An endocytic mechanism for haemoglobin-iron acquisition in Candida albicans
Summary
The fungal pathogen Candida albicans is able to utilize haemin and haemoglobin as iron sources. Haem-iron utilization is facilitated by Rbt5, an extracellular, glycosylphophatidylinositol (GPI)-anchored, haemin- and haemoglobin-binding protein. Here, we show that Rbt5 and its close homologue Rbt51 are short-lived plasma membrane proteins, degradation of which depends on vacuolar activity. Rbt5 facilitates the rapid endocytosis of haemoglobin into the C. albicans vacuole. We relied on recapitulation of the Rbt51-dependent haem-iron utilization in Saccharomyces cerevisiae to identify mutants defective in haemoglobin utilization. Homologues of representative mutants in S. cerevisiae were deleted in C. albicans and tested for haemoglobin-iron utilization and haemoglobin uptake. These mutants define a novel endocytosis-mediated haemoglobin utilization mechanism that depends on acidification of the lumen of the late secretory pathway, on a type I myosin and on the activity of the ESCRT pathway.
Introduction
Candida albicans is a commensal microorganism that can cause deep-seated, life-threatening infection among immunocompromised or debilitated patients (Richardson and Warnock, 1997). In order to survive in the host, C. albicans, like all microbial pathogens, needs to acquire essential nutrients. Among others, pathogens have evolved mechanisms to acquire iron, an essential but scarce element in the host environment, from the host tissues (Payne, 1993). Fungal iron uptake systems have been best characterized in Saccharomyces cerevisiae. Low-affinity elemental iron transport depends on the ferric reductases Fre1 and Fre2 (Georgatsou and Alexandraki, 1994) and the permease Fet4 (Dix et al., 1994; 1997). High-affinity iron import depends on the permease Ftr1 (Stearman et al., 1996) in conjunction with the cell-surface multicopper ferroxidase, Fet3 (Askwith et al., 1994; De Silva et al., 1995), which acts downstream of the reductases. Biogenesis of Fet3 occurs in a trans-Golgi compartment and requires the activity of an intracellular copper transporter, Ccc2 (Yuan et al., 1995). Iron can also be acquired in a siderophore-bound form. S. cerevisiae contains four characterized siderophore transporters (Lesuisse et al., 1998; Heymann et al., 2000; Yun et al., 2000). Many C. albicans orthologues of the components of the S. cerevisiae high-affinity iron import system have been identified. Two high-affinity iron permeases were cloned, Ftr1 and Ftr2; Ftr1, a plasma membrane protein, is required for growth in limiting iron, whereas Ftr2 may be a vacuolar protein (Ramanan and Wang, 2000). A multicopper iron ferroxidase (Eck et al., 1999) and a Ccc2 copper transporter orthologue (Weissman et al., 2002) are also required for growth under iron-limited conditions. In addition, C. albicans contains a single characterized siderophore transporter, CaArn1 (Ardon et al., 2001; Hu et al., 2002).
Candida albicans, like many bacterial pathogens, is able to utilize haemin and haemoglobin as iron sources, and this pathway is distinct from the high-affinity iron uptake pathway (Weissman et al., 2002). By screening a C. albicans genomic library, RBT51/PGA10 was identified as a gene able to confer to S. cerevisiae the ability to utilize haemoglobin (Weissman and Kornitzer, 2004). RBT5 is a close homologue of RBT51 that is strongly induced by iron limitation, such as is induced by addition of the iron chelator ferrozine. Deletion of RBT5 strongly impairs the ability of C. albicans to utilize haemin and haemoglobin as iron sources, indicating a central role for this protein in haem-iron utilization. Rbt5 and Rbt51 carry a CFEM domain, characterized by eight cysteine residues of conserved spacing, a domain found in many fungal membrane protein, mostly of unknown function (Kulkarni et al., 2003). Characterization of Rbt5 and Rbt51 indicated that they function as extracellular glycosylphophatidylinositol (GPI)-anchored haem and haemoglobin receptors (Weissman and Kornitzer, 2004). Another gene, CaHMX1, was identified as encoding an intracellular haem oxygenase that breaks down iron-protoporphyrin IX to α-biliverdin and is required for haem-iron utilization (Santos et al., 2003; Pendrak et al., 2004).
The mechanism by which the iron atom is extracted from haemoglobin and taken up into the fungal cell is unknown. Rbt5/51-mediated haemoglobin-iron utilization could involve either extracellular extraction of the haem from haemoglobin, and tethering of the haem at the membrane, followed by uptake by a membrane haem receptor; or internalization of the haemoglobin via endocytosis, and intracellular extraction of the haem iron. The data that we present here distinguish between these possibilities, and genetically define a new endocytic pathway of haem-iron utilization.
Results
Rbt5-facilitated uptake of haemoglobin into the vacuole
To obtain insight into the mechanism by which haemoglobin, a large macromolecule, can be utilized as iron source by the fungal cell, we fluorescently tagged haemoglobin with rhodamine B (RB-Hb), and followed its interaction with the cells by epifluorescence microscopy. C. albicans cells were grown in iron-depleted medium, a treatment that induces Rbt5, and incubated with RB-Hb. As shown in Fig. 1A, at low RB-Hb concentrations (0.1 μM, 0.5 μM), the wild-type cells displayed rim staining, suggesting binding to the cell envelope, as well as an accumulation of the label within an intracellular compartment. The rbt5−/−rbt51−/− mutant, in contrast, did not bind or accumulate the label at low RB-Hb concentrations. As this mutant is able to utilize haemoglobin at higher concentrations (Weissman and Kornitzer, 2004), we also tested label incorporation with 2.5 μM RB-Hb. At these concentrations, intracellular staining was visible within the mutant cells as well as in the wild-type cells, but rim staining was significantly weaker in the mutant cells (Fig. 1A).
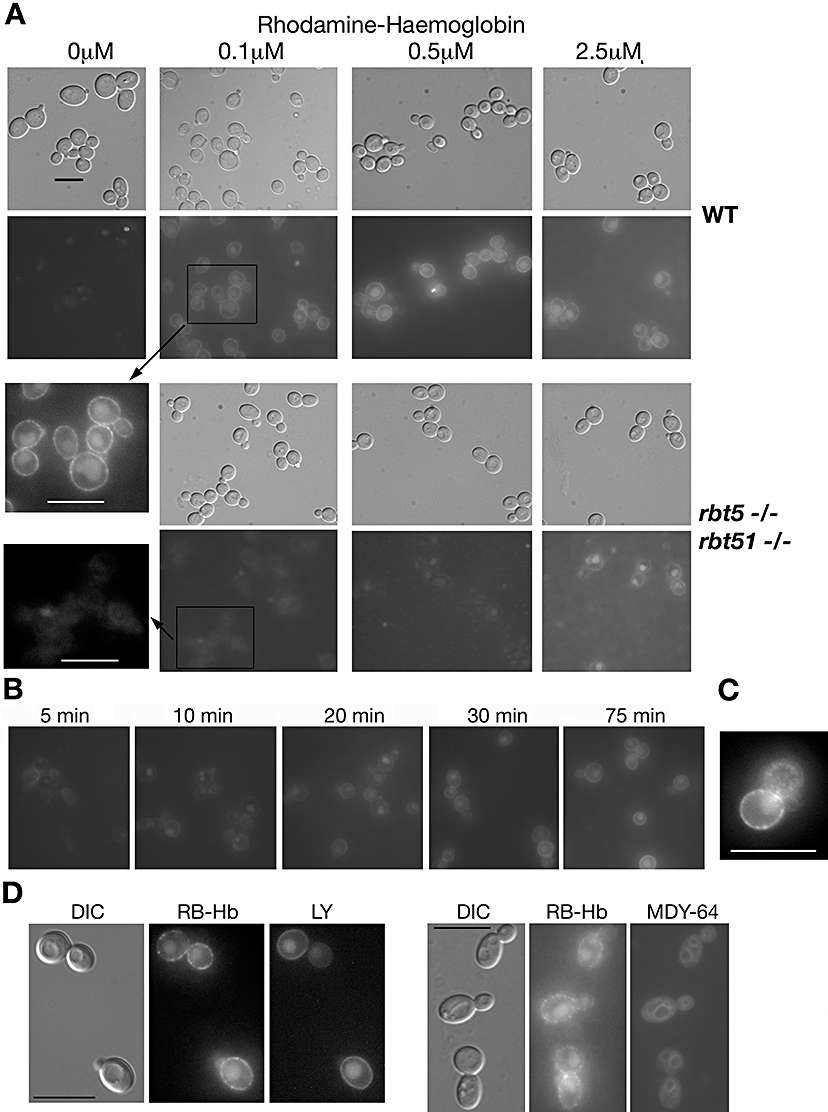
Haemoglobin uptake by C. albicans.A. Wild-type (CAI4) or rbt5−/−rbt51−/− cells, as indicated, were incubated for 2 h with the indicated concentrations of rhodamine-tagged haemoglobin (RB-Hb). The exposure of the epifluorescence micrographs was identical (8 s) except for the 2.5 μM RB-Hb samples, which were exposed for 14 s. To better compare the wild-type and mutant cells exposed to 0.1 μM RB-Hb, part of the two epifluorescence micrographs were blown up and contrast-enhanced to the same extent (insets).B. Wild-type C. albicans cells exposed to 0.25 μM RB-Hb were visualized at the indicated times.C. Higher magnification of a wild-type cell exposed for 2 h to 0.25 μM RB-Hb. The contrast was adjusted to make the punctate staining more apparent.D. Wild-type C. albicans cells were exposed for 60 min to 0.25 μM RB-Hb and either 1 mg ml−1 Lucifer yellow (LY) or MDY-64 (1 μM). The cells were visualized by epifluorescence with the rhodamine channel (RB-Hb) or with the GFP channel (LY, MDY-64).Bars = 10 μm.
