Partners in crime: phosphotransfer profiling identifies a multicomponent phosphorelay
Summary
The first multicomponent phosphorelay, regulating stalk biogenesis, has been identified in Caulobacter crescentus using a bioinformatic screen, targeted disruptions of each histidine kinase and response regulator, and a new technique called phosphotransfer profiling, in which a purified histidine kinase or histidine phosphotransferase is simultaneously assayed for the ability to phosphorylate each purified response regulator protein from one organism. This powerful combination of approaches will allow future researchers to map the interactions among all two-component signal transduction proteins in genetically tractable bacteria with sequenced genomes.
Two-component signal transduction systems are a major mechanism used by bacteria to sense and respond to external or internal conditions by changing, for example, metabolism, chemotactic behaviour, or gene expression (reviewed in Stock et al., 2000). The upstream component of such systems is the histidine kinase, which senses a signal and autophosphorylates on a conserved histidine residue. The phosphoryl group is then transferred to an aspartate residue in the receiver domain of the downstream component or response regulator. Phosphorylation of the receiver domain elicits cellular responses by one of several mechanisms, altering protein–protein interactions of the receiver domain itself or affecting the enzymatic activity or DNA-binding ability of a separate output domain within the response regulator.
It is relatively straightforward to identify genes encoding histidine kinases and response regulators because each group shares conserved sequence motifs necessary for catalysis as well as a similar overall fold. Furthermore, histidine kinases and response regulators that function together in vivo are often encoded adjacent to each other or within the same operon. Researchers have leveraged this information to identify and characterize signal transduction pathways governing a variety of cellular behaviours.
Despite these advantages, significant challenges remain in the study of two-component signal transduction. First, although many histidine kinases and response regulators are encoded as genome pairs, many other two-component genes are ‘orphans’, having no functional partner encoded nearby. Second, verifying that a particular histidine kinase is the bona fide partner of a particular response regulator is complicated by the fact that many two-component proteins have promiscuous activity in vitro, with individual kinases phosphorylating more than one regulator and individual response regulators accepting a phosphoryl group from more than one kinase. Third, some two-component signalling pathways (called phosphorelays) are expanded to include intermediate receiver domains and histidine phosphotransferase proteins, the latter of which have been difficult to identify using bioinformatic methods. Finally, as many signalling pathways (∼10–100) in each bacterium use these two conserved modules with apparently promiscuous in vitro activity, we must discern how signals are kept straight within the cell, so that specific stimuli elicit specific responses.
The report by Biondi et al., in this issue of Molecular Microbiology represents a comprehensive attack on all of these challenges and a significant advance in the study of two-component signal transduction. The authors characterize the first phosphorelay in the dimorphic alpha-proteobacterium Caulobacter crescentus, a pathway controlling the synthesis of a polar stalk at a specified time during the cell division cycle. The most notable aspect of this study, however, is its use of genome-wide genetic and biochemical analyses of two-component proteins, enabling the authors to identify unambiguously the components of a phosphorelay that interact in vivo.
In a recent report (Skerker et al., 2005), Laub and colleagues introduced a new technique called phosphotransfer profiling (Fig. 1), in which one purified histidine kinase is simultaneously assayed for its ability to phosphorylate each of the response regulators from the same organism. By profiling Escherichia coli histidine kinases with known functional partners, the authors showed that while each kinase phosphorylates multiple E. coli regulators at longer reaction times (1 h), each kinase specifically phosphorylates only its in vivo partner in very short reactions (10 s). They observed the same phenomenon for several Caulobacter histidine kinases when each was profiled against all of the Caulobacter response regulators.
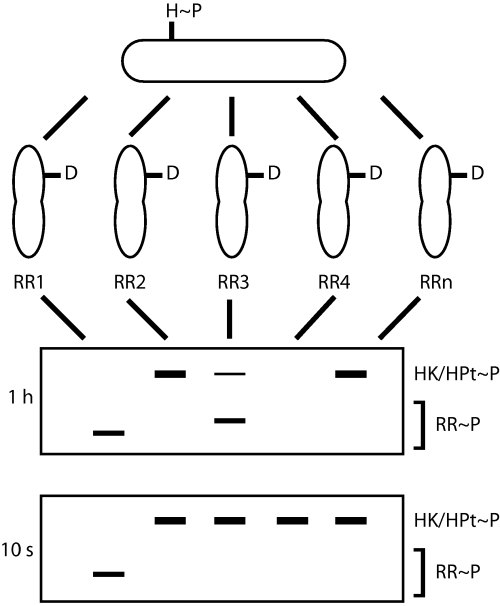
Phosphotransfer profiling. A purified histidine kinase is autophosphorylated, or a histidine phosphotransferase is phosphorylated by a hybrid kinase, using [γ-32P]-ATP. The reaction is then split and mixed with each purified response regulator encoded in the genome. Reactions are allowed to proceed for long (1 h) or short (10 s) times before analysis by SDS-PAGE and phosphorimaging. At longer reaction times, a histidine kinase or phosphotransferase may phosphorylate multiple response regulators, seen as the appearance of a radiolabelled response regulator band (RR1, RR3) and/or the disappearance of the radiolabelled HK/HPt band (RR4). At short reaction times, the kinase or phosphotransferase displays kinetic preference for its in vivo target, in this case RR1.
