The non-typeable Haemophilus influenzae Sap transporter provides a mechanism of antimicrobial peptide resistance and SapD-dependent potassium acquisition
Summary
We have shown that non-typeable Haemophilus influenzae (NTHI) resists killing by antimicrobial peptides (APs). A mutant defective in expression of the sap (sensitivity to antimicrobial peptides) gene cluster product SapA is sensitive to killing by APs and is significantly attenuated in its ability to survive in a chinchilla model of otitis media compared with the parent strain. In NTHI, SapA is believed to function as the periplasmic solute binding protein of an ABC transporter. Here, we demonstrated that recombinant chinchilla beta defensin-1 specifically interacted with recombinant SapA and that AP exposure increased expression of the sap operon. We further demonstrated that the putative Sap transporter ATPase protein, SapD, was required for AP resistance as well as potassium uptake in NTHI strain 86-028NP. Loss of SapD additionally abrogated NTHI survival in vivo. Complementation of the sapD mutation restored the ability to grow in potassium-limited medium, resistance to AP-mediated killing and survival in vivo. Collectively, these data support a mechanism of Sap system-mediated resistance to APs that depends on Sap-dependent transport of APs and a Sap-dependent restoration of potassium homeostasis. Thus, NTHI required a functional Sap system to mediate bacterial survival and pathogenesis in vivo.
Introduction
Non-typeable Haemophilus influenzae (NTHI) is a benign nasopharyngeal commensal microorganism, but also an opportunistic invader of the normally sterile middle ear space (Infante-Rivard and Fernandez, 1993). As such, NTHI predominates in both chronic otitis media (OM) with effusion (Klein, 1997) and plays a key role in acute OM as well (Kilpi et al., 2001). Furthermore, NTHI strains principally have a role in other localized respiratory diseases such as acute sinusitis, community-acquired pneumonia and have important consequences in patients with chronic obstructive pulmonary diseases and cystic fibrosis (St Geme, 2000; Sethi and Murphy, 2001; Murphy, 2003; Roman et al., 2004). The pathogenic potential of NTHI is dictated by concomitant viral infection or possibly alterations in the host microenvironment such as changes in levels of specific inflammatory mediators or decrease in host antimicrobial peptides (APs) (Bakaletz, 2002; Heikkinen and Chonmaitree, 2003; G. McGillivary, unpublished).
The diversity of mechanisms bacteria have evolved to resist killing by APs suggests an important role for this component of the innate host response. APs are typically cationic, amphipathic molecules of 12–50 amino acids in length that interact with the bacterial cytoplasmic membrane, comprised primarily of negatively charged phospholipids (Hoffmann et al., 1999). These peptides are thought to insert into the cytoplasmic membrane and form channels that result in leakage of cytoplasmic contents and subsequent cell death (White et al., 1995; Hancock and Chapple, 1999; Peschel, 2002). One group of these peptides, the beta-defensins, are expressed by both a human middle ear epithelial cell line and middle ear mucosae recovered from patients with chronic OM with effusion (Lee et al., 2002; 2004; Moon et al., 2002). Recently, it was demonstrated that lysozyme and beta-defensin can inhibit the growth of clinical isolates of OM pathogens via bacterial membrane damage (Lee et al., 2004). These data suggest an important role for APs in maintaining the health of the uppermost airway and perhaps the prevention of OM. Bacteria, having infected the middle ear cavity, will likely possess mechanisms to resist the barrage of antimicrobial weapons of the host immune response.
It is clear that products of the Salmonella sap operon (sensitivity to antimicrobial peptides) are necessary for resistance to APs (Parra-Lopez et al., 1993; Lopez-Solanilla et al., 1998). These investigators demonstrated that mutants deficient in sap operon proteins exhibited hypersensitivity to melittin and crude extracts from human neutrophils (Parra-Lopez et al., 1993). Similarly, the Sap proteins confer resistance to killing by APs in NTHI and are required for survival of NTHI in a chinchilla model of OM (Mason et al., 2005). The sap gene products (SapABCDFZ) share homology to a family of ‘ATP-binding-cassette’ (ABC) family of transporters that are diverse in substrate binding and uptake (Hiles et al., 1987; Abouhamad et al., 1991; Perego and Hoch, 1991; Parra-Lopez et al., 1993). Higgins and colleagues showed that bacterial transporters containing a periplasmic solute binding protein usually import solutes (Higgins, 1992). Although the substrate for transport remains undefined, SapA is predicted to function as a periplasmic solute-binding protein; SapB and SapC as inner membrane permease proteins; SapD and SapF as ATPase subunits; whereas the function of SapZ remains unknown. The mechanism(s) that underlie AP resistance in NTHI remain undefined.
In addition to resistance to host clearance mechanisms, bacterial survival depends upon the ability to maintain cell turgor pressure via potassium release and/or uptake from the cell upon exposure to changes in medium osmolarity or acute stressors, including exposure to APs (Csonka, 1989; Stumpe and Bakker, 1997). In Escherichia coli, three potassium uptake systems have been described. The main system, Trk (composed of TrkH and TrkG in association with the cytoplasmic protein TrkA), is constitutively expressed and transports potassium at a high rate but with low affinity (Dosch et al., 1991). When potassium is limited, the cells utilize the high-affinity potassium translocating Kdp-ATPase (Rhoads et al., 1976). The third system, Kup, is expressed constitutively and transports potassium relatively slowly, but with a higher affinity than the Trk system (Rhoads et al., 1976; Rhoads and Epstein, 1977). Mutations in the trkH and trkA genes, as well as sapD in S. typhimurium confer sensitivity to the prototypic AP protamine, and further, a TrkA mutant was attenuated for virulence in mice (Groisman et al., 1992). The TrkA protein is thus required for transport of potassium in the presence of TrkH, and may contribute to AP resistance. AP association with the bacterial membrane likely results in the rapid efflux of potassium (Matsuzaki et al., 1991; Aspedon and Groisman, 1996; Bengoechea and Skurnik, 2000; Kim et al., 2004; Ohmizo et al., 2004), which is predicted to be mediated via the glutathione-regulated potassium efflux protein homologue, KefB (Meury and Robin, 1990; Douglas et al., 1994; Harrison et al., 2005). Because exposure to APs results in a rapid loss of internal potassium (Stumpe and Bakker, 1997), rapid recovery of potassium homeostasis is critical for bacterial survival when exposed to sublethal concentrations of AP. Upon inspection of the genome, NTHI strain 86-028NP appears to possess one specific potassium uptake system, the TrkH/TrkA system (Harrison et al., 2005). Regulation of potassium transport via this system would therefore, be critical to the survival of this important causative agent of OM.
In this work, we showed that the Sap transporter confers to NTHI the ability to detect the presence of APs, subsequently upregulate expression of the sap genes, and mediate potassium-uptake that was dependent upon SapD. This mechanism of Sap system-mediated resistance to APs and Sap-dependent restoration of potassium homeostasis was critical for NTHI survival in vivo, and further equipped this benign commensal with the ability to resist host defence mechanisms, a function crucial in its transition to pathogen of the upper and lower respiratory tract.
Results
Non-typeable Haemophilus influenzae exposure to AP upregulated expression of the sap operon
We previously demonstrated early, transient expression of the NTHI sap operon in vivo during acute OM (Mason et al., 2005). In addition, we showed expression of chinchilla beta defensin-1 (cBD-1) and a cathelicidin-related antimicrobial peptide (cCRAMP) in the upper respiratory tract of a chinchilla model of NTHI-induced OM (Harris et al., 2004). We further showed that cBD-1 is expressed at sites of NTHI colonization, suggesting a role for APs in prevention of OM (Harris et al., 2004). As a mutation in the sapA gene renders NTHI strain 86-028NP sensitive to AP-mediated killing, we hypothesized that exposure of NTHI to APs would regulate expression of the sap operon, thereby mediating AP resistance (Mason et al., 2005). In order to address this question, we incubated a reporter NTHI strain, containing the lux operon under the control of the sap promoter, in the presence of increasing, yet sublethal concentrations of recombinant chinchilla beta defensin-1 [(r)cBD-1] and monitored sap promoter activity by measuring luminescence (Fig. 1B). sap promoter activity increased in a dose- and time-dependent manner when NTHI was exposed to (r)cBD-1, suggesting the presence of a resistance mechanism in NTHI that is upregulated upon exposure to APs. Similar results were observed when NTHI was exposed to human beta-defensin 3 [(r)hBD-3] and the human cathelicidin LL-37 (data not shown) suggesting that our observations were not restricted to an AP of chinchilla origin.