We measured the kinetics of RB-Hb uptake by visualizing wild-type C. albicans cells at various time points after exposure to 0.25 μM RB-Hb. As shown in Fig. 1B, within a few minutes, rim staining was visible, and at the 10 min time point, vacuolar staining was already clearly visible in the cells. Intracellular staining increased in intensity throughout the 75 min time-course.
The rim staining of the cells appears, upon closer examination, to be punctate rather than uniform. In Fig. 1C, a micrograph of a wild-type cell exposed to RB-Hb was magnified and contrast-enhanced to make this punctate staining more apparent. Note also that the larger bud of this cell is in a different focal plane, making a surface punctate staining of the bud apparent. This suggests that RB-Hb might be concentrated in vesicles located near the plasma membrane.
The RB-Hb stain accumulates in an intracellular compartment that, by DIC microscopy, appears to correspond to the vacuole. In order to confirm this localization, we exposed the cells to RB-Hb together with Lucifer yellow (LY), a dye that was shown to accumulate in the vacuole by fluid-phase endocytosis (Riezman, 1985), or with MDY-64, a styryl dye that preferentially stains the vacuolar membrane. As shown in Fig. 1D, LY and RB-Hb colocalized, and MDY-64 preferentially stained the membrane of the compartment containing RB-Hb, confirming that this compartment corresponds to the vacuole.
Membrane localization of Rbt5
In order to understand the role of Rbt5 in haemoglobin-iron utilization in C. albicans, it was necessary to first determine its precise localization. Rbt5, a predicted GPI-anchored protein, was previously localized by immunofluorescence to the cell envelope, and additional experiments indicated that the protein is, at least in part, membrane-bound (Weissman and Kornitzer, 2004). However, in fungi, the GPI moiety can serve either to anchor a protein to the membrane, or to cross-link it covalently to the cell wall. In proteomic analyses of C. albicans, Rbt5 and Rbt51/Pga10 were identified as a cell wall proteins (de Groot et al., 2004; Sosinska et al., 2008). Furthermore, a GFP fusion protein containing the N- and C-termini of Rbt5 was found predominantly linked to the cell wall (Mao et al., 2003). In contrast, we had previously shown by SDS-PAGE and Western blotting that zymolyase treatment of purified cell walls, sufficient to release a control protein, did not release Rbt5 (Weissman and Kornitzer, 2004). However, as GPI-anchored proteins are linked via a β-1,6-glucan linkage, and as zymolyase is a β-1,3 endoglucanase, it remained conceivable that zymolyase digestion would leave extended glucan chains attached to Rbt5, preventing penetration of the protein in the gel and/or electrophoretic transfer to the membrane. To rule out this possibility, and to quantify more accurately the relative fractions of Rbt5 found at the cell wall versus the membrane, immunoblotting of whole-cell extracts was carried out. A pellet of wild-type, rbt5−/− or rbt5−/−rbt51−/− mutant C. albicans cells grown in iron-limited conditions was extracted with SDS, to solubilize cytoplasmic and membrane proteins, and the remaining cell wall pellet was in part solubilized with zymolyase, and in part transferred to the nitrocellulose membrane as a suspension. The different fractions were directly blotted onto nitrocellulose and reacted with anti-Rbt51 antiserum (Rbt5 and Rbt51 cross-react antigenically; Weissman and Kornitzer, 2004). An autoradiogram of the dot-blot is shown in Fig. 2. Comparison of the signal in the rbt5−/− and rbt51−/− mutants confirmed that Rbt5 is the predominant species in iron-starved cells. Direct quantification of the ECL signal in the different fractions (see Experimental procedures) indicated that over twofold more Rbt51-reactive protein was located in the SDS-soluble fraction than in the cell wall pellet, confirming that Rbt5 is mainly membrane-anchored.
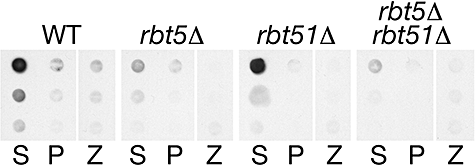
Immunoblotting of fractionated C. albicans extracts with anti-Rbt5/Rbt51 antibody. Proteins from wild-type and mutant cells, as indicated, grown in YPD + 1 mM ferrozine, were fractionated and extracted as described in Experimental procedures. The three rows represent sequential fivefold dilutions of the extracts. S = supernatant after extraction with 2% SDS; P = pellet; Z = zymolyase digestion of pellet.
Rapid turnover of Rbt5 and Rbt51
Rbt5 in C. albicans is only detectable under iron-limited conditions (Weissman and Kornitzer, 2004). In order to follow the biogenesis of Rbt5, wild-type cells grown in the presence of the iron chelator ferrozine were pulse-labelled for a short time with radioactive methionine, then chased with cold methionine, and Rbt5 was immunoprecipitated from extracts taken at different time points. As shown in Fig. 3A, sequential appearance of Rbt5 species can be detected. We presume that the ∼39 kDa species is the unmodified precursor, that the ∼55 kDa species represents the precursor with added GPI anchor, and that the ∼81 kDa species represents the fully mature, O-mannosylated form. Strikingly, the 81 kDa protein band then disappeared rapidly, with a half-life of under 5 min, suggesting that Rbt5 is an extremely unstable protein.
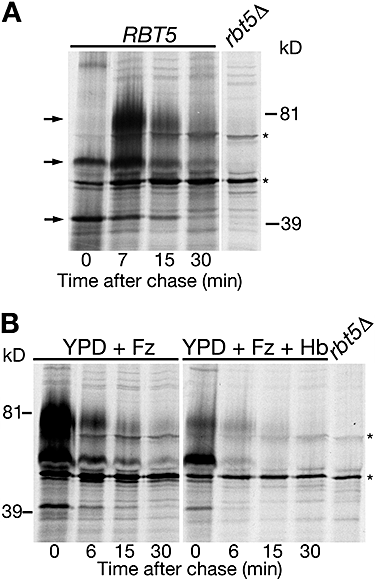
Pulse-chase analysis of Rbt5 in C. albicans.A. Biogenesis and degradation of Rbt5 in C. albicans. Cells were grown in YPD + 1 mM ferrozine, and pulse-labelled for a short time (2–3 min). The arrows show the three forms of Rbt5, from bottom to top (presumably): precursor, precursor + GPI anchor, fully O-mannosylated.B. Degradation of Rbt5 in cells grown in YPD + 1 mM ferrozine (Fz), with or without 5 μM haemoglobin (Hb). The asterisks indicate non-specific bands.
As Rbt5 is a haemoglobin receptor, we tested the effect of added haemoglobin to the turnover of Rbt5. The pulse-chase experiment depicted in Fig. 3B indicates that in the presence of added haemoglobin, the fully mature protein becomes less prominent, compared with the precursors, and may disappear even more rapidly, suggesting that presence of haemoglobin either accelerates the turnover of Rbt5 or inhibits its full maturation.
Rbt51/Pga10 is the Rbt5 homologue that is able to confer to S. cerevisiae the ability to utilize haemoglobin iron (Weissman and Kornitzer, 2004). In S. cerevisiae strain W303, we find that Rbt51 is normally stable; however, under iron starvation conditions, Rbt51 is rapidly degraded (Fig. 4A). Like Rbt5 in C. albicans, addition of haemoglobin to the iron-poor medium affects turnover and/or maturation of Rbt51, to the extent that the fully mature protein became barely detectable during the time-course of the experiment (Fig. 4A). Surprisingly, we found that in the EUROSCARF strain background BY4741, an S288C-derived strain, Rbt51 is constitutively stable, including in the presence of haemoglobin (Fig. S1). Nonetheless, in this strain background, Rbt51 can still promote haemoglobin-iron utilization (see below).
