Concerted action of plasmid maintenance functions: partition complexes create a requirement for dimer resolution
Summary
Partition of prokaryotic DNA requires formation of specific protein–centromere complexes, but an excess of the protein can disrupt segregation. The mechanisms underlying this destabilization are unknown. We have found that destabilization by the F plasmid partition protein, SopB, of plasmids carrying the F centromere, sopC, results from the capacity of the SopB–sopC partition complex to stimulate plasmid multimerization. Mutant SopBs unable to destabilize failed to increase multimerization. Stability of wild-type mini-F, whose ResD/rfsF site-specific recombination system enables it to resolve multimers to monomers, was barely affected by excess SopB. Destabilization of plasmids lacking the rfsF site was suppressed by recF, recO and recR, but not by recB, mutant alleles, indicating that multimerization is initiated from single-strand gaps. SopB did not alter the amounts or distribution of replication intermediates, implying that SopB–DNA complexes do not create single-strand gaps by blocking replication forks. Rather, the results are consistent with SopB–DNA complexes channelling gapped molecules into the RecFOR recombination pathway. We suggest that extended SopB–DNA complexes increase the likelihood of recombination between sibling plasmids by keeping them in close contact prior to SopA-mediated segregation. These results cast plasmid site-specific resolution in a new role – compensation for untoward consequences of partition complex formation.
Introduction
The systems that ensure faithful inheritance of low-copy-number bacterial plasmids are generally thought to act independently of one another. Replication, multimer resolution and partition into daughter cells are each specified by a genetic module which operates efficiently when transferred alone to vector plasmids (Austin et al., 1981; 1986; Ogura and Hiraga, 1983; Lane et al., 1986). We report here results which indicate that plasmid stability relies on a greater degree of interdependence among these functions than previously supposed.
Our investigation began as an attempt to understand how an excess of one of the proteins essential to partition of the F plasmid of Escherichia coli causes the plasmid to be lost from dividing cells. Partition of F is typical of that of low-copy-number plasmids. It is governed by the sop locus, which specifies a Walker-box ATPase, SopA, a centromere-binding protein, SopB, and the centromere itself, sopC, composed of 12 tandem 43 bp repeats each with a SopB binding site (Hiraga, 2000). sopA and sopB constitute an operon whose promoter is repressed by SopA with the help of corepression by SopB and sopC (Mori et al., 1989; Biek and Strings, 1995; Yates et al., 1999). How these components ensure mitotic segregation is not yet clear, but studies of Sop-directed partition and of a number of analogous partition systems allow us to discern the mechanism in outline. Following replication, SopB protein binds to sopC on each sibling molecule to form a partition complex (Hayakawa et al., 1985; Mori et al., 1989); partition complexes interact to pair plasmid copies (Jensen et al., 1998; Edgar et al., 2001); and recognition of the paired complexes by SopA-ATP stimulates assembly of a dynamic SopA polymer which splits the pair and moves the copies into each new cell-to-be (Gordon et al., 1997; Bouet and Funnell, 1999; Ebersbach and Gerdes, 2001; Libante et al., 2001; Lim et al., 2005). Whatever the mechanism, it only works if it is presented with enough plasmid molecules, and can be subverted if plasmid siblings recombine to form multimers. Plasmids deal with this eventuality by resolving multimers back to the monomer form (Sternberg et al., 1981). In the case of the F plasmid, this is done by the ResD recombinase which catalyses crossing over at copies of a specific site, rfsF (Lane et al., 1986; O'Connor et al., 1986; Disque-Kochem and Eichenlaub, 1993).
Kusukawa et al. (1987) observed that a mini-F plasmid was lost during growth of cells following the introduction of a multicopy plasmid carrying the sopB gene, a phenomenon they termed IncG. The centromere-binding proteins of the P1 and R1 plasmids behaved similarly (Funnell, 1988; Dam and Gerdes, 1994). However, the explanations advanced – abnormal (non-functional) partition complexes (Kusukawa et al., 1987), sequestration of plasmids in unpartitionable aggregates (Funnell, 1988), overrepressed synthesis of the cognate partition ATPase (Dam and Gerdes, 1994) – were not further explored. The subsequent discovery that the SopB and ParB (of plasmid P1) proteins normally extend their partition complexes by spreading along the DNA for several kilobases on either side of their centromeres (Biek and Strings, 1995; Lynch and Wang, 1995; Rodionov et al., 1999) led to another suggestion, that excess SopB protein destabilizes mini-F by interfering with its replication (Kubo et al., 2002). It appeared that testing these ideas might well provide insights into the nature and activity of the SopB–sopC partition complex.
We were encouraged to probe the mechanism of IncG incompatibility by the availability of two sopB mutants that are unable to exert it. While trying to identify the functional defects of the two mutant proteins we found unexpectedly that normal partition complexes stimulate plasmid multimerization and threaten plasmid stability. Our experiments were accordingly redirected towards analysing the specific contribution that site-specific resolution makes to F plasmid stability.
Results
SopB mutants unable to exert IncG incompatibility
The two available IncG-defective SopB mutants were SopBP28L, found during a direct screen for inability to destabilize mini-F (Kusukawa et al., 1987), and SopBE215K, identified from a screen for unstable mini-F mutants as being able to increase plasmid linking number, indicating normal SopB binding and spreading, but defective in corepression (Yates, 1997) and in IncG (our results; see below). Figure 1 summarizes our attempts to identify an alteration in the properties of the SopB mutants which would provide clues to the basis of SopB-induced destabilization. The mutants have distinct phenotypes (Fig. 1A). SopBE215K is slightly defective in partition but is unable to help repression of the sop promoter by SopA, whereas SopBP28L strongly destabilizes its mini-F while remaining a good corepressor. However, these differences are not reflected by alterations in readily observed properties of the proteins. With respect to binding to sopC DNA (Fig. 1B), increase of sopC-plasmid linking number (Fig. 1C), return to normal linking number upon production of SopA (data not shown) and silencing of a distant promoter (Fig. 1D), the two mutants do not differ significantly from wild-type SopB. We therefore turned to a direct characterization of the IncG phenotype, using the mutants as IncG-negative controls.
