Genetic rescue of a Toxoplasma gondii conditional cell cycle mutant
Summary
Growth rate is a major pathogenesis factor in the parasite Toxoplasma gondii; however, how cell division is controlled in this protozoan is poorly understood. Herein, we show that centrosomal duplication is an indicator of S phase entry while centrosome migration marks mitotic entry. Using the pattern of centrosomal replication, we confirmed that mutant ts11C9 undergoes a bimodal cell cycle arrest that is characterized by two subpopulations containing either single or duplicated centrosomes which correlate with the bipartite genome distribution observed at the non-permissive temperature. Genetic rescue of ts11C9 was performed using a parental RH strain cDNA library, and the cDNA responsible for conferring temperature resistance (growth at 40°C) was recovered by recombination cloning. A single T. gondii gene encoding the protein homologue of XPMC2 was responsible for genetic rescue of the temperature-sensitive defect in ts11C9 parasites. This protein is a known suppressor of mitotic defects, and in tachyzoites, TgXPMC2–YFP localized to the parasite nucleus and nucleolus which is consistent with the expected subcellular localization of critical mitotic factors. Altogether, these results demonstrate that ts11C9 is a conditional mitotic mutant containing a single defect which influences two distinct control points in the T. gondii tachyzoite cell cycle.
Introduction
While healthy individuals usually control Toxoplasma infections, toxoplasmosis is a dangerous disease in patients undergoing cancer chemotherapy or organ transplant, and in people with AIDS (Dubey, 1986; Chan et al., 1995). The tissue damage that results from parasite growth during infection of immunocompromised individuals is cumulative and ultimately fatal if not prevented. Type I strains cause a disproportionate share of severe congenital (Fuentes et al., 2001) and ocular disease which may be related to their fast growing phenotype (Grigg and Boothroyd, 2001). Growth rate is a common virulence trait in protozoan pathogens (Diffley et al., 1987; Turner et al., 1996; Taylor et al., 2002) and evidence suggests that increased parasite burden, resulting from either a rapid growth rate and/or the inability to switch into the slow growing (or growth arrested) bradyzoite form, is a major contributor to Toxoplasma pathogenesis (>100-fold parasite burden in mice is seen with fast growing type I strains) (Sibley et al., 2002). Despite the importance of growth mechanisms in Toxoplasma-caused disease, we know very little about the regulation of the parasite cell cycle or how growth mechanisms are linked to parasite development. It has long been established that differentiation of tachyzoites-to-bradyzoites is accompanied by a change to a slower parasite growth rate (Soete et al., 1993; 1994; Bohne et al., 1994) which is programmed (Jerome et al., 1998; Radke et al., 2003) and obligatory to development as fast growing mutant strains do not spontaneously differentiate but will do so if their growth is slowed by drug or stress treatment (Soete et al., 1993; 1994; Bohne et al., 1994). These and other findings (Jerome et al., 1998) indicate that molecular switches link the growth rate and number of parasite divisions to the developmental fate of this organism, and therefore, studies of the parasite cell cycle are crucial to understand chronic as well as acute disease.
Apicomplexan parasites undergo three types of replication. The most common process is schizogony which is characterized by repeated rounds of DNA synthesis (S) and mitosis (M) [(S-M)n–C-G1] yielding a multinucleated cell. The budding of daughter parasites (cytokinesis = C) is delayed until the last round of mitosis (merozoites of Toxoplasma gondii replicate by schizogony) (Dubey and Carpenter, 1991). In endopolygeny [(S-M)n–nuclear division/C–G1] multiple rounds of DNA synthesis and chromosome segregation (S. Vaishnava and B. Striepen, unpublished results) occur without nuclear division thereby generating a single polyploid nucleus that subsequently undergoes concerted nuclear division and parasite budding (Canning and Sinden, 1973; Speer and Dubey, 1981). The third type of replication is represented by T. gondii endodyogeny [S-M–C-G1] which has been examined in detail by electron microscopy (Ogino and Yoneda, 1966; Sheffield and Melton, 1968) and recently, using fluorescent markers to allow the visualization of centrosome, daughter and nuclear division (Radke et al., 2001; Hu et al., 2002). Endodyogeny is a binary process with a single chromosome replication followed by concurrent mitosis and parasite budding. In cell cycle terms, an endodyogenic cycle is divided into three phases G1, S and mitotic/cytokinetic phases, while G2 phase is either short or non-existent (Radke et al., 2001). Mitosis is endomitotic (nuclear membrane remains intact) and daughter cell budding initiates late in S phase and is closely co-ordinated with and intimately linked to mitotic progression (Sheffield and Melton, 1968; Striepen et al., 2000; Hu et al., 2002).