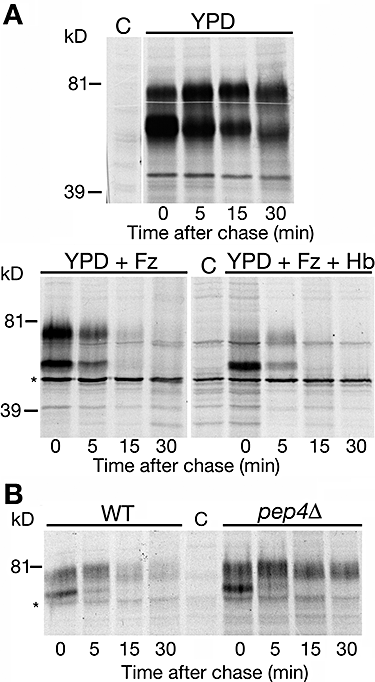
Pulse-chase analysis of Rbt51 in S. cerevisiae.A. In strain W303 transformed with plasmid KB1189, Rbt51 is normally stable, but rapidly degraded under conditions of iron starvation, and when haemoglobin is added to the medium. + Fz = 1 mM ferrozine added. + Hb = 5 μM haemoglobin added.B. Rbt51 is stabilized in the W303 pepΔD mutant. Wild-type and pepΔD mutant cells (KY1175) carrying plasmid KB1189 were grown in medium supplemented with 1 mM ferrozine. Asterisks indicate non-specific bands.
As GPI-anchored proteins are anchored to the topographically external side of cell membranes, their turnover is expected to involve the vacuole. To test this with Rbt51 in S. cerevisiae, we followed degradation of Rbt51 under iron starvation conditions in the W303 pep4Δ strain, a mutant that is defective in most proteolytic activities of the vacuole (Ammerer et al., 1986). In this mutant, Rbt51 is significantly stabilized (Fig. 4B), pointing to an involvement of the vacuole in its degradation.
The rapid disappearance of the Rbt5 protein in C. albicans would be expected, by analogy, to also be due to vacuolar degradation. To test this, we constructed a deletion mutant of the PEP4 homologue of C. albicans. Unlike Rbt51 in the S. cerevisiae pep4Δ strain, however, Rbt5 was not stabilized in the C. albicans pep4−/− strain (Fig. S2). This suggested that the vacuole is not required for Rbt5 degradation in C. albicans. Alternatively, it remained possible that Rbt5 degradation in C. albicans still depends on the vacuole, and that the lack of Rbt5 stabilization in Capep4−/− is due to the fact that the role of PEP4 as an activator of the other vacuolar preproteases is not conserved in C. albicans. In support of the latter possibility, we subsequently identified a mutant affecting vacuolar activity that is defective in Rbt5 degradation (see below).
Large-scale screen for haemoglobin-iron utilization in S. cerevisiae
In order to genetically analyse the components of the pathway responsible for internalization of haemoglobin into the C. albicans cells, we took advantage of the fact that haemoglobin-iron utilization was recapitulated in S. cerevisiae cells expressing RBT51 (Weissman and Kornitzer, 2004). However, S. cerevisiae growth on haemoglobin as iron source, even in the presence of RBT51, is weak, compared with that of C. albicans, and requires the presence of RBT51 on a high-copy-number plasmid. In an attempt to ameliorate haemoglobin utilization in S. cerevisiae, we expressed RBT51 under the control of the strong HSP150 promoter. HSP150 was found in global expression analysis experiments to be both strongly expressed under standard growth conditions, and less affected by adverse growth conditions, compared with primary metabolism genes (our unpublished data). We found that when expressed under the HSP150 promoter, the ability of RBT51 to confer haemoglobin utilization to S. cerevisiae, although not ameliorated, is retained even when expressed from a single-copy plasmid (data not shown). This enabled us then to construct a strain where the high-affinity iron transporter FTR1 is replaced by the HSP150p-RBT51 construct together with the prototrophic URA3 marker. Thus, while the ftr1::HSP150p-RBT51 strain is unable to grow in the presence of the iron chelator ferrozine due to lack of the high-affinity iron transporter, growth is restored to some extent in the presence of haemoglobin.
In order to identify S. cerevisiae mutants of this strain unable to utilize haemoglobin iron, the ftr1::HSP150p-RBT51 URA3 locus was then systematically crossed to a subset of the yeast deletion strain collection according to the Synthetic Genetic Analysis (SGA) method (Tong et al., 2001). A total of 279 strains mutated in genes related to endosome function (Schluter et al., 2008), and another 93 randomly picked strains, were subjected to this procedure, in duplicate. The doubly marked segregants were isolated, streaked on medium containing 1 mM ferrozine and 2 μM haemoglobin, and scored for haemoglobin utilization in five categories according to colony size (Table S1). The results showed a good concordance between the duplicates: out of 384 starting strains (372 distinct mutants, several wild-type controls and some duplicate mutant strains), 348 doubly marked segregants were recovered in both sets. Of these duplicate sets, 230 (66%) obtained identical scores, and of the remainder, only 13 (3.7%) were scored more than one category apart. Table 1 contains the list of the 28 mutants defective in haemoglobin-iron utilization, i.e. those mutants where one strain of the duplicate set exhibited strongly impaired growth on haemoglobin (indicated by ‘−’ in Table S1), and where either the second strain exhibited at least moderately impaired growth (‘+/−’), or no viable double segregant had been recovered in the second set (‘/’).
| Gene/ORF | Function/complex |
|---|---|
| VMA1 | Vacuolar H+ ATPase |
| VMA11 | Vacuolar H+ ATPase |
| YOR331C∼VMA4 | Vacuolar H+ ATPase |
| VMA6 | Vacuolar H+ ATPase |
| VMA7 | Vacuolar H+ ATPase |
| VMA10 | Vacuolar H+ ATPase |
| VMA11 | Vacuolar H+ ATPase |
| VMA12 | Vacuolar H+ ATPase |
| VMA13 | Vacuolar H+ ATPase |
| VMA16 | Vacuolar H+ ATPase |
| VMA21 | Vacuolar H+ ATPase |
| VMA22 | Vacuolar H+ ATPase |
| VPS22/SNF8 | ESCRT II complex |
| VPS32/SNF7 | ESCRT III complex |
| VPS16 | HOPS complex |
| VPS41 | HOPS complex |
| VAM3 | t-SNARE |
| VAM7 | t-SNARE |
| VPS6/PEP12 | t-SNARE |
| COD3/COG1 | Conserved oligomeric Golgi complex |
| SNF1 | AMP-activated S/T protein kinase |
| DBF2 | S/T protein kinase; mitotic exit network |
| NBP2 | Protein involved in HOG pathway |
| PMR1 | Ca2+/Mn2+ Golgi P-Type ATPase |
| PMT1 | Protein O-mannosyltransferase |
| RAI1 | rRNA maturation |
| RPL40A | Large ribosomal subunit protein; ubiquitin |
| MDM20 | N-terminal protein acetyltransferase |
The largest class of mutants is composed of 12 genes encoding subunits of the vacuolar ATPase (VMA genes), responsible for acidification of the vacuole and the endosome. The strain set we tested contained 16 VMA mutants; thus, although most VMA genes in the set we tested were scored as being defective in haemoglobin-iron utilization, one yielded inconsistent results (VMA5) and three did not show any defect (VMA2, VMA4, VMA8). This indicates that the screen yielded a proportion of false-negatives. We believe this reflects the limited stringency of our screen, due to the relatively weak growth of S. cerevisiae on haemoglobin iron, even in the presence of Rbt51. Conversely, we believe the screen yielded few false-positives, as most C. albicans orthologues of mutants identified in the S. cerevisiae screen were confirmed as having a haemoglobin utilization defect (see data below).
The second class of mutants shown in Table 1 represents components of the ESCRT system, a set of protein complexes that participate in internalization of monoubiquitinated membrane proteins (Hurley and Emr, 2006). Only two mutants, VPS22/SNF8 and VPS32/SNF7, members of the ESCRT II and ESCRT III complexes, respectively, were defective in haemoglobin utilization, although additional ESCRT complex members were tested. VPS20, which forms with VPS32 a subcomplex of ESCRT III (Babst et al., 2002a), shows a moderate defect in haemoglobin-iron utilization in one set of SGA strains, whereas it did not survive in the second set. The second ESCRT III subcomplex members, VPS2 and VPS24, did not show any impairment in haemoglobin utilization. The ESCRT II complex is composed, in addition to VPS22, of VPS36 and of VPS25 (Babst et al., 2002b). VPS36 was not recovered in either repeat of the SGA procedure, and VPS25 displayed moderately impaired growth in one repeat only. The ESCRTI complex is composed of VPS23 and VPS37 (Katzmann et al., 2001), which did not show any growth impairment, and of VPS28, which was not recovered in the SGA selection.
