Meningococcal biofilm formation: structure, development and phenotypes in a standardized continuous flow system
Summary
We show that in a standardized in vitro flow system unencapsulated variants of genetically diverse lineages of Neisseria meningitidis formed biofilms, that could be maintained for more than 96 h. Biofilm cells were resistant to penicillin, but not to rifampin or ciprofloxacin. For some strains, microcolony formation within biofilms was observed. Microcolony formation in strain MC58 depended on a functional copy of the pilE gene encoding the pilus subunit pilin, and was associated with twitching of cells. Nevertheless, unpiliated pilE mutants formed biofilms showing that attachment and accumulation of cells did not depend on pilus expression. Mutation and complementation analysis revealed that the type IV pilus-associated protein PilX, which was recently shown to mediate interbacterial aggregation, indirectly supported microcolony formation by contributing to pilus expression. A large number of PilX alleles was identified among genetically diverse meningococcal strains. PilX alleles differed in their propensity to support autoaggregation of cells in suspension, but not in their ability to support microcolony formation within biofilms in the continuous flow system.
Introduction
Neisseria meningitidis contributes significantly to childhood and adolescent morbidity and mortality (Rosenstein et al., 2001). Asymptomatic nasopharyngeal carriage of the organism is frequent among healthy individuals (Cartwright et al., 1987). Phase variation of the meningococcal capsule expression has been described (Hammerschmidt et al., 1996a), and constitutively unencapsulated variants have been shown to account for a significant proportion of isolates recovered from healthy carriers (Claus et al., 2005). Meningococcal carriage is of pivotal importance for the development of naturally acquired immunity to meningococcal disease (Goldschneider et al., 1969). Interestingly, microcolonies have been described in tonsillar tissue, and it may be assumed that meningococcal carriage is underestimated in studies relying on retropharyngeal swabbing (Sim et al., 2000). There are, however, currently no data available on the physiological state and antigen expression in tonsillar microcolonies.
Many bacterial species besides Staphylococci and Pseudomonas species exhibit the propensity to form biofilms on solid surfaces in vitro. In vitro biofilm formation has been demonstrated for Vibrio cholerae (Wai et al., 1998), Salmonella typhimurium (Römling and Rohde, 1999),Salmonella enteritidis (Korber et al., 1997), Escherichia coli K-12 (Vidal et al., 1998), Haemophilus influenzae (Murphy and Kirkham, 2002) and Klebsiella pneumoniae (Wentland et al., 1996). Biofilm formation has also been investigated in pathogenic Neisseria. Greiner and coworkers demonstrated the ability of Neisseria gonorrhoeae to form biofilms in a biofilm flow system, and over primary urinary tract epithelial cells, the latter investigated under static conditions (Greiner et al., 2005). Furthermore, it has been shown that piliated and non-piliated N. meningitidis strains formed biofilms in a static model, i.e. without the application of shear forces by a continuous media flow (Yi et al., 2004). Biofilm formation could be observed with different meningococcal strains and was inhibited by polysaccharide capsules.
It is intriguing to speculate that loss of encapsulation during carriage by a variety of phase variable and irreversible mechanisms (Hammerschmidt et al., 1996a,b; Claus et al., 2002; Dolan-Livengood et al., 2003) coincides with the formation of nasopharyngeal biofilms on the mucosa and within tissues. These multicellular structures might display increased tolerance against penicillin, which has been observed to be inefficient for eradication of meningococcal carriage (Abramson and Spika, 1985). Furthermore, prolonged carriage of distinct meningococcal strains despite of the induction of a type specific immunity (Jordens et al., 2004) might be an effect of biofilm forma tion. If loss of encapsulation is of benefit for carriage of the bacteria, why is the propensity to express capsules maintained in most meningococcal lineages? This cannot be explained by the rare event of invasion of the blood stream and the cerebrospinal fluid by encapsulated meningococci, which is a dead-end of evolution. It has been speculated (but not proven yet) that the meningococcal capsule evolved to support between-host transmission of the bacteria, possibly by prevention of desiccation during the aerosol route (Virji, 1996). This assumption, however, is not in contrast to the speculation of a life in biofilms in the unencapsulated state on the mucosal surface: Occasional phase variation to encapsulation possibly results in release of cells from biofilms, which are then transmitted between hosts. Taken together, understanding the molecular mechanisms of in vitro biofilm formation by meningococci might contribute to our understanding of the complex lifestyle of meningococci comprising carriage, transmission and disease. The initial studies by Yi et al. however, did not utilize standardized flow systems combined with non-invasive, real-time confocal laser scanning microscopy (CLSM), which have become the gold standard in biofilm research (Christensen et al., 1999). Such continuous flow systems not only guarantee continuous nutrient supply, but also apply shear forces mimicking natural dynamic conditions encountered by sessile bacteria. The flow-cell system secures reproducibility of biofilm development (Heydorn et al., 2002). The present study describes the establishment of a standardized continuous flow biofilm model for meningococci. Differences in biofilm structure, development and phenotypes were analysed for this highly variable pathogen. Molecular mechanisms of biofilm formation were elucidated.
Results
Only capsule deficient meningococci form biofilms
Biofilm formation of meningococci under static conditions was analysed on a glass surface using a modified Neisseria defined medium (NDM) as growth medium. Encapsulated meningococcal strains MC58 [serogroup B, sequence type (ST)-32 complex], 2120 (serogroup C, ST-11) and 2594 (serogroup A, ST-5) as well as their unencapsulated derivatives (i.e. MC58siaD-, 2120siaD-, 2594mynB-) were tested for biofilm formation. According to results published recently (Yi et al., 2004), unencapsulated derivatives were very potent biofilm formers, whereas encapsulated parental strains formed no appreciable biofilm. Similar results were obtained with a polystyrene microtitre plate assay using GC-medium. Furthermore, unencapsulated derivatives of nine carrier isolates and three invasive isolates (Table 1) efficiently formed biofilms (data not shown). These findings demonstrate that biofilm formation is a general trait of unencapsulated meningococci. We investigated whether this also held true in a continuous flow system which is described in detail in the following paragraph. For this purpose the apathogenic serogroup Y strain Y2220 was compared with its unencapsulated siaD mutant (Ram et al., 2003; Madico et al., 2006). The wild-type strain did not develop substantial amounts of biofilm mass, in contrast to the unencapsulated derivative, which was highly effective in this respect (Fig. 1B).