In mammalian cells (Roscoe et al., 1973; Talavera and Basilico, 1977), bacteria (Roscoe et al., 1973), viruses (Fields and Joklik, 1969; Roscoe et al., 1973) and yeast (Hartwell et al., 1970; 1973; Hartwell, 1974), loss-of-function mutants have been utilized to explore the regulation of events within the eukaryotic cell cycle. In T. gondii, tachyzoite temperature-sensitive mutants have been generated using a chemical mutagenesis strategy (Pfefferkorn and Pferfferkorn, 1976; Radke et al., 2000). From 40 temperature-sensitive mutants recently isolated, eight (20%) were found to have a cell cycle defect (Radke et al., 2000). A limiting factor in the large-scale pursuit of T. gondii tachyzoite ts mutants was the absence of a complementation strategy. Recently, two strategies were developed for complementing mutants: (i) an episomal vector that contains large genomic fragments (Black and Boothroyd, 1998) and (ii) an approach that combines high frequency insertion of cDNAs (Donald and Roos, 1993) with the power of phage recombination (Striepen et al., 2002). In this work, we present the further characterization and genetic complementation of Toxoplasma mitotic mutant ts11C9 (Radke et al., 2000).
Results
Characterization of centrosome duplication in cell cycle mutant ts11C9 and synchronized RHTK+ parasites
Cell cycle control is not well understood in apicomplexan protozoa; however, parasites uniformly arrested in G1, at the G1/S boundary, or in mitosis have been demonstrated experimentally in T. gondii (Radke and White, 1998; Radke et al., 2000) suggesting that cell cycle mechanisms are co-ordinated in these parasites. In order to further characterize the timing of cell cycle events, we examined centrosomal duplication in synchronously growing tachyzoites (Radke and White, 1998). RHTK+ parasites treated with thymidine for 4 h are blocked in late G1/early S and are synchronized within a 2 h cell cycle window. Upon release from thymidine media they progress immediately through S phase, enter mitosis 3–4 h post-thymidine release and then complete daughter budding beginning at 5 h post release (Radke and White, 1998; Radke et al., 2001). Analysis of thymidine-blocked RHTK+ parasites using anti-centrin antibodies demonstrated that ≈75% of these parasites contained two centrosomes that were closely associated (Fig. 1A, blocked). These observations indicate that centrosome duplication occurs at the G1/S phase boundary. Centrosome migration in conjunction with mitosis was evident at 4 h post-thymidine release (Fig. 1A, line graph) which paralleled the rise in immature daughter forms (budding) in these populations (8% daughter forms in thymidine-block; 36% daughter forms at 4 h post release in these experiments) (Radke et al., 2001). In the 6 h post-release population (Fig. 1A, R6), completion of cytokinesis is reflected by the stepwise increase in vacuole size (not graphed) and reduction in centrosome number. Twenty per cent of vacuoles contain four parasites in blocked, 2 and 4 h populations (mean vacuole size 2.1 parasites) and this increased to 38% fours at 6 h post release. As expected, the emergence of new parasites is matched by a corresponding increase in parasites containing a single centrosome (Fig. 1A, R6).