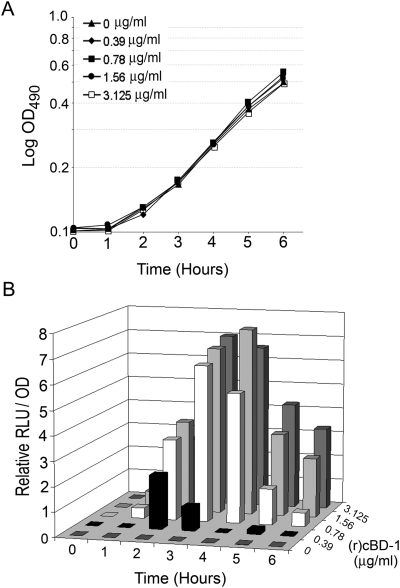
Activity of the NTHI sap promoter following exposure to antimicrobial peptide as measured via detection of relative luminescence.A. NTHI strain 1885/pKMLN-01 was grown in the presence of increasing, yet sublethal concentrations of (r)cBD-1.B. The ratio of relative light units (RLU) to optical density at 490 nm (OD490) is presented when NTHI strain 1885/pKMLN-01 was exposed to increasing concentrations of (r)cBD-1 for the indicated times. RLU/OD490 was calculated by dividing each sample ratio by the RLU/OD490 value obtained for each AP dilution at time zero, then subtracting from this value the value obtained for cultures not containing AP. Shown is the result of one of three independent experiments, which gave similar results.
These data indicated that, although deficient in PhoP–PhoQ and PhoP–PhoQ-dependent systems similar to those described in Salmonella (Guo et al., 1997; Ernst et al., 1999; Moss et al., 2000; Derzelle et al., 2004; Rebeil et al., 2004), NTHI-mediated AP resistance was dependent upon the Sap transporter whose expression was regulated by concurrent exposure to APs. Similarly, Bengoechea and Skurnik showed that Yersinia resist the action of APs via a temperature-regulated efflux pump/potassium antiporter system that is itself regulated by exposure to an AP (Bengoechea and Skurnik, 2000). Our data thereby suggested that NTHI mediated AP resistance by sensing the presence of the AP and initiating signalling events that lead to increased sap gene expression and production of Sap proteins required for resistance to killing by APs.
(r)cBD-1 associated with (r)SapA protein; evidence supporting direct interaction
As we observed AP-dependent regulation of sap gene expression, we attempted to determine whether AP directly associated with the SapA protein, thereby serving as a ligand for possible transport by the Sap transporter. Recently, Bader and colleagues demonstrated a direct association between the bacterial sensor kinase PhoQ and the alpha helical AP C18G, suggesting a greater specificity of AP interaction with the bacterial cell than previously described (Bader et al., 2005). In this work, we hypothesized that NTHI mediates AP resistance, in part, by direct association with the periplasmic-binding protein SapA. To test this, we overexpressed the complete sapA gene in a Champion pET Directional TOPO expression vector (Invitrogen, Carlsbad, CA) and purified recombinant SapA His-tagged fusion protein [(r)SapA] from a soluble fraction by nickel-nitrilotriacetic acid (Ni-NTA) affinity chromatography. We then assayed for a direct association of (r)SapA with (r)cBD-1 by Far-Western immunoblot analysis. We demonstrated specific association of (r)cBD-1 with (r)SapA (68 kDa), but not with recombinant thioredoxin A protein [(r)TrxA; 18 kDa] (Fig. 2A, left panel). No reactivity was observed in the absence of AP, indicating specific detection of (r)cBD-1 in association with (r)SapA (data not shown). The presence of recombinant protein was confirmed by probing for the His-tag moiety (Fig. 2A, right panel). To confirm the specificity of this association, equimolar amounts of (r)SapA or (r)TrxA proteins were captured via the His-tag to a Ni-NTA biosensor chip surface and monitored for (r)cBD-1 binding by Biacore analysis. (r)cBD-1 showed greater association with (r)SapA than the control protein (r)TrxA (compare RU values of 3668–2786; difference of 882 RU) during AP injection across the chip surface. Preferential binding to (r)SapA was also stable, as demonstrated by a lack of return to baseline, for 10 min following injection (Fig. 2B). Although we observed low-level binding of (r)cBD-1 to the protein-free chip surface (438 RU), this did not affect its specific relative association with (r)SapA (902RU) compared with binding with (r)TrxA (529 RU), still observable 10 min following the end of AP injection. Binding was not due to charge attraction alone because the predicted isoelectric point (pI) of (r)TrxA was 6, compared with a pI of 7.2 for (r)SapA, indicating that at the neutral pH of buffers used here (pH = 7.4) (r)TrxA would have a greater net negative charge. We further demonstrated (r)cBD-1 association with (r)SapA by immunoprecipitation (Fig. 2C). We detected the presence of (r)cBD-1 only in samples containing the recombinant SapA protein as determined by Western blot analysis. Samples containing (r)TrxA or controls lacking (r)cBD-1 did not show any reactivity (Fig. 2C). Collectively these data indicated for the first time, direct interaction between an AP and an NTHI protein, thereby supporting a direct mechanism for Sap transporter-mediated uptake of AP.
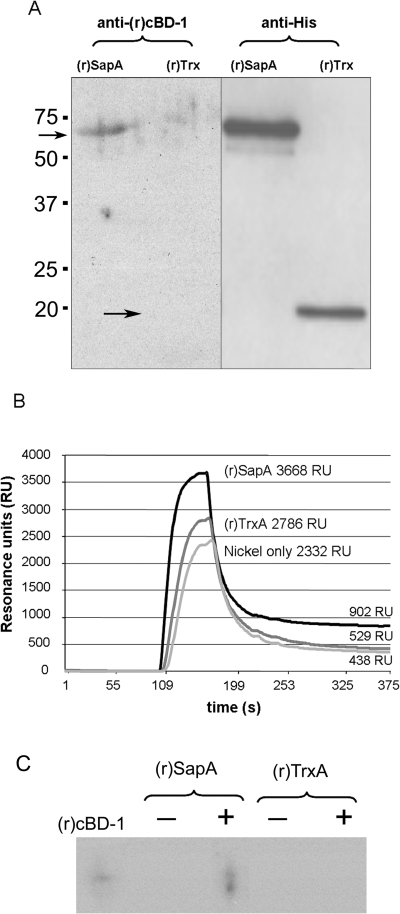
Recombinant SapA binds AP.A. Far-Western blot analysis for AP adherence. Left panel: Equimolar amounts of (r)SapA or (r)TrxA were separated by 18% SDS-PAGE, transferred to nitrocellulose, incubated with (r)cBD-1 and detected by Western blot analysis as described in Experimental procedures. Right panel: The presence of recombinant proteins was confirmed by probing for the histidine (His) tag.B. Biosensor analysis to monitor AP binding. Equimolar concentrations of (r)SapA or (r)TrxA were captured via His-tag to a Ni-NTA biosensor chip surface. AP binding was monitored by increase in surface plasmon resonance and plotted as total resonance units (RU). Greater recognition and binding was observed to the (r)SapA protein compared with the control protein or chip surface. Shown is the result of one of three independent experiments, which gave similar results.C. Immunoprecipitation to detect AP adherence to (r)SapA. Equimolar amounts of (r)SapA-His or (r)TrxA-His were incubated with (r)cBD-1 and protein complexes were precipitated by antibody labelling of the His-tag moiety and subsequent binding to sepharose beads. Immune complexes were separated by AU-PAGE and (r)cBD-1 was detected by Western blot analysis. Immunoblot for (r)cBD-1 alone served as a mobility standard.
