-ring assembly is preceded by the collapse of a helical intermediate (Thanedar and Margolin, 2004; Monahan et al., 2009). Thus, the study of the early events of Z-ring assembly is likely to yield novel insights into how cell division is controlled.
Besides the above-mentioned essential cell division factors, two additional factors that are recruited early to the Z-ring have been identified.
ZapA is a widely conserved bacterial division protein that colocalizes with FtsZ and stimulates Z-ring formation (Gueiros-Filho and Losick, 2002). ZapA was identified in a screen for factors that counteracted MinCD, a cell division inhibitor that interferes with Z-ring formation via a direct interaction with FtsZ. ZapA is not essential in wild-type cells but becomes indispensable at reduced levels of FtsZ. ZapA interacts directly with FtsZ and, consistent with its ability to antagonize MinCD overproduction in vivo and promotes the polymerization of FtsZ in vitro. Remarkably, a ZapA-like protein is also present in the mitochondrial division apparatus (Yoshida et al., 2009).
The crystal structure of ZapA revealed that the monomer consists of a C-terminal coil-coiled domain and an N-terminal globular domain (Low et al., 2004). The monomer forms parallel dimers that can associate into anti-parallel dimers of dimers. In solution, ZapA exists in a dimer–tetramer equilibrium that is strongly correlated with concentration. Electrostatic surface mapping and sequence conservation suggested that ZapA dimers interact with FtsZ via the N-terminal globular domains (Low et al., 2004).
Recently we identified ZapB, a novel cell division factor that stimulates Z-ring assembly and cell division (Ebersbach et al., 2008). Deletion of zapB resulted in delayed cell division and the formation of ectopic Z-rings and spirals. Localization of ZapB to the divisome depended on FtsZ and time-lapse microscopy showed that ZapB–GFP was present at mid-cell in a pattern very similar to that of FtsZ. However, it is not known how ZapB is recruited to the Z-ring or how ZapB stimulates Z-ring formation and cell division.
Here, we show that ZapB interacts with ZapA and that ZapA is required for the recruitment of a ZapB–GFP fusion to the Z-ring. Using double labelling and conventional fluorescence microscopy, we show that ZapB is an early cell division protein that colocalizes with the Z-ring during all stages of its assembly and maturation. Unexpectedly, we find evidence that ZapB fusion proteins are located on the inner surface of the contracting ring, consistent with the proposal that ZapA functions as a bridging molecule between FtsZ proto-filaments. Unexpectedly, deletion of zapB from cells lacking ZapA delayed cell division and Z-ring assembly further, indicating that ZapB can stimulate Z-ring assembly even in the absence of ZapA.
Results
ZapB colocalizes with FtsZ and is recruited early to the divisome
To investigate the timing of ZapB recruitment to the Z-ring we used double fluorescence labelling. FtsZ and ZapB were fused to mCherry and GFP, respectively, and coexpressed (Fig. 1). All cells with a visible FtsZ–mCherry signal at mid-cell exhibited a colocalizing ZapB–GFP signal (Fig. 1A). A time-lapse experiment confirmed that ZapB–GFP and FtsZ–mCherry colocalized throughout the entire cell cycle and that ZapB was associated with FtsZ during the transition from a helical structure into a ring (Fig. S1 and Movie S1). Importantly, the two proteins localized simultaneously at the new division sites of the daughter cells showing that ZapB belongs to the class of early cell division proteins.

ZapB colocalizes with FtsZ and ZapA.A. MC1000/pEG3a (PBAD::zapB::gfp)/pQW59 (Plac::ftsZ::mCherry).B. MC1000/pEG9 (PBAD::zapB::mCherry)/pNG53 (Plac::yfp::zapA). Cells were grown at 30°C in M9 minimal medium supplemented with glucose (0.2%), casamino acids (0.1%) and appropriate antibiotics. Cells were visualized by combined phase-contrast and fluorescence microscopy. For the Plac fusions, the leakiness of the Plac promoter resulted in a sufficient level of expression while the PBAD fusions required a pulse of transcription (0.2% arabinose was added for 5′ for pEG3a and 25′ for pEG9). The experiment is described further in Experimental procedures. Scale bar = 2 µm.
We also compared the ZapB–mCherry and GFP–FtsK signals in the same cell. In this case it was not possible to obtain informative time-lapse series because of the limited signal emitted by the GFP–FtsK fusion protein. However, from static images arranged as a pseudo-time-lapse (Fig. 2), it was evident that the ZapB–mCherry signal condensed from ring-like structures (Fig. 2A) to dots (Fig. 2B) and moved to new cell division sites (Fig. 2C–E) before the GFP–FtsK signal (Fig. 2A′–E′). ZapB–mCherry, as previously seen with ZapB–GFP, exhibited helical localization patterns when moving to the new cell division sites whereas these structures were not visible with GFP–FtsK. This furtherly suggests that ZapB localizes at the cell division site before FtsK and strengthens the conclusion that ZapB is recruited early to the divisome.
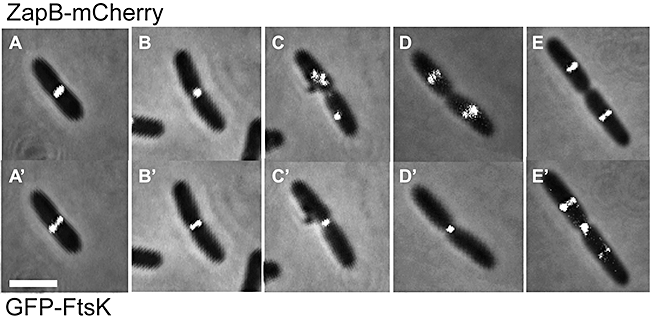
ZapB is recruited to the divisome before FtsK. Combined phase-contrast and fluorescence microscopy images showing ZapB–mCherry and GFP–FtsK localization. MC1000/pEG9 (PBAD::zapB::mCherry)/pFX158 (PBAD::gfp::ftsK) cells were grown in M9 minimal medium supplemented with glucose (0.2%) and casamino acids (0.1%) at 30°C. Expression of both fusion proteins was induced with a 25′ pulse of 0.2% arabinose as described in further detail in Experimental procedures. Representative cells were sampled from liquid culture to generate a pseudo-time-lapse series. (A–E): ZapB–mCherry localization; (A′–E′): GFP–FtsK localization. Scale bar = 2 µm.