The ESCRT complexes are involved in transporting membrane proteins, tagged with a monoubiquitin adduct, to the multivesicular body (MVB) compartment, and from there to the vacuole, where these proteins are degraded (Hurley and Emr, 2006). The HOPS complex (Seals et al., 2000), of which Vps16 (Peterson and Emr, 2001) and Vps41 are members, is involved, together with the tSNAREs Vam3, Vam7 (Sato et al., 1998) and Pep12 (Becherer et al., 1996), in targeting vesicles to the vacuole. Deletions of VPS41, VPS16, VAM3, VAM7 and PEP12 were also identified in the screen. Thus, the majority of the genes recovered in our screen indicate a role for the vacuole in haemoglobin-iron utilization, corroborating the morphological data shown above.
Of the remaining nine genes that came up in our screen, we note PMT1, which encodes a protein O-mannosyltransferase previously shown to be necessary for full Rbt51 maturation (Weissman and Kornitzer, 2004); RPL40A, encoding a ribosomal protein, but also one of the ubiquitin gene precursors, which might be linked to the role of the ESCRT complexes in the trafficking of monoubiquitinated proteins; and PMR1, a Golgi calcium transporter (Antebi and Fink, 1992). The C. albicans PMR1 orthologue was recently described (Bates et al., 2005); however, we found that the Capmr1−/− strain is not defective in haemoglobin-iron utilization (data not shown).
Homologous genes affect haemoglobin utilization in C. albicans
Haemoglobin utilization in C. albicans could occur via a pathway similar to that of S. cerevisiae expressing Rbt51, or via an altogether different pathway. If a similar cellular pathway is involved, then at least some of the C. albicans genes homologous to those identified in the S. cerevisiae screen should also be required for efficient haemoglobin-iron utilization. We first tested the role of the vacuolar ATPase, the protein complex represented by the most hits in the S. cerevisiae screen, in haemoglobin-iron utilization in C. albicans. We identified orf19.6538 as the C. albicans orthologue of VMA11/TFP3, encoding the c′ subunit of the V0 subcomplex of the ATPase (Graham et al., 2003). Both alleles of this open reading frame were deleted, and growth was tested at different haemoglobin concentrations. As shown in Fig. 5A, the C. albicans vma11−/− mutant was strongly defective in haemoglobin-iron utilization in desferrated RPMI1640 medium. This mutant also grew about twofold slower than the wild type in regular RPMI1640 (Fig. S3). This is not surprising, as RPMI1640 is buffered to neutral pH, and the vacuolar ATPase mutants in S. cerevisiae exhibit growth defects at neutral pH (Graham et al., 2003). Importantly, however, this difference in growth rate is not sufficient to account for the almost complete inability of the Cavma11−/− strain to grow in the presence of haemoglobin as only iron source.
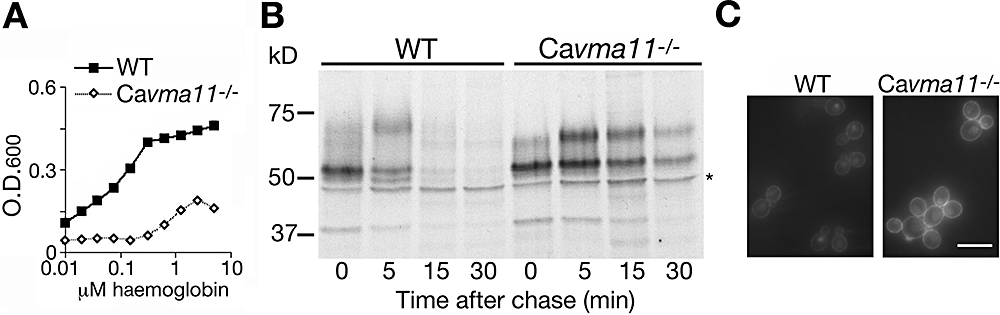
Phenotype of the C. albicans vacuolar ATPase mutant Cavma11−/−.A. Growth of the mutant versus wild-type strain in iron-depleted RPMI medium supplemented with the indicated amounts of haemoglobin. The optical density was measured after 3 days of growth at 30°C.B. Pulse-chase analysis of Rbt5 turnover in the wild-type and Cavma11−/− mutant cells. Cells were grown under iron-limited conditions (1 mM ferrozine). The asterisk indicates a non-specific band.C. Incorporation of RB-Hb into wild-type versus Cavma11−/− mutant cells. Cells were visualized after 2 h of incubation with 0.1 μM RB-Hb. Bar = 10 μm.
We also tested the Cavma11−/− mutant for Rbt5 turnover and haemoglobin uptake. Pulse-chase analysis showed that Rbt5 was significantly stabilized in the mutant cells (Fig. 5B). Haemoglobin uptake was tested by incubating the cells with RB-Hb. As shown in Fig. 5C, significantly stronger rim staining was detected in the mutant cells after 2 h of incubation with RB-Hb. This may correspond to the higher stability of the haemoglobin receptor Rbt5, which is expected to lead to higher steady-state levels of the protein. In addition, some vacuolar staining was visible in the mutant strain, indicating that haemoglobin import into the vacuole is not completely blocked.
The next C. albicans homologue tested was that of VPS41, a HOPS complex gene, absence of which was previously shown to lead to fragmentation of the vacuole, and to cause defects in iron utilization (Nakamura et al., 1997; Radisky et al., 1997). We identified the VPS41 orthologue in C. albicans as orf19.4858 and deleted both alleles. The Cavps41−/− strain was partially defective in haemoglobin-iron utilization (Fig. 6A). Microscopic examination of the cells indicated that, although RB-Hb was taken up, it was found in numerous intracellular vesicles, rather than in a single compartment. DIC microscopy suggested that, like the homologous S. cerevisiae mutant, Cavps41−/− cells lack a single large vacuole, but rather contain numerous smaller compartments (Fig. 6B).
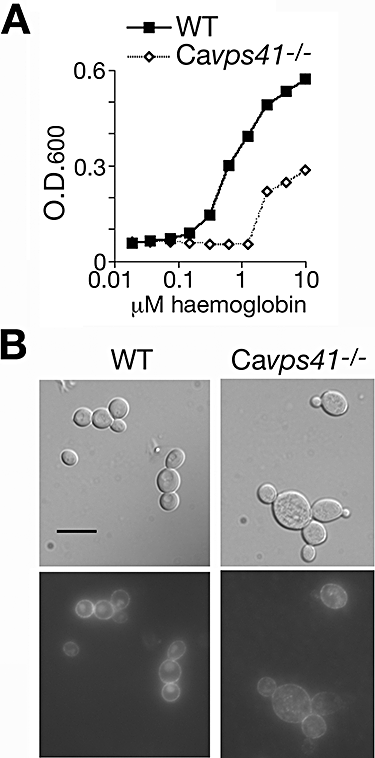
Phenotype of the C. albicans HOPS complex mutant Cavps41−/−.A. Growth of the mutant versus wild-type strain in iron-depleted RPMI medium supplemented with the indicated amounts of haemoglobin. The optical density was measured after 3 days of growth at 30°C.B. Incorporation of RB-Hb into wild-type versus Cavps41−/− mutant cells. Cells were visualized after 2 h of incubation with 0.1 μM RB-Hb. Bar = 10 μm.
The role of the ESCRT complexes on haemoglobin-iron utilization was tested in C. albicans, taking advantage of a series of C. albicans ESCRT complex mutants created by Xu et al. (2004). The mutants tested represent members of all three ESCRT complexes; the data show that all mutants have defects, of varying severity, in haemoglobin-iron utilization (Fig. 7A). After 3 days of incubation, most mutants only grew at the higher haemoglobin concentrations, and reached lower densities than the wild-type strain, whereas the snf7−/− mutant did not grow at all; after 6 days of incubation, all mutants had reached the same density as the wild-type strain at high haemoglobin concentrations, but still had not grown at the lower concentrations. We also tested RB-Hb uptake, and found that although some level of intracellular staining was detectable for almost all mutants, it was significantly lower than that of the control wild-type strain (Fig. 7B and C). Rim staining appeared similar to wild type for most mutants, except for mutants vps28−/− and snf7−/−. A subset of these mutants was also tested for Rbt5 turnover; however, none of the mutants tested showed any defect at this level (Fig. S4).