The sapD mutant was attenuated for survival in vivo
We have demonstrated that sap promoter activity was specifically upregulated by exposure to (r)cBD-1 as well as specific binding of this AP to SapA. These data support the hypothesis that Sap-mediated AP resistance is an important NTHI virulence mechanism.
To further define the role of the Sap transporter in NTHI, and specifically AP-mediated resistance, we extended our mechanistic analysis to the SapD ATPase protein, the ATP-binding subunit of the Sap transporter, that is predicted to provide energy for ligand transport. Work in other microorganisms suggests that the sapD gene is upregulated when Pasteurella is exposed to iron-containing compounds (Paustian et al., 2002), and that SapD is required for resistance to polymyxin B and contributes to LPS modifications in Proteus species (McCoy et al., 2001), and mediates potassium uptake in E. coli (Harms et al., 2001).
We reasoned that a mutation in the sapD gene would disrupt the function of the Sap transporter, thereby allowing us to ascertain the involvement of SapD in NTHI survival in a chinchilla model of OM. In order to determine the biological consequences of disruption of the sapD gene, we inoculated the parent, sapD mutant or complemented sapD mutant strain either alone or in competition with the parent strain into the nares and middle ears of chinchillas (3, 4).
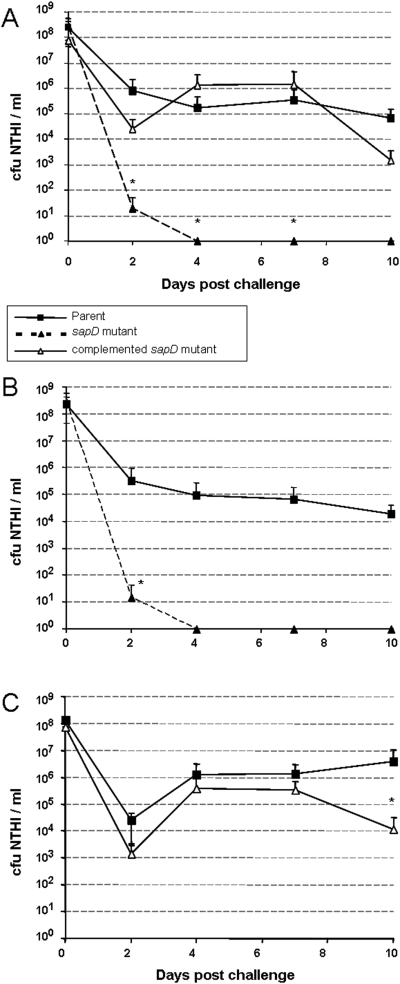
NTHI strain 86-028NP, the sapD mutant and complemented sapD mutant were inoculated into the nasopharynx (NP) of adult chinchillas to assay relative survivability and/or ability to colonize in this host.A. Comparison of relative abilities of individual strains to colonize the NP. The sapD mutant (▴, dotted line) was not recovered from nasopharyngeal lavage fluids 4 days following inoculation. Complementation of the sapD mutation restored the strains ability to colonize the NP (▵), similar to the parent strain (▪).B. The parent strain (▪) and the sapD mutant (▴, dotted line) were coinoculated into chinchillas to measure the relative ability of these strains to colonize the NP. The sapD mutant was unable to compete with the parent strain and was not recovered from NP lavage fluids 4 days following inoculationC. The parent strain (▪) and the complemented sapD mutant (▵) were coinoculated into chinchillas to measure the relative ability of these strains to colonize the NP. Complementation of the sapD mutation restored the ability of the strain to compete with the parent.All data (A–C) represent mean values of five cohorts of four animals each (n = 4 per cohort) and error bars represent standard deviation values. Statistically significant differences were determined as described in Experimental procedures and were noted by an asterisk.
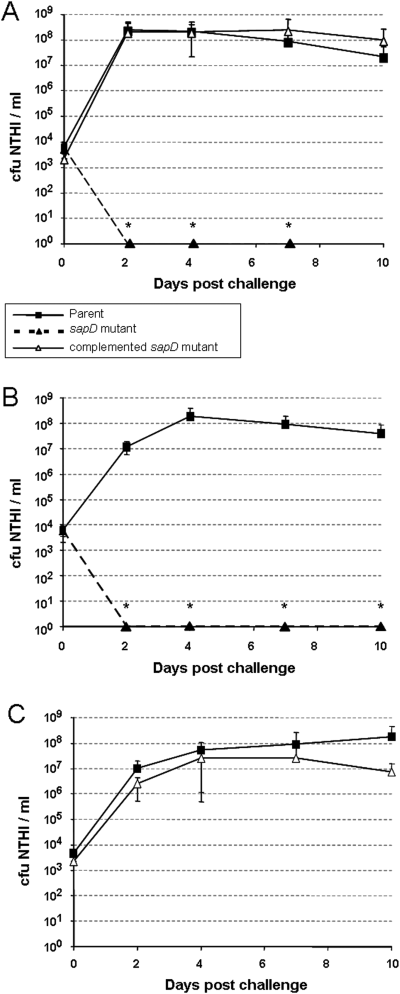
NTHI strain 86-028NP, its sapD mutant and complemented sapD mutant were inoculated into middle ears of adult chinchillas to assay relative survivability in this niche.A. Comparison of relative abilities of individual strains to survive in the middle ear. The sapD mutant (▴, dotted line) was not recovered from middle ear effusions (MEE) 2 days following inoculation. Complementation of the sapD mutation (▵) restored survivability similar to that of the parent strain (▪).B. The parent strain (▪) and the sapD mutant (▴, dotted line) were coinoculated into chinchillas to measure the relative ability of these strains to infect the middle ear. The sapD mutant was unable to compete with the parent strain and was not recovered from MEE 2 days following inoculation.C. The parent strain (▪) and the complemented sapD mutant (▵) were coinoculated into chinchillas to measure the relative ability of these strains to infect the middle ear. Complementation of the sapD mutation restored the ability of this strain to compete with the parent strain.All data (A–C) represent mean values of five cohorts containing four animals each (8 ears per cohort; n = 8) and error bars represent standard deviation values. Statistically significant differences were noted by an asterisk.
We first assessed survival of the sapD mutant in the nasopharynx compared with the parent strain. The sapD mutant was immediately impaired in its ability to colonize the nasopharynx, and showed a significant reduction in relative cfu NTHI ml−1 recovered from nasopharyngeal lavage fluid compared with the parent strain (P = 0.029). Moreover, the sapD mutant was completely eradicated from this site 4 days after inoculation (Fig. 3A). Complementation of the sapD mutation restored the ability of this strain to colonize the nasopharynx at a level similar to wild type (Fig. 3A). In order to further evaluate the loss in relative pathogenesis displayed by the sapD mutant, we coinoculated both the parent and mutant strains into the nasopharynx and monitored them for competitive fitness (Fig. 3B). The sapD mutant was unable to compete with the wild-type strain and demonstrated a significant reduction in amount of bacteria recovered from nasopharyngeal lavage fluids 2 days following inoculation, and was cleared by 4 days after challenge (P = 0.029). Likewise, complementation of the sapD mutation restored colonization to wild-type level (Fig. 3C).
The inability of the sapD mutant to survive in this host was even more pronounced in the middle ear microenvironment. Wild-type NTHI steadily increased from an inoculum of approximately 2500 cfu to approximately 1.0 × 108 cfu NTHI ml−1 middle ear fluid within 48 h. In contrast, the sapD mutant was unable to survive and was completely cleared from the middle ear in the same period of time (Fig. 4A, P = 0.006). Similarly, the sapD mutant was unable to compete with the wild-type strain for survival in the middle ear (Fig. 4B, P < 0.001). The inability of the mutant to survive was due to the absence of SapD, because complementation with the sapD gene fully restored wild-type growth, but also the ability for the mutant strain to compete equally with the wild-type strain (Fig. 4A and C). Although we previously demonstrated that a mutation in the sapA gene also attenuates survival in the middle ear, the sapA mutant was not cleared from the chinchilla middle ear until at least 8 days after inoculation, suggesting that the sapD gene product may provide a multifunctional role in NTHI pathogenesis, particularly in the acute phase.