ZapB also colocalizes with ZapA
We used a YFP–ZapA fusion (Goehring et al., 2006) to study ZapA localization, and ZapB was again labelled with mCherry. Because of rapid photobleaching of the ZapB–mCherry fusion protein, we were only able to analyse static images. Figure 1B shows cells in which ZapB–mCherry and YFP–ZapA colocalized at the division site. For all cells observed (n = 2000), ZapB and ZapA invariably colocalized.
ZapB constricts inside the Z-ring
In the above-described time-lapse experiment, ZapB–GFP and FtsZ–mCherry colocalized during the entire cell division process in most of the cells. However, careful scrutiny of the images from the time-lapse series revealed that occasionally, the ZapB–GFP ring at mid-cell constricted into a dot just ahead of the FtsZ–mCherry ring (Fig. S1, 40′ to 55′). To increase resolution, we analysed the localization of the two proteins in static images. In particular, we paid close attention to predivisonal cells on the verge of septation. We found that in ≈2% of the cells, ZapB–GFP formed one distinct focus that localized between two dots of FtsZ–mCherry (Fig. 3A), suggesting that the ZapB signal was ahead of that of FtsZ.
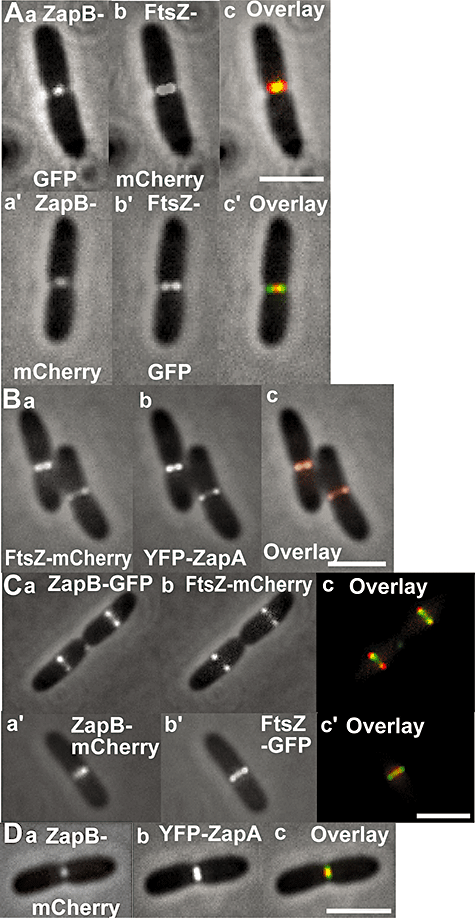
ZapB can constrict ahead of FtsZ. Cells of MC1000 carrying the plasmids listed below were grown in M9-glucose medium supplemented with casamino acids at 30°C and FPs induced as described in Experimental procedures. The Figure shows combined phase-contrast and fluorescence microscopy images.A. Upper panel: MC1000/pEG3a (PBAD::zapB::gfp)/pQW59 (Plac::ftsZ::mCherry). Inserts: (a) ZapB–GFP; (b) FtsZ–mCherry; (c) overlay of (a) and (b). Lower panel: MC1000/pEG9 (PBAD::zapB::mCherry)/pEG12 (Plac::ftsZ::gfp): (a′) ZapB–mCherry; (b′),FtsZ–GFP; (c′) overlay of (a′) and (b′).B. MC1000/pEG4 (PBAD::ftsZ::mCherry)/pNG53 (Plac::yfp::zapA). (a) FtsZ–mCherry; (b) YFP–ZapA; (c) overlay of (a) and (b).C. Upper panel: MC1000/pEG3a (PBAD::zapB::gfp)/pQW59 (Plac::ftsZ::mCherry). Inserts: (a) ZapB–GFP; (b) FtsZ–mCherry; (c) overlay of (a) and (b). Lower panel: MC1000/pEG9 (PBAD::zapB::mCherry)/pEG12 (Plac::ftsZ::gfp). Inserts: (a′) ZapB–mCherry; (b′) FtsZ–GFP; (c′) overlay of (a′) and (b′).D. MC1000/pEG9 (PBAD::zapB::mCherry)/pNG53 (Plac::yfp::zapA). (a) ZapB–mCherry; (b) YFP–ZapA; (c) overlay of (a) and (b). Scale bar = 2 µm.
To ensure that this phenomenon was not due to an artefact produced by the fluorescent proteins, we swapped fluorophores and repeated the experiment. Using the swapped combination, the ZapB–mCherry signal was now ahead of the FtsZ–GFP signal in ≈4% of the cells (Fig. 3A). As FtsZ is the first to assemble and the principal component of the Z-ring, it was unexpected that ZapB was ahead of FtsZ in a significant fraction of the cells. To investigate if this characteristic was specific for ZapB, we analysed ZapA in a similar way using the YFP–ZapA and FtsZ–mCherry fusion proteins. However, in no cases, we observed any significant difference in the patterns of the two flourescent proteins – that is – the proteins colocalized in all cells that we observed (Fig. 3B). Furthermore, in almost all pre-divisional cells, in which septum indentations were not yet visible, the FtsZ and ZapA signals were adjacent to the membrane whereas the ZapB signal was slightly shifted towards the internal part of the cell. This was true for both of the fluorophore combinations that we used (Fig. 3C).
To substantiate that ZapB formed a smaller ring enclosed by FtsZ, we processed Z-stacks of FtsZ–GFP and ZapB–mCherry by deconvolution and reconstructed a 3-D model of the two proteins in the same cell. Indeed, in all the cells processed (n = 27), FtsZ invariably encircled ZapB (the same result was obtained using the pair FtsZ–mCherry/ZapB–GFP). When the diameter of the Z-ring became smaller, ZapB was seen as a dot at the middle of the contracting ring. Figure 4A shows such images tilted at three different angles, and Movies S2–S4 yield further examples of such cells. Z-stacks of FtsZ–mCherry and YFP–ZapA were processed in the same way and the two proteins formed rings of identical dimensions in all the cells analysed (n = 20) (Fig. 4B and Movie S5). As a control, ZapB–mCherry and YFP–ZapA were studied in a similar way: in ≈2% of the cells (n = 3000) ZapB–mCherry formed one distinct focus that localized between two dots of YFP–ZapA (Fig. 3D). In the 3-D reconstructed models, ZapB was now encircled by ZapA in all cells processed (n = 30) (Fig. S2 and Movies S6 and S7) with the exception of one cell in which the two rings had the same diameter.