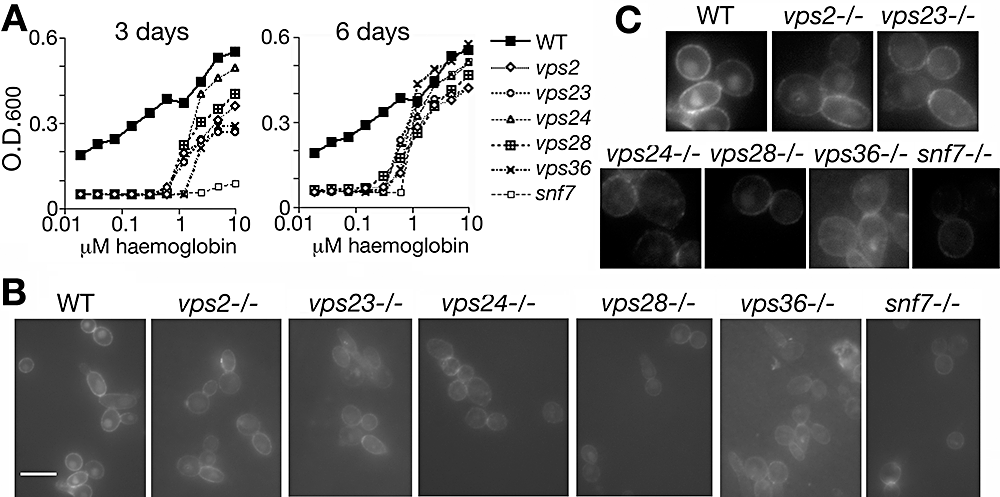
Phenotype of C. albicans ESCRT complex mutants.A. Growth of the mutant versus wild-type strain in iron-depleted RPMI medium supplemented with the indicated amounts of haemoglobin. The optical density was measured after 3 or 6 days of growth at 30°C, as indicated.B. Incorporation of RB-Hb into wild-type versus indicated mutant strains. Cells were visualized after 2 h of incubation with 0.1 μM RB-Hb. Bar = 10 μm.C. Portions of the images in (B) were blown up and contrast-enhanced to better visualize the differences between the different strains.
Candida albicans Myo5 is a type I myosin involved, among other functions, in cortical actin patch organization at hyphal tips (Oberholzer et al., 2002). As the Camyo5−/− mutant is defective in fluid-phase endocytosis (Oberholzer et al., 2004), and as endocytosis of haemoglobin appears to be central to its utilization, we tested the capacity of this mutant to utilize haemoglobin iron. We found that the Camyo5−/− mutant is relatively defective in haemoglobin-iron utilization (Fig. 8A). No significant defect was detected in the kinetics of Rbt5 turnover, but the different migration of the mature form of Rbt5 in the Camyo5−/− strain suggests that the protein is differentially modified in this mutant (Fig. 8B). Microscopic examination indicated that uptake of RB-Hb into the vacuole did take place in this mutant; however, very little rim staining was visible, and the staining appeared as a few discrete points on the plasma membrane (Fig. 8C).
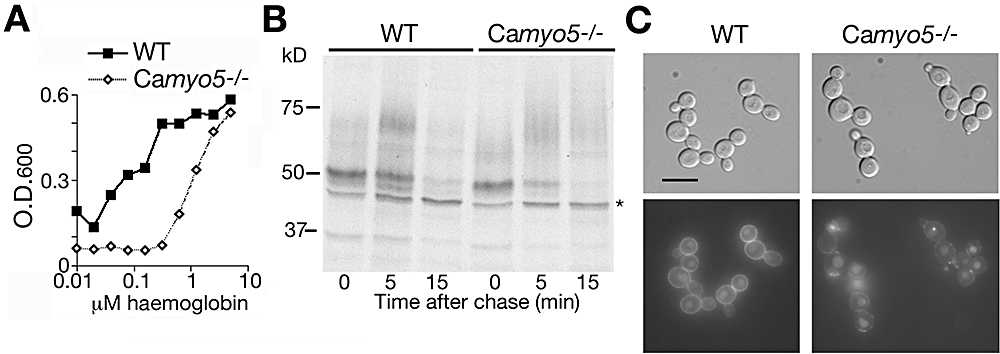
Phenotype of the C. albicans type I myosin mutant Camyo5−/−.A. Growth of the mutant (COU42) versus wild-type (CAI4) strain in iron-depleted RPMI medium supplemented with the indicated amounts of haemoglobin. The optical density was measured after 3 days of growth at 30°C.B. Pulse-chase analysis of Rbt5 turnover in the wild-type and Camyo5−/− mutant strain. Cells were grown under iron-limited conditions (1 mM ferrozine).C. Incorporation of RB-Hb into wild-type versus the Camyo5−/− mutant strain. Cells were visualized after 2 h of incubation with 0.25 μM RB-Hb. Bar = 10 μm.
Candida albicans Sla2 is a functional homologue of S. cerevisiae End4/Sla2 that was shown to play a role in cellular morphogenesis (Asleson et al., 2001). S. cerevisiae End4/Sla2 is an actin-binding protein that is required for endocytosis (Holtzman et al., 1993; Raths et al., 1993), prompting us to test whether the C. albicans orthologue is involved in haemoglobin-iron utilization. The Casla2−/− strain indeed appeared highly defective in growth on haemoglobin as iron source (Fig. 9A). The RB-Hb uptake assay showed much reduced incorporation in the Casla2−/− mutant, suggesting that CaSla2 is involved in haemoglobin uptake (Fig. 9B). Furthermore, pulse-chase analysis indicated that Rbt5 turnover in the Casla2−/− mutant was reduced in comparison with the rate in the wild type (Fig. 9C). We calculated the half-life of Rbt5 to be 4 min in the mutant, versus 2 min in the wild type.
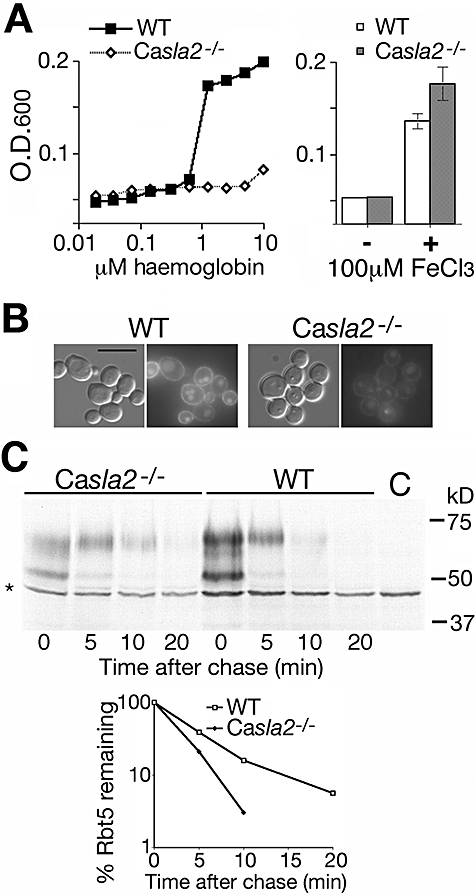
Phenotype of the Casla2−/− mutant.A. Left: Growth of the mutant (YJB7674) versus wild-type (BWP17) strain in iron-depleted RPMI medium supplemented with the indicated amounts of haemoglobin. The optical density was measured after 3 days of growth at 30°C. Right: Growth of the wild-type and mutant strains in the same medium re-supplemented with elemental iron.B. Incorporation of RB-Hb into wild-type versus Casla2−/− cells. Cells were visualized after 1 h of incubation with 0.25 μM RB-Hb. Bar = 10 μm.C. Pulse-chase analysis of Rbt5 turnover in the wild-type and Casla2−/− strain. The graph represents the quantification of the aggregate intensities of both the lower (precursor) and the upper (mature) Rbt5 bands.
Role of vacuolar iron transporters in haemoglobin-iron utilization
The data shown above indicate that haemoglobin-iron utilization involves import of haemoglobin into the vacuole where, presumably, extraction of the haem from the globin polypeptides also occurs. For the final step, we assume either that the extracted haem is transported to the cytoplasm, where it can be utilized as such or used as iron source; or that the haem is degraded in the vacuole, after which the released elemental iron is transported from the vacuole into the cytoplasm. To distinguish between these two possibilities, S. cerevisiae mutants of two characterized vacuolar iron transporters were tested: Smf3 (Portnoy et al., 2000) and Fth1 (Urbanowski and Piper, 1999). We constructed the single and the double mutants in the ftr1::HSP150p-RBT51 background and tested them for the ability to utilize haemoglobin iron. As shown in Fig. 10, whereas either single mutant was still able to utilize haemoglobin iron, the double mutant was unable to do so. This result further supports the central role of the vacuole in the pathway of haemoglobin-iron utilization, and suggests that in S. cerevisiae, the haem is degraded in the lumen of the vacuole, followed by transport of elemental iron via either Smf3 or Fth1.