| Straina | Serogroup | Sequence type (parental strain) | Genotype | Plasmid content | Resistanceb |
|---|---|---|---|---|---|
| 2135 (syn. MC58) | B | ST-74 | wt (invasive isolate) | – | – |
| 3240 | Unencapsulated | (MC58) | siaD- | – | CM |
| 3349 | Unencapsulated | (MC58) | siaD-, gfp+ | pEG2-Ery | CM, Ery |
| 3618 | Unencapsulated | (MC58) | siaD-, gfp+, pilX- | pEG2-Ery | CM, Ery, Kana |
| 3641 | Unencapsulated | (MC58) | siaD-, cfp+ | pGH53 | CM, Ery |
| 3644 | Unencapsulated | (MC58) | siaD-, yfp+ | pGH52 | CM, Ery |
| 3704 | Unencapsulated | (MC58) | siaD-, gfp+, pilX-, pilXMC58+ | pEG2-Ery, pHC33 | CM, Ery, Kana, Spc |
| 3663 | Unencapsulated | (MC58) | siaD-, gfp+, pilX-,pilX2120+ | pEG2-Ery, pHC34 | CM, Ery, Kana, Spc |
| 3847 | Unencapsulated | (MC58) | siaD-, gfp+, pilX-, pilX2594+ | pEG2-Ery, pHC35 | CM, Ery, Kana, Spc |
| 3854 | Unencapsulated | (MC58) | siaD-, gfp+, pilE- | pEG2-Ery | CM, Ery, Kana |
| 3861 | Unencapsulated | (MC58) | siaD-, gfp+, pilE-, pilEMC58+ | pEG2-Ery, pHC36 | CM, Ery, Kana, Spc |
| 2120 | C | ST-11 | wt (invasive isolate) | – | – |
| 2517 | Unencapsulated | (2120) | siaD- | – | CM |
| 3379 | Unencapsulated | (2120) | siaD-, gfp+ | pEG2-Ery | CM, Ery |
| 3620 | Unencapsulated | (2120) | siaD-, gfp+, pilX- | pEG2-Ery | CM, Ery, Kana |
| 3772 | Unencapsulated | (2120) | siaD-, gfp+, pilX-, pilXMC58 | pEG2-Ery, pHC33 | CM, Ery, Kana, Spc |
| 3773 | Unencapsulated | (2120) | siaD-, gfp+, pilX-, pilX2120 | pEG2-Ery, pHC34 | CM, Ery, Kana, Spc |
| 2594 | A | ST-5 | wt (invasive isolate) | – | – |
| 2668 | Unencapsulated | (2594) | mynB- | – | CM |
| 3380 | Unencapsulated | (2594) | mynB-, gfp+ | pEG2-Ery | CM, Ery |
| 3623 | Unencapsulated | (2594) | mynB-, gfp+, pilX- | pEG2-Ery | CM, Ery, Kana |
| α14 | Unencapsulated (cnl) | ST-53 | wt (carrier isolate) | – | – |
| α62 | Unencapsulated (cnl) | ST-845 | wt (carrier isolate) | – | – |
| α724 | Unencapsulated (cnl) | ST-845 | wt (carrier isolate) | – | – |
| α111c | Y | ST-23 | wt (carrier isolate) | – | – |
| α171c | Y | ST-60 | wt (carrier isolate) | – | – |
| 2220c | Y | ST-23 | wt (carrier isolate) | – | – |
| α278c | 29E | ST-60 | wt (carrier isolate) | – | – |
| α378c | 29E | ST-60 | wt (carrier isolate) | – | – |
| α425c | B | ST-32 | wt (carrier isolate) | – | – |
| H44/76c | B | ST-32 | wt (invasive isolate) | – | – |
| DE8797c | B | ST-41 | wt (invasive isolate) | – | – |
| DE8823c | C | ST-8 | wt (invasive isolate) | – | – |
| 3870 | Y | (2220) | gfp+ | pEG2-Ery | Ery |
| 3871 | Unencapsulated | (2220) | siaD-, gfp+ | pEG2-Ery | Ery, Cm |
- a. Numbers according to the strain collection of the IHM Würzburg.
- b. CM, chloramphenicol; Ery, erythromycin; Kana, kanamycin; Spc, spectinomycin.
- c. Genetically engineered unencapsulated variants of these strains were also used, but are not explicitly named here.


A. Spatial distribution of biofilm formation of strains MC58siaD-/gfp+, 2120siaD-/gfp+ and 2594mynB-/gfp+ and the respective pilX mutants. Biofilms were grown in flow chambers. Biofilm development was monitored by CLSM 1, 6 and 12 h after inoculation. Micrographs represent simulated three-dimensional images.B. Biofilm experiments in flow chambers with strain MC58siaD-/gfp+/pilX- complemented with pilX2120 and pilX2594 respectively; with strain MC58siaD-/gfp/pilE- and its complemented derivative; and with the encapsulated serogroup Y strain Y2220 and its unencapsulated derivative.
Biofilm development of capsule deficient meningococci in a continuous flow cell system
To investigate biofilm development and specific biofilm structures we constructed capsule deficient derivatives of several meningococcal strains harbouring plasmids for the expression of the red-shifted green fluorescent protein (rsGFP). This allowed us to monitor biofilm development in a non-destructive mode in continuous flow chamber cultures by CLSM. The strains were cultivated for up to 120 h on glass surfaces in the continuous flow cell system irrigated with modified NDM. The supplementation of NDM with 5 mM NaHCO3 and PolyViteX was indispensable to yield persistent and reproducible biofilms in the flow cell system. All capsule deficient strains formed biofilms, and the biofilms of each strain could be maintained for at least 96 h. Figure 1A shows the different biofilm architectures produced by strains MC58siaD-/gfp+, 2120siaD-/gfp+ and 2594mynB-/gfp+. After inoculation, single cells were equally distributed over the glass surface. For reasons of the experimental set-up, these data cannot be shown. Within 1 h after inoculation without medium flow small microcolonies consisting of only a few cells were observed for strain MC58siaD-/gfp+. We suggest that these cell clusters are the product of aggregation and growth immediately after inoculation of the channels. Six hours after inoculation, strains MC58siaD-/gfp+ and 2120siaD-/gfp+ formed dense regularly shaped microcolonies whereas strain 2594mynB-/gfp+ formed a flat biofilm without the presence of these regularly shaped microcolonies. Strain 2594mynB-/gfp+ appeared to cover much more of the substratum than the two other strains. From 1 h to 12 h a consistent increase of biomass and biofilm thickness was visible (Fig. 1). The CLSM images were analysed by the COMSTAT computer program (Heydorn et al., 2000), which comprises 10 different features for quantifying three-dimensional biofilm image stacks. As already seen by visual inspection (Fig. 1A), 2594mynB-/gfp+ covered more than 80% of the substratum, whereas MC58siaD-/gfp+ and 2120siaD-/gfp+ covered less than 40%. In conclusion, unencapsulated meningococci readily formed biofilms under conditions of continuous medium flow. Differences in biofilm architecture between strains became apparent. Therefore, a further set of experiments was designed to elucidate the molecular mechanisms of microcolony formation in meningococcal biofilms.
PilX mutation and complementation and autoaggregation studies
Recently, PilX has been described as a type IV pilus-associated pilin-like protein that mediates aggregation of meningococcal cells in liquid cultures and on epithelial cells without influencing the adhesive properties to epithelial cells (Helaine et al., 2005). PilX was necessary for bacterial aggregation in static fluid suspensions, and pilX negative mutants did not form microcolonies on epithelial cells. We therefore investigated the biofilm formation of pilX mutant strains in the continuous flow biofilm model. The pilX gene was inactivated in the strain MC58 by insertion of a kanamycin resistance cassette (Fig. 2A). The knock-out was confirmed by polymerase chain reaction (PCR), by Southern blotting, and phenotypically by an aggregation assay (Helaine et al., 2005) (Fig. 2B, C and F), and by reverse transcription (RT)-PCR (data not shown). RT-PCR demonstrated that the pilX mutation did not affect transcription of NMB889 (Fig. 2D), which has recently been shown to be necessary for pilus assembly (Carbonnelle et al., 2005). Furthermore, Western blot analysis using pilin–specific antibodies suggested that pilus subunit expression was not reduced by the PilX mutation (Fig. 2E). We also generated PilX-specific antisera by immunizing rabbits with purified PilXMC58-GST fusion protein. Only truncated proteins representing the globular domain could be expressed in sufficient amounts. His-tagged fusions could not be expressed in sufficient amounts in E. coli. The GST fusion proteins were not soluble at various temperatures. Antibodies were elicited in the rabbit immunized with the MC58 protein, but not in the rabbit immunized with the 2120 protein (data not shown). Very weak expression of PilX was seen in the MC58 parental strain, whereas in the complemented mutant harbouring the pilX gene under control of a porA promoter, PilX was clearly visible (Fig. 2G). Unfortunately, numerous cross-reactive bands were observed. There was no cross-reactivity of the serum with strains 2120 and 2594. This might be due to extensive antigenic variability (see below).