ZapB ring is enclosed by the Z-ring. Cells of MC1000 carrying (A) pEG9 (PBAD::zapB::mCherry) and pEG12 (Plac::ftsZ::gfp) and (B) pEG4 (PBAD::ftsZ::mCherry) and pNG53 (Plac::yfp::zapA) were grown in M9-glucose medium supplemented with casamino acids at 30°C and FPs induced as described in Experimental procedures. All images were processed by deconvolution imaging. The same cell is shown tilted at three different angles (a–c) labelled with FtsZ–GFP or ZapB–mCherry or the overlay of the two for (A) and FtsZ–mCherry or YFP–ZapA or the overlay of the two for (B).
ZapA is required for recruitment of ZapB to the divisome
The results described above indicated that ZapA and ZapB associate with the Z-ring by different molecular mechanisms and raised the possibility that the interaction between ZapB and FtsZ could be indirect. This possibility was strengthened by the fact that all our attempts to detect a direct interaction between the two proteins in vitro (such as pull down, pelletting and surface plasmon resonance assays) were inconclusive (data not shown). Therefore, we tested if known cell division factors were involved in the recruitment of ZapB to mid-cell. ZapB–GFP localization was analysed in E. coli strains lacking minCD, slmA or zapA. Interestingly, the ZapB–GFP localization pattern was lost in ΔzapA cells (Fig. 5Ac) but not in ΔminCD or ΔslmA cells (Fig. 5Aa and b). Western blot analyses showed that the ZapB–GFP fusion was not destabilized in cells lacking ZapA (Fig. S3).
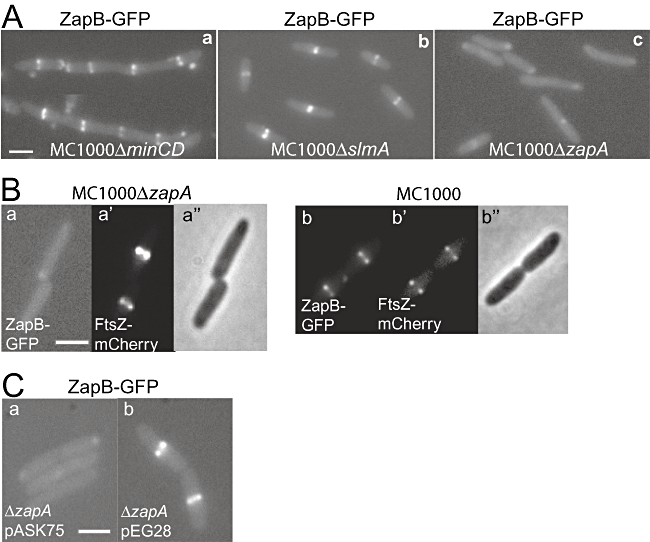
ZapA recruits ZapB to the Z-ring. Localization of ZapB–GFP and FtsZ–mCherry in living cells of strains MC1000 and MC1000ΔzapA. Cells were grown at 30°C in M9-glucose medium supplemented with casamino acids. Expressions of ZapB–GFP from plasmid pEG3a, FtsZ–mCherry from plasmid pQW59 and ZapA from plasmid pEG28 were induced as described in Experimental procedures.A. ZapB–GFP localization in (a) MC1000ΔminCD, (b) MC1000ΔslmA and (c) MC1000ΔzapA.B. Localization of (a and b) ZapB–GFP, (a′ and b′) FtsZ–mCherry and (a″ and b″) phase contrast in living cells of strains MC1000ΔzapA (a, a′ and a″) and MC1000 (b, b′ and b″).C. Localization of ZapB–GFP in (a) MC1000ΔzapA/pEG3a/pASK75 and (b) MC1000ΔzapA/pEG3a/pEG28. Scale bar = 2 µm.
Next, we investigated Z-ring formation in the ΔzapA strain. As shown in Fig. 5B (a′ and b′), the Z-ring was able to form in ΔzapA cells, whereas ZapB required the presence of ZapA to be recruited to the ring (Fig. 5Ba and b). Finally, we tested if ZapB–GFP localization could be restored in ΔzapA cells by ectopic production of ZapA. Indeed, as shown in Fig. 5C, ZapA donated from a plasmid in ΔzapA cells resulted in regeneration of a ZapB–GFP pattern indistinguishable from that of wild-type cells. Taken together, these results show that ZapA is required for the recruitment of ZapB to the divisome.
ZapA and ZapB interact in the bacterial two-hybrid (BTH) assay
Using the BTH approach (Karimova et al., 1998), we showed previously that ZapB yielded a positive response with FtsZ, with itself and, to a lesser extent, with FtsA (Ebersbach et al., 2008). Our new findings prompted us to repeat the BTH experiments in ΔzapA cells. As shown in Fig. 6, deletion of zapA reduced the BTH signals between ZapB–FtsZ and ZapB–FtsA pairs, whereas no such reduction was seen for the ZapB–ZapB or ZapB–ZapA pairs. These observations support that ZapB interacts with ZapA and that ZapB recruitment to the Z-ring occurs indirectly via ZapA.
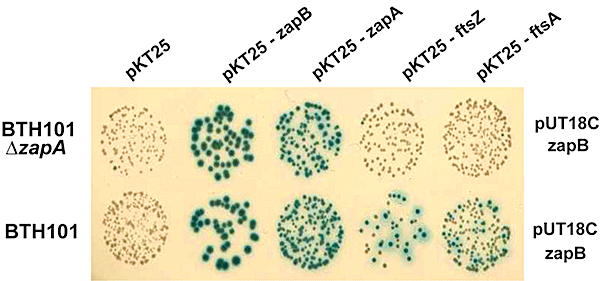
ZapA and ZapB interact in the bacterial two-hybrid assay. Protein interaction detected by bacterial two-hybrid tests. E. coli wild-type strain BTH101 and mutant strain BTH101ΔzapA were co-transformed with two-hybrid vector plasmids (pUT18C and pKT25) expressing fusions to various cell division protein genes as indicated. Transformants were spotted onto selective plates containing X-Gal and incubated at 30°C for 48 h. Blue coloration indicates a positive interaction.
ZapA and ZapB interact in vitro
To directly test this contention, we used Sucrose Gradient Ultracentrifugation of purified FtsZ, His6-ZapA and ZapB-His6. As shown in Fig. 7A, FtsZ and ZapA remained at the top of the gradient (Fraction F#1) whereas ZapB was present in the fractions with a higher sucrose concentration (from F#3 to F#6). The formation of ZapB high-molecular-weight (HMW) complexes (as indicated by the high sedimentation rate) is consistent with ZapB's propensity to spontaneously form polymers (Ebersbach et al., 2008). We then repeated the experiment mixing ZapA, ZapB and FtsZ (in a molar ratio of 3:3:1, respectively) in all the possible combinations: when ZapA and ZapB were mixed together, 10% of ZapA co-sedimented with ZapB – that is – the two proteins were present at the bottom of the gradient (F#6). Strikingly, when the three proteins were mixed, they co-sedimented (10% of ZapA and 10% of FtsZ were present in F#6). In contrast, neither ZapA nor ZapB alone had any effect on FtsZ sedimentation in this assay (Fig. 7B). The experiments were performed in the absence of GTP, precluding the formation of HMW FtsZ–ZapA complexes when ZapA and FtsZ were mixed together. These results demonstrate that ZapA and ZapB interact directly in vitro and that ZapB forms a complex with ZapA and FtsZ.