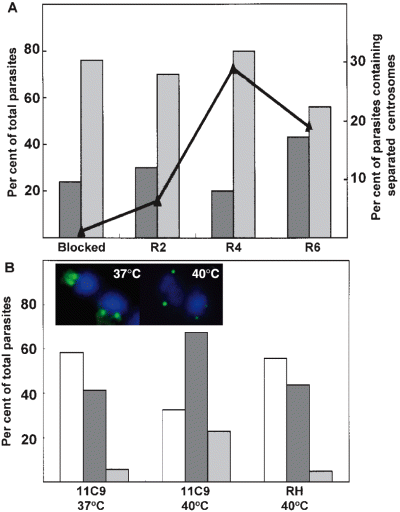
Centrosomal replication in synchronous and mutant tachyzoites. A. RHTK+ parasites were treated with 10 µM thymidine for 4 h (blocked) and then washed to release parasites to transit synchronously through at least one cell cycle. At 2, 4 and 6 h release from thymidine (R2, R4 and R6) parasites were co-stained with anti-centrin monoclonal antibody 26-14.1 to visualize centrosome and DAPI to stain nuclear DNA. The number of parasites containing a single or double centrosomes and the fraction of parasites whose centrosomes had clearly migrated apart were quantified in at least 100 vacuoles. Grey bars, parasites that contain a single centrosome; hatched bars, parasites with recently doubled adjacent centrosomes; line graph, parasites with two centrosomes that have migrated apart. B. Asynchronous RHΔhxgprt parental strain and mutant ts11C9 parasites in HFF cells were incubated at 37°C or 40°C for 24 h and then fixed as above and co-stained with monoclonal 26-14.1 and DAPI. A total of 100 vacuoles were selected at random and the fraction containing a single centrosome (open bars), double centrosome (hatched bars) and those parasites containing centrosomes which had migrated apart (grey bars) was determined. Average vacuole size in each of these populations: ts11C9 grown at 37°C ts11C9 = 7.9 parasites per vacuole or grown at 40°C = 1.7 parasite per vacuole and parental RHΔhxgprt grown at 40°C = 16 parasites per vacuole. Inset micrograph: representative merged fluorescence images (FITC anti-centrin and DAPI) demonstrating the centrosomal pattern (green) with respect to the nucleus (blue) in 37°C and 40°C cultured ts11C9 parasites. Note that centrosomal migration in ts11C9 parasites grown at 40°C has a greater range than parasites grown at 37°C.
Centrosomal duplication, migration and reduction offer a promising new temporal context for the analysis of the T. gondii cell cycle. Previous studies of T. gondii mutant ts11C9 provisionally assigned a mitotic defect to this parasite, and thus, we explored whether the changes in centrin fluorescence would support this conclusion. At the non-permissive temperature (40°C) ts11C9 tachyzoites growth arrest with a single nucleus containing either ≈1 N and ≈2 N genomic contents with parasites containing immature daughters constituting a subpopulation that was equivalent in size to the 2 N fraction (Radke et al., 2000). In asynchronous ts11C9 populations grown at permissive temperatures, the fraction of parasites with a single centrosome (58.6%; Fig. 1B, 11C9 37°C) was consistent with the estimated G1 fraction (based on DNA content) (Radke et al., 2000) while the fraction with duplicated centrosomes (41.4%) was a reasonable match to the combination of S phase plus mitosis (G2 is short or non-existent) (Radke et al., 2001). Upon shift of ts11C9 to the non-permissive temperature (24 h at 40°C), there was an increase in parasites containing duplicated centrosomes (65.3%; Fig. 1B, hatched bar, 11C9 40°C) which corresponded with a decrease in single centrosomes (open bar, 32.7%), and a dramatic rise in the fraction of parasites containing separated centrosomes (grey bars, 5% at 37°C versus 21% at 40°C) as well as a greater range of migration distance (see Fig. 1B, inset micrographs for representative examples). These results indicate that the spindle apparatus is intact and at least partially active in the 2 N growth-arrested subfraction. The centrosomal patterns in ts11C9 populations did not result from non-specific temperature effects as parental RHΔhxgprt parasites grown at 40°C displayed a normal distribution of single and double centrosomes (Fig. 1B). Centrin staining taken together with earlier evidence demonstrates that the ts11C9 2 N subfraction at 40°C is arrested at a point just before and/or in early mitosis. The ts11C9 subpopulation containing a haploid DNA content at high temperature appears to possess a single centrosome, and thus, these parasites are likely stopped at a G1 checkpoint which is different from thymidine-blocked RHTK+ parasites which are also haploid but have largely duplicated their centrosomes.