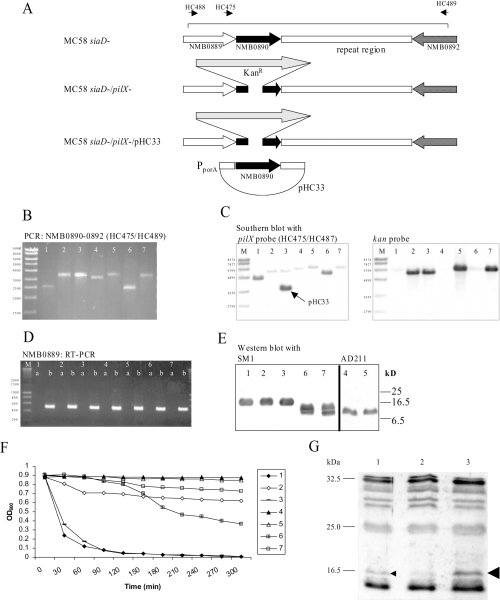
PilX mutagenesis: pilX was inactivated by transformation of meningococci with plasmid pHC32 comprising a PCR product obtained with primers HC488/HC489.A. Genetic organization of the pilX region in strains MC58 and respective mutants. The pilX gene NMB0890 is preceded by the gene NMB0889 possibly involved in Pilus assembly (Carbonnelle et al., 2005). Downstream of NMB0890, there is a complex repeat region also comprising in MC58 one small putative open reading frame (ORF) of unknown function transcribed in opposite direction (ORF not shown). The location of primers HC488/HC489 is shown.B. Analysis of pilX and the pilX downstream repeat region by a PCR spanning NMB0890 to NMB0892 using primers HC475/HC489. Throughout the figure the strains were numbered as follows: 1, MC58siaD-/gfp+ 2, MC58siaD-/gfp+/pilX-; 3, MC58siaD-/gfp+/pilX-/pHC33; 4, 2120siaD-/gfp+ 5, 2120siaD-/gfp+/pilX-; 6, 2594mynB-/gfp+ 2594mynB-/gfp+/pilX-. The PCR analysis shows that strain 2120 harboured a larger pilX downstream repeat region, which was truncated by mutagenesis with pHC32.C. Southern blot analysis using a pilX probe and a kan probe. The Southern blot confirms correct insertion of the kanamycin resistance determinant. Furthermore, in lane three the pilX gene carried on pHC33 is visible.D. RT-PCR demonstrating transcription of NMB0889. (a) PCR with total RNA as a template. (b) RT-PCR with total RNA as a template.E. Detection of pilin, the basic unit of type IV pili, by immunoblot using the antibodies SM1 and AD211 respectively. The nature of the double band seen in strain 2594 is unclear.F. Determination of autoaggregation by measuring the absorbance at 600 nm over time in non-agitated liquid cultures. Values are means of three independent experiments.G. Detection of PilXMC58 in whole cell lysates using a polyclonal antiserum directed against recombinant PilXMC58-GST fusion protein.
The pilX knock-out mutant of strain MC58siaD-/gfp+ showed a clear delay in autoaggregation, and complementation in trans of PilX expression under the control of a meningococcal porA promoter restored autoaggregation confirming that PilX was necessary for the phenotype (Fig. 2F). Also strain 2594mynB-/gfp+ exhibited autoaggregation, which was absent in the isogenic pilX mutant strain, again demonstrating the role of PilX. Surprisingly, strain 2120siaD-/gfp+ displayed no interbacterial aggregation. We cannot rule out the possibility that the strain 2120siaD-/gfp+ showed only weak PilX protein expression. However, when MC58siaD-/pilX- was complemented with the pilX gene from strain 2120 by transformation with plasmid pHC34, autoaggregation was also not restored in significant contrast to complementation with the homologous pilX variant (Fig. 3, P = 0.0006). In contrast to strain MC58, strain 2120 could not be transferred to an autoaggregating phenotype by either pilX variant, suggesting that PilX was necessary, but not sufficient for autoaggregation (Fig. 3).
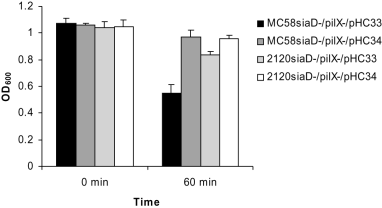
Bacterial autoaggregation assay. Autoaggregation was measured by determining the absorbance at 600 nm after 60 min in non-agitated liquid cultures. Values are means of five independent experiments. The standard deviation is provided.
We sequenced pilX genes of a variety of meningococcal isolates and compared the deduced amino acid sequences (Fig. S2). Until now, we identified 35 alleles with an average of 163 amino acids. Thirty-nine segregating sites were identified (0.24 segregating sites per site). These sites are clearly concentrated in proposed globular domain of the protein. Strain 2120 harboured one of six alleles with an eight amino acid insertion (position 107–114), whose function is yet unclear. Preliminary analysis suggest an increase of the dN/dS ratio in the globular domain, and weak signs of recombination at the C-terminus (M. Reinhardt, pers. comm.). The PilX2594 and PilX2120 differ from PilXMC58 in 11 and 20 amino acids respectively.
PilX mutation and meningococcal biofilm architecture
We next asked whether the diverse effects of meningococcal PilX with regard to autoaggregation might be the reason for differing biofilm architectures. Therefore, biofilm formation by pilX knock-out mutants was recorded by CLSM under continuous flow conditions. The pilX mutation abrogated microcolony formation of the unencapsulated derivative of MC58, resulting in a biofilm with a high surface coverage (1, 4). Microcolony formation by the mutant was restored by pilX complementation in trans. COMSTAT analysis confirmed that biofilms of a pilX knock-out mutant showed increased substratum coverage for MC58siaD-/gfp+ and 2120siaD-/gfp+, but not for 2594mynB-/gfp+ (data shown for strain MC58siaD-/gfp+ in Fig. 4). Biofilm formation for the pilX mutant remained unaffected. Microcolony formation was also observed in strain 2120siaD-/gfp+. Again, the pilX mutation abrogated microcolonies without affecting biofilm formation itself. This finding was interesting, because strain 2120siaD-/gfp+ was incapable of autoaggregation. We conclude that autoaggregation mediated by PilX is not a sufficient explanation for microcolony formation in the biofilm flow system, which was supported by the observation that the autoaggregating strain 2594mynB-/gfp+ did not display microcolonies.
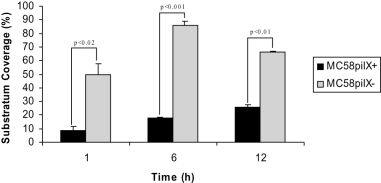
COMSTAT analysis of biofilm parameters. Substratum coverage of biofilms of strain MC58siaD-/gfp+ and its pilX knock-out mutant was determined by the COMSTAT program at three different time points. Values are means of data from 18 image stacks (six image stacks from three independent channels). Standard deviations are indicated by the error bars.
We therefore asked the question, how PilX affected microcolony formation, as it was obviously not linked to autoaggregation. Visual inspection of early phase biofilms and short time-lapse movies demonstrated that MC58siaD-/gfp+ and 2120siaD-/gfp+ showed a higher degree of twitching motility compared with both strain 2594mynB-/gfp+ and the pilX knock-out mutants. The data are presented for strain MC58 as time-lapse movies (see Fig. S1). Twitching of strain MC58siaD-/pilX-/gfp+ could be restored by complementation in trans. Thus, the pilX mutation reduced twitching motility of cells in the continuous flow biofilm model. The twitching motility phenotype was associated with microcolony formation (Table 2).
| Strain | Autoaggregation | Twitching motility | Microcoloniesa |
|---|---|---|---|
| MC58 siaD- | + | + | + |
| MC58 siaD-/pilX- | – | – | – |
| MC58 siaD-/pilX-/pHC33 | + | + | + |
| 2120 siaD- | – | + | + |
| 2120 siaD-/pilX- | – | – | – |
| 2594 mynB- | + | – | – |
| 2594 mynB-/pilX- | – | – | – |
- a. All strains formed biofilms, but differed in the biofilm architecture with regard to microcolonies.