SapD was required for resistance to killing by APs
We then determined whether the sapD mutant was more sensitive to killing by (r)cBD-1 compared with the parent strain. Using a bactericidal assay, we assessed the ability of this AP to kill NTHI strain 86-028NP, and its sapD mutant (Fig. 5). The mutation rendered the bacterium more sensitive to the antimicrobial activity of (r)cBD-1 over a concentration range of 0.5–3.8 μg (r)cBD-1 ml−1 compared with killing of the parent. Specifically, when incubated with 0.5 μg (r)cBD-1 ml−1, we observed a 16-fold increase in killing of the sapD mutant compared with killing of the parent strain. Further, the sapD mutant was sensitive to killing by human beta defensin-3, hBD-3 and the human cathelicidin, LL37 (data not shown). These data indicated that the NTHI SapD protein functions, at least in part, to protect this microorganism from the lethal effects of AP.
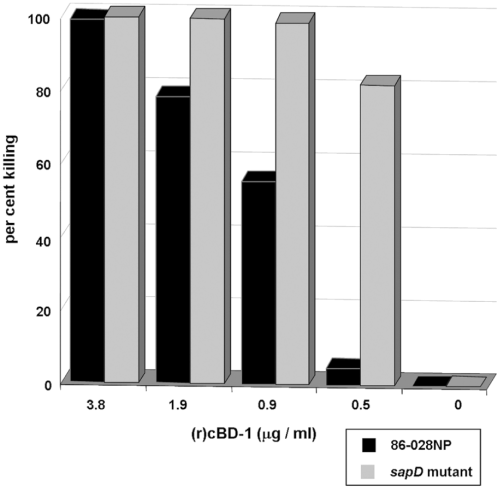
Sensitivity of the sapD mutant to AP. NTHI strain 86-028NP or its sapD mutant were incubated with increasing concentrations of (r)cBD-1 for 1 h and then plated on chocolate II agar for determination of viable bacteria. Per cent killing values were determined by dividing the number of viable bacteria after AP exposure by the number of viable bacteria in cultures not exposed to AP for the same period of time. Shown is the result of one of three independent experiments, all of which yielded similar results.
SapD was required for NTHI growth in potassium-limited conditions
Recent studies suggest that the sapD gene product may play a role in multiple microbial functions. McCoy et al. (2001) demonstrates that sapD is required for LOS modifications in Proteus species by an as-yet undefined mechanism. Further studies in Pasteurella indicate that sapD expression was upregulated when this microbe was exposed to specific iron sources (Paustian et al., 2002). SapD also mediates potassium uptake in E. coli K-12 by providing ATP dependence to the TrkG/TrkH potassium uptake system in this strain (Harms et al., 2001). As we demonstrated that an NTHI strain unable to produce SapD was sensitive to killing by AP, and previous literature suggests that AP killing results in potassium release by the exposed bacterium, we reasoned that SapD likely plays a role in potassium acquisition in NTHI. In order to investigate the relationship between sapD expression and potassium acquisition in NTHI, we monitored our sapD mutant for growth when cultured on different media. We initially observed a slight attenuation in growth on chocolate and GC agars (which both contain haem as the iron source) and further, we noted that the sapD mutant failed to grow on BHI agar supplemented with haem and NAD. We determined that the addition of 50 mM potassium phosphate to sBHI agar rescued the no-growth phenotype of the sapD mutant (Fig. 6A). Complementation of the sapD mutation (e.g. complemented sapD mutant) restored wild-type growth, even under potassium limited conditions (sBHI agar). We further defined this potassium requirement by monitoring growth of the wild type, sapD mutant and complemented sapD mutant strains following potassium starvation and transfer to a potassium-free chemically defined iron source (DIS) medium that contained increasing amounts of potassium (0.003 mM−50 mM). We showed that the sapD mutant required a 1000-fold increase in extracellular potassium to support even minimal growth when compared with either the wild type or complemented sapD mutant strain (Fig. 6B and C). These data indicate that SapD is required for NTHI growth in potassium-limited environments.
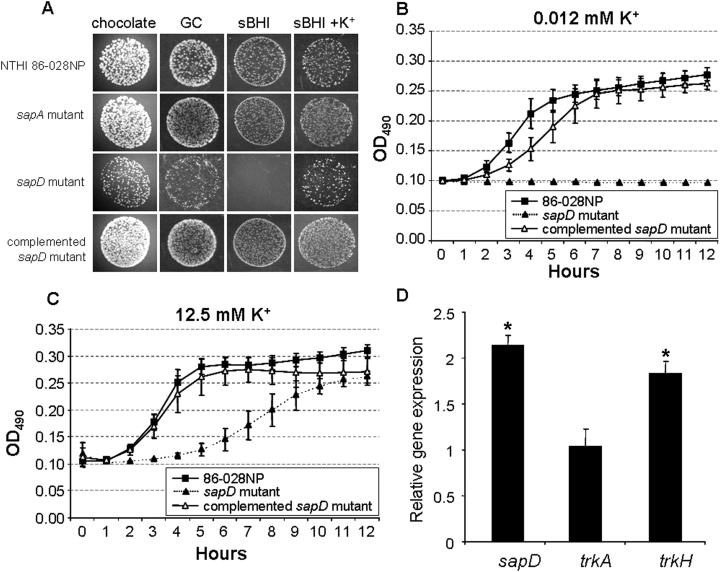
The role of SapD in growth of NTHI in potassium-limited conditions.A. NTHI strain 86-028NP, its sapA and sapD mutant, and the complemented sapD mutant were spotted (10 μl each) on various agar surfaces and monitored for growth after 18 h incubation. Images were obtained from matched dilutions at the same magnification.B and C. Potassium-starved bacteria were cultured in potassium-free DIS medium containing low (B) or high (C) concentrations of potassium phosphate. A concentration of 12.5 mM potassium phosphate was required to rescue growth of the sapD mutant. Shown are the mean values from three independent experiments performed on different days. Error bars represent standard deviation.D. Abundance of gene transcript levels under potassium-starved conditions. RNA was isolated from NTHI strain 86-028NP grown in medium supplemented with either high or low concentrations of potassium phosphate and monitored for changes in relative transcript levels by quantitative RT-PCR analysis. Changes in relative gene expression levels were determined by normalization to constitutive gene expression and fold increase determined by dividing expression level in medium containing a low concentration of potassium by the value obtained in medium containing a high concentration of potassium. Error bars represent the standard deviation of four independent experiments performed in triplicate. Statistical significance was determined by Student t-test and significant differences were noted by an asterisk (sapD, P = 0.01; trkH, P = 0.029).
Because we showed that SapD was required for NTHI growth in potassium-depleted media, we further reasoned that these environmental conditions would upregulate sapD expression. To test this, NTHI was depleted of potassium by overnight growth in DIS medium and this culture was used to inoculate fresh DIS media that contained either high (25 mM) or low (25 μM) concentrations of potassium. By reverse transcriptase polymerase chain reaction (RT-PCR) we observed an approximate twofold upregulation of sapD transcription in cultures that contained a low concentration of potassium (Fig. 6D, sapD: P = 0.01). This increase was not observed in cultures containing millimolar (mM) concentrations of potassium. These data, along with our evidence indicating that SapD was required for growth in potassium-limited environments strongly suggested that the SapD protein, may be necessary for transport of potassium by NTHI. We next wanted to determine whether the NTHI trkA and trkH genes were differentially regulated when strain 86-028NP was grown in media supplemented with a low concentration of potassium as described above. We demonstrated by quantitative RT-PCR that, similar to sapD expression, trkH gene expression was upregulated twofold when NTHI was grown in potassium-limited conditions (Fig. 6D, trkH: P = 0.03), compared with trkH gene expression grown in the presence of a high concentration of potassium. We did not observe a similar upregulation in trkA gene expression but observed however, that trkA transcript levels were 10-fold greater than trkH transcript levels under all growth conditions tested (data not shown). Although the mechanism of potassium transport may not be entirely dependent upon the amount of transporter present, our data indicated that potassium acquisition by NTHI strain 86-028NP via the TrkA/TrkH system may depend, in part, upon trkH gene regulation and increase in the gene product TrkH. These data suggest that sap regulation may be dependent upon the presence of microenvironmental cues, specifically those relevant to in vivo conditions such as iron and potassium availability as well as the presence of APs.