Genetic rescue of cell cycle mutant ts11C9
To characterize ts11C9 further, we attempted to genetically complement the defect in ts11C9 (Striepen et al., 2002) (see Fig. 2, flow diagram). Mutant parasites were transformed with an RH strain cDNA library and selected in 1 µM pyrimethamine at the non-permissive temperature (40°C). (Radke et al., 2000). Inserts were recovered from the primary temperature-resistant population by recombination cloning (BP-recombination) (Hartley et al., 2000; Striepen et al., 2002) and following a second recombination (LR-recombination) (Hartley et al., 2000) to shuttle the inserts back into a Toxoplasma expression context, the recovered cDNA inserts were transformed again into ts11C9 mutant parasites. The number of temperature-resistant parasites increased >200-fold after three rounds of enrichment (Fig. 2). Restriction digest of plasmids recovered from the tertiary round revealed a relatively few distinct cDNA inserts (Fig. 2A). Single-pass sequencing demonstrated that the 2300 bp inserts were identical. To determine whether any of these inserts would be further enriched, a fourth transformation of ts11C9 was performed using the 3°-LR library. To reduce the number of multiple integrations the amount of library DNA was lowered and the restriction enzyme was omitted from the transfection. Temperature-resistant parasites were again selected, the cDNA inserts recovered by recombination and the colonies analysed by polymerase chain reaction (PCR). Figure 2B shows that the 2300 bp cDNA insert was further enriched and retransformation of ts11C9 parasites with the plasmid containing the this cDNA insert confirmed that it was responsible for conferring temperature resistance (Fig. 2C).
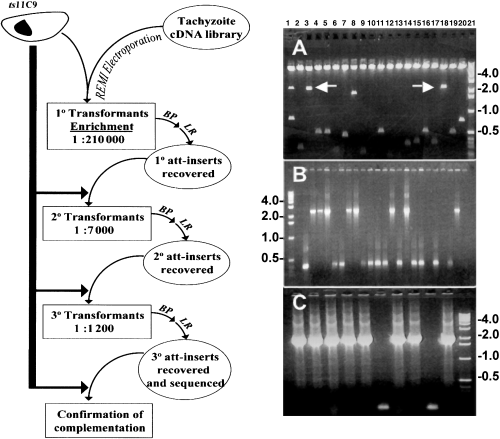
Genetic rescue of cell cycle mutant ts11C9. The flow diagram of the sequential transformations and cDNA insert recovery represents the number of temperature-resistant parasites (at 40°C) as a function of the total viable parasites plated (34°C) as measured by plaque assay in HFF monolayers. One in 210 000 viable parasites from this primary screen were temperature resistant after transformation with the cDNA library (spontaneous revertant frequency <1 in 106). A second and third round of enrichment resulted in 1:7000 and 1:1200 viable parasites respectively. BP = recombinational cloning of cDNA inserts from parasite genomic DNA. LR = second recombinational cloning to move recovered cDNA inserts into T. gondii expression vectors. A. SfiI digest of 3°-LR library plasmids revealed three large inserts of 2700 bp (combined fragments in lane 1); 2300 bp (lanes 3 and 18); 1900 bp (lane 8) and several inserts of <500 bp. B. PCR of bacterial colonies (4°-BP) recovered from ts11C9 parasites complemented with 5 µg of the 3°-LR library plasmid DNA demonstrated that 8 of 20 colonies contained the 2300 bp insert. C. Complementation of ts11C9 with the plasmid containing the 2300 bp cDNA insert resulted in the re-recovery of this insert in 8 of 10 BP plasmids.