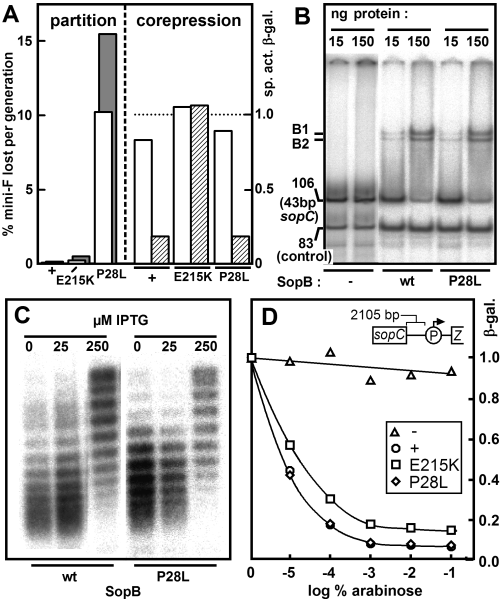
Properties of IncG mutant SopB proteins.A. Partition: the stability of mini-F plasmids carrying the indicated sopB alleles under natural sop promoter control (pDAG114, pJYB76, pJYB77; open bars) was measured as the rate of loss from strain DLT1215, as described in Experimental procedures; to avoid effects of variable corepression, stability of mini-F with ptet substituted for psop (pDAG173, pJYB93, pJYB94) was also measured (shaded bars). Corepression: strains DLT1472 (sopB+), 1473 (sopBE215K) and 1474 (sopBP28L) carrying pDAG196, pDAG301 (sopA+, open bars) or pDAG310 (sopA+sopC+, hatched bars) were grown exponentially in the presence of 1 mM IPTG for six generations then sampled for assay of β-galactosidase. Specific activities were normalized to the unrepressed values in pDAG196-carrying cells (∼2600 Miller units) indicated by the dotted line.B. Affinity for sopC: phosphor image of gel retardation by SopB proteins in crude extracts of a 32P-labelled fragment (106 bp) carrying a single 43 bp sopC unit, with a second fragment (83 bp) as control. The physical difference between the two retarded species, B1 and B2, is unknown. The result for SopBE215K was the same (not shown).C. Chloroquine-agarose gel separation of topoisomers of pZC204 (sopC) from cells synthesizing SopB proteins at different rates; the mid-points of the distributions of topoisomers at 0 and at 250 μM IPTG differ by ΔLk∼6. The result for SopBE215K was the same (not shown).D. Silencing by SopB proteins of paadA linked to sopC. Strain DLT2067 (λRS45-sopC-paad::lacZ) harbouring plasmids carrying wild-type (pDAG607) or mutant (E215K pDAG609; P28L pDAG611) sopB genes under para control were grown for about 12 generations in the presence of arabinose at various concentrations, and samples then assayed for β-galactosidase. Circles, sopB+; squares, sopBE215K; diamonds, sopBP28L; triangles, no sopB (vector only). β-Galactosidase-specific activities shown at the right are normalized to those at 0% arabinose (90–110 Miller units).
Destabilization of sopC plasmids by SopB (IncG)
To observe and measure IncG incompatibility, we established a stable, controlled source of the wild-type and IncG mutant SopB proteins by integrating their respective genes into the lacZ gene of strain DLT1215, thus placing sopB transcription under wild-type lacOP/LacI control. Cells of the three resulting strains grown in Luria–Bertani (LB) medium with 1 mM IPTG maintained their SopB proteins at a steady-state concentration 5.6-fold (± 0.7 SD) higher than that of SopB in cells harbouring wild-type mini-F (pDAG114; data not shown). At this concentration, wild-type SopB caused loss of the sopC target plasmid, pZC204, at over 300-fold the rate seen in cells lacking SopB (Table 1, line 1). Stability of the vector without sopC (pACYC184) was unaffected (line 5). As expected, loss of pZC204 in response to the E215K and P28L mutant SopB proteins was much reduced, about 25-fold (Table 1, line 1). Coproduction of SopA protein at its natural concentration moderated the destabilization, and overproduction abolished it (lines 2, 3). These observations suggest that IncG destabilization of pZC204 is dependent on and subject to the normal interactions that SopB protein experiences in partition. Indeed, the partition complexes formed on pZC204 by SopB's binding to sopC and spreading along flanking DNA should be of about the same extent as those formed naturally on mini-F: the copy number of pZC204 in our conditions is approximately eight times that of mini-F (J. Rech, unpubl. data), giving a SopB:sopC ratio in cells carrying pZC204 about 0.7-fold the wild-type mini-F ratio. The behaviour of SopB–sopC complexes on pZC204 should therefore reflect that of complexes on mini-F. The experiment depicted in Fig. 2A confirmed that pZC204 copy number is unaffected by induction of sopB. Over 16 generations, the ratio of pZC204 DNA to DNA of a coresident pSC101-based plasmid without sopC, pAM238, underwent the same slight decline whether IPTG was present (SopB at 5.6 times the level characteristic of cells carrying mini-F) or absent (SopB undetected, < 0.2 times the mini-F level).
| Name | Plasmid | % plasmid loss per generationaExtra SopB | ||||||
|---|---|---|---|---|---|---|---|---|
| sopC | SopAb | SopB | – (DLT1215) | wt (DLT1472) | E215K (DLT1473) | P28L (DLT1474) | ||
| 1 | pZC204 | + | – | – | < 0.007 | 2.3 ± 0.13 | 0.09 ± 0.06 | 0.09 ± 0.06 |
| 2 | pZC204 | + | 1× | – | – | 1.4 | – | – |
| 3 | pZC204 | + | 60× | – | – | < 0.02 | – | – |
| 4 | pDAG114 | + | 1× | 1× | 0.006 ± 0.003 | 0.04 ± 0.02 | 0.03 ± 0.01 | 0.05 ± 0.01 |
| 5 | pACYC184 | – | – | – | – | < 0.06 | – | – |
- a. Values ± standard deviation are the averages of at least three determinations, < values are averages of at least two, and the value for 60 × SopA was determined once.