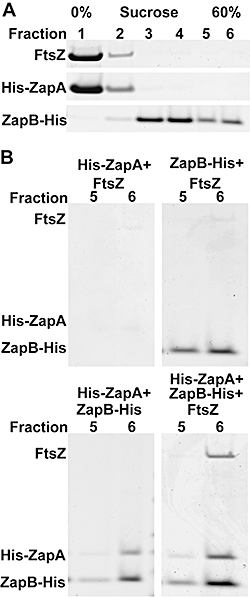
ZapB interacts with ZapA in vitro. SDS-PAGE of sucrose gradient fractions after centrifugation. Purified proteins were mixed in a 50 mM PIPES pH 6.5 buffer and incubated for 30 min at room temperature before loading onto sucrose gradients.A. The gradient was divided into six fractions from the lowest (F#1, 0%) to the highest (F#6, 60%) sucrose concentration. FtsZ and His6-ZapA localized predominantly in F#1, ZapB-His6 from F#3 to F#6.B. The three proteins were mixed together in all the possible combinations and the last two fractions of the gradient (F#5 and F#6) are shown. His6-ZapA in presence of ZapB-His6 partially precipitated in the last fraction (F#6) and FtsZ sedimented at the bottom of the gradient only when His6-ZapA and ZapB-His6 were both present.
Morphological analysis of cell division mutants
We combined deletions of zapB, zapA, slmA and minCD in all possible permutations and examined the resulting mutant strains for defects in cell growth, morphology and FtsZ localization. These data are summarized in Table 1 and Fig. S4A. The combination ΔslmAΔminCD is synthetic lethal (Bernhardt and de Boer, 2005) and was not included in our analysis. In rich and minimal liquid media, the mass-doubling times of all the deletion strains were indistinguishable from that of the wild-type strain (Fig. S5A).
| Strain | Cell length (µm)a | Mutant/wild-type ratio | FtsZ–GFP mislocalizedb,c |
|---|---|---|---|
| MC1000 | 2.80 ± 0.04 | 1 | 2% |
| ΔzapA | 3.89 ± 0.07 | 1.4 | 25% |
| ΔzapB | 3.93 ± 0.07 | 1.4 | 21% |
| ΔzapAΔzapB | 4.70 ± 0.11 | 1.7 | 35% |
| ΔzapBΔminCD | 6.89 ± 0.24 | 2.5 | 61% |
| ΔzapAΔminCD | 9.34 ± 0.36 | 3.3 | 94% |
| ΔzapAΔzapBΔminCD | 11.92 ± 0.64 | 4.3 | 95% |
| MG1655 (= TB28) | 3.15 ± 0.05 | 1 | ND |
| ΔzapA | 4.52 ± 0.05 | 1.4 | ND |
| ΔzapB | 4.32 ± 0.10 | 1.4 | ND |
| ΔzapAΔzapB | 5.69 ± 0.07 | 1.8 | ND |
- a. Standard error is indicated.
- b. Immunoblotting on the appropriate strains using α-FtsZ antibodies was used to confirm that FtsZ–GFP was present in equal amounts in all the strains and that FtsZ–GFP mislocalization was not due to differences in the levels of expression (Fig. S7).
- c. A classification of FtsZ mislocalization for each strain is summarized in Fig. S4B and C.
- All the strains were grown in LB at 37°C; at an OD450 value of ∼0.4 samples were collected and cell lengths measured. FtsZ–GFP was expressed from plasmid pEG12 as described in Experimental procedures and samples analysed 1 h after induction. For all entries except the last four, 250 cells were measured for every strain. For the last four entries, 100 cells were measured for every strain. FtsZ–GFP localization was analysed in ∼1000 cells for each strain.
- ND, no data.
Deletion of zapA or zapB resulted in a ≈40% increase in cell length in both cases and a significant increase in the fraction of cells with mislocalized Z-rings (25% and 21% respectively). Deletion of slmA had a milder effect (30% increase in cell length and 12% cells with mislocalized Z-rings). Deletion of minCD resulted in a considerably larger increase in cell length (120%) and more than 50% mislocalized Z-rings.
Introduction of the slmA deletion into the single ΔzapA and ΔzapB strains only marginally affected cell length and Z-ring localization (Fig. S4A). However, unexpectedly, the ΔzapAΔzapB double deletion exhibited a 70% increase in cell length and 35% of such cells had mislocalized Z-rings. This result raises the possibility that ZapA and ZapB may have separate functions in cell division even though they interact (see Discussion). In further support of this hypothesis, 2% of cells lacking ZapB have polar Z-rings or structures (Fig. S6), whereas this was not the case for cells lacking ZapA.
Cells of the triple ΔzapAΔzapBΔminCD mutant had a significantly increased cell length, grew slower than the other strains on solid medium (Fig. S5C) and displayed a plating defect of one order of magnitude (Fig. S5B). In liquid media, this strain produced very long cells (330% increase) but its mass-doubling rate was not affected. In contrast, deleting slmA from the ΔzapAΔzapB strain did not seriously affect cell growth or division. Again, it is noteworthy that the ΔzapAΔzapBΔminCD strain produced cells longer than theΔzapAΔminCD strain (330% vs. 230% increase in cell length), supporting the idea that ZapB may have an effect on cell division that is independent of ZapA.
To assess if the phenotypes described above were background-dependent, the ΔzapA and ΔzapB alleles were transduced into MG1655, a fully sequenced standard E. coli K-12 strain. Cell length measurements confirmed that the relative increases in cell lengths were very similar in MG1655 and MC1000, showing that the phenotypes were not strain-specific (Table 1).