SapD was required for potassium transport
Because our data suggested that SapD was required for NTHI growth in a potassium-limited environment, we reasoned that a functional SapD protein was required to mediate potassium uptake by this organism. NTHI strain 86-028NP, the sapD mutant and the complemented sapD mutant strain were grown in DIS medium containing 10 μg haem ml−1 to which we added 40 mM potassium phosphate to support growth of the sapD mutant. Mid-log phase cells were removed from culture and depleted of intracellular potassium by Tris/EDTA treatment as described in Experimental procedures. Potassium-depleted cells were then spiked with a 1 mM potassium chloride solution and monitored for potassium uptake by inductively coupled plasma atomic emission spectroscopy. Strain 86-028NP demonstrated rapid uptake of potassium, reaching an intracellular saturation level of 75 nanomoles potassium per milligram of dry weight cells in 5 min (Fig. 7). In contrast, the sapD mutant strain was clearly deficient in its ability to transport potassium during the 10 min incubation period. Importantly, complementation of this mutant strain with SapD restored its ability to transport potassium to a saturation level of 54 nanomoles potassium per milligram of dry weight cells in 5 min. The sapA mutant was not impaired in its ability to transport potassium indicating that SapA is not required for potassium uptake and further suggesting that potassium uptake in not largely the physiological role of the Sap transporter. These data indicated a direct involvement of the SapD protein in potassium uptake by NTHI.
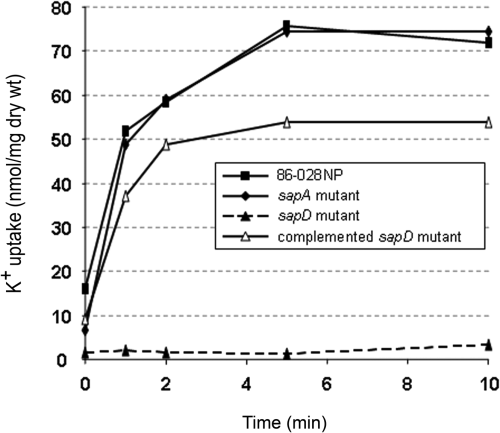
SapD was required for potassium transport. Exponentially grown NTHI strain 86-028NP (▪), its sapA mutant (◆), sapD mutant (▴, dotted line), or the complemented sapD mutant strain (▵) were depleted of internal potassium by Tris/EDTA treatment and maintained viable in potassium-free DIS medium. Following the addition of 1 mM potassium chloride, potassium uptake was monitored over a 10 min period and intracellular potassium levels were determined as described in Experimental procedures. Shown are the data from a single experiment that was repeated with similar results.
Discussion
We have previously demonstrated that an NTHI sapA mutant was significantly attenuated for survival in a chinchilla model of NTHI-induced OM, and was also highly susceptible to killing by APs (Mason et al., 2005). Importantly, we showed both upregulation of the sap promoter in vivo and expression of cBD-1 in the upper respiratory tract in the chinchilla model of NTHI-induced OM, suggesting an important role for the Sap-system in response to components of the host innate immune response (Mason et al., 2003; Harris et al., 2004). APs are cationic, amphipathic molecules that interact with and disrupt the bacterial outer membrane. Once APs gain access to the periplasmic space, they exert their lethal effect on the cytoplasmic membrane which is comprised, in part, of negatively charged phospholipids. It is generally believed that these cationic peptides insert into the cytoplasmic membrane and form channels that result in the leakage of cytoplasmic contents (White et al., 1995; Hancock and Chapple, 1999; Peschel, 2002). Gram-positive and Gram-negative bacteria limit the effectiveness of APs by various mechanisms, including removal of APs via an energy-dependent efflux pump; altering cytoplasmic membrane fluidity, cleavage with surface associated proteases (Guo et al., 1998; Shafer et al., 1998; Peschel et al., 1999; Bayer et al., 2000; Guina et al., 2000; McCoy et al., 2001) or reducing the net negative charge of the cell envelope via surface modifications that contribute to the repulsion of cationic APs (Martinez de Tejada et al., 1995; Freer et al., 1996; Guo et al., 1997; Gunn et al., 1998).
Many Gram-negative organisms mediate some of these modifications via the PhoP–PhoQ two-component system (Guo et al., 1997; Ernst et al., 1999; Moss et al., 2000; Derzelle et al., 2004; Rebeil et al., 2004), which plays a role in the control of virulence determinants of many microorganisms (Fields et al., 1989; Moss et al., 2000; Derzelle et al., 2004). H. influenzae however, lacks a PhoP–PhoQ regulatory system, and thus must possess alternative mechanisms to regulate resistance mechanisms to AP-mediated killing. Recent literature suggests that AP interaction with the bacterial cell may trigger the upregulation of other AP resistance determinants, allowing survival of the bacterium in AP-rich environments (Stumpe and Bakker, 1997; Stumpe et al., 1998; Bengoechea and Skurnik, 2000). Here, we present evidence of a novel AP resistance mechanism in NTHI by describing an interaction between a component of a bacterial transporter complex and an AP. These data support a mechanism to counteract the lethal effects of APs on the bacterial cytoplasmic membrane.
The Sap transporter was first described in Salmonella typhimurium and shown to harbour a periplasmic component that plays a role in virulence (Parra-Lopez et al., 1993). It is clear that products of the sap operon play a significant role in resistance to APs (Parra-Lopez et al., 1993; Lopez-Solanilla et al., 1998). However, the exact mechanism(s) conferring this resistance remain unclear. In this work, we defined a role for the NTHI Sap transporter system in mediating resistance to the lethal effects of APs. We demonstrated that SapA, the putative periplasmic binding protein of this ABC transporter, binds at least one AP, and that NTHI association with this and other APs upregulated sap operon gene expression. It is possible that AP interaction with the putative periplasmic binding protein SapA mediates resistance via transport of APs to the bacterial cytoplasm where they are targeted for degradation.
Recent evidence also indicates a role for the sap gene product, SapD, in potassium homeostasis in bacteria. Harms and coworkers demonstrated that the role of the sap operon, with respect to protamine resistance in an E. coli K-12 strain, involves potassium transport via the Trk system (Stumpe and Bakker, 1997; Stumpe et al., 1998). The Trk system requires both proton motive force and ATP for potassium uptake (Rhoads and Epstein, 1977; Stewart et al., 1985; Harms et al., 2001). SapD is required for potassium uptake in this microbe and is dependent upon the ATP-binding domain (Harms et al., 2001). Further work in Salmonella serovar typhimurium demonstrates that SapD confers to Trk its previously documented ATP dependence (Parra-Lopez et al., 1994). Mutants deficient in expression of the genes encoding SapD, as well as TrkH and TrkA (components of the low affinity, high rate potassium transporter in Salmonella) are more sensitive to the prototypic AP protamine (Parra-Lopez et al., 1994). Further, a TrkA mutant is attenuated for virulence in mice (Groisman et al., 1992). In contrast, potassium uptake by the Trk system in Vibrio alginolyticus does not appear to be dependent upon SapD (Nakamura et al., 1998). Here, we studied the role of the putative sap transporter ATPase, SapD, in potassium transport in NTHI. We constructed an NTHI sapD mutant and demonstrated that SapD was required for potassium uptake in NTHI strain 86-028NP. Complementation of the sapD mutation restored NTHI's ability to grow in micromolar (low) concentrations of potassium. SapD also conferred resistance to killing by APs. Importantly, SapD complementation completely restored survival of the sapD mutant in both the nasopharynx and middle ear in a chinchilla model of NTHI-induced OM. By sequence homology, we showed the presence of trkH and trkA genes in the NTHI strain 86-028NP genome, but no genes encoding a high affinity potassium transport system homologous to those described in E. coli (Bossemeyer et al., 1989; Dosch et al., 1991; Schlosser et al., 1995; Ness and Booth, 1999) were identified. We hypothesize therefore, that SapD confers ATP dependence to the TrkAH potassium transport system in NTHI. We further propose a hypothetical model of the Sap transporter and its relationship to Trk potassium uptake in NTHI, by sharing of ABC transporter ATPase function with the potassium uptake system (Fig. 8).