- b. The arabinose-inducible sopA gene was present on plasmid pDAG127 (Lemonnier et al., 2000). The 1× concentration of SopA protein, equivalent to wild-type mini-F, is provided by basal (uninduced) expression, the 60-fold excess by growth in the presence of 0.0025% arabinose.
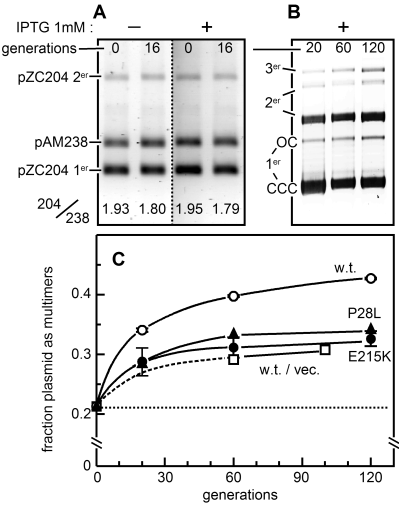
Effects of excess SopB on the sopC plasmid pZC204.A. Maintenance of copy number. DLT1472 carrying the stable reference plasmid pAM238 was transformed with pZC204. Three independent transformants were grown with selection for pZC204 then diluted into non-selective medium with or without IPTG to induce SopB synthesis. Samples were removed for quantification of plasmid DNAs. The results for the three transformants were essentially the same. Numbers below the bands are the ratios of pZC204 monomer (1er) plus dimer (2er) to pAM238 DNA.B. Multimer formation. Calcium-treated cells of the plac::sopB strain DLT1472 were transformed with pZC204 DNA, but after a 90 min incubation to allow expression of cat, growth was continued in liquid medium in the presence of chloramphenicol and IPTG. Plasmid DNAs were extracted at intervals, fractionated on an agarose gel and quantified. CCC, covalently closed circular; OC, open circular.C. Evolution of multimer formation. DNA of pZC204 from strains producing wild-type, E215K and P28L SopB proteins, or of pACYC184 producing wild-type SopB, was isolated and processed as in (B). Monomer and multimer forms were quantified using ImageQuant (Molecular Dynamics). The multimer fractions at 0 generations (dotted baseline) are those of the transforming DNA preparations. Data are from two experiments for wild-type and E215K SopBs, and from one for the P28L mutant and the vector control. The first part of the curve for the latter is broken to indicate uncertainty resulting from lack of a 30-generation sample.
In view of the strong SopB-induced destabilization of mini-F reported by Kusukawa et al. (1987), we were surprised to find that the same concentration of SopB protein that strongly destabilized pZC204 caused only a modest (approximately sevenfold) increase in mini-F loss rate (Table 1, line 4). The source of this slight instability appears to be different from that which destabilizes pZC204, as the two mutant SopBs also provoked it. While it had seemed possible that the concentrations of SopB protein (not reported) used by Kusukawa et al. were higher than ours, accounting for destabilization of their mini-F plasmid, it was an inconsistency in our pZC204 destabilization data that provided the key to the discrepancy.
IncG is correlated with plasmid multimerization
We noticed that SopB-induced destabilization of pZC204 varied according to the history of the cells used for the loss rate experiments. Where the pZC204 transformant had been extensively purified and subcultured (∼80 generations altogether) prior to the measurement, substantial plasmid loss was consistently observed, as in Table 1: where cultures directly inoculated with the transformant colony were used, rates of plasmid loss were extremely variable. We examined electrophoretic profiles of pZC204 DNA in samples taken at various times after inoculating medium with transformant colonies. These revealed that multimer forms of the plasmid accumulated over time, as illustrated in Fig. 2B and C. The increase in the fraction of multimers was significantly higher in cells producing wild-type SopB protein than the background level in those carrying the pACYC184 vector, without sopC (Fig. 2C). Moreover, in cells producing either of the IncG mutant SopB proteins, the fraction of multimer forms above this background level was much reduced. These data suggested that the complex formed by SopB protein on sopC stimulates multimer formation beyond the level characteristic of the vector, to the point where a significant proportion of dividing cells have too few plasmid molecules to assure transmission to both offspring.
Resolution by ResD/rfsF suppresses IncG
Unlike pZC204, mini-F can resolve its multimers to monomers through the action of its ResD recombinase at a specific site, rfsF, near the primary origin of replication (Lane et al., 1986). To assess the role of multimerization in SopB-induced destabilization, we deleted the resD gene from mini-F plasmids with or without sopC and measured the effects of SopB on the stability of the resulting plasmids (Table 2). The rate of plasmid loss caused by excess SopB protein increased markedly following deletion of resD (pJYB74; line 3), to about 40-fold the slight instability of wild-type mini-F (pDAG114; line 1). This destabilization took place through the partition system, because the normal instability of the ΔsopC derivative (pDAG209; lines 6, 7) was little altered by deletion of resD (pJYB75; line 5). It also reflected IncG incompatibility, because the P28L and E215K mutant SopB proteins had no effect (lines 1, 3). These results matched the effects of the SopB proteins on stability of pZC204 (Table 1). SopB-induced destabilization of mini-F is thus prevented by the ResD/rfsF site-specific recombination system. This suggests a simple explanation for the strong destabilization reported by Kusukawa et al. (1987) – their mini-F (pXX704) did not carry resD/rfsF.