Molecular standards in kb are indicated to the left and right of the panels.
The 2300 bp cDNA insert was sequenced completely and a blast analysis identified the cDNA insert as encoding a protein with high similarity to XPMC2 from Xenopus laevis (Su and Maller, 1994; 1995). The T. gondii XPMC2 (TgXPMC2) cDNA encodes a protein of 361 amino acids with a predicted molecular weight of ≈40 kDa and an isoelectric point of 9.59 (accession number AY841997). The N-terminal half of the protein encodes a putative exonuclease domain (residues 60–219) conserved in all XPMC2-related genes (Moser et al., 1997) (Fig. 3). In order to determine whether the TgXPMC2 allele in ts11C9 was mutated, reverse transcription polymerase chain reaction (RT-PCR) was used to clone the coding region from this mutant and three independent cDNA clones completely sequenced. The nucleotide sequence of TgXPMC2 from ts11C9 was identical to that obtained by genetic complementation from wild type (RH strain cDNA library). We did not examine sequences outside the coding region, however, northern analysis of TgXPMC2 expression in ts11C9 showed that the mRNA level was unaffected by growth temperature (data not shown) indicating that the regulation of TgXPMC2 mRNA was not defective in ts11C9 parasites. These results indicate that TgXPMC2 is not mutated, but acts as a suppressor of the cell cycle defect in ts11C9.
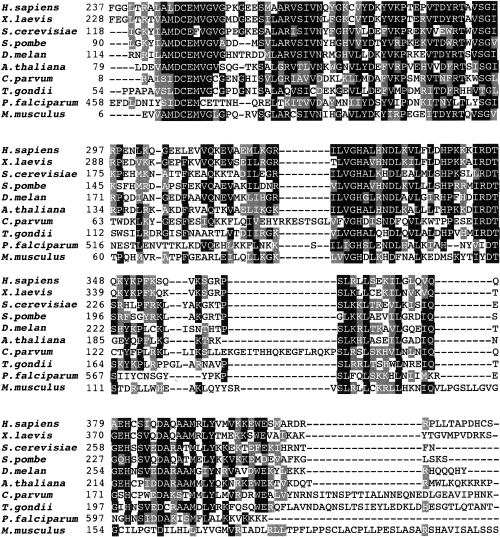
Amino acid alignment of XPMC2 exonuclease domains. Amino acid alignments of the conserved exonuclease domain (using human XPMC2 to root the comparison) from the eukaryotic XPCM2 homologues. Black boxes indicate amino acid identity while grey boxes indicate significant conservation. Species represented are: H. sapiens; Xenopus laevis; Saccharomyces cerevisiae; Schizosaccharomyces pombe; D. melan; A. thaliana; C. parvum; Toxoplasma gondii; Plasmodium falciparum; M. musculus.
Cell cycle analysis of TgXPMC2 transformants
A series of experiments were performed to compare the replication of ts11C9 to a temperature-resistant clone genetically rescued with TgXPMC2 (TgXPMC2-tr11C9). Growth was measured at 35°C, 37°C and 40°C by calculating the average vacuole size 36 h post inoculation. DNA content of asynchronous growing parasites was analysed by flow cytometry as previously described (Radke and White, 1999; Radke et al., 2001). Based on average vacuole size, there was no difference between the growth rate of RHΔhxgprt parental controls and TgXPMC2-tr11C9 parasites at any of the temperatures evaluated (Fig. 4A). The DNA content of asynchronously growing TgXPMC2-tr11C9 parasites was also unaffected by temperature (Fig. 4B and C, filled histogram) and was indistinguishable from RHΔhxgprt parasite profiles (data not shown) (Radke et al., 2000; 2001) whereas ts11C9 DNA profiles (bold trace overlay) displayed the characteristic bimodal 1 N and 2 N distributions when shifted to 40°C (Fig. 4C). The 2 N assignment in FACS plots of ts11C9 populations growth-arrested at 40°C (second PI peak) is validated here by the shift in the DNA content of growing TgXPMC2-tr11C9 parasites (see second peak of solid histogram in Fig. 4C) to the unusual near-diploid genome (≈1.8 N) content previously described in asynchronous tachyzoite populations (Radke and White, 1998).