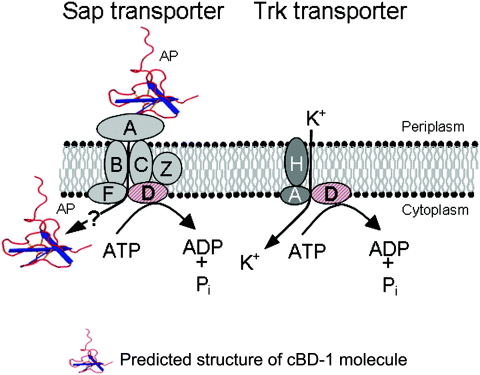
Proposed model of the Sap transporter and its relationship to Trk-mediated potassium uptake. SapA binds and likely transports AP for proteolytic cleavage. SapD energizes this process, but is also required for high affinity potassium uptake in NTHI, possibly energizing the Trk transport system. The cBD structure is based upon the known structure of hBD-3 (Schibli et al., 2002).
We hypothesize that SapD provides energy to components of a high-rate potassium transporter system, resulting in increased intracellular levels of potassium, possibly countering the rapid loss of potassium that occurs after exposure to APs (Aspedon and Groisman, 1996; Matsuzaki et al., 1997; 1999; Bengoechea and Skurnik, 2000; Ohmizo et al., 2004). This mechanism would equip NTHI to survive in the host, possibly in part by coupling transport and destruction of APs with Sap-dependent maintenance of potassium homeostasis.
In future work, we plan to assess whether association of APs with the periplasmic SapA protein results in the delivery of peptide to the integral membrane permease proteins SapB and SapC followed by transport to the cytoplasm, presumably for subsequent peptide degradation. This mechanism of AP transport and cytoplasmic degradation concomitant with regulation of potassium uptake would confer a selective advantage for NTHI survival in the host, an environment rich in innate immune molecules but also limited in nutrient availability, as this normally commensal microorganism transitions to an opportunistic pathogen of the middle ear or other sites of the respiratory tract.
The present work defined, for the first time, a molecular mechanism by which NTHI resist killing by APs. Our results also showed that SapD was required for potassium uptake, possibly important as a consequence of exposure to APs. The Sap transporter provides a critical and necessary function in NTHI to mediate survival in response to attack by key components of the host's innate immune response. We propose that the presence of effectors of innate immunity in the host induces expression of a more resistant NTHI phenotype, that thereby equips this commensal microorganism for enhanced survival against host defence mechanisms in vivo, thus promoting the long-term colonization phenotype that is a hallmark of this member of the normal flora of the human airway.
Experimental procedures
Bacterial strains and growth conditions
The parental NTHI strain 86-028NP is a minimally passaged clinical isolate obtained from a paediatric patient who underwent tympanostomy and tube insertion for chronic OM with effusion at Columbus Children's Hospital. This strain has been extensively characterized in chinchilla models of OM. The genome of strain 86-028NP has been sequenced and annotated [GenBank Accession number CP000057; (Harrison et al., 2005)]. This strain has been maintained frozen in skim milk containing 20% glycerol (v/v). NTHI was grown on chocolate II agar (Becton Dickinson and Co., Sparks, MD) or in brain heart infusion broth (BHI broth; Becton Dickinson and Co.) supplemented with 2 μg of haemin chloride ml−1 (dissolved in 20 mM NaOH; Sigma-Aldrich, St. Louis, MO) and 2 μg of NAD ml−1 (Sigma-Aldrich) (sBHI broth) at 37°C in 5% CO2. Strains containing plasmids containing antibiotic resistance genes were grown on chocolate agar prepared in house using GC agar base (Fisher Scientific), supplemented with 2% haemoglobin (Fisher Scientific), IsoVitaleX® (BBL, Kansas City, MO), 200 mM l-glutamine (Cellgro, Herndon, VA), NAD 2.5 μg ml−1, Sigma and 20 μg kanamycin ml−1 or 200 μg spectinomycin ml−1, as appropriate. NTHI strain 1885MEE is also a low-passage-number clinical isolate obtained from a child with chronic OM and has been used in a chinchilla model of OM (Sirakova et al., 1994; Kennedy et al., 2000; Munson et al., 2004). For luminescence, potassium growth and potassium transport assays, we used a chemically defined medium (Coleman et al., 2003), an RPMI 1640-based medium that was further modified by treatment with 6% Chelex 100 (Sigma, St. Louis, MO) overnight with stirring to remove divalent cations, adjusted to a pH of 7.4 and filtered through a 0.22 μm GP Express PLUS membrane filter (Millipore, Billerica, MA) and then supplemented with 0.07 mM CaCl2, 0.7 mM MgSO4 and 2 μg haemin chloride ml−1 (Sigma Aldrich, St. Louis, MO), generating a DIS medium.
Measuring sapA promoter activity by monitoring luminescence
Non-typeable Haemophilus influenzae strain 1885/pKMKLN-01 (Mason et al., 2005) was grown for 18 h on chocolate agar and colonies were suspended in sterile saline to obtain an OD490 of 0.65. Bacterial suspensions (1:100) were added to a 96-well, white, clear bottom plate (Costar, Corning, NY) containing 200 μl of BHI broth supplemented with 2 μg NAD ml−1 and 10 μg haemoglobin ml−1 (Fluka; Buchs, Switzerland) and increasing concentrations of (r)cBD-1. Microtiter plates were incubated statically at 37°C, 5% CO2 and sapA promoter activity was monitored via detection of relative luminescence. Relative light units (RLU) were determined using a GENios plate reader (Tecan, Durham, NC) and ratios of RLU per unit of optical density (OD) were calculated.
Expression and purification of recombinant SapA protein
The sapA gene was amplified from 86-028NP genomic DNA by PCR using Pfu Turbo Hotstart DNA polymerase (Stratagene, La Jolla, CA), the forward primer 5′-CACCATGTTACGTCTAAATCTGAGA-3′, and the reverse primer 5′-GTGTTTCTCAATAAAATATAAGGTGGAAAAATCTAA-3′. The amplicon was cloned into the pET101/D-TOPO® vector contained in the Champion pET Directional TOPO® Expression kit (Invitrogen, Carlsbad, CA) according to manufacturer's protocol. A portion of the cloning reaction was used to transform One Shot® TOP10 competent E. coli and a plasmid with the correct restriction map and sequence was saved as pSAP6. To express the (r)SapA protein, we transformed pSAP6 into E. coli One Shot® BL21 STAR (DE3) (Invitrogen), and the appearance of an IPTG-inducible protein band of appropriate molecular mass was evaluated in Coomassie brilliant blue-stained SDS-PAGE gels of total cell lysates.
For isolation of recombinant SapA, two litres of cells grown to mid-log phase in Luria–Bertani (LB) broth were induced overnight with 1 mM IPTG to express the fusion protein. Bacterial cells were pelleted by centrifugation at 8000 g for 10 min and the cell pellet was suspended in a mixture of Bugbuster® reagent (Novagen) (5 ml per gram of wet weight), 20 μg lysozyme per ml, and 1/1000 volume Benzonase® nuclease (Novagen). Cell lysis was allowed to occur for 15 min at room temperature and insoluble debris was separated from soluble protein by centrifugation at 20 000 g for 20 min. The supernatant containing soluble protein was applied to a prepared Ni2 ± NTA column according to the manufacturers suggestions for soluble proteins (Invitrogen). The His-rich proteins were allowed to bind to the Ni2+-agarose slurry for 1 h at room temperature and loosely bound protein was stripped from the column using four separate washes of 1 × Native Wash buffer (10 mM sodium phosphate buffer, 500 mM NaCl and 20 mM imidazole pH 8.0). Proteins bound to the Ni2+-agarose slurry were then eluted with Elution buffer (10 mM sodium phosphate buffer, 500 mM NaCl and 200 mM imidazole pH 8.0). One milliliter fractions were collected and fractions containing recombinant protein were pooled and dialysed overnight at 4°C against 1 × Native purification buffer (Native wash buffer without imidazole).