| Name | Plasmid | Strain rec | % loss rate – per generationExtra SopB | ||||||
|---|---|---|---|---|---|---|---|---|---|
| sopB | sopC | resD | – | + | E215K | P28L | |||
| 1 | pDAG114 | + | + | + | + | 0.006 ± 0.003 | 0.04 ± 0.02 | 0.03 ± 0.01 | 0.05 ± 0.01 |
| 2 | pDAG114 | + | + | + | F | 0.050 ± 0.03 | 0.14 ± 0.09 | 0.09 ± 0.01 | 0.09 ± 0.03 |
| 3 | pJYB74 | + | + | Δ | + | 0.02 ± 0.01 | 1.7 ± 0.5 | 0.02 ± 0.01 | 0.02 ± 0.01 |
| 4 | pJYB74 | + | + | Δ | F | 0.08 ± 0.07 | 0.20 ± 0.11 | 0.08 ± 0.03 | 0.16 ± 0.01 |
| 5 | pJYB75 | + | – | Δ | + | 1.7 ± 0.34 | 2.2 ± 0.26 | ||
| 6 | pDAG209 | – | + | + | + | 2.5 ± 0.3 | 2.4 ± 0.5 | ||
| 7 | pDAG209 | – | + | + | F | – | 2.3 ± 0.4 | ||
IncG phenotype requires the RecFOR pathway
Plasmid multimers arise mainly via recombination mediated by RecF protein (James et al., 1982; Kolodner et al., 1985) in concert with RecO and RecR (Umezu et al., 1993). To determine whether SopB stimulates multimer formation by this pathway, we measured mini-F stability in recF mutant derivatives of the SopB-producing strains (Table 2). In the absence of RecF, wild-type mini-F was slightly but consistently less stable than in wild-type strains, whether or not extra SopB protein was present (line 2). But more importantly, the pronounced instability of the ΔresD mini-F, pJYB74, in the presence of excess wild-type SopB protein was strongly suppressed (approximately ninefold) by the recF mutation (line 4), almost back to the level observed for wild-type mini-F, pDAG114, in the recF mutant (line 2). The rates of pDAG114 and pJYB74 loss provoked by excess SopB protein in recO, recR and recA mutants were found (in a single determination) to be similar, within a factor of two, to those observed for the recF mutant: in contrast, introduction of a ΔrecBC allele did not reduce the instability of pJYB74 caused by excess SopB (data not shown). Reduced loss rate in the recF strain was specific for instability related to failure to resolve multimers; the instability of the resD+ plasmid pDAG209, which results from lack of the sopAB operon, is not alleviated by absence of RecF (Table 2, lines 6, 7). These results indicate that the RecFOR pathway is responsible for most of the multimers that potentiate loss of mini-F. We next asked how excess SopB might stimulate recombination via the RecFOR pathway.
Does SopB generate RecFOR substrates?
RecFOR recombination begins at single-strand (ss) gaps in DNA. In principle, these could arise either by selective ss degradation in response to DNA damage or by removal of nascent lagging strands at paused replication forks (Kuzminov, 1995). Centromere-binding proteins are not known to damage DNA, but SopB does interfere with DNA gyrase (Lynch and Wang, 1995) and in excess might distort this enzyme's cleavage-rejoining activity to create nicks which trigger ss gap formation. If so, IncG mutant SopBs should be less able than wild type to generate the extended complexes with DNA which block gyrase and reduce plasmid supercoil density (Biek and Strings, 1995; Lynch and Wang, 1995). Topoisomer analysis showed that this is not the case (Fig. 1B). Moreover, promoter silencing, another index of centromere-binding protein spreading (Lynch and Wang, 1995; Rodionov et al., 1999), was similar for the wild-type and mutant SopBs (Fig. 1C). Hence, we doubt that a fivefold excess of SopB damages DNA, at least via interference with DNA gyrase. Action via paused replication forks appears more likely.
If excess SopB increases the frequency of replication fork pausing, by forming complexes which act as physical barriers for example, it would be expected to increase the frequency of partly replicated plasmid molecules. We therefore compared the concentration of pZC204 replication intermediates in cells with excess SopB protein (wild type and mutant) with that in cells without SopB. Total plasmid DNA isolated from exponentially growing cells was cleaved at a unique restriction site (Fig. 3A), and branched molecules were resolved by two-dimensional neutral agarose gel electrophoresis, in which one dimension separates on the basis of size and the other largely according to shape, and revealed by Southern blot hybridization (Brewer and Fangman, 1987). The various electrophoretic species were assigned structures on the basis of the analyses of similar plasmids by Martin-Parras et al. (1991) and Kuzminov et al. (1997) and by comparison with DNAs of known structure separated on a gel run in parallel (not shown). These assignments are shown schematically in Fig. 3E. Figure 3B depicts replication intermediates expected to contribute to the patterns seen.
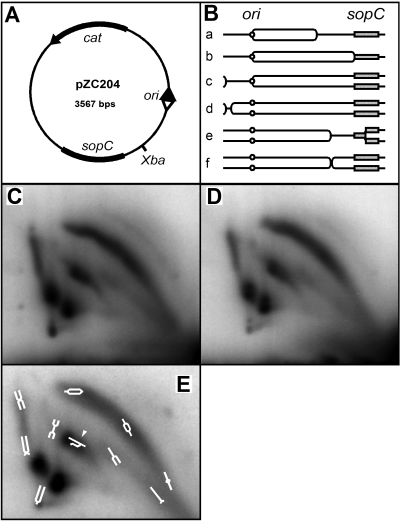
Lack of effect of SopB on pZC204 replication.A. Relevant characteristics of pZC204. The origin of delayed bidirectional replication (Kuzminov et al., 1997) is depicted as arrowheads, filled for the primary fork, open for the delayed fork.B. Structures of molecules after XbaI cleavage of replication intermediates produced by (a–c) unidirectional (d) delayed bidirectional, and (e and f) delayed bidirectional replication with premature fork arrest. Origins are shown as small circles, sopC as a shaded rectangle.C. Radioautograph of pZC204 DNA from DLT1790 (no sopB) fractionated by two-dimensional neutral agarose gel electrophoresis and detected by Southern blotting and hybridization. Over 95% of the probe binds to linear monomer which migrates below and rightward of the species shown and is uninformative.D. As for (C), pZC204 DNA from DLT1501 (plac::sopB+).E. As for (C), from DLT1503 (plac::sopBP28L+) overlaid with schematic interpretation. The central comet-like spot includes broken replicative intermediates (arrowhead).