Elegant, detailed kinetic studies comparing the reactions between cognate kinase-regulator pairs and non-cognate (cross-talk) reactions have shown that these proteins can have strong kinetic preference for interacting with their relevant partners over other highly similar signalling proteins (Fisher et al., 1996; Grimshaw et al., 1998). Laub and colleagues have extended these observations showing that in vitro, each histidine kinase prefers its in vivo response regulator partner over every other response regulator from the same organism. This result implies that phosphotransfer profiling can be used to identify the likely interacting partners of orphan histidine kinases and response regulators. It also implies that in the cell, unwanted cross-talk between signalling pathways is prevented by the kinetic preference of individual signalling proteins for their relevant partners.
As a complementary approach to phosphotransfer profiling, Laub and colleagues disrupted each of the genes encoding two-component signalling proteins in Caulobacter and performed preliminary phenotypic characterization of these 106 strains. They noted that only two mutants specifically lacked the polar stalk and hypothesized that the two affected genes participate in a signal transduction pathway governing stalk biogenesis. In the current issue of Molecular Microbiology, Biondi et al. pursue this initial observation to establish the first multicomponent phosphorelay in Caulobacter.
The two stalkless mutants contained disruptions in CC0138, encoding a hybrid histidine kinase and CC3315, the DNA-binding response regulator TacA (Marques et al., 1997). Hybrid histidine kinases are so named because each protein possesses a receiver domain at its C-terminus, in addition to domains required for histidine phosphorylation. Hybrid kinases have been shown to participate in phosphorelays, in which the phosphoryl group is passed intramolecularly from the conserved histidine to the conserved aspartate in the receiver domain, thence to a histidine phosphotransferase protein or domain, and finally to the response regulator that generates a cellular output (Burbulys et al., 1991). Reasoning that CC0138 and TacA might be linked by an unknown histidine phosphotransferase protein, Biondi et al. used a novel computational strategy to identify putative histidine phosphotransferases encoded in the Caulobacter genome, using filters for gene size, alpha-helical content and a small conserved sequence motif including the phosphorylated histidine residue.
One candidate identified by this approach, CC1114, was purified and studied in detail by a modified version of phosphotransfer profiling. CC1114 was phosphorylated in vitro by CC0138 and then split into reactions with each purified response regulator from Caulobacter. Comparison of long and short reaction times revealed that CC1114 was most efficient at phosphorylating the response regulator TacA. In a similar experiment where CC1114 was phosphorylated and mixed with the purified receiver domain of each hybrid histidine kinase, CC1114 displayed preferential phosphotransfer to the receiver domain of CC0138. Together, these experiments suggested that CC1114 mediates phosphotransfer between CC0138 and TacA in vivo. Like the interaction between histidine kinases and response regulators, it has previously been observed in a limited number of cases that the histidine phosphotransferase protein of a phosphorelay is highly specific for the receiver domains within its pathway (Perraud et al., 1998). Here again, the phosphotransfer profiling technique strongly suggests that kinetic preference in the reactions between phosphorelay components is genome-wide and underlies the observed signalling specificity.
To verify the in vivo role of CC1114, the authors disrupted the gene and observed a stalkless phenotype reminiscent of strains lacking CC0138 or TacA. The stalkless phenotype of ΔCC114 could be complemented by the wild-type gene carried on a plasmid, but not by a gene carrying a histidine-to-alanine substitution at the predicted site of phosphorylation. Furthermore, the mutant tacA(D54E), which is predicted not to be dependent upon phosphorylation for activity (Klose et al., 1993), complemented the phenotypes of each deletion of a phosphorelay component, ΔCC0138, ΔCC1114 and ΔtacA itself, implying that the primary function of CC0138 and CC1114 in vivo is to generate phosphorylated TacA.
Phosphotransfer profiling has the potential to clarify many ambiguous signal transduction pathways, particularly in cases where genomic and phenotypic analyses fail to yield a single, likely interacting partner for a histidine kinase or response regulator. Over many years the Caulobacter research community has found that most two-component proteins involved in cell-cycle progression and cellular asymmetry are orphans and create pleiotropic effects when mutated, hindering efforts to delineate signal transduction pathways (reviewed in Skerker and Laub, 2004). Furthermore, when studied in biochemical detail, there are instances where more than one kinase may phosphorylate the same response regulator as well as instances where one kinase phosphorylates a regulator and another kinase predominantly dephosphorylates the same regulator. Phosphotransfer profiling will help not only to determine the functions of two-component proteins that have never been studied, but also to generate an interaction map of the known two-component proteins governing complex processes such as the Caulobacter developmental cycle.
There are many genetically tractable species in which it seems feasible to disrupt each of the two-component signalling genes and also to purify each of these proteins for phosphotransfer profiling experiments similar to those described by Laub and colleagues (Yamamoto et al., 2005). Using these tools, it is within our grasp to map the two-component signalling interactions that orchestrate growth, development, and the responses to stimuli for a host of bacterial species. In addition, as the capacity for signal insulation or signal cross-talk appears to reside mainly within the histidine kinases and response regulators themselves, it should also be possible to use these approaches in concert with bioinformatics to identify specific sequences that account for the genome-wide kinetic preference of histidine kinase-response regulator pairs.
The combined approaches of comprehensive gene disruption and comprehensive biochemical analysis, when applied to a functionally related group of proteins, can yield startling leaps in our understanding of both high-level biological problems (in this case signal insulation in two-component pathways) and specific biological processes (in this case Caulobacter stalk biogenesis). In this type of ‘medium-throughput’ analysis, not every gene is deleted and not every protein–protein interaction is detected, but comprehensive, genome-level conclusions can be drawn about a large and important class of molecules, and researchers can move quickly from broad, preliminary analyses of knockout libraries and biochemical profiles into detailed studies that deepen our understanding of a specific biological problem.