Far-Western blot analysis
Equimolar (2 μM) amounts of His-tagged recombinant SapA (r)SapA-His, or recombinant thioredoxin A (r)TrxA-His, were separated on a 12% polyacrylamide gel and transferred (1 h, 100 V) to 0.45 μm nitrocellulose (Bio-Rad, Hercules, CA). Membranes were then blocked overnight with Blocking Buffer (TBS, 0.05% tween-20, 5% skim milk) before incubation [room temperature (RT), 4 h] with 100 ng of recombinant chinchilla beta-defensin 1 (r)cBD-1, diluted in Blocking Buffer. Membranes were washed three times (20 min each wash) in Blocking Buffer. Western blot analysis was then performed using rabbit polyclonal anti(r)cBD-1 (Proteintech Group, Chicago, Ill) or Penta-His antibody (Qiagen) probes and bound antibody was detected with Goat anti-rabbit IgG (H + l)-HRP (Xymed, San Francisco, CA) followed by chemiluminescent detection (ECL detection reagents, Amersham Biosciences, Piscataway, NJ).
Surface plasmon resonance
Analysis of interaction between recombinant proteins was performed with a BIAcore 3000 instrument (Biacore AB, Uppsala, Sweden) as previously described (Novotny et al., 2000). Briefly, an NTA sensor chip was used to bind the histidine–tagged proteins for interaction analysis in the Biacore system. The surface consists of a carboxymethylated dextran matrix preimmobilized with nitrilotriacetic acid (NTA) for capture of histidine-tagged molecules via Ni2+/NTA chelation. The chip was saturated with nickel by injecting a 1 min pulse (5 μl min−1) of 500 μM NiCl2 in Running Buffer (0.01 M Hepes, 0.15 M NaCl, 50 μM EDTA, 0.005% Surfactant P20, pH 7.4) across the chip surface to attain a baseline Resonance Unit (RU) of approximately 60 RU. Equimolar (2 μM) concentrations of (r)SapA-His or (r)TrxA-His were then injected and bound to individual quadrants of the nickel-loaded NTA chip surface (2000 RU and 333 RU respectively). Next, a 2 μM stock of (r)cBD-1 was injected (5 μl min−1) for 1 min across flow cells 1, 2 and 3 containing immobilized (r)SapA, (r)TrxA or nickel-bound NTA (negative control) respectively. The amount of ligand bound was determined by a change in refractive index at the chip surface and was expressed as relative RU, which are plotted against time (Karlsson et al., 1991). Peak RU values were obtained immediately following injection of ligand across the chip surface, and a secondary RU value was obtained 2.5 min later. The NTA sensor chip was regenerated by stripping nickel from the surface with Regeneration Solution (0.01 M HEPES, 0.15 M NaCl, 0.35 M EDTA, 0.005% Surfactant P20, pH 8.3). Ligand binding was performed at least three times on separate days and a representative graph is shown.
Immunoprecipitation
Equimolar amounts of (r)SapA-His or (r)TrxA-His were combined with 0.2 μg Penta-His antibody ml−1 (Qiagen) in 1 ml of 10 mM sodium phosphate buffer (pH 7.2) in 1.5 ml Eppendorf tubes and incubated on a rotating platform at 4°C for 7 h. Following incubation, (r)cBD-1 was added to each tube to a final concentration of 0.1 μM and incubated at 4°C overnight. Each tube then received a 10 μl volume of GammaBind Plus Sepharose (Amersham Biosciences, Piscataway, NJ) that had been previously washed once and resuspended to one fourth the volume in 10 mM sodium phosphate buffer, pH 7.2. The tubes were incubated for 8 h at 4°C on a rotating platform and subsequently were centrifuged at 2500 r.p.m. for 3 min to pellet the Sepharose beads. The beads were washed three times with a 1 ml volume of sodium phosphate buffer, pH 7.2. Following the final wash, the bead pellet was suspended in 40 μl of 10 mM sodium phosphate buffer, pH 7.2. Ten microlitre volumes were subsequently analysed for the presence of (r)cBD-1 by Acid-Urea polyacrylamide gel electrophoresis (AU-PAGE) according to published protocols (Wang et al., 1997; Porter et al., 1998).
SapD mutant and complemented sapD mutant construction
The sapD gene in strain 86-028NP, as well as an additional 1 kb sequence upstream and downstream, was amplified from 86-028NP genomic DNA by a PCR using Pfu Turbo Hotstart DNA polymerase (Stratagene, La Jolla, CA), the forward primer 5′-GTATGCAAGATAAAGAACCTGATGAAT-3′, and the reverse primer 5′-CCAAAAGTAAGGCGAAAACCA-3′. After purification (Qiagen) and the introduction of an A tail, the PCR product was ligated into pGEM-T Easy (Promega, Madison, WI) and transformed into One Shot® TOP10 Chemically Competent E. coli cells (Invitrogen, Carlsbad, CA). A plasmid with the correct restriction fragments was saved as pMEB3, and the DNA was sequenced for verification. For insertional inactivation of sapD, pMEB3 was linearized by EcoRV digestion and then gel purified using a QIAEX II Agarose Gel Extraction kit (Qiagen). The kanamycin resistance gene was obtained from SmaI-digested pUC18K3 plasmid, a derivative of pUC18K that contained two extra nucleotides for in-frame cloning (kindly provided by J. Kaper), was ligated overnight with the linearized pMEB3 plasmid generated as described above by the use of T4 DNA ligase (Invitrogen, Carlsbad, CA). The ligation products were then used to chemically transform high-efficiency One Shot® TOP10 Chemically competent E. coli cells. Plasmid DNAs were purified from kanamycin-resistant clones, and a plasmid with the correct restriction map was saved and named pMEB4. One microgram of pMEB4 was linearized with ApaI and used to transform NTHI strain 86-028NP according to a modified MIV transformation protocol (Mason et al., 2005). Transformants were selected on chocolate agar supplemented with 20 μg of kanamycin ml−1, and the correct allelic exchange was determined by Southern analysis and DNA sequencing and saved as mutant strain 86-028NP sapD::kan.
Complementation was performed with vector pSPEC1, whose construction has been previously described (Bakaletz et al., 2005). We amplified the promoter region for the sap gene cluster from pKM33C10, a GFP-reporter plasmid (Mason et al., 2003) using primer A: 5′-GCGCGCATGCTGATGAATGTTGATTA-3′ and primer B: 5′-GCGCGGATCCGTAATTCTACACTTTG-3′ (SphI and BamHI restriction sites are underlined respectively). The sapD gene was amplified from pMEB3 using primer C: 5′-GCGCGGATCCATGGCACTTTTAGA-3′ and primer D: 5′-GCGCGAATTCTTATTCATTTCCTTTGC-3′ (BamHI and EcoRI restriction sites are underlined respectively). The amplicons were then purified, digested with BamHI, and ligated for 1 h at room temperature. Following purification of the ligated product, the sapA promoter-sapD gene containing product was amplified by PCR using primers A and D described above. The generated amplicon and vector pSPEC1 were digested with SphI and EcoRI and ligated for 1 h at room temperature. The ligation product was electroporated into H. influenzae strain Rd and a plasmid with the correct restriction map and sequence was saved as pSAP5 and subsequently transformed into strain 86-028NP sapD::kan.
Southern hybridization
Southern hybridization was performed as previously described (Mason et al., 2005). Briefly, two micrograms of genomic DNAs were purified from the parent strain 86-028NP and from two candidate sapD mutants using a Puregene DNA isolation kit (Gentra Systems, Minneapolis, MN), digested with MfeI, and the fragments were resolved in a 0.8% agarose gel and then blotted onto a Nytran SuPerCharge membrane by use of a Turbo Blotter kit (Schleicher and Schuell BioScience, Keene, NH). Membranes were hybridized to a probe generated by PCR amplification of the coding sequence of the 86-028NP sapD gene with the primers 5′-GTATGCAAGATAAAGAACCTGATGAAT-3′ and 5′-CCAAAAGTAAGGCGAAAACCA-3′. The amplicon was purified using a QIAquick PCR purification kit (Qiagen) and was labelled with horseradish peroxidase using an ECL direct nucleic acid labelling and detection system (Amersham Biosciences, Piscataway, NJ). All procedures were performed according to the manufacturers' directions, and developed blots were exposed to Fuji Super Rx X-ray film (Fuji Photo Film, Tokyo, Japan).