The two-dimensional gel patterns shown in Fig. 3 were obtained from rec+ cells, so that some of the species, particularly the X-forms, may include recombination as well as replication intermediates. However, the top arc of bubble forms represents intermediates of unambiguously replicative origin. Comparison of the patterns reveals essentially no differences in either the absolute or relative concentrations of branched molecules in cells without SopB (Fig. 3C) and with excess SopB (Fig. 3D). IncG mutant SopB patterns were the same (not shown). Although fork arrest by SopB–DNA complexes would not necessarily have given rise to a strong, specific zone on the arc, owing to possible heterogeneity resulting from spreading, the absence of any significant change in the arc of the SopB-minus sample indicates that excess SopB protein has no net effect on the replication intermediate population. The patterns of intermediates from recA strains with and without SopB likewise showed no significant differences.
In the absence of evidence for a source of increased ss gap formation, it appeared possible that it is the processing, rather than the creation, of stalled forks that is affected by excess SopB.
Effect of copy number on IncG
Rescue of replication by recombination, rather than by simple restitution of the pre-existing fork, could occur by crossing over either in cis, behind the fork, or in trans with a sister copy of the replicon. We know of no estimate of the relative frequencies of cis and trans plasmid recombination, but it is possible that in the case of a small plasmid such as pZC204 replication might often resume and finish before crossing over in cis had taken place, thus pre-empting recombination. If so, recombination in trans could be the more important contributor to IncG destabilization. Unlike recombination in cis, it would also be sensitive to substrate concentration, i.e. plasmid copy number. If excess SopB protein promotes dimerization by channelling stalled forks into the RecFOR recombination pathway, increased plasmid copy number would then be predicted to raise SopB-induced recombination frequency. Higher copy number itself, however, might overcome IncG instability, preventing its use as an indicator of increased dimerization. We therefore tested the effect of copy number on IncG instability by growing all cultures in minimal salts-glycerol medium (doubling time 125 min) to reduce the number of mini-F copies per cell. Mini-F copy number mutants were obtained by deleting the incC replication control locus; they were estimated from loss rates of ΔsopC derivatives to have approximately fourfold increased copy number, in agreement with previous estimates (Bergquist et al., 1981; Tsutsui et al., 1983).
The results (Table 3) show that whereas excess SopB destabilized wild-type mini-F in slowly growing cells (line 1) by about the same moderate factor (fivefold) as in rapidly growing cells (Table 1, line 4), it had a significantly reduced effect on the ΔresD mini-F, increasing loss rate only 10-fold (line 3) rather than eightfold (Table 2, line 3). Deletion of incC caused the moderate increase in intrinsic stability of partition-competent mini-Fs expected from an increase in copy number (lines 1 and 4, 3 and 6). More striking were the responses of the ΔincC plasmids to additional SopB: the resD+ mini-F (pJYB106) became effectively immune to SopB-induced destabilization (line 4), while the ΔresD mini-F (pJYB108) became three to fourfold more unstable than its normal copy number parent (lines 3 and 6). Raising mini-F copy number clearly aggravates IncG destabilization, consistent with the proposal that high SopB concentrations increase the probability that a ss gap, once formed, will initiate recombination between sibling mini-F molecules.
| Plasmid | sopC | resD | incC | % loss per generation ± SD (no. of measurements) | ||
|---|---|---|---|---|---|---|
| Extra SopB | Increase in IncG owing to lack of ResD | |||||
| – | + | |||||
| pDAG114 | + | + | + | 0.05 ± 0.03 (3) | 0.27 ± 0.07 (4) | |
| pDAG115 | Δ | + | + | 11.8 ± 3.9 (3) | 11.3 ± 3.0 (3) | |
| pJYB74 | + | Δ | + | 0.06 ± 0.04 (5) | 0.58 ± 0.44 (5) | 2.1 |
| pJYB106 | + | + | Δ | < 0.03 (2) | < 0.01 (2) | |
| pJYB107 | Δ | + | Δ | < 0.05 (2) | < 0.04 (2) | |
| pJYB108 | + | Δ | Δ | < 0.04 (3) | 2.1 ± 1.1 (3) | > 210 |
Discussion
The importance to replicon stability of remaining monomeric has long been appreciated (Austin et al., 1981; Sternberg et al., 1981). Site-specific recombination mechanisms that ensure the resolution of multimers to monomers and so provide discrete molecules in sufficient number for segregation have since proved to be specified by many plasmids and chromosomes (Hakkaart et al., 1982; Summers and Sherratt, 1984; Garnier et al., 1987; Grinter et al., 1989; Clerget, 1991; Kuempel et al., 1991; Cornet et al., 1994), and certain of them are understood in considerable molecular detail (Barre and Sherratt, 2002; van Duyne, 2002). What has been less clear is the nature of the events which stimulate multimerization in the first place. Our study reveals that in the case of the mini-F plasmid the segregation apparatus itself can provoke multimerization, so threatening the very stability it is there to ensure, and that one task of the plasmid's multimer resolution system is to eliminate this threat. Resolution can thus be considered as an accessory, corrective component of the partition system.