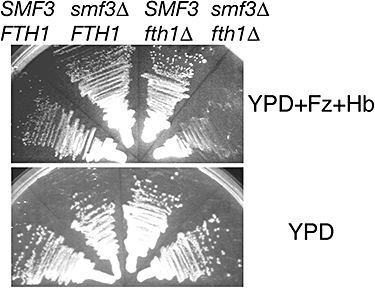
Role of vacuolar iron transporters in haemoglobin-iron utilization. S. cerevisiae strains carrying the ftr1Δ::HSP150p-RBT51 construct, and the indicated mutations, were streaked on YPD + 1 mM ferrozine + 2 μM haemoglobin and incubated at 30°C for 3 days (top) or streaked on YPD and incubated for 36 h (bottom).
In C. albicans, a previously characterized iron transporter, Ftr2, was suggested to be the orthologue of the vacuolar transporter Fth1 (Ramanan and Wang, 2000). In addition, we identified orf19.2069 as the closest bidirectional homologue of SMF3, and called it CaSMF3. Deletion of both alleles of CaSMF3 in either wild-type or in ftr2−/− background did not result in any defect in haemoglobin-iron utilization (data not shown). Interestingly, we were unable to delete the second CaSMF3 allele in the double ftr1−/−ftr2−/− background, or in the single Caccc2−/− background. CaCcc2 is a Golgi copper transporter necessary for the biogenesis of the ferroxidases associated with both plasma membrane (Ftr1) and vacuolar (Ftr2) high-affinity iron transporters. Thus, it is possible that in C. albicans, in the absence of functional high-affinity iron transporters Ftr1 and Ftr2, Smf3 becomes essential.
Comparison of ferrichrome and haemoglobin utilization pathways
The data shown above suggest that unlike elemental iron, which is taken up at the cell membrane, haemoglobin iron is utilized via an endocytic pathway. Ferrichrome is a siderophore that can be utilized as iron source by both S. cerevisiae and C. albicans. The specific ferrichrome transporter was identified as Arn1 in S. cerevisiae (Yun et al., 2000) and as CaArn1 in C. albicans (Ardon et al., 2001; Hu et al., 2002). Ferrichrome utilization may involve a cycle of endocytosis of the ligand–transporter complex in S. cerevisiae (Kim et al., 2002), and a similar mechanism may apply in C. albicans (Hu et al., 2002). To test whether the mechanisms of hemoglobin and ferrichrome uptake have overlapping requirements, we assayed ferrichrome utilization in the mutants characterized above, by monitoring growth on a medium depleted of iron with the iron chelator bathophenanthroline disulphonate (BPS) and re-supplemented with iron-loaded ferrichrome. As shown in Fig. 11, the only mutants that were unable to grow under these conditions were Cavma11−/− and Cavps41−/−. The ESCRT pathway and Camyo5−/− mutants were indistinguishable from wild type in ferrichrome utilization. These data support an involvement of the vacuole in ferrichrome utilization, but indicate that the pathways of haemoglobin- and ferrichrome-iron utilization are otherwise distinct.
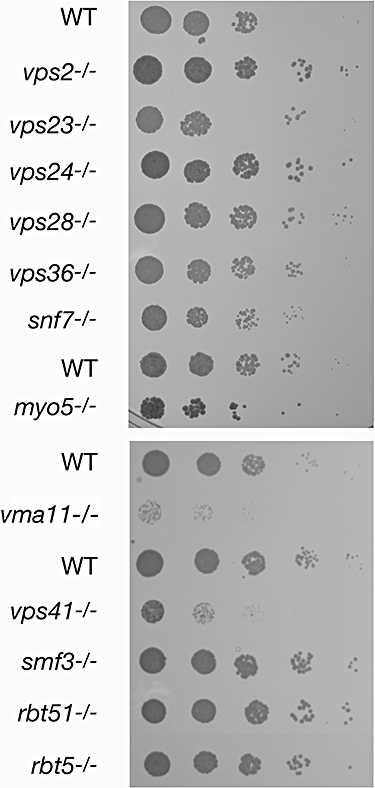
Ferrichrome utilization displays distinct genetic requirements from haemoglobin utilization. Fivefold dilutions of the indicated C. albicans strains were spotted on YPD plates supplemented with 1 mM iron chelator BPS and 5 μM iron-ferrichrome. The plates were incubated for 5 days at 30°C. See Fig. S5 for growth of the same strains on the control medium.
Discussion
The two main questions that were addressed here regard the pathway of haemoglobin-iron utilization in C. albicans, and the role of the GPI-anchored haemoglobin receptor Rbt5 in this pathway. A combination was used of microscopy with fluorescently tagged haemoglobin and genetic interrogation of a reconstituted haemoglobin utilization pathway in S. cerevisiae. In spite of its limited stringency, the S. cerevisiae screen served as a useful guide to identify C. albicans genes potentially involved in haemoglobin-iron utilization. These genes were then analysed by reverse genetics methods in C. albicans.
The pathway of haemoglobin-iron uptake
Microscopic evidence shows that RB-Hb is taken up by endocytosis into the vacuole (Fig. 1). The requirement for Myo5, a type I myosin that may be involved in endocytic vesicle scission (Jonsdottir and Li, 2004), and the paucity of punctate membrane staining in the Camyo5−/− mutant, support a role for endocytic vesicle formation in the haemoglobin utilization pathway. The requirement of CaSla2 provides additional evidence for the role of the endocytic pathway for haemoglobin uptake and utilization. The involvement of the vacuole is further demonstrated genetically by the requirement for an active vacuolar ATPase, and of CaVps41.
In the Cavma11−/− mutant, haemoglobin is still imported into the vacuole. However, in this mutant, Rbt5 is stabilized, suggesting that vacuolar proteases are largely inactive. To the extent that the globin chains may need to be hydrolysed to release the haem, this by itself would be sufficient to explain the requirement for the vacuolar ATPase. We noticed, however, that in S. cerevisiae, haemoglobin utilization is unaffected by deletion of PEP4, i.e. does not require vacuolar proteolytic activity (data not shown). Furthermore, although the rapid degradation of Rbt5 in C. albicans and of Rbt51 in S. cerevisiae W303 suggests concomitant degradation of the receptor and its ligand in the vacuole, we also found that S. cerevisiae strain BY4741, while able to utilize haemoglobin iron, does not degrade Rbt51. It is possible that hydrolysis of Rbt5/51 and of the globin chains is not required for haem extraction, but that the acidity of the vacuole is sufficient to release the ligand from its receptor, and to cause denaturation of the haemoglobin molecule and release of the haem (Griffith and Kaltashov, 2003).
The requirement for vacuolar iron transporters (but not of the plasma membrane high-affinity iron uptake system), at least in S. cerevisiae, suggests that in this organism, elemental iron is released in the vacuole. By analogy, this would suggest that in C. albicans as well, haem degradation by the haem oxygenase CaHmx1 (Santos et al., 2003; Pendrak et al., 2004) may occur in the vacuole. However, we found that the C. albicans vacuolar iron transporters CaFtr2 and CaSmf3 are not required for haemoglobin-iron utilization. One possibility is that a third putative vacuolar iron transporter detectable in the C. albicans genome sequence, CaFTH2, a homologue of ScFTH1, is responsible for iron uptake from the vacuole after haem breakdown in the double Caftr2−/− Casmf3−/− mutant. Alternatively, we cannot exclude at this stage that C. albicans contains a vacuolar haem transporter that transfers it into the cytoplasm to be utilized as such, or to be degraded there by the haem oxygenase.
Role of Rbt5/51 in haemoglobin-iron utilization
Rbt5/51 are receptors that mediate binding of haemin (Weissman and Kornitzer, 2004) and haemoglobin (Fig. 1) to the cell envelope. These proteins are predicted from sequence data to be GPI-anchored, a prediction supported by metabolic pulse-and-chase analysis of Rbt5, which indicates sequential modification of the protein (Fig. 3A). One possible role for Rbt5/51 is that they function as receptors that mediate internalization of their ligand, haemoglobin. However, GPI-anchored proteins are often covalently bound to the cell wall in fungi, in which case they would not be internalized. Indeed, the rapid disappearance of Rbt5 in pulse-chase analyses could have been due to covalent cross-linking to the cell wall, which would remove Rbt5 from the pool of soluble proteins. Therefore, we performed an immunoblot experiment that showed that at steady state, at least twofold more Rbt51-reactive protein is found in the SDS-soluble fraction than in the cell wall fraction. As the soluble fraction has a half-life of about 5 min, given the generation time of C. albicans (60–70 min under optimal conditions), and assuming that the cell wall-bound fraction is largely stable, one can estimate that less than 0.5% of the synthesized Rbt5 is cross-linked to the cell wall. Degradation of the bulk of the protein occurs in the vacuole, as shown by the stabilization of Rbt5 and Rbt51 in vacuolar mutants.