Animal infection model: biological evaluation of the sapD mutant
Twenty (five cohorts of four animals each) healthy adult chinchillas (Chinchilla lanigera; Rauscher Chinchillas, LaRue, OH) with no evidence of middle ear infection by either video otoscopy or tympanometry were used to assess the biological consequences of colonizing or infecting with an NTHI strain defective in expression of the sapD gene. This chinchilla model of NTHI-induced OM has been well characterized (Sirakova et al., 1994; Suzuki and Bakaletz, 1994; Kennedy et al., 2000). We used this model to assay the relative ability of the sapD mutant to colonize the nasopharynx and to multiply and survive in the middle ear compared with the parental isolate. We also assayed the ability of the sapD mutant to compete with the parent in the nasopharynx and the middle ear microenvironments. Briefly, NTHI strain 86-028NP and the sapD mutant were grown overnight on chocolate agar or chocolate agar supplemented with 20 μg of kanamycin ml−1, respectively, and were then adjusted to an optical density of 0.65 at 490 nm in 0.9% (wt/vol) sodium chloride in water. For assessment of survival of the mutant strain alone, one chinchilla cohort was inoculated both intranasally and transbullarly with approximately 1.0 × 108 or 2500 cfu of the sapD mutant respectively. Similarly, an additional two cohorts were inoculated with the parent strain alone or the complemented sapD mutant strain alone. The actual inocula received were confirmed by plate counts. For competition experiments, parent and mutant bacteria were diluted to a concentration of approximately 2500 cfu per ear (for TB challenge) and 1.0 × 108 cfu per naris (for IN challenge), and equal volumes were combined. A 300 μl volume of the TB challenge mixture (4800 cfu parent; 3900 cfu sapD mutant) was used to inoculate the left and right transbullar cavities, and 200 μl volume of the IN challenge mixture (1.4 × 108 cfu parent; 1.3 × 108 cfu sapD mutant) was used for NP inoculation. This procedure was similarly repeated with another cohort to assess survival of the complemented sapD mutant in competition with the parental isolate in the middle ear (4800 cfu parent; 2280 cfu complemented sapD mutant) or the nasopharynx (1.4 × 108 cfu parent; 7.6 × 107 cfu complemented sapD mutant). Animals were evaluated by video otoscopy and tympanometry every 2 or 3 days from the time of bacterial inoculation until clearance. Nasopharyngeal lavage was performed every 2 days by passive inhalation of 500 μl of pyrogen-free sterile saline with recovery of lavage fluid from the contralateral naris as fluid was exhaled (Bakaletz et al., 1997). Epitympanic taps were attempted when an effusion was considered to be of sufficient volume to be retrieved. Nasopharyngeal lavage and epitympanic tap fluids were maintained on ice until serially diluted. Dilutions of nasopharyngeal lavage fluids were then cultured on chocolate agar plates containing ampicillin (15 μg ml−1) to limit the growth of other normal nasopharyngeal flora and on chocolate agar containing ampicillin and 20 μg of kanamycin ml−1 in order to select for the sapD mutant or on chocolate agar containing 20 μg kanamycin ml−1 and 200 μg spectinomycin ml−1 to select for the complemented sapD mutant. Similarly, dilutions of epitympanic tap fluids were cultured on chocolate agar plates (Becton Dickinson and Co.) and on chocolate agar plates containing 20 μg of kanamycin ml−1 or containing kanamycin and 200 μg spectinomycin ml−1.
Bactericidal assays
Sensitivity of strains 86-028NP and 86-028NP sapD::kan to AP killing were performed as previously described (Mason et al., 2005).
RNA isolation and RT-PCR analysis
RNA was isolated from strain 86-028NP and assessed for purity and integrity as previously described (Mason et al., 2005). Gene expression was assessed by RT-PCR using the two-step RT-PCR kit (Qiagen) according to the manufacturer's protocol. Using primers generated to a short intergenic region of sapD, 5′-CGATCGCTTTCGTTTTCAC-3′ and 5′-TCAAGGCAAGATAAGGGATTTT-3′; trkA, 5′-TTTTGGGTGCAGGTCAGGTAGGAA-3′ and 5′-ACGAAGCACTTTTGGAGATGATGG-3′; and trkH, 5′-CCTCTTTTATTGTTCGATTCACCA-3′ and 5′-ATCCCCATACCACCTAACCATTGT-3′, mRNA was reverse transcribed and amplified by RT-PCR. Controls were analysed in parallel to verify the absence of DNA in the RNA preparation (– RT control) as well as the absence of primer dimers in control samples lacking template RNA. RT-PCR products were analysed by gel electrophoresis and, in all cases, a single product was observed at the appropriate size.
Potassium transport measurements
The method to assess potassium uptake in NTHI strains was adapted from published work in E. coli strains (Bakker and Harold, 1980; Bakker and Mangerich, 1981; Richey et al., 1987; Tholema et al., 1999; 2005; Matsuda et al., 2004; Kraegeloh et al., 2005) with the following modifications. NTHI strain 86-028NP, its sapD mutant and the complemented sapD mutant strain were grown overnight on chocolate II agar at 37°C, 5% CO2. Bacteria were suspended in DIS medium supplemented with 2 μg haemin chloride ml−1 and 40 mM potassium phosphate, adjusted to an OD490 of 0.65, diluted (1:6) into 250 ml of prewarmed DIS medium supplemented as described above, and grown statically to mid-log phase at 37°C, 5% CO2. Exponentially growing cells (OD490 = 0.5) were collected by centrifugation and resuspended in 10 ml of 120 mM Tris-HCl (pH 8) containing 1 mM EDTA and incubated with agitation (at 180 r.p.m.) for 10 min at 37°C to deplete surface and internal potassium stores, as previously described for other microorganisms (Bakker and Mangerich, 1981; Tholema et al., 1999). Cells were collected by centrifugation and washed twice with potassium-free DIS medium, then resuspended in the same buffer for 20 min at room temperature. Cell concentrations were adjusted to an OD578 of 3.0 corresponding to approximately 1 mg dry weight ml−1 of NTHI. Potassium chloride (1 mM) was added to each culture and 1 ml volumes were removed at 0, 1, 2, 5 and 10 min and immediately centrifuged through 300 μl of silicon oil AR100 (14 000 g; Fluka, density = 1.05). The aqueous supernatant and oil were carefully aspirated, and the pellet was resuspended in 1 ml of trichloracetic acid (5%) followed by heating at 90°C for 10 min. Supernatants were clarified by centrifugation (10 min, 2700 g) and analysed for potassium content using a Perkin Elmer 5300 DV Inductively Coupled Plasma-Atomic Emission Spectrometer (ICP/AES; Huffman Laboratories, Golden, CO).
Statistical methods
The Biometrics Laboratory of The Ohio State University's College of Medicine and Public Health conducted all statistical analyses. P-values were obtained, for direct comparisons between the parent, sapD mutant and complemented sapD mutant groups alone, and in competition, in both the nasopharynx and the middle ear, by exact Wilcoxon Rank Sum tests. An alpha level of 0.05 was accepted as significant. Competitive fitness data were compared (median cfu ml−1) by group and day then analysed by exact Wilcoxon Rank Sum tests.
Acknowledgements
We thank Joe A. Jurcisek for assistance with figure preparation, Stephanie R. Hill for Biosensor analysis, Glen McGillivary for helpful discussions and Jennifer Neelans for assistance with manuscript preparation. This work was supported by Grants R01 DC03915 and R01 DC05847 to LOB and an NRSA to K.M.M. from NIDCD/NIH.