Two results provided the most direct evidence for this proposition: the loss of the resolvase-negative mini-F at a rate fourfold higher than the wild type in the presence of a fivefold excess of SopB protein (Table 2), and the reduction of multimerization to near-background levels when the SopB proteins were defective in destabilization (Fig. 1C). The latter finding was based on use of a surrogate resolvase-negative, sopC+ plasmid of naturally high copy number. In addition to raising the quantity of DNA isolated and so facilitating its analysis, use of this plasmid had two advantages. One was to increase the sensitivity of multimer detection and measurement resulting from the enrichment of multimers that accompanies their preferential replication (Summers et al., 1993): dimers of mini-P1, and presumably of other plasmids whose replication is repressed by handcuffing such as mini-F, are less frequently replicated than monomers because intramolecular handcuffing of replication regions is kinetically favoured over intermolecular handcuffing of monomers, and this counteracts the strictly probabilistic preference for initiating replication at dimer origins (Park and Chattoraj, 2001).
The other advantage was the establishment of a SopB:sopC ratio comparable to that present in mini-F-carrying cells, so that the partition complexes were, in principle, of normal extent and of similar destabilization potential to those on wild-type mini-F. The limited excesses of SopB protein we have used here are thus likely to be closer to the variations normally experienced by the F plasmid, implying that the generation of multimers and the need for their resolution are biologically relevant. The conditions of centromere-binding protein synthesis described in previous studies of IncG-like behaviour probably resulted in concentrations of centromere-binding proteins far higher than those encountered during normal growth. Silencing of replication, proposed by Kubo et al. (2002), is an unlikely cause of destabilization in our conditions, because copy number per plasmid-carrying cell is maintained in the presence of destabilizing levels of SopB protein (Fig. 1). Overrepression of partition ATPase synthesis (Dam and Gerdes, 1994) is also unlikely, in view of the failure of approximately fivefold increased sopB gene dosage to further corepress the normally repressed sop promoter (Ravin et al., 2003). The proposal that interactions among overabundant P1 ParB molecules destabilize parS plasmids by clumping them (Funnell, 1988) parallels our finding of multimer formation, for both multimers and clumps (‘phenotypic multimers’) limit the units available for segregation. Increased multimerization was not observed in Dr Funnell's study, presumably because the strain used was a recA mutant.
How does excess SopB stimulate multimer formation? The observation that sopC is also needed for IncG (Table 1) implies that the nucleoprotein partition complex is needed. The most obvious way for the SopB–DNA complex to stimulate multimerization appeared to be physical blockage of replication forks (Kuzminov, 1995), which could prolong exposure of the lagging strand template to RecFOR-mediated loading of RecA protein (Courcelle et al., 1997; Morimatsu and Kowalczykowski, 2003). Analysis of chromosome dimer resolution by XerCD/dif in rec mutant strains indicated that RecFOR pathway contributes substantially to chromosome dimerization in E. coli (Steiner and Kuempel, 1998). However, our failure to detect an increase of any species of forked molecule in either rec+ or recA cells containing excess SopB protein (Fig. 3) argues against this mechanism. We are thus led to consider that SopB–DNA might act by increasing the frequency with which ss gaps, whatever their origin, enter the recombination pathway rather than undergo repair. In theory it could do this by inhibiting gap repair, which by prolonging the lifetime of gaps would favour recombination. There is no evidence for such a mechanism, although the possibility that SopB binds to ss DNA has not been excluded. Some of our observations suggest an alternative. Coproduction of SopA counteracted SopB-induced destabilization (Table 1), indicating that SopA-induced disruption of the extended SopB–DNA complex (Lemonnier et al., 2000) could hasten the onset of segregation and so reduce recombination. We also found that reducing mini-F copy number reduced SopB-induced destabilization, while raising copy number (Table 3) aggravated it. Just how multimerization of mini-F predominates over raised copy number in determining the level of stability is unclear. It is possible that the intramolecular handcuffing referred to above reduces mini-F replication frequency in cells containing multimers to create a subpopulation with very few partitionable units. In any case, multimerization, and the destabilization it causes, is enhanced by factors which favour proximity of recombination partners, in particular the multiplicity of plasmid copies and the time that the copies spend together prior to segregation. This notion is illustrated in Fig. 4. Normal SopB concentrations allow rapid separation of sibling plasmids, consistent with the brief period between replication and partition reported by Onogi et al. (2002). High SopB concentrations result in a more extensive nucleoprotein complex which takes longer to disperse, favouring the encounter between an ss gap and its target.
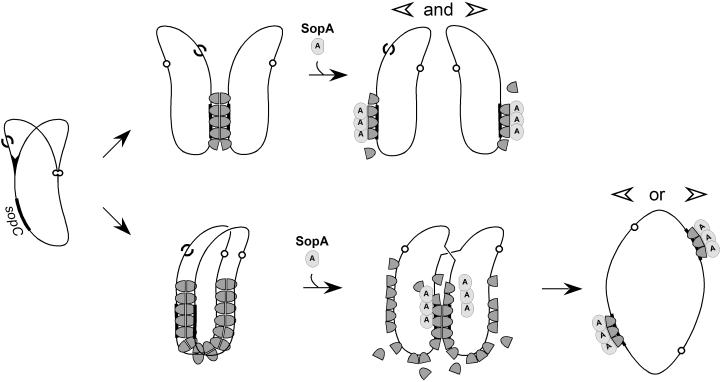
Hypothesis for promotion of dimer formation by SopB. During replication of a sopC plasmid (at left) one of replicas acquires a single-strand gap (S), possibly following a brief interruption in the progress of the replication fork, which can either be repaired or act as a recombination substrate. At normal concentrations of SopB (dark grey thimble) the replicas are connected mainly through paired SopB–sopC complexes and are readily accessible to SopA (light grey knob) which segregates them rapidly (top row). At elevated SopB concentrations interactions within and between the more extensive SopB–DNA complexes keep the DNA replicas in closer contact, and SopA at its normal concentration takes longer to segregate them, favouring the encounter of a RecA-primed recombination substrate with its twin sequence. High concentrations of SopA suppress enhancement of recombination (Table 1) because they prevent formation of the extended complex (Lemonnier et al., 2000).