Twitching furthermore resulted in extensive motility of cells even after microcolonies were formed. When we mixed CFP- and YFP-labelled cells of strain MC58siaD-, microcolonies consisted of homogeneously mixed blue and yellow cells (Fig. 5). In contrast, the pilX mutant cells formed a flat biofilm with very distinct zones of blue and yellow cells with no obvious mixing. We therefore suggest that twitching motility results in intensive cell mixture in biofilms.
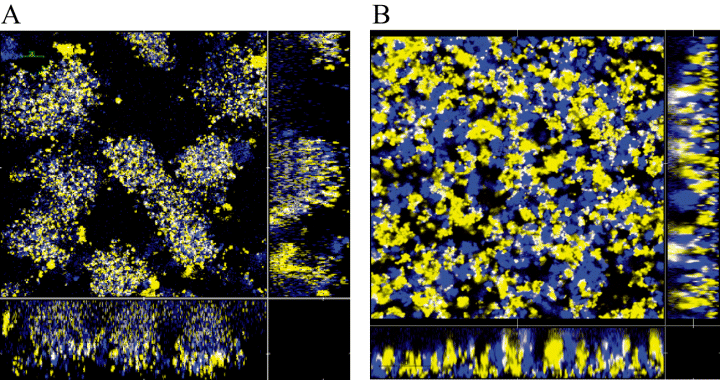
Biofilm formation by CFP- and YFP-labelled derivatives of strain MC58. The biofilm was initiated with a 1:1 mixture of yellow fluorescent and cyan fluorescent bacteria of strain MC58siaD-, and (B) strain MC58siaD-/pilX-. Biofilm formation was monitored by CSLM 12 h after inoculation. Horizontal and vertical sections are provided. The size of the area shown is 235 μm × 235 μm.
Why was twitching motility reduced in pilX mutants? Twitching in pathogenic Neisseria is mediated by type IV pili composed of the pilin subunit, which is encoded by the pilE gene. Immunofluorescence analysis of pilX mutants and the respective derivatives complemented in trans using the pilin-specific antibodies SM1 and AD211, respectively, revealed a significant reduction of diplococci with attached pili in the pilX mutants. PilX complementation restored full piliation in all derivatives (Fig. 6). Ten digital microscopic images were taken for each strain, and the ratio of pilated to unpiliated diplococci was determined. Of 459 diplococci 4.36% were piliated in strain MC58siaD-/gfp+, whereas 0.9% of 660 diplococci were piliated in the pilX mutant (two-tailed Fisher's exact test, P = 0.001). PilX complementation of the pilX mutant resulted in a frequency of piliated diplococci of 6.5% of 569 diplococci. Similar results were obtained with the other meningococcal strains [2120siaD-/gfp+, 9.9% of 638; 2120siaD-/gfp+/pilX-, 1.3% of 639; 2120siaD-/gfp+/pilX-/pHC34 (complemented), 15.1% of 602; 2594mynB-/gfp+, 17.5% of 555; 2594mynB-/gfp+/pilX-, 1.2% of 500; 2594mynB-/gfp+/pilX-/pHC35 (complemented), 7.8% of 580]. The reduction of piliated cells was seen both for meningococci taken directly from agar plates and after intermediate growth in broth. We did not observe changes in the proportion of cell-attached pili to unattached pili. Using ELISA, Helaine et al. saw a ratio of piliation of 1.5 if a wild-type strain and the corresponding pilX mutant were compared with regard to pilus formation, indicating a barely measurable effect of PilX on piliation (Helaine et al., 2005). It should be taken into account that the strain used by these authors was encapsulated and belonged to a different genotype. In our hands the pilX mutation resulted in reduced piliation, reduced twitching motility in Neisseria biofilms, and as a result abrogated the formation of distinct microcolonies.

Immunofluorescence study of pilus expression in PilX mutants. Class II pili were stained with mAb AD211. Representative pictures are shown for derivatives of strain 2120.
We finally asked the question inasmuch PilX variability contributes to differences in biofilm architecture. We expressed the PilX alleles of strains 2120 and 2594 in trans in the pilX knock-out of MC58siaD-. We clearly observed a complementation of the mutation with regard to microcolony formation irrespective of the pilX allele (Fig. 1B). This supported the idea that pilX irrespective of the allele was needed for pilus assembly, twitching motility and consecutively microcolony formation. We then knocked out the gene encoding the subunit of pili, i.e. pilE, and observed loss of microcolony formation despite of the presence of a functional copy of the pilX gene (Fig. 1B). The mutation was complemented if PilE was expressed from a plasmid (Fig. 1B). This demonstrated the importance of pili for microcolony formation under continuous flow conditions. Of note, pili were not necessary for formation of a biofilm (Fig. 1B).
Meningococcal biofilms are more susceptible to rifampin and ciprofloxacin than to penicillin
Following the analysis of the development of meningococcal biofilms, we focused on biofilm phenotypes, the most important of which being the resistance to antibiotics. It is well established that penicillin, albeit being highly efficient in treatment of invasive meningococcal disease, is ineffective for eradication of meningococci from the nasopharynx of healthy carriers (Abramson and Spika, 1985), in contrast to ciprofloxacin (Cuevas et al., 1995) and rifampin (Schwartz et al., 1988). With the newly established biofilm model, we tested the antimicrobial susceptibility of meningococci in biofilms. After 24 h of biofilm growth in flow chambers, 10 times the minimal inhibitory concentration (MIC) of either penicillin, ciprofloxacin, rifampin, was added to the medium. Propidium iodide (PI) in the medium was used for continuous monitoring of the viability of the biofilm cells. The biofilms were recorded with CLSM after 4 h and 24 h to follow the antibiotic killing. Representative data are shown for strain 2120siaD-/gfp+ and 2594mynB-/gfp+ (Fig. 7). After 4 h of antibiotic treatment only the biofilm treated with rifampin was slightly affected. Cells of the apical biofilm layers took up the red PI stain, indicating cell death. The biofilms treated with penicillin or ciprofloxacin as well as the control were completely unaffected. After 24 h of antibiotic treatment the biofilms incubated with rifampin and ciprofloxacin were almost completely dead, whereas in the biofilm treated with penicillin only the upper layers of the biofilm were dead. The deeper layers expressed GFP, and did not take up PI, suggesting that the cells here were alive. The strains displayed identical susceptibility profiles despite of their differing biofilm architectures.
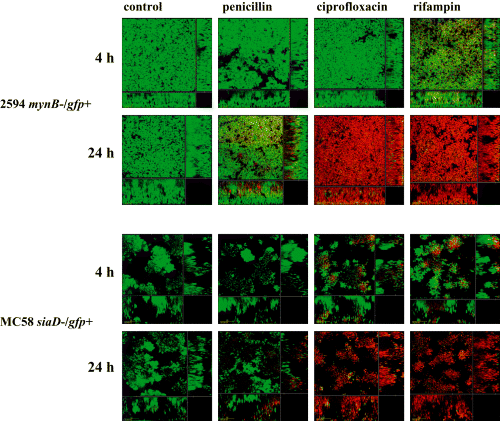
Antibiotic treatment of meningococcal biofilms. Strains 2120siaD-/gfp+ and 2594mynB-/gfp+ were grown as a biofilm in flow chambers for 24 h. Thereafter, 10 times MIC of either penicillin, ciprofloxacin, or rifampin, respectively, was added to the medium. Simultaneously, propidium iodide was added to the biofilm medium to monitor the killing of biofilm cells. The micrographs represent biofilms treated for 4 and 24 h respectively. The size of the area shown is 235 μm × 235 μm.