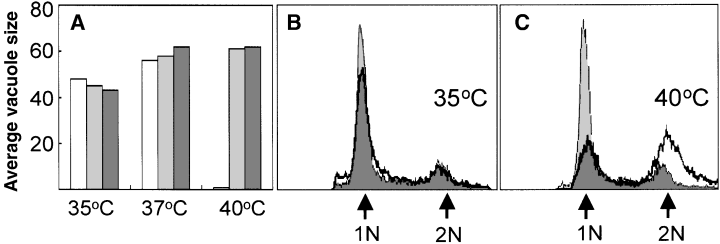
Phenotypic rescue of ts11C9 by TgXPMC2. A. The average vacuole size attained after 36 h of growth in HFF cells at three different temperatures (35°C, 37°C and 40°C) was determined for mutant ts11C9 parasites (open bars), parental RHΔhxgprt (hatched bars) and TgXPMC2-tr11C9 (solid bars) parasites. Note average vacuole size indicated applies to (A) only. B and C. The DNA content (propidium iodide-stained parasites analysed by FACS) of asynchronously growing TgXPMC2-tr11C9 parasites (filled histogram) was compared with ts11C9 (bold trace overlay) at 35°C (B) and 40°C (C). Note that the histogram scale used in (B) and (C) are identical and the genomic equivalents in each graph are indicated by arrows.
In order to visualize organellar division, parental ts11C9 parasites were transformed with a plasmid encoding the apicoplast marker ACP–GFP fusion protein (Waller et al., 1998). The TgXPMC2 cDNA was introduced in a subsequent transfection. ACP–GFP transgenic parasites that were temperature sensitive or temperature resistant by virtue of TgXPMC2 expression were compared. Similar to centrosomes in these parasites (Fig. 1B), ACP–GFP-labelled plastids undergo replication and segregation in a segment of the 40°C-arrested ts11C9 parasites (Fig. 5), although the plastids are significantly larger and more elongated than in parental parasites and in some plastids fission seems to have occurred. In contrast, plastids in TgXPMC2-complemented parasites are normal sized and divide just before or simultaneously with nuclear division (Fig. 5, bottom) as previously described for RH parasites (Striepen et al., 2000). The timing of daughter budding and maturation was also restored to the parental phenotype in ts11C9 parasites complemented with TgXPMC2 (data not shown). Thus, TgXPMC2 appears capable of fully rescuing the cell cycle defect in ts11C9 parasites.
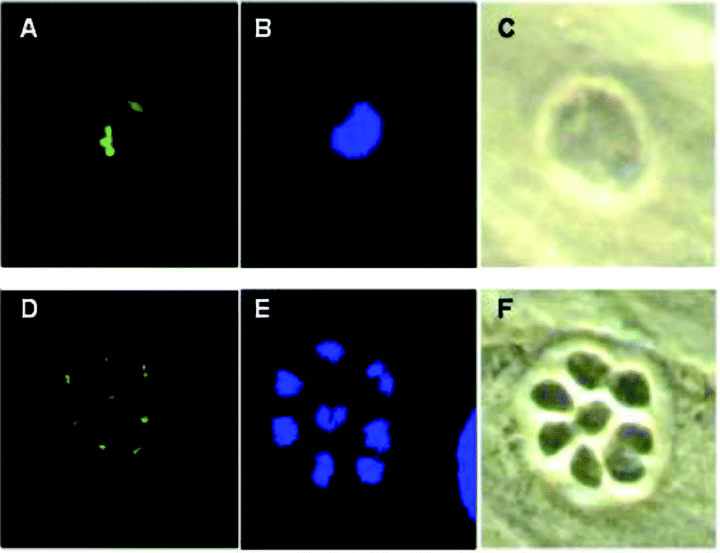
Plastid replication in mutant ts11C9 expressing ACP–GFP. The plastid marker ACP–GFP was introduced into ts11C9 followed by a second transformation with a plasmid carrying TgXPMC2. A–C. Representative fluorescent (A and B) and phase (C) micrographs of a clone of ts11C9 transgenic for ACP–GFP that was grown for 24 h at 40°C in HFF cells and then fixed and stained with rabbit polyclonal anti-GFP (A) and DAPI (B). D–F. Fluorescent (D and E) and phase (F) micrographs of a ts11C9 clone double transgenic for ACP–GFP and TgXPMC2 and now temperature resistant. HFF monolayers infected with this transgenic line for 24 h at 40°C and then stained for GFP and DAPI show normal nuclear and plastid division.