We had expected that identifying a defect shared by the mutant SopB proteins would indicate the mechanism of IncG destabilization. In the event, neither mutant showed significant loss of promoter silencing or partition complex extension in cis (Fig. 1), activities that mutation might be expected to diminish if enlarged complexes underlie plasmid destabilization. On the other hand, the weak instability of the E215K mutant and the strong destabilizing activity of the P28L mutant are consistent with a loss of partition complex interaction in trans (pairing) in the former case and with unduly tight interaction in the latter. In our hands the supercoil-trap procedure which allowed (Edgar et al., 2001) to demonstrate in vivo pairing of ParB–parS complexes did not generate positively supercoiled sopC plasmids upon production of wild-type SopB, precluding a rigorous test of altered pairing properties (J. Rech and D. Lane, unpubl. data). However, the P28L mutant showed very strong positive supercoil production in this assay, indicating an abnormally strong pairing ability which would explain its highly destabilizing behaviour. These observations are compatible with the notion that the E215K mutant curtails enhancement of recombination by reducing interplasmid contact while the P28L mutant does so by physical interference with recombination itself. This idea remains speculative for the moment.
Regardless of the mechanism involved, the ability of SopB to enhance sibling plasmid recombination shown here reveals that the functional autonomy of the Sop partition system does not prevent it from influencing other processes in which its replicon engages. While we have used small plasmids to reveal the phenomenon, it is possible that the parental F plasmid and other large naturally occurring plasmids, with their more varied potential for arresting replication forks, are more subject to dimerization and thus more dependent on dimer resolution systems such as ResD/rsfF. It will be important to consider this aspect in future studies of plasmid maintenance, especially where the absolute or relative amounts of partition proteins deviate from their natural values.
Experimental procedures
Bacterial strains and plasmids
All strains are derivatives of E. coli K-12 W1485 and are listed, together with plasmids, in Table 4. Most strain and plasmid construction is detailed in Supplementary material. The substitution of pLtetO-1 and an inefficient translation signal in the mini-F pDAG173 results in constitutive production of Sop proteins at about threefold the level in cells harbouring wild-type mini-F. Copy number mutants of the mini-F plasmids pDAG114, pDAG115 and pJYB74 were made by replacing the small EcoRV fragment that includes the last seven codons of repE and the first four of the five incC iterons with annealed oligonucleotides constituting the repE 3′ end and stop codon. pDAG310 was derived by replacement of the distal half of sopB in the low-copy-number pSC101 mutant plasmid, pDAG221 (Ravin and Lane, 1999), with a fragment carrying sopC and cat, together with deletion of kan.
| Strain | Genotype/relevant propertiesa | Source |
|---|---|---|
| DLT1215 | F–, thi, leu, thyA, deoB, supE, Δ(ara-leu)7696, zac3051::Tn10, rpsL812; spontaneous SmR mutant of DLT812a | Bouet et al. (2005)This work |
| DLT1472 | DLT1215 lacZΩsopB+ | Bouet et al. (2005) |
| DLT1473 | DLT1215 lacZΩsopBE215K | This work |
| DLT1474 | DLT1215 lacZΩsopBP28L | This work |
| DLT1501 | DLT1472/pZC204 | This work |
| DLT1790 | DLT1215/pZC204 | This work |
| DLT1815 | DLT1215 recF400::Tn5; P1 transduction from JJC451a | This work |
| DLT1816 | DLT1472 recF400::Tn5; as DLT1815 | This work |
| DLT1817 | DLT1473 recF400::Tn5; as DLT1815 | This work |
| DLT1818 | DLT1474 recF400::Tn5; as DLT1815 | This work |
| DLT1900 | araD139, Δ(ara-leu)7679, ΔlacX74, galU, galK, rpsL, thi, hsdR2, mcrB, Δ(araFGH), Φ(ΔparaE, pcp18::araE); arabinose transport mutant of MC1061a | This work |
| DLT2067 | DLT1900 [λimm21 sopC-paadA::lac(ZYA)+] | This work |
| Plasmid | Relevant characteristics | Source |
|---|---|---|
| pACYC184 | ori p15A tet +, cat+ | Chang and Cohen (1978) |
| pAM238 | 523 bp lacOPZ'Ωmcs in EcoRI-HindIII interval of pGB2a | Gil (1990) |
| pDAG114 | mini-F; repFIA+, ccdB–, resD+, rfsF+, sop+, cat+ | Ravin and Lane (1999) |
| pDAG127 | ori pMB1 araC-paraBAD::sopA+, kan+ | Lemonnier et al. (2000) |
| pDAG173 | pDAG114 with sopAB control replaced by ptet and a low efficiency translation signal | Libante et al. (2001) |
| pDAG196 | oripSC101 repmmp6, pLtetO-1, cat+ | Ravin et al. (2003) |
| pDAG209 | pDAG114 ΔsopAB | Ravin and Lane (1999) |
| pDAG301 | pDAG196, pLtetO-1::sopA+ | Ravin et al. (2003) |
| pDAG310 | pDAG196, pLtetO-1::sopA+sopBΔ(codons 204–323), sopC+ | This work |
| pDAG607a | pGB2 araC-paraBAD::sopAΔ(codons 92–375) sopB+ | This work |
| pDAG609 | pDAG607 but sopBE215K | This work |
| pDAG611 | pDAG607 but sopBP28L | This work |
| pJYB74 | pDAG114 ΔresD | This work |
| pJYB75 | pDAG114 ΔsopC, ΔresD | This work |
| pJYB76 | pDAG114 sopBE215K | This work |
| pJYB77 | pDAG114 sopBP28L | This work |
| pJYB93 | pDAG173 sopBE215K | This work |
| pJYB94 | pDAG173 sopBP28L | This work |
| pJYB106 | pDAG114 incCΔiterons(1–4) | This work |
| pJYB107 | pDAG115 incCΔiterons(1–4) | This work |
| pJYB108 | pJYB74 incCΔiterons(1–4) | This work |
| pZC204 | pACYC184 ΔtetAΩsopC+, cat+ | Yates et al. (1999) |
- a. Parental strains or plasmids described in Supplementary material.