Complementation analysis
To further confirm that ZapB has a role in cell division that is independent of ZapA, we repeated the cell length analyses of zapA, zapB and zapA zapB deletion strains in the presence of plasmids that produced ZapA, ZapB or ZapA plus ZapB. Table 2 summarizes the results of the complementation analysis. Copies of ZapA or ZapB on plasmids fully restored the wild-type cell length phenotype. Strikingly, however, ZapB was able to significantly mitigate the cell length defect of the zapA zapB double mutant – that is – ZapB remains seemingly functional even in the absence of ZapA.
| Strain | Cell length (µm)a | Mutant/wild-type ratio |
|---|---|---|
| MC1000/pOU82 (vector) | 3.70 ± 0.06 | 1 |
| ΔzapA/pOU82 (vector) | 4.80 ± 0.30 | 1.3 |
| ΔzapA/pEG83 (PzapA::zapA) | 3.80 ± 0.07 | 1 |
| ΔzapB/pOU82 (vector) | 4.80 ± 0.13 | 1.3 |
| ΔzapB/pEG82b (PzapB::zapB) | 3.80 ± 0.08 | 1 |
| ΔzapAΔzapB/pOU82 (vector) | 5.90 ± 0.03 | 1.6 |
| ΔzapAΔzapB/pEG83 (PzapA::zapA) | 5.30 ± 0.20 | 1.4 |
| ΔzapAΔzapB/pEG82b (PzapB::zapB) | 5.10 ± 0.20 | 1.3 |
| ΔzapAΔzapB/pEG82 (PzapB::zapB zapA) | 4.00 ± 0.06 | 1.1 |
- a. Standard error is indicated.
- All the strains were grown in LB at 37°C; at an OD450 value of ∼0.4 samples were collected and cell lengths measured. For all the entries, 100 cells were measured for every strain (2 replicates each).
Discussion
We showed previously that ZapB is recruited to the Z-ring in a pattern very closely resembling that of FtsZ itself. Moreover, the simultaneous inactivation of FtsA and ZipA, which prevents Z-ring assembly (Pichoff and Lutkenhaus, 2002), abolished ZapB recruitment to mid-cell, showing that ring assembly was required for efficient recruitment. To gain further insight, we used double labelling of FtsZ and ZapB. We found that ZapB consistently colocalized with FtsZ (Fig. 1 and Movie S1) and that ZapB was recruited to the divisome earlier than FtsK (Fig. 2). Remarkably, high-resolution microscopy showed that ZapB was slightly ahead of FtsZ during constriction (3, 4 and Movies S2–S4).
The careful analysis of the pairs ZapA/FtsZ and ZapA/ZapB using the same high-resolution technique showed that ZapB was also ahead of ZapA (Figs. 3 and S2 and Movies S6 and S7) whereas ZapA and FtsZ colocalized in all samples inspected (3, 4 and Movie S5).
ZapB recruitment to the Z-ring was indirect: it depended on ZapA (Fig. 5A and B) and consistent with these findings, ZapB interacted with ZapA in the BTH assay (Fig. 6). In contrast, ZapA recruitment to the Z-ring did not depend on ZapB (Fig. S8). These results support the proposal that ZapB is recruited to the Z-ring via its interaction with ZapA.
This interpretation is further supported by our in vitro interaction studies. In the sucrose gradient assay, ZapB spontaneously formed HMW complexes that were able to pull ZapA down to the bottom of the gradients (Fig. 7). Moreover, ZapA and ZapB together made a HMW complex with FtsZ, whereas neither protein alone was able to pull FtsZ down. Several previous reports have shown that ZapA and FtsZ interact directly (Small et al., 2007; Mohammadi et al., 2009). Indeed, affinity chromatography analysis of our preparations of FtsZ and ZapA revealed that they did interact (Fig. S9). Thus, the lack of a detectable ZapA–FtsZ co-sedimentation at the bottom of our gradients indicates that FtsZ and ZapA did not form HMW complexes in the experimental conditions we used, even though they probably did interact.
Interestingly, in addition to the Z-ring, FtsZ also forms dynamic helices (Ben-Yehuda and Losick, 2002; Thanedar and Margolin, 2004; Grantcharova et al., 2005; Chauhan et al., 2006; Thanbichler and Shapiro, 2006). In B. subtilis, a long helical filament of FtsZ moving from pole to pole was present at all stages of growth and collapsed into a ring in pre-divisional cells (Peters et al., 2007). In newborn cells, FtsZ moved randomly and dynamically within the extended helical pattern. However, at a later stage of the cell cycle, the helix was remodelled so that the majority of FtsZ molecules, while still localized in a helical pattern, were restricted to a small central region of the cell. This central intermediate helix persisted for a considerable period, before finally reorganizing into the Z-ring at mid-cell (Peters et al., 2007). A similar helical and dynamic FtsZ pattern has been observed in E. coli (Thanedar and Margolin, 2004). These observations raise the possibility of the existence of factors that facilitate or control the Z helix-to-ring transition. Interestingly, a recent report proposed that ZapA promotes lateral interactions between FtsZ proto-filaments and thereby stimulates polymer association during the transition from helical filament to mid-cell ring (Monahan et al., 2009). This suggestion was supported by biochemical studies (Mohammadi et al., 2009).
In the following section, we present a simple model describing the function of ZapA and ZapB in Z-ring assembly (Fig. 8). ZapA interacts directly with FtsZ (Small et al., 2007; Mohammadi et al., 2009) and promotes lateral interaction between proto-filaments, thereby facilitating the helix-to-ring transition and/or stabilization of the Z-ring. ZapB forms spontaneous filaments (Ebersbach et al., 2008) and interacts with ZapA. The cross-interaction between ZapB and individual ZapA molecules decorating FtsZ proto-filaments stabilizes the lateral interactions between individual, adjacent filaments. The relatively high copy number of ZapB in the cell (≈13 000 per cell) (Ebersbach et al., 2008), two to four copies per FtsZ monomer (Pla et al., 1991; Rueda et al., 2003) is sufficient to form short filaments that connect adjacent FtsZ proto-filaments decorated with ZapA. Even though purified FtsZ has been shown to be able to generate a constriction force in liposomes (Osawa et al., 2008), it is plausible that additional factors, such as ZapA and ZapB, help to form a more stable Z-ring structure in vivo and thereby facilitate Z-ring constriction. The fact that ZapB forms a ring that seems to be located inside the Z-ring may be explained by the fact that ZapB (or ZapA) may be able to form filament protrusions that connect the two rings and keep them constantly spaced. ZapB and FtsZ in the time-lapse experiments always localize at and leave the septum at the same time, meaning that their relocation to the new cell division sites is co-ordinated.