We quantified and confirmed the above results by treating static biofilms with antibiotics. For that purpose we used 24-well cell culture dishes in which a 15 mm round glass cover slip served as the substratum. After 24 h the biofilms were treated with 10 times MIC penicillin, ciprofloxacin and rifampin for another 24 h. Thereafter, the biofilm cells were resuspended, and serial dilutions were plated out on GC agar to determine the colony-forming units (cfu) of surviving bacteria. Without antibiotic treatment 107 cfu well−1 (Fig. 8) were recovered from the biofilm. In contrast to the lack of growth after treatment with ciprofloxacin and rifampin for 24 h, counts of 104−105 cfu were obtained after treatment with penicillin for 24 h.
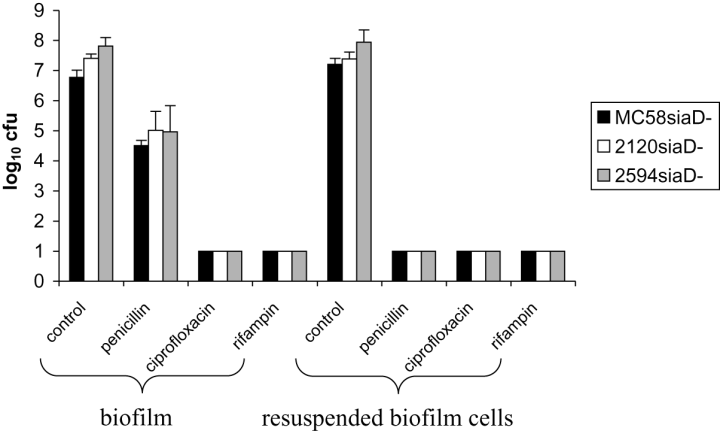
Viability of static biofilms and planktonic cultures treated with antibiotics. Twenty-four-hour-old biofilms of strains MC58siaD-/gfp+, 2120siaD-/gfp+ and 2594mynB-/gfp+ were treated for 24 h with either 10 times MIC of penicillin, ciprofloxacin, or rifampin respectively. Furthermore, biofilms were disintegrated, cells were resuspended and then treated with either antibiotics.
As a control, we resuspended untreated 24 h old biofilms in medium and added 10 times MIC of penicillin, ciprofloxacin, or rifampin respectively. Subsequently, after additional 24 h shaking, these cultures were plated out. Resuspended biofilm cells were completely sensitive to any of the three drugs (untreated resuspended biofilm cells were viable). Thus, outside the biofilm the cells were sensitive to penicillin as suggested before by MIC determination.
Discussion
In this study we established a biofilm model, in which N. meningitidis was grown under flow conditions in a chemically defined biofilm medium to guarantee stable nutritional conditions and high reproducibility. We used a modification of the defined growth medium NDM (Archibald and DeVoe, 1978). NDM was modified by addition of PolyViteX and 5 mM NaHCO3 to yield more reproducible biofilms under flow conditions than with the original NDM. Importantly, the resulting modified NDM led to a slower biofilm formation and more defined biofilm structures in comparison to GC medium (data not shown), which has been used in a static meningococcal biofilm model described recently by Yi and coworkers. GC medium induced clogging of the silicon tubes of the biofilm flow system within less than 24 h. The use of a defined and potentially modifiable medium will be beneficial for future experiments on meningococcal biofilm formation to address the question of biofilm growth requirements.
Biofilm formation could be achieved with a large variety of meningococcal strains, either constitutively unencapsulated or rendered unencapsulated by genetic manipulation. Thus, biofilm formation was a general trait of unencapsulated meningococci, which are frequently observed in the nasopharynx. Biofilm formation alters the biology of the bacteria, as demonstrated by the antibiotic treatment experiments shown in this study. The reduced susceptibility to penicillin found herein cannot be explained by genetic mutation which requires the acquisition of plasmids or horizontally transferred DNA from resistant strains (Spratt et al., 1992; Backman et al., 2000). Not surprisingly, nothing is known about the extent and composition of the extracellular polymeric substances (EPS) in meningococcal biofilms, so it can only be speculated that meningococcal EPS might serve as a barrier of antibiotics as in other bacteria (Nichols et al., 1988). Many recent publications reinforce the theory that the physiological state of the biofilm bacteria is responsible for an antibiotic resistant phenotype (Huang et al., 1995; Spoering and Lewis, 2001; Anderl et al., 2003; Sufya et al., 2003; Walters et al., 2003; Fux et al., 2004; Keren et al., 2004). This could also hold true for the action of penicillin on meningococci in biofilms, due to the effect of penicillin on only actively dividing cells. It is tempting to speculate that the in vitro assembled biofilms resemble meningococcal microcolonies reported recently (Sim et al., 2000), and represent a penicillin tolerant carrier state. Furthermore, given the different degrees of hydrophobicity of the antibiotics studied, it will be of interest to determine their diffusion into the biofilms in future experiments.
We focused our investigation of meningococcal biofilm architecture on the role of PilX. The study design consequently addressed the genetic diversity of meningococcal strains by the inclusion of three different strains. We could reproduce the finding of Helaine et al. that PilX mediated interbacterial aggregation in an autoaggregation assay (Helaine et al., 2005). However, not all strains were autoaggregating. There are two possible explanations for this phenomenon: one is the extensive antigenic diversity of PilX observed in this study; the other hypothesis is that PilX expression varies between strains. The large number of non-synonymous mutations of PilX might indicate that this protein is under immune selection as a pilus-associated protein exposed to the host–bacterial interface. It will be interesting to see whether carriage elicits antibodies blocking PilX function thereby altering the colonization status. Furthermore, the finding that the ST-11 isolate 2120 harboured a non-aggregating copy of PilX raises the question whether the low carriage rate particularly of ST-11 meningococci, which we have recently attributed to lacking capsule phase variation (Claus et al., 2005), might also be caused by a lack of autoaggregation. Extensive population biology and functional studies are needed to provide sufficient information to back up this hypothesis.
Interestingly, twitching motility, not autoaggregation mediated by PilX, could be correlated with the formation of microcolonies within the biofilm. In contrast to Helaine and coworkers who used an encapsulated isolate of different clonal background, we monitored twitching in continuous flow biofilms, and not in a static assay. One should consider that, although the investigation of individual cell twitching has been very fruitful (Merz et al., 2000), twitching motility can also be viewed as a social activity of bacteria involving cell-cell contact (Mattick, 2002). Thus, different assay conditions might explain the contradicting result that twitching was dramatically impaired by pilX mutation in our hands. Twitching in meningococci is promoted by type IV pili (Henrichsen, 1975). PilT provides the energy for pilus retraction (Wolfgang et al., 1998). It is unclear whether pilX mutations affects the expression or function of PilT. Therefore, pilT knock-out analysis in the continuous flow biofilm system would be desirable to directly address twitching on microcolony formation. Unfortunately, pilT mutations have been shown to result in hyperpiliation (Wolfgang et al., 1998). This is the reason, why it was not possible to dissect autoaggregation and twitching in this study. Reduced twitching could be attributed to the fact that in our hands piliation of the bacteria was reduced. More data are needed to explain why PilX reduces piliation, as Western blot analysis in this study did not suggest any reduction of the pilus subunit PilE.
One might ask why twitching is so efficient in promoting microcolony formation, if only relatively few cells are piliated. Two explanations can be offered, one of which comes from high magnification movies we produced (data not shown). These movies clearly show that movements in biofilms usually comprised several cells sticking together. Thus, piliation of few cells might be sufficient to move a whole cell cluster composed mostly of unpiliated, non-motile cells. Secondly, one has to consider that we might have enriched piliated cells by initial attachment to the surface of the flow chamber. This would increase the number of piliated cells. This hypothesis could not be investigated until now.