Media and growth conditions
Cultures were grown at 37°C with aeration in LB medium containing thymine (10 μg ml) and antibiotics (Sigma) as appropriate (μg ml−1): kanamycin (Km, 50), chloramphenicol (Cm, 20), streptomycin (Sm, 200), tetracycline (Tc, 10), spectinomycin (Sp, 20 in liquid medium, 30 in solid). LB was supplemented with 1.5% agar (Difco) for solid medium.
DNA manipulations and related procedures
Enzyme reactions, DNA preparation, agarose gel electrophoresis and transformation with plasmid DNA were carried out using standard procedures, according to suppliers' recommendations where applicable (restriction endonuclease, DNA polymerase and ligase, New England Biolabs; PCR with Pfu, Stratagene; plasmid and gel-fractionated fragment DNAs, Qiagen). For quantifying DNA in bands, gels were stained with SYBR Green 1 (Sigma) and data analysed using Image Quant (Molecular Dynamics).
Plasmid stability and psop activity assays
Stability of mini-F and p15A derivatives was assayed by plating culture samples on LB agar medium, replica plating to medium with Cm, and calculating loss rates from the fractions of each sample resistant to Cm, as described (Lemonnier et al., 2000). Corepression was measured by assaying β-galactosidase (Lane et al., 1994) in cultures of psop::lacZ reporter strains DLT2054, -5, -6, -7 freshly transformed to CmR with pDAG196 (no sopA), pDAG301 (sopA) and pDAG310 (sopA sopC), as described by Ravin et al. (2003).
Gel mobility shift assays
A 106 bp XbaI-XhoI fragment of pJYB55, containing a single 43 bp unit of sopC, and an 83 bp SacI-XhoI fragment of pBSKS+ (Stratagene), serving as the control, were purified, labelled with 32P by end-filling with Klenow polymerase, and combined. Binding reaction mixtures were assembled on ice by adding the combined fragments at ∼2 nM (final concentration) to 25 mM HEPES-KOH, pH 7.8, 100 mM KCl, 5 mM MgCl2, 100 μg ml BSA, 100 μg ml salmon sperm DNA, 10% glycerol, 1 mM DTT, and binding begun by addition of SopB protein extract FrI (see Supplementary material). The mixtures were incubated for 10 min at 30°C, and fractionated by electrophoresis on 6% polyacrylamide gels in TBE (90 mM Tris-borate, 1 mM EDTA) buffer at 200 V for 5 h at 4°C. The gels were dried on Whatman DE81 paper and exposed to a PhosphorImager screen (Fugi).
Topoisomer analysis
Cells from fresh overnight cultures were diluted 2000-fold in LB containing the appropriate antibiotics and incubated at 37°C, with induction of SopA synthesis as required (Lemonnier et al., 2000). At OD600∼0.25, 40 ml of culture was mixed rapidly with 40 ml of cold stop solution (75% ethanol, 21 mM sodium acetate, pH 5.5, 2% phenol, 2 mM EDTA). The cells were centrifuged for 12 min at 4°C and washed once in 20 ml of cold TNE (50 mM Tris-HCl pH 8.0, 50 mM NaCl, 1 mM Na3EDTA), and plasmid DNA was purified by the Qiagen miniprep procedure. Plasmid topoisomers were resolved by agarose gel electrophoresis in the presence of 2 μg ml chloroquine, then revealed and analysed as previously described (Lemonnier et al., 2000).
Transcription silencing assay
DLT2067 was transformed to SpR with SopB producer plasmids and cultures started by resuspending fresh transformant colonies in LB + Sp. After four to five generations of exponential growth to OD600∼0.2, the cells were induced to produce SopB protein by 1000-fold dilution into pre-warmed medium containing arabinose at various concentrations, and incubation continued for about 12 generations before sampling for assay of β-galactosidase.
Two-dimensional gel electrophoresis
Replication and recombination intermediates were analysed by two-dimensional gel electrophoresis of plasmid DNA in cleared lysates, essentially according to Friedman and Brewer (1995) with modifications (Kuzminov et al., 1997). DLT1501 (sopB+/pZC204) and DLT1790 (sopB–/pZC204) were grown at 37°C in 120 ml LB containing chloramphenicol and 1 mM IPTG to OD600∼0.5, and the cells were chilled on ice. Plasmid DNA was prepared by the cleared lysate procedure of Polard and Chandler (1995) as modified by Normand et al. (2001). About 1 μg of each DNA was digested with XbaI. The digests were subjected to electrophoresis on a 0.4% agarose gel in TBE buffer at 1 V cm−1 for 22 h at room temperature. After ethidium bromide staining, lanes containing plasmid DNA migrating between 3.5 kb and 25 kb linear markers were excised and embedded in a 1.1% agarose gel in TBE buffer for electrophoresis in the second dimension at 5 V cm−1 for 5 h at 4°C. DNAs were transferred to nylon membrane (Qbiogene) by Southern blotting, hybridized with 32P-labelled pZC204 DNA and exposed to a Phosphorimager screen (Fuji).
Acknowledgements
We thank Bénédicte Michel and Richard Fekete for strains, Sota Hiraga and Don Biek for the sopB mutant plasmids, Christian Lesterlin for his contribution to early stages of this work, Jérôme Rech for help with the silencing experiments and François Cornet for helpful discussions. This work was supported by the soutien de base du LMGM.