Once we established that a large fraction of Rbt5 remains at the membrane, it became conceivable that it functions as a receptor for haemoglobin internalization. This suggests the following working model for the pathway haemoglobin-iron utilization: extracellular membrane receptors (Rbt5/51) bind haemoglobin (and/or haemin), which is then internalized, as a complex with Rbt5/51, in plasma membrane-proximal vesicles that are eventually delivered to the vacuole. Supporting this model is the rapid vacuole-dependent turnover of Rbt5, and the partial stabilization of Rbt5 in the Casla2−/− mutant. It should be noted, however, that in some mutants that are defective in RB-Hb internalization, such as the ESCRT mutants, Rbt5 degradation is unaffected, whereas in the Casla2−/− mutant, degradation is delayed, but not abolished. This is in contradiction with the simple model that places Rbt5 at the plasma membrane prior to internalization and degradation. It is possible, however, that in the ESCRT mutants, a block in endocytosis re-routes the newly synthesized Rbt5 molecules directly to the vacuole. It is also possible that even in wild-type cells, a significant portion of Rbt5 molecules never reaches the plasma membrane, but rather functions in the endosomes to tether the haemin released after haemoglobin dissociation or degradation. The data showing that in the Casla2−/− mutant, part of the labelled Rbt5 pool persists longer than in the wild type, does support that at least part of the Rbt5 pool normally traverses the plasma membrane en route to the vacuole.
Another difficulty with the suggestion that Rbt5 mediates haemoglobin internalization is that Rbt5 is a GPI-anchored protein, and has therefore no cytoplasmic domain. There are a few precedents for facilitation of ligand internalization by GPI-anchored receptors, e.g. the human folate receptor (Sabharanjak and Mayor, 2004). The mechanism by which such receptors mediate internalization of a ligand is unclear. One possibility is that Rbt5/51 facilitates haemoglobin uptake by tethering the protein at the membrane, thereby increasing the local concentration of haemoglobin, which is then taken up in endocytic vesicles by fluid-phase endocytosis. The morphological evidence of these vesicles is the punctate membrane staining of RB-Hb in wild-type cells. Conceivably, free haemoglobin, being a bivalent ligand [haemoglobin dissociates to αβ dimers at low concentration (Nagel and Gibson, 1971)], could promote the formation of such vesicles via clustering of the receptors. Moreover, Pendrak et al. (2000) found that C. albicans cells caused free haemoglobin to form aggregates on the cell surface. Such aggregates, which are able to induce a signalling function of haemoglobin (Pendrak et al., 2000), could also conceivably cause receptor clustering and internalization.
Function of ESCRT system in haemoglobin utilization
Our genetic data indicate that transport of these vesicles to the vacuole requires the activity of the ESCRT complexes. Another iron acquisition system, the Arn1-dependent ferrichrome uptake system, was suggested to involve endocytosis as well (Hu et al., 2002; Kim et al., 2002). Our data, which show a requirement for CaVma11 and CaVps41, support a role for the late secretory pathway in ferrichrome utilization. However, the ESCRT mutants did not show any defect in ferrichrome utilization, indicating that the haemoglobin and ferrichrome pathways are genetically distinct.
There is ample genetic and structural evidence that the ESCRT system is dedicated to the targeting of monoubiquitinated membrane proteins to the vacuole (Hurley and Emr, 2006). As the haemoglobin receptors Rbt5/51 are GPI-anchored proteins that lack a cytoplasmic domain, the role of the ESCRT system in the uptake of haemoglobin is unexpected. While it is possible that in C. albicans, haemoglobin uptake requires, in addition to Rbt5, a specific transmembrane receptor that could serve as target for the ESCRT system, it is unlikely that such a receptor exists in S. cerevisiae. As an alternative, we suggest that the endocytic vesicles contain transmembrane proteins, unrelated to haemoglobin per se, that serve to target these vesicles to the vacuole, in effect allowing the GPI-anchored proteins and their ligands to piggyback on the ESCRT system. Regardless of the mechanism, the dramatic effect of iron starvation on vacuole-dependent turnover of Rbt51 in S. cerevisiae W303 suggests that bulk endocytosis of vesicles originating from the plasma membrane may be part of an evolutionarily conserved system for scavenging iron from the environment.
Experimental procedures
Strains and plasmids
Saccharomyces cerevisiae strains used were W303 (R. Rothstein) and the S288C-based strains BY4741 and BY4742 (EUROSCARF). All gene deletions were obtained from the systematic deletion project consortium via EUROSCARF. Strain KY1175 (W303 pep4Δ::KANr) was generated by transforming W303 with a PCR fragment amplified from the EUROSCARF pep4Δ::KANr strain. C. albicans strains (listed in Table 2) were derivatives of CAI4 (Fonzi and Irwin, 1993), BWP17 (Wilson et al., 1999) or SN78 (Noble and Johnson, 2005). Strains KC280 (Cavma11Δ::HIS3/Cavma11Δ::LEU2) and KC281 (Capep4Δ::HIS3/Capep4Δ::LEU2) were constructed in SN78 background following the described protocol (Noble and Johnson, 2005). CaVMA11/TFP3 and CaPEP4 were identified by homology in the C. albicans genomic database as orf19.5886 and orf19.6538 respectively. Strain KC283 (Cavps41Δ::hisG-URA3-hisG/Cavps41Δ::hisG) was built in CAI4 background, using plasmids KB1658-1 and KB1658-2 for deleting the two alleles of CaVPS41 (orf19.4858). Strains KC295 (Casmf3Δ::hisG-URA3-hisG/Casmf3Δ::hisG) and KC318 (Caftr2Δ::hisG/Caftr2Δ::hisG Casmf3Δ::hisG-URA3-hisG/Casmf3Δ::hisG) were generated with CaSMF3 (orf19.2069) deletion plasmids KB1660, KB1661 in strains CAI4 and Caftr2−/− (Ramanan and Wang, 2000) respectively.