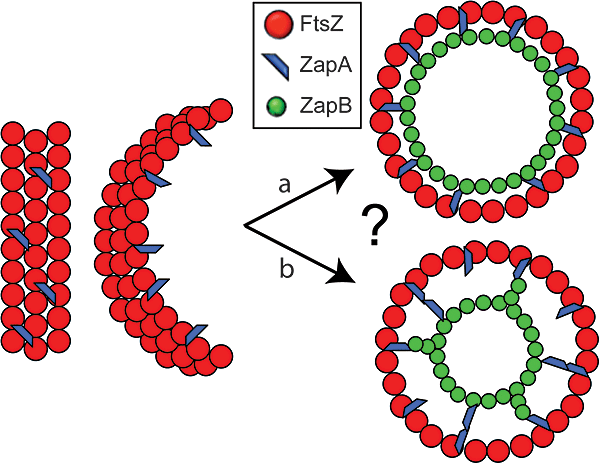
Working model explaining Z-ring stabilization by ZapA and ZapB. ZapA strengthens lateral interactions between individual FtsZ proto-filaments in the Z-ring. (a) ZapB bridges ZapA molecules (or short polymers) that interact with FtsZ and thereby strengthens the lateral interactions, forming a ring scaffold that supports and stabilizes the formation of the Z-ring. (b) The fact that ZapB forms a ring that seems located further inside in the cell compared with the one of FtsZ and ZapA is explained by the fact that ZapB (or ZapA) may be able to form filament protrusions that connect the two rings and keep them constantly spaced.
An alternative possibility is that ZapB directly interacts with ZapA during relocation to mid-cell, when FtsZ transitions from a helical-like structures to a ring-like structure, but once the Z-ring is formed, ZapB may be able to generate an independent ring located further inside at the septum. We do not, of course, exclude other explanations.
The model proposed above is consistent with our cytological observations and in vitro interaction data. However, unexpectedly, our genetic analysis showed that ZapB can stimulate Z-ring assembly independently of ZapA. Thus, the deletion of zapB increased cell length and Z-ring mislocalization, irrespective of the presence or absence of zapA (Table 1). Furthermore, expression of ZapB in a ΔzapAΔzapB double deletion strain mitigated the cell length defect (Table 2). To reconcile these observations with the recruitment of ZapB–GFP to the Z-ring via ZapA, we note the possibility that ZapB may be able to affect Z-ring formation by two different mechanisms: directly via ZapA and indirectly by an as yet unknown mechanism.
Previously, we showed that overexpression of ZapB leads to highly compacted and mislocalized nucleoids (Ebersbach et al., 2008). In an attempt to understand the ZapA-independent effect of ZapB, we further characterized the effects of ZapB overproduction. But the results included in the Supporting Information, most likely, suggest that nucleoid condensation by ZapB overproduction is an artefact (Fig. S10).
In B. subtilis, deletion of zapA caused no recognizable phenotype (Gueiros-Filho and Losick, 2002). However, we show here that the deletion of zapA in E. coli increased cell length reproducibly (Tables 1 and 2) and is consistent with data presented recently (Mohammadi et al., 2009). In B. subtilis, the combination of a zapA deletion and a mutation in divIVA, responsible for the polar localization of MinCD, had a synergistic effect and resulted in highly filamentous cells (Gueiros-Filho and Losick, 2002). This effect is reminiscent of that seen with E. coli cells lacking ZapA and MinCD (Table 1).
In E. coli, the ΔzapB and ΔzapA phenotypes are very similar: the deletions resulted in similar increases in cell length, FtsZ double bands and helix-formation at mid-cell. These phenotypes are consistent with a less stable or incorrectly organized Z-ring. The main difference however was that ≈2% of ΔzapB cells had polar Z-rings, which was not seen with ΔzapA cells.
In B. subtilis, deletion of the early cell division protein SepF resulted in septa with an abnormal and incomplete morphology (Hamoen et al., 2006; Ishikawa et al., 2006). To see if this was also the case with ZapB of E. coli, we investigated septum morphology by EM. However, our EM analysis did not show any altered morphology of the division septum, consistent with an early recruitment of ZapB to the divisome (data not shown).
In summary, we show here that ZapA recruits ZapB to the Z-ring and that the three proteins form a complex in vitro. ZapB localize to the inner face of the Z-ring forming an open ring with a smaller diameter compared with the one od FtsZ. To our knowledge, this is the first time that a protein recruited to a sub-cellular structure in bacteria has been discriminated cytologically from the structure itself (i.e. the Z-ring). It will be interesting to learn if Z-ring dynamics or kinetics is regulated by ZapA and/or ZapB.
Experimental procedures
Strains and plasmids
Strains and plasmids are listed in Table 3 and oligonucleotides in Table S1.
| Strain or plasmid | Relevant genotype/description | Resistance | Reference/source |
|---|---|---|---|
| Strains | |||
| BL21 A1 | Arabinose-inducible gene expression from T7 promoter | Invitrogen | |
| BL21DE3 | IPTG-inducible gene expression from T7 promoter | Invitrogen | |
| BTH101 | F-, cya-99 araD139 galE15 galK16 rpsL1 hsdR2 mcrA1 mcrB1 | Karimova et al. (1998) | |
| BTH101ΔzapA | BTH101 in which the zapA gene is deleted | This work | |
| C41 | F-, ompT hsdSB (rB- mB-) gal dcm | ||
| MC1000 | Δ(ara-leu) Δlac rpsL150 | Casadaban and Cohen (1980) | |
| MC1000ΔminCD | MC1000 minCD::kan | Kan | Ebersbach et al. (2008) |
| MC1000ΔslmA | MC1000 ΔslmA | Ebersbach et al. (2008) | |
| MC1000ΔzapA | MC1000 ΔzapA | Ebersbach et al. (2008) | |
| MC1000ΔzapB | MC1000 ΔzapB | Ebersbach et al. (2008) | |
| MC1000ΔminCDΔzapA | MC1000 ΔzapA minCD::kan | Kan | This work |
| MC1000ΔminCDΔzapB | MC1000 ΔzapB minCD::kan | Kan | Ebersbach et al. (2008) |
| MC1000ΔslmAΔzapB | MC1000 ΔzapBΔslmA | Ebersbach et al. (2008) | |
| MC1000ΔzapAΔslmA | MC1000 ΔslmAΔzapA | This work | |