As discussed above, twitching motility was associated with microcolony formation in meningococcal biofilms. Only strains or mutants with reduced twitching motility formed a homogeneous biofilm covering the whole of the substratum without defined microcolonies. Studies on Pseudomonas aeruginosa and Vibrio cholerae (O'Toole and Kolter, 1998; Watnick and Kolter, 1999) also suggested a role of motility driven aggregation for microcolony formation in static biofilms systems. These findings were contradicted by the work of Heydorn and coworkers (Heydorn et al., 2002) who could show for P. aeruginosa grown in a continuous flow of citrate minimal medium that after initial formation of microcolonies, there was a dispersal of microcolonies, which was dependent on twitching motility (Klausen et al., 2003a). In glucose minimal medium, an alternative biofilm morphology, i.e. heterogeneous ‘mushroom shaped’ biofilms, was observed (Klausen et al., 2003b). After initial attachment a non-motile subpopulation of the bacteria formed the mushroom stalks by clonal growth, while another subpopulation of motile bacteria spread out on the substratum in a type IV pili-dependent manner. The motile subset of the bacteria climbed up the stalks forming mushroom caps. Thus, in different bacterial systems, twitching motility can have opposite effect, either promoting microcolony and mushroom formation, or the dispersal thereof. The influence of media composition needs to be highlighted in this context, and should be addressed in future studies of meningococcal biofilms. Interestingly, twitching of meningococci caused a complete mixture of cyan and yellow fluorescent bacteria, whereas two different subpopulations of P. aeruginosa are maintained in the stalks and caps of mushrooms respectively (Klausen et al., 2003b). This indicates: (i) that in meningococci there is only one population of cells; and (ii) that within the meningococcal microcolonies the level of motility of single bacteria is high. The discussion of bacterial twitching motility and its impact on biofilm architecture demonstrates that it is impossible to extrapolate from a given biofilm morphology the molecular mechanisms behind it, and that experimental systems including growth media and the model organisms have to be carefully taken into account when interpreting and comparing results.
In conclusion, biofilm formation is a trait which is inherent to unencapsulated meningococci. Several morphological and functional observations were obtained in this study. With the knowledge accumulated until now, further questions such as nutrient requirements, extracellular substances, DNA exchange, phase variation and protein expression in meningococcal biofilms can now be addressed. Data derived from this model will contribute to a better understanding of the biology of meningococcal carriage. Thus, we will follow the in vitro biofilm model as a robust and reproducible surrogate system to study human adapted bacteria for which there is no entirely satisfying animal model.
Experimental procedures
Bacterial strains and culture conditions
Invasive meningococcal wild-type strains were used, i.e. strain MC58 (serogroup B; ST-74) (ST-32 complex) (UK) (kindly provided by E.R. Moxon) (Dunn et al., 1995), strain 2120 (serogroup C; ST-11) (Germany) (Vogel et al., 1998) and strain 2594 (serogroup A; ST-5) (Germany) (kindly provided by Ingrid Erhard). Derivatives of those wild-type strains used in this study are listed in Table 1. GC agar (BD DifcoTM, Heidelberg, Germany) was used for standard cultivation, and was supplemented with PolyViteX (bioMerieux, Nürtingen, Germany). When appropriate, erythromycin (7 μg ml−1), kanamycin (100 μg ml−1), chloramphenicol (7 μg ml−1) and spectinomycin (100 μg ml−1), respectively, were added to the medium. For liquid cultures and biofilm experiments a modification of the NDM (Archibald and DeVoe, 1978) was used. Modified NDM was supplemented with PolyViteX and 5 mM NaHCO3 to achieve reproducible growth of biofilms under flow conditions. Despite of the fact that pEG2-Ery derivatives proved to be stable for several days of biofilm culture without addition of antibiotics, 7 μg ml−1 erythromycin was added to all biofilm media in this study except for the media used for antimicrobial treatment experiments. All experiments were performed at 37°C. CO2 enriched atmosphere was used for GC agar cultures only.
Recombinant DNA techniques
Restriction enzymes and DNA-modifying enzymes were purchased from New England Biolabs (Frankfurt/Main, Germany). Chromosomal DNA of N. meningitidis was purified with the Qiagen® Genomic tips system (Hilden, Germany) according to the manufacturer's instructions. Southern blot hybridizations were performed as described previously with digoxigenin-labelled probes (Roche, Mannheim, Germany) (Hilse et al., 1996). Recombinant plasmids were isolated with QIAprep® Spin miniprep kit (Qiagen, Hilden, Germany). Transformation of meningococci was performed as described previously. Oligonucleotides were purchased from Sigma-Genosys (Steinheim, Germany). PCR was performed on a thermal cycler obtained from Biometra (Göttingen, Germany). The thermostable Taq DNA polymerase was purchased from New England Biolabs.
Insertional inactivation of meningococcal capsule genes
Plasmids and primers used for construction of meningococcal mutants are listed in Table 3, and Table S1 respectively. For construction of a serogroup A capsule deficient mutant the mynB gene, hypothesized to encode the capsular polymerase of serogroup A meningococci (Swartley et al., 1998), was insertionally inactivated. The mynB gene was amplified with primers NT2 and NT4, and the PCR product was cloned into the vector pCR®2.1-TOPO (Invitrogen, Karlruhe, Germany), resulting in pNT3. After deletion of a 253 bp HincII fragment (position 477–729 of mynB) from pNT3 the blunt ended HindIII fragment of pTnMax5 (Kahrs et al., 1995) comprising the chloramphenicol resistance cassette was inserted resulting in plasmid pNT5. Subsequently, strain 2594 was transformed with pNT5. The inactivation of the capsule was confirmed by PCR and by ELISA using mAb932 (kindly provided by D. Bitter-Suerbaum, Medical School Hannover, Germany) specific for N. meningitidis serogroup A capsule polysaccharide (data not shown). The capsule deficient mutants of the serogroup C strain 2120, and of the serogroup B strain MC58 have been described previously (Ram et al., 2003; Kurzai et al., 2005).