| Name | Genotype | Origin |
|---|---|---|
| KC2=CAI4 | ura3Δ::imm434/ura3Δ::imm434 | Fonzi and Irwin (1993) |
| KC35 | KC2 Caccc2Δ/Caccc2Δ | Weissman et al. (2002) |
| KC65 | ura3Δ::imm434/ura3Δ::imm434 Caftr1Δ/Caftr1Δ | Ramanan and Wang (2000) |
| KC289 | ura3Δ::imm434/ura3Δ::imm434 Caftr2Δ/Caftr2Δ | Ramanan and Wang (2000) |
| KC290 | KC65 Caftr2Δ/Caftr2Δ | Ramanan and Wang (2000) |
| BCa18-2 | ura3Δ::imm434/ura3Δ::imm434 rbt5Δ::hisG/rbt5Δ::hisG-URA3-hisG | Braun et al. (2000) |
| KC85 | KC2 rbt51Δ::hisG/rbt51Δ::hisG-URA3-hisG | Weissman and Kornitzer (2004) |
| KC100 | KC85 rbt51Δ::hisG/rbt51Δ::hisG-URA3-hisG | Weissman and Kornitzer (2004) |
| BWP17 | ura3Δ/ura3Δhis1Δ/his1Δarg4Δ/arg4Δ | Wilson et al. (1999) |
| YJB7674 | BWP17 sla2Δ::ARG4/sla2Δ::HIS1 | Asleson et al. (2001) |
| DAY286 | BWP17 URA+ ARG+ | Xu et al. (2004) |
| KC249 | BWP17 vps2::URA3/vps2::ARG4 | Xu et al. (2004) |
| KC250 | BWP17 vps23::URA3/vps23::ARG4 | Xu et al. (2004) |
| KC251 | BWP17 vps24::URA3/vps24::ARG4 | Xu et al. (2004) |
| KC252 | BWP17 vps28::URA3/vps28::ARG4 | Xu et al. (2004) |
| KC253 | BWP17 vps36::URA3/vps36::ARG4 | Xu et al. (2004) |
| KC254 | BWP17 snf7::URA3/snf7::ARG4 | Xu et al. (2004) |
| KC255=CAI4 | ura3Δ::imm434/ura3Δ::imm434 | Oberholzer et al. (2002) |
| KC256=COU42 | KC255 Camyo5::hisG/Camyo5::hisG | Oberholzer et al. (2002) |
| KC271=SN78 | ura3Δ::imm434/ura3Δ::imm434 his1/his1 leu2/leu2 | Noble and Johnson (2005) |
| KC280 | KC271 Cavma11::HIS1/Cavma11::LEU2 | This work |
| KC281 | KC271 Capep4::HIS1/Capep4::LEU2 | This work |
| KC283 | KC2 Cavps41Δ::hisG/Cavps41Δ::hisG-URA3-hisG | This work |
| KC295 | KC2 Casmf3Δ::hisG/Casmf3Δ::hisG-URA3-hisG | This work |
The CaVPS41 deletion plasmids were constructed as follows: in a first stage, the CaVPS41 (ORF length: 1929 nt) genomic region was cloned as a SacI–HindIII PCR fragment extending from position −800 to +2850 relative to the CaVPS41 start site into pBSII SK+ (Stratagene) to generate KB1648. The BamHI–BglII CaURA3 blaster fragment (Fonzi and Irwin, 1993) was cloned in two opposite orientations in KB1648 digested with BglII (position +348 of CaVPS41) and BamHI (position +1778), generating two disruption plasmids that remove 75% of the CaVPS41 ORF. The CaSMF3 deletion plasmids were constructed as follows: the 5′ and 3′ regions of CaSMF3 were amplified as a SacI–SpeI and a PstI–HindIII fragment, respectively, and cloned sequentially into KB985, KB986 (Atir-Lande et al., 2005) to generate KB1660, KB1661. Plasmid KB1544 (HSP150p-RBT5) and KB1546 (HSP150p-RBT51) were constructed by PCR amplification of the promoter region of S. cerevisiae HSP150 from −560 to −1 relative to the translation start site as a SacI–EcoRI fragment, and PCR amplification of C. albicans RBT5 and RBT51 as EcoRI–SalI fragments extending from −1 to 70 nucleotides beyond the respective stop codons. The PCR fragments were ligated, re-amplified and cloned into p426GAL1 (Mumberg et al., 1994) digested with SacI and Sal1.
To substitute FTR1 with HSP150p-RBT51, the HSP150p-RBT51 URA3 region of KB1546 was amplified with oligonucleotides containing 40 bp of homology to the sequences immediately adjacent to the FTR1 open reading frame: 5′FTR1-pRS4xxSacI (5′-ACGTTGTCGAAATCTTCTCTCAGCAGGTCATCACACATATaagggaacaaaagctggagc) and 3′FTR1-pRS4xx (5′-GAAAATAGGTGGAAAACTCCCACCCTGTGCTAGACTTCATgcagattgtactgagagtgc). This PCR fragment was used to transform S. cerevisiae strain YPH1581 (BY4742 mfa1::MFApr-HIS3), to generate KY1081.
Media and growth experiments
Standard yeast media are described in Sherman et al. (1986). To elicit iron starvation, YPD was supplemented with 1 mM ferrozine (Sigma), an iron chelator. For synthetic low-iron media, iron- and copper-depleted YNB was obtained from Bio101, and re-supplemented with 0.4 μM CuSO4. Iron-depleted RPMI1640 medium supplemented with apo-transferrin was described before (Weissman et al., 2002). The various strains were inoculated, in triplicates, at OD600 = 0.005 in 96-well plates containing 100 μl of this medium supplemented with increasing amounts of haemoglobin, Growth was measured as optical density with an ELISA reader. The growth data shown represent the averages of the triplicate data points. Standard deviations did not exceed 10%, except at very low densities. A control experiment showed that most strains grew equally well as wild type in RPMI1640 medium supplemented with 2% glucose (Fig. S3).
SGA strain construction and testing
Introduction of the ftr1ΔHSP150p-RBT51 construct into the G418r-marked secretion mutants collection (Schluter et al., 2008) was carried out by crossing strain KY1081 (BY4742 mfa1::MFApr-HIS3 ftr1Δ::<HSP150p-RBT51 URA3>) to the secretion mutants arrayed in 384-colony format, using a Virtek automated colony arrayer (Bio-Rad, Hercules, CA). Sequential pinning to appropriate selection plates enabled to select for diploids, and subsequently to sporulate and select for doubly marked haploids, according to Tong et al. (2001). The double mutant haploids were then tested for haemoglobin-iron utilization by streaking to individual colonies on YPD + 1 mM ferrozine and 2 μM haemoglobin.
Immunoblotting
Total soluble and membrane proteins were extracted by heating to 95°C in 2% SDS. The cell extract was separated from the insoluble cell wall material by spinning at 14 000 r.p.m. for 10 min. The pellet was extracted once more with 1% SDS, washed twice with PBS and re-suspended in an equal volume to the original extract. One-half of the suspension was incubated overnight at 30°C with Zymolyase 100T (Takara), 1 mg ml−1 final concentration, in the presence of antiproteases (1 mM phenylmethylsulphonylfluoride, leupeptin, antipain, chymostatin, 10 μg ml−1 each). This completely solubilized the suspension. The different fractions were serially diluted in PBS, and blotted onto 0.2 μm nitrocellulose using a vacuum dot-blot device. The membrane was reacted with rabbit anti-Rbt51 (Weissman and Kornitzer, 2004) followed by a secondary HRP-conjugated goat anti-rabbit antibody (Sigma). The signals were recorded both by autoradiography, and using a Chemdoc digital imaging device (Bio-Rad). The digital images were used to quantify the signal intensities, taking care to use those exposures and extract concentrations that fell within the linear range of intensities.
Pulse-chase analysis
The protocol used for both C. albicans and S. cerevisiae was as described (Kornitzer, 2002), with the following modification: to achieve iron starvation, cell were pre-grown in YPD medium supplemented with 1 mM ferrozine, then washed in iron-depleted YNB medium supplemented with amino acids as required by the respective strain's auxotrophies, labelled with ‘Easytag’ (Perkin Elmer), a 35S-methionine–cysteine mix, for 5 min, and chased in YPD + ferrozine supplemented with 10 mM methionine, 10 mM cysteine.
Rhodamine-haemoglobin conjugation
All the reagents were obtained from Sigma biochemicals. Throughout the procedure, haemoglobin concentration was followed by absorbance at 410 nm. Bovine haemoglobin was dissolved to 0.5 mM in 0.2 M Na2CO3, pH 8.5, and purified from possible contaminants by passage through a Sephadex G-25 (fine) column equilibrated with the same buffer. Rhodamine B-isothiocyanate was dissolved to 5 mg ml−1 in anhydrous DMSO, and added to the haemoglobin solution at a sixfold molar excess, i.e. 5% rhodamine B/haemoglobin, w/w. The reaction mix was incubated at room temperature, protected from light, for 1 h while tumbling. Finally, the reaction mix was loaded on a Sephadex G-25 (fine) column, equilibrated with PBS, to separate the RB-Hb from the unincorporated rhodamine B. The RB-Hb solution was filter-sterilized and kept at 4°C. The ability of the wild-type C. albicans cells to utilize RB-Hb versus native haemoglobin as iron source was identical.
Microscopy
Cells pre-grown in YPD + 1 mm ferrozine, to elicit Rbt5 synthesis, were exposed to the indicated concentrations of RB-Hb for the indicated amounts of time, spun down and washed twice with PBS, and immediately placed on a microscope slide and visualized. Lucifer yellow staining was performed as described (Dulic et al., 1991). MDY-64 (Invitrogen) was used at 1 μM. A Zeiss Axio-Imager M1 microscope with a 100× plan-apochromat objective, NA 1.40, was used for both DIC and epifluorescence microscopy. The Zeiss rhodamine filter set was used for RB-Hb visualization, and the GFP filter set was used for Lucifer yellow and for MDY-64 staining. Images were acquired with a Zeiss Axiocam MrM digital camera, via the Axiovision software.
Acknowledgements
We are grateful to Aaron Mitchell, Ursula Oberholzer, Yue Wang, Steve Bates and Judy Berman, for C. albicans strains, to Phil Hieter for S. cerevisiae SGA starting strains, to Piet de Groot for advice on analysis of cell wall proteins, and to Cayetana Schluter and Tsvia Gildor for expert technical assistance. D.K. thanks Phil Hieter for hosting him in his laboratory during the initial phases of this project. This research was supported by grants from the Israeli Ministry of Health's Chief Scientist Office and from the Wolfson Center of Excellence for the Study of Protein Turnover to D.K., and from Canadian Institutes of Health Research and Michael Smith Foundation for Health Research to E.C.