| MC1000ΔzapAΔzapBΔslmA | MC1000 ΔzapAΔzapBΔslmA | This work | |
| MC1000ΔzapAΔzapBΔminCD | MC1000 ΔzapBΔzapA minCD::kan | Kan | This work |
| TB28 | MG1655 ΔlacIZYA | Laboratory collection | |
| TB28ΔzapA | TB28 ΔzapA | This work | |
| TB28ΔzapB | TB28 ΔzapB | This work | |
| TB28ΔzapAΔzapB | TB28 ΔzapAΔzapB | This work | |
| Plasmids | |||
| pASK75 | Cloning vector, contains PoxyTc promoter | Amp | Skerra (1994) |
| pBAD24 | Cloning vector, contains PBAD promoter | Amp | Guzman et al. (1995) |
| pBAD33 | Cloning vector, contains PBAD promoter | Cml | Guzman et al. (1995) |
| pCA24N-zapA+ | pT5lac::his-zapA | Cml | Kitagawa et al. (2005) |
| pEGFP | GFP fusion vector | Amp | Clontech |
| pEG3a | PBAD::zapB::gfp | Cml | Ebersbach et al. (2008) |
| pEG4 | PBAD::ftsZ::mCherry | Cml | This work |
| pEG6 | Plac::zapB::mCherry | Amp | This work |
| pEG7 | Plac::ftsZ::gfp | Amp | This work |
| pEG9 | PBAD::zapB::mCherry | Cml | This work |
| pEG12 | Plac::ftsZ::gfp | Amp | This work |
| pEG28 | POxyTc::zapA | Amp | This work |
| pEG82b | PzapB::zapB | Amp | This work |
| pEG82 | PzapB::zapB zapA | Amp | This work |
| pEG83 | PzapA::zapA | Amp | This work |
| pEG86 | PzapB::zapB::gfp | Amp | This work |
| pEG88 | PzapB::zapB::his | Amp | This work |
| pEG89 | PzapA::his::zapA | Amp | This work |
| pEG90 | PzapB::zapB::mCherry | Amp | This work |
| pFX158 | PBAD::gfp::ftsK | Amp | Dubarry and Barre (2009) |
| pMFV56 | Plac::ftsZ | Kan | Rivas et al. (2000) |
| pNDM220 | Cloning vector, contains Plac promoter | Amp | Gotfredsen and Gerdes (1998) |
| pGE604 | PBAD::zapB | Cml | Ebersbach et al. (2008) |
| pNG53 | PTrc99a::yfp::zapA, IPTG-regulated | Amp | Goehring et al. (2006) |
| pOU82 | Amp | Laboratory collection | |
| pQW58 | mCherry fusion vector | Amp | Gerdes laboratory |
| pQW59 | Plac::ftsZ::mCherry | Amp | Gerdes laboratory |
| pKT25 | T25 fusion vector | Kan | Karimova et al. (1998) |
| pKT25-FtsA | Plac::T25::ftsA | Kan | Karimova et al. (2005) |
| pKT25-FtsZ | Plac::T25::ftsZ | Kan | Ebersbach et al. (2006) |
| pKT25-ZapA | Plac::T25::zapA | Kan | This work |
| pKT25-ZapB | Plac::T25::zapB | Kan | Ebersbach et al. (2008) |
| pUT18C | T18 fusion vector | Amp | Karimova et al. (1998) |
| pUT18C-FtsA | Plac::T18::ftsA | Amp | Karimova et al. (2005) |
| pUT18C-FtsZ | Plac::T18::ftsZ | Amp | Ebersbach et al. (2006) |
| pUT18C-ZapA | Plac::T18::zapA | Amp | This work |
| pZapBHis | PT7::zapB::His6 | Amp | Ebersbach et al. (2008) |
- Kan, kanamycin; Amp, ampicillin; Cml, chloramphenicol.
Construction of plasmids
Purified MG1655 chromosomal DNA was used as the template for PCR reactions.
Plasmid pEG4 carries ftsZ::mCherry downstream of the arabinose-inducible PBAD promoter of vector pBAD33. A BamHI(blunted)-HindIII fragment from pQW59 carrying ftsZ::mCherry was inserted into SmaI-HindIII sites of pBAD33.
Plasmid pEG6 carries zapB::mCherry downstream of the IPTG-inducible Plac promoter of vector pQW58. zapB was amplified by PCR with primers: ForZapB–RFP + RevZapB–RFP. The PCR fragment was cut with restriction enzymes BamHI and SacI and cloned into the corresponding sites of pQW58. Subsequently, an EcoRI(blunted)-HindIII fragment carrying zapB::mCherry was inserted into the SmaI-HindIII sites of pBAD33, creating pEG9.
Plasmid pEG7 carries ftsZ::gfp downstream of the IPTG-inducible Plac promoter of vector pEGFP. ftsZ was amplified by PCR with primers: ForFtsZ–GFP + RevFtsZ–GFP. The PCR fragment was cut with restriction enzymes SmaI and NcoI and cloned into the corresponding sites of pEGFP. Subsequently, a BamHI-StuI fragment carrying ftsZ::gfp was inserted into BamHI-PmlI sites of pNDM220, creating pEG12.
Plasmid pEG28 is a pASK75 derivative that carries zapA downstream of the oxyTc-inducible promoter. zapA was amplified by PCR with primers: zapA-3 + zapA-4. The PCR product was cut with BamHI and HindIII and cloned into the same sites of pASK75.
Plasmids used in the BTH system. Plasmids were constructed that express in frame fusions of zapA and zapB to the C-termini of the two complementary fragments T18 (vector pUT18C) and T25 (vector pKT25) of the catalytic domain of the Bordetella pertussis adenylate cyclase. zapA and zapB were amplified by PCR with the following primers: zapA: zapA-1 + zapA-2. The PCR fragments were cloned between the SalI and KpnI sites of pUT18C, and between the BamHI and KpnI sites of pKT25.
Plasmids pEG82b and pEG83 are derivatives of pOU82. Plasmid pEG82b carries zapB under its own native promoter: zapB was amplified by PCR with primers: zapB-82 + zapB-83. The PCR fragment was cut with restriction enzymes EcoRI and BamHI and cloned into the corresponding sites of pOU82. Plasmid pEG83 carries zapA under its own native promoter; zapA was amplified by PCR with primers: pEG83-For + pEG83-Rev. The PCR fragment was cut with restriction enzymes EcoRI and BamHI and cloned into the corresponding sites of pOU82.
Plasmid pEG82 is a derivative of pEG82b; zapA was cloned immediately after zapB; zapA was amplified by PCR with primers: zapA-82 + zapA-83. The PCR fragment was cut with restriction enzymes Asp718 and BamHI and cloned into the corresponding sites of pEG82b.
The functionality of ZapB and ZapA fusion proteins is analysed in Table S2.