| Plasmid | Description | References |
|---|---|---|
| pNT3 | PCR product NT2/NT4 comprising mynB encoding the putative serogroup A capsule polymerase cloned into pCR®2.1-TOPO | This study |
| pNT5 | Insertion of the CAT gene of pTnMax5 between the HincII sites ofmynB in pNT3 | This study |
| pGH15 | Insertion of the CAT gene of pTnMax5 into the SpeI site of the serogroup B siaD gene, encoding the α-2,8-polysialyl transferase, in plasmid pUE3 | Kurzai et al. (2005) |
| pHC10 | Insertion of the mini-transposon TnMax5 harbouring the CAT gene at position 370 of the serogroup C siaD gene, encoding the α-2,9-polysialyl transferase, in plasmid pHC1 | Ram et al. (2003) |
| pEG2-Ery | Neisserial expression plasmid for rs-GFP derived from pEG2(Christodoulides et al., 2000). The ampicillin resistance cassette (bla)of pEG2 was exchanged by an erythromycin resistance cassette (ermC). | Christodoulides and van der Ley (unpublished) |
| pAP1 | Neisserial expression vector derived from pEG2-Ery by deletion of rs-gfp | This study |
| pAP2-1 | Neisserial expression vector derived from pAP1. The erythromycin resistance cassette was exchanged by a spectinomycin resistance cassette. | This study |
| pSM236.1 | Delivery plasmid containing ecfp from pECFP (Clontech) | Lambertsen et al. (2004) |
| pSM236.2 | Delivery plasmid containing eyfp from pEYFP (Clontech) | Lambertsen et al. (2004) |
| pGH52 | Neisserial expression plasmid for YFP derived from pAP1and pSM236.2 | This study |
| pGH53 | Neisserial expression plasmid for CFP derived from pAP1 and pSM236.1 | This study |
| pHC31 | PCR product HC488/HC489 comprising pilX cloned into pCR-Script® Amp SK(+) | This study |
| pHC32 | Insertion of the kanamycin resistance cassette of pUC4K into pilXafter inverse PCR with HC492/HC493 of pHC31 | This study |
| pHC33 | PCR product HC511/HC512 comprising MC58 pilX cloned between the SpeI and the EcoRI sites of pAP2-1 | This study |
| pHC34 | PCR product HC511/HC512 comprising 2120pilX cloned between the SpeI and the EcoRI sites of pAP2-1 | This study |
| pHC35 | PCR product HC511/HC535 comprising 2594pilX cloned between the SpeI and the EcoRI sites of pAP2-1 | This study |
| pHC36 | PCR product HC530/HC531 comprising MC58 pilE cloned between the SpeI and the EcoRI sites of pAP2-1 | This study |
| pHC37 | PCR product HC526/527 comprising MC58 pilE cloned into pCR®2.1-TOPO | This study |
| pHC38 | Insertion of the kanamycin resistance cassette of pUC4K into pilEafter inverse PCR with HC539/540 of pHC37 | This study |
| pTnMax5 | Vector harbouring a mini-transposon comprising the CAT gene with a gonococcal opa promoter and an fd-terminator on an HindIII DNA fragment | Kahrs et al. (1995) |
| pUC4K | Vector containing an aminoglycoside 3′-phosphotransferasase gene conferring resistance to kanamycin | GE Healthcare |
| pHP45Ω | Vector containing an omega fragment harbouring the aadA gene from plasmid R100.1 conferring resistance to spectinomycin | Prentki and Krisch (1984) |
| pCR-Script® Amp SK(+) | PCR product cloning vector | Stratagene |
| pCR®2.1-TOPO | PCR product cloning vector | Invitrogen |
Labelling of meningococcal strains with fluorescence proteins
The hybrid shuttle vector pEG2 harbours the red-shifted gfp (rs-gfp) gene under the control of a porA promoter (Christodoulides et al., 2000). This plasmid has been modified by replacing the ampicillin resistance cassette (bla) with an erythromycin resistance cassette (ermC), resulting in pEG2-Ery (kindly provided by M. Christodoulides and P. van der Ley, unpublished). The capsule deficient strains 3240 (MC58siaD-), 2517 (2120siaD-) and 2668 (2594mynB-) were transformed with plasmid pEG2-Ery to generate green fluorescent meningococci. For the construction of cyan and yellow labelled meningococci the rs-gfp gene was deleted from plasmid pEG2-Ery by partial restriction with KpnI and subsequent restriction with SacII. After incubation with T4-DNA polymerase the remaining DNA fragment was religated resulting in pAP1. The genes encoding the cyan fluorescent protein (ecfp) and the yellow fluorescent protein (eyfp) were excised from pSM236.1 and pSM236.2 (Lambertsen et al., 2004), respectively, with XbaI and HindIII and cloned between the SpeI and EcoRV sites of pAP1 resulting in pGH53 and pGH52 respectively. Capsule deficient strains MC58siaD-, 2120siaD- and 2594mynB- were transformed with plasmids pGH53 and pGH52. Stable expression of rs-GFP, CFP and YFP was confirmed by fluorescence microscopy (Zeiss LSM510 Confocal Laser Scanning Microscope (Carl Zeiss, Jena, Germany) at 508 nm, 483 nm and 542 nm respectively.
Insertional inactivation and complementation of the pilX and pilE genes
For cloning of the pilX gene, a 2.9 kb DNA fragment was amplified from strain MC58 with primers HC488 and HC489 and ligated into pCR-Script® (Stratagene, Amsterdam, the Netherlands), resulting in plasmid pHC31. Using as a template plasmid pHC31, an inverse PCR was performed with primers HC492 and HC493. The PCR product was restricted with NsiI and ligated with the PstI DNA fragment of pUC4K comprising the kanamycin resistance cassette resulting in plasmid pHC32. The unencapsulated strains expressing GFP, i.e. strains 3349, 3379 and 3380 were transformed with plasmid pHC32 to generate a pilX knock-out. Complementation of the pilX knock-out was achieved by cloning the pilX gene into a derivative of pAP1. Firstly, the erythromycin resistance cassette in pAP1 was excised with SacI and NsiI. The resulting DNA fragment was incubated with T4 DNA polymerase and ligated with the 2.2 kb SmaI restriction fragment of pHP45Ω harbouring the aadA gene conferring spectinomycin resistance to the new plasmid pAP2-1. Subsequently, the pilX gene was amplified from strain MC58 with the primers HC511 and HC512. The PCR product was cloned between the SpeI and the EcoRI restrictions sites of pAP2-1 resulting in plasmid pHC33. The MC58 pilX knock-out mutant was transformed with pHC33. The pilX knock-out and the subsequent complementation of the knock-out were confirmed by PCR and Southern blot hybridization. Additionally, inactivation of the pilX gene was indirectly confirmed by an aggregation assay (Helaine et al., 2005). The pilE gene (NMB0018) with 500 bp upstream and downstream sequences, respectively, of strain MC58 was amplified with primers HC526 and HC527, and cloned into the vector pCR®2.1-Topo® (Invitrogen, Karlsruhe, Germany), resulting in plasmid pHC37. To delete the pilE gene from pHC37 an inverse PCR was performed using primers HC539 and HC540. After restriction of the resulting PCR product with MfeI, it was ligated with the 1.2 kb EcoRI fragment of pUC4K comprising the kanamycin resistance cassette. The resulting plasmid pHC38, in which the pilE sequence was replaced by a kanamycin cassette, was used for transformation of strain 3349. Complementation of the pilE mutation by PilE expression in trans could only be accomplished, if an expression plasmid was introduced first, because pilE mutation abolished natural competence. PilE expression in trans was achieved by transformation of meningococci with the expression plasmid pHC36. For construction of pHC36, the pilE gene of strain MC58 was amplified with primers HC530 and HC531. The PCR product was restricted with SpeI and EcoRI and cloned into the respective restriction sites of pAP2-1 resulting in plasmid pHC36.
RT-PCR and DNA sequencing of NMB0889
Expression of NMB0889 mRNA was tested performing RT-PCR with primers HC475r and HC514 using Qiagen® OneStep RT-PCR kit. RT-PCR parameters were used as described by Claus et al. (2004) with the following exception: Extension at 72°C was performed for 2 min. Automated DNA sequencing was performed with the dye-deoxy terminator cycle method on a Applied Biosystems 377 sequencer.
Western blot
Pilin (PilE) expression was assayed by Western blot analysis using mAB SM1 (anti class I pili, kindly provided by M. Virji; Bristol, UK) and mAB AD211 (anti class II pili, kindly provided by Mark Achtmann; Berlin, Germany). For detection, a goat anti-mouse IgG conjugated with horseradish peroxidase was used.
Immunofluorescence
Immunofluorescence of meningococci was performed on a Zeiss Axio Imager Z1 fluorescence microscope (Carl Zeiss, Jena, Germany) using standard protocols (Hammerschmidt et al., 1996b). For staining class I pili, the monoclonal antibody SM1 was used, for staining of class II pili mAB AD211. Alexa Fluor 488 nm goat anti-mouse IgG antibody (Molecular Probes®) was used for detection.
Flow chamber experiments
The experiments were performed at the Center for Biomedical Microbiology of the Danish Technical University. Biofilms were cultivated at 37°C in three-channel flow cells with individual channel dimensions of 1 × 4 × 40 mm. Stable growth temperature was maintained by using a culture room at 37°C. The flow system was assembled and prepared as previously described (Christensen et al., 1999). A 24 × 50 mm glass cover slip (Knittel Gläser, Braunschweig, Germany) was used as substratum for biofilm growth. Before each experiment, the system was sterilized by pumping a 0.5% (wt/vol) hypochlorite solution into the system and leaving it there for 4 h. The system was consecutively flushed with 2 l of sterile water. Thereafter, the flow chamber was rinsed with modified NDM overnight at 37°C. Inocula were prepared as follows: meningococcal cultures on GC agar plates grown for less than 8 h were resuspended in modified NDM, diluted to a density of 108 cells ml−1, and incubated at 37°C for 5–6 h shaking. Two hundred microlitres portions of these cultures with 1.0 × 108 cells ml−1 were injected into each channel of the flow cell. The flow cell was turned upside down for 1 h to allow for adhesion of cells to the glass surface without flow. After 1 h the flow was resumed. During growth of biofilms the medium was pumped through the flow cells at a constant rate of 0.2 mm s−1 by using a peristaltic pump (model 205S; Watson Marlow, Calmouth, UK).
Microscopy and image analysis
All microscopic observations and image acquisitions were performed on a Zeiss LSM510 Confocal Laser Scanning Microscope (Carl Zeiss, Jena, Germany). Images were obtained using a 40×/1.3 Plan-Neofluar oil objective. Multi-channel simulated fluorescence projection images, and vertical and horizontal cross sections through the biofilm were generated by using the IMARIS software package (Bitplan AG, Zürich, Switzerland) running on an Indigo2 workstation (Silicon Graphics, Mountain View, USA). Images were further processed for display by using Photoshop (Adobe, Mountain View, USA). Biofilm development was quantified with the COMSTAT computer program (Heydorn et al., 2000). Strains were grown in three separate channels. From each channel six image stacks were acquired randomly down through the channel at different time points. The image stacks for each time point were gained from independent experiments, because the experimental procedure of image acquisition for COMSTAT analysis strongly interferes with meningococcal biofilm development at early time points.
Antibiotic treatment
The MIC of penicillin G, ciprofloxacin and rifampin (Sigma-Aldrich, München, Germany) was determined for the strains 3349 (MC58siaD-/gfp+), 3379 (2120siaD-/gfp+) and 3380 (2594mynB-/gfp+) using Etest® (AB Biodisk, Solna, Sweden). Meningococci grown on Columbia agar were harvested with a sterile cotton swab and resuspended in 0.9% NaCl solution. The optical density of the bacterial suspension was adjusted to McFarland 0.5 using a Vitek Densicheck (bioMerieux, Marcy l'Etoile, France). Bacteria were streaked out on Mueller-Hinton agar supplemented with 5% sheep blood to achieve subconfluent growth after incubation at 37°C for 18–24 h in the presence of an Etest strip. Etest results were evaluated according to the manufacturers instructions. For strains MC58siaD-, 2120siaD- and 2594mynB-, the penicillin MICs (μg ml−1) were 0.032, 0.047 and 0.047, respectively, the ciprofloxacin MICs were 0.003 for all strains, the rifampin MICs were 0.004, 0.004 and 0.012 respectively. Antibiotic susceptibility was assessed for biofilms grown under flow and static conditions as well as for planktonic cells. Biofilm susceptibility to penicillin, ciprofloxacin and rifampin in the biofilm flow system was tested by supplementing the modified NDM with 10 times MIC of the respective antibiotics. Twenty-four-hour-old biofilms were treated with antibiotics under constant flow conditions for 4 h, and 24 h respectively. Bacterial viability in biofilms was estimated by adding with 1 μM propidium iodide (PI) to the medium, which selectively stains dead cells. PI in conjunction with GFP has been successfully used as a dead cell stain for P. aeruginosa (Hentzer et al., 2001). Live GFP-stained and dead PI-stained bacteria were visualized by CLSM. Antibiotic susceptibility in a static biofilm model was assessed in 24-well cell culture dishes on round 15 mm glass cover slips. One millilitre with 108 cells ml−1 of bacterial suspension was seeded per well to initiate biofilm growth. The medium of a 24 h old biofilm was replaced by fresh medium containing 10 times MIC of penicillin, ciprofloxacin and rifampin respectively. After another 24 h the biofilm was rinsed twice with medium without antibiotics. Then the biofilms were harvested with cotton swabs and vortexed for 1 min to disintegrate the biofilm and resuspend the bacteria. The quantity of viable biofilm cells was determined by serial dilutions and plating on GC agar. In the same set of experiments, the antibiotic susceptibility of planktonic cells was tested. Static biofilms were grown as described above. The biofilm medium of a 24 h old biofilm was replaced by fresh medium containing 10 times MIC of penicillin, ciprofloxacin and rifampin respectively. Then the biofilm was harvested with cotton swabs to disintegrate the biofilm and vortexed for 1 min to resuspend the biofilm bacteria. These planktonic cultures were incubated for 24 h at 37°C and 200 r.p.m. Then the planktonic cells were washed twice in antibiotic-free medium and plated on GC agar to determine viable cells.
Aggregation assay
To quantify bacterial aggregation, we used a modification of an assay that was designed for N. meningitidis (Helaine et al., 2005). Bacteria grown for less than 10 h on GC agar were thoroughly resuspended in modified NDM or p.p.m., respectively, and adjusted to an OD600 of 0.1 in 10 ml. Bacteria were then grown in glass test tubes under constant agitation at 200 r.p.m. until OD600 of 0.9 was reached. Subsequently, the suspensions were incubated at room temperature without shaking and the OD600 of the suspensions close to the liquid air interface was measured at 30 min intervals.
Crystal violet assay
Biofilm growth in a static model was assessed in modified NDM in 24-well cell culture dishes on round 15 mm glass cover slips. One millilitre with 1.0 × 108 cells ml−1 was seeded per well to initiate biofilm growth. After 24 h, the biofilm was washed with 1 ml of distilled water per well. To stain the adherent bacteria, 250 μl of 0.05% Crystal Violet (CV) (Difco, Augsburg, Germany) were added for 10 min. After two washes with each 1 ml of distilled water, the CV stain of the biofilm was dissolved in 96% ethanol and quantified by measuring optical density at 570 nm.
Production of rabbit antibodies
For expression of a PilX fusion with glutathione S-transferase (GST), the GST Gene Fusion System (Amersham Biosciences Europe, Freiburg, Germany) was used. For this purpose, the pilX gene of strains MC58 and 2120 was amplified by PCR omitting the 5′-sequence encoding the first 62 residues of the PilX preprotein. For strain MC58, the primers ML20 and ML27 were used, for strain 2120 primers ML22 and ML27. Primers contained a restriction site for BamHI. PCR products were restricted with BamHI and cloned into the BamHI restricted plasmid pGEX-3X. The proteins were expressed in E. coli DH5α at 27°C by induction with 1 mM isopropyl-beta-d-thiogalactopyranoside (IPTG). Bacteria were lysed using the BugBusterTM Master Mix (Novagen, Darmstadt, Germany). The insoluble proteins were purified by separation using sodium dodecyl sulphate (SDS)-polyacrylamide gel electrophoresis, and excision of coomassie blue stained bands, which were consecutively elctroeluted using the BIOTRAP system (Schleicher und Schuell, Dassel, Germany). Rabbits were immunized by immunoGlobe Antikörpertechnik (Himmelstadt, Germany).
Statistical analysis
Substratum coverage was calculated by the COMSTAT computer program (Heydorn et al., 2000). Structural biofilm parameters were evaluated with Microsoft Excel. Statistical evaluation was performed using two-tailed paired Student's t-test. COMSTAT data of six images obtained from a single channel were combined to a single mean value, which served as input for the t-test.
Acknowledgements
This study was supported by the Deutsche Forschungsgemeinschaft by Grant VO718/3 to U.V. and Matthias Frosch, and by the graduate college 520 immune modulation (University of Würzburg; speaker, Thomas Hünig; project leader, U.V.). We thank the Boehringer Ingelheim Fond for travel support to M.L. We gratefully acknowledge Mumtaz Virji (Bristol, UK) and Mark Achtmann (Berlin, Germany) for kindly providing monoclonal antibodies, and Myron Christodoulides (Southhampton, UK) and Peter van der Ley (Bilthoven, the Netherlands) for supporting us with plasmid pEG2-Ery. We thank Gabi Heinze and Christine Meinhardt for expert technical assistance. Markus Reinhardt (Würzburg, Germany) is gratefully acknowledged for help with the analysis of pilX sequences. We thank Susse Kirkelund Hansen (Lyngby, Denmark) for most helpful discussions.