Microscopy
For phase-contrast and fluorescence microscopy 1–3 µl of a culture sample was placed on a microscope slide coated with a thin agarose (1%) layer and covered with a coverslip. For membrane or DNA staining a 10 µl culture sample was mixed with 1 µl of Nile Red (Molecular Probes) (12.5 µg ml−1) or 1 µl of DAPI (Sigma) solution (1 µg ml−1) respectively. Images were acquired with a Sony Cool-Snap HQ cooled CCD camera (Roper Scientific) attached to a Zeiss Axiovert 200 M microscope. The images were acquired and analysed with METAMORPH version 6 software. Final image preparation was performed in Adobe Photoshop 6.0 (Adobe Systems Incorporated). For the Z-stacks and 3-D reconstruction images were acquired with a Deltavision microscope (Applied Precision). The images in the Z series were recorded with step size of 0.2 µm and deconvolved using software softWoRx version 3.5.1. The 3-D models were rendered using software softWoRx version 3.5.1.
Growth and analysis of deletion strains
The growth of all deletion and wild-type strains was performed in Luria–Bertani (LB) at 30°C and in M9-MM and LB at 37°C. Overnight cultures were diluted 500-fold into fresh medium. To follow cell growth, samples were collected at regular intervals for optical density OD450 measurements. At OD450 values of about 0.4, cells were mounted for microscopy and the cell length measured.
Fluorescent fusion proteins expression and localization
Cells were grown at 30°C or 37°C in M9 minimal medium supplemented with 0.2% glucose, 1 µg ml−1 thiamine, 0.1% casamino acids and the specific antibiotic [30 µg ml−1 chloramphenicol (pEG3a, pEG4, pEG9), 100 µg ml−1 ampicillin (pFX158, pNG53, pQW59), 30 µg ml−1 ampicillin (pEG12, pEG82b, pEG82, pEG83)]. ZapB–GFP induction and localization is described in Ebersbach et al. (2008). FtsZ–mCherry from pEG4: at an OD450 value of about 0.2, the cells were harvested by centrifugation followed by resuspension in pre-heated medium without glucose. Arabinose (0.2%) was added in order to induce expression of FtsZ–mCherry. After 1 min, induction was stopped by adding 0.2% glucose. ZapB–mCherry from pEG9 and GFP–FtsK from pFX158: at an OD450 value of about 0.2, the cells were harvested by centrifugation followed by resuspension in pre-heated medium without glucose. Arabinose (0.2%) was added in order to induce expression of ZapB–mCherry and GFP–FtsK. After 25 min induction was stopped by adding 0.2% glucose. YFP–ZapA from pNG53 and FtsZ–mCherry from pQW59: the leakiness of the promoter was sufficient for localization studies. FtsZ–GFP from pEG12: at an OD450 value of about 0.2 FtsZ–GFP was induced by 200 µM IPTG. After induction, cells were allowed to grow for at least another 30 min before the samples were collected for microscopy. If not differently specified in the Results session, all the localization analyses were performed using fusions expressed from a plasmid in addition to the native untagged copy on the chromosome.
Construction of E. coli strains carrying deletions of zapA, zapB, slmA or minCD
zapA::kan, zapB::kan, slmA::cml and minCD::kan were introduced into the respective receiver strain by P1 transduction using strains carrying a single gene deletion as donors. Colonies were tested for the insertion of the resistance gene by using colony-PCR with oligoes that anneal outside of the deleted chromosomal regions: zapA-up + zapA-down, zapB-up + zapB-down, slmA-up + slmA-down and minC-up + minD-down, respectively for zapA, zapB, slmA and minCD. The kanamycin and the chloramphenicol resistance genes were finally eliminated by transforming the deletion strains with the temperature-sensitive pCP20 helper plasmid that encodes the FLP recombinase, which acts on target sites flanking the resistance genes. Because of temperature-sensitive replication, the pCP20 plasmid could be eliminated by incubation at 42°C. It was not possible to eliminate the kanamycin resistance gene from the strains carrying minCD::kan.
ZapB-His, His-ZapA and FtsZ purification
FtsZ was expressed and purified as described in Rivas et al. (2000). ZapB-His was expressed and purified as described in Ebersbach et al. (2008). His-ZapA was overexpressed from plasmid pCA24N-zapA+ in E. coli C41 cells. Cells were grown at 37°C in 2xTY medium supplemented with 30 µg ml−1 chloramphenicol. At mid-exponential growth, protein expression was induced by the addition of 1 mM IPTG. After 5 h of induction, the cells were harvested, resuspended in buffer A (50 mM Tris-HCl, pH 7; 300 mM NaCl; 15 mM imidazole) containing 5 µg ml−1 DNase I and 1 mg ml−1 lysozyme and lysed by sonication. The lysate was cleared by centrifugation for 30 min at 20k r.p.m. The cleared lysate was loaded onto a 1 ml HisTrap (GE Healthcare) column, and protein was eluted with a buffer B (buffer A + 1M imidazole) gradient. Purified His-ZapA was dialysed into buffer C (20 mM Tris-HCl; 10% glycerol), concentrated, snap-frozen in liquid nitrogen and stored at −80°C.
Sucrose gradient centrifugation
For interaction studies between His-ZapA, ZapB-His and FtsZ a step gradient of 10%, 20%, 30%, 40%, 50% and 60% sucrose was used. Centrifugation was carried out for 2 h (25k r.p.m., 4°C) in a Beckman Rotor SW50.1/55 using 0.8 ml tubes (5 × 41 mm) and suitable adapters. Purified proteins (10–15 µg each) were mixed in the presence of BSA (the absence of BSA did not change the sedimentation patterns) in a 50 mM PIPES pH 6.5 buffer and incubated for 30 min at room temperature before loading onto sucrose gradients. The sucrose gradients were prepared using the same buffer. After centrifugation, the gradients were fractionated in 100 µl samples that were analysed by SDS-PAGE and Coomassie staining.
Bacterial two-hybrid analysis
Bacterial two-hybrid analysis was performed as described by Karimova et al. (1998). A 15 µl aliquot from each transformation reaction was spotted onto nutrient agar plates containing 100 µg ml−1 ampicillin, 50 µg ml−1 kanamycin, 0.1 mM IPTG and 0.004% X-Gal. Pictures were taken after 48 h of growth at 30°C.
Acknowledgements
We would like to thank Jeff Errington for stimulating discussion and Leendert Hamoen for useful comments. We thank David Adams for critical reading of the manuscript, German Rivas for gift of the plasmid pMFV56, Nathan Goehring for plasmid pNG53, Francois-Xavier Barre for plasmid pFX158 and Qing Wang for plasmids pQW58 and pQW59. This work was supported by the Biotechnology and Biological Sciences Research Council and E.G. has partially been funded by a Marie Curie Early Stage Research Training Fellowships (ATP-BCT).