To localize TgXPMC2 in RH strain tachyzoites, the coding region was PCR amplified and cloned as an N-terminal fusion with either YFP or a 5X repeat of the myc epitope tag (Evan et al., 1985; Longtine et al., 1998) using in vitro recombination (YFPdest/sagCAT destination vector; Gubbels et al., 2004; [myc]5dest/HXGPRT destination vector; M.W. White, M. Behnke and M. Guerini, unpubl.). Plasmids containing epitope tagged or TgXMPC2 fusion with YFP were introduced into parasites by electroporation and stable transformants selected in chloramphenicol (for TgXPMC2–YFP) (Striepen et al., 1998) or mycophenolic acid (TgXPMC2-myc5) (Donald et al., 1996). In either context (YFP or myc5), TgXPMC2 fusion proteins were equally capable of complementing ts11C9 (data not shown) and transgenic parasites displayed an indistinguishable labelling for either fusion protein. Figure 6 shows representative images of transgenic parasites expressing the TgXPMC2–YFP fusion protein. The parasites in these images possess a single centrosome and plastid indicating they are in the G1 phase of the tachyzoite cell cycle. TgXPMC2–YFP is concentrated in a subcellular structure that is distinct from the centrosome (Fig. 6C and D) or plastid (elongated blue staining in Fig. 6D) and is exclusively contained within the parasite nucleus (Fig. 6D). Colocalization of TgXPMC2–YFP with fibrillarin (Yang et al., 2001) identified this structure as the parasite nucleolus (Fig. 7) which is a known repository for cell cycle factors (Visintin et al., 1999; Cerutti and Simanis, 2000).
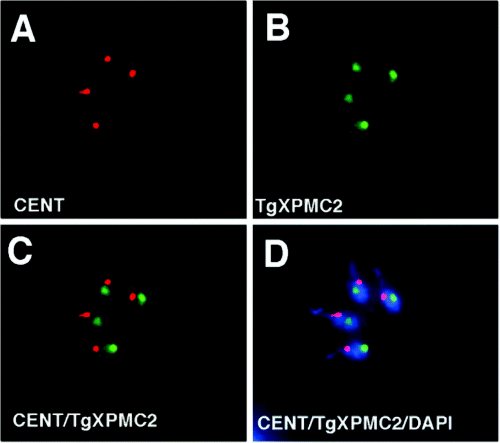
Localization of TgXPMC2–YFP in asynchronously growing tachyzoites. In order to localize TgXPMC2 in T. gondii a fusion of TgXPMC2 and YFP was constructed and transformed into parental RH strain parasites. The resulting fusion protein was fully capable of rescuing the temperature defect of ts11C9 (not shown). (A) Staining of TgXPMC2–YFP transgenic parasites with anti-centrin antibody. (B) Localization of TgXPMC2–YFP with anti-GFP antibodies demonstrated that the fusion protein was concentrated in a body that is distinct from the centrosome (C; merge of anti-centrin and anti-GFP staining) and contained within the parasite nucleus as revealed by colocalization of anti-GFP and DAPI staining (D, merge of anti-centrin, anti-GFP, and DAPI staining). Note that genomic and plastid (elongated body adjacent to larger DAPI concentration) DNA are stained in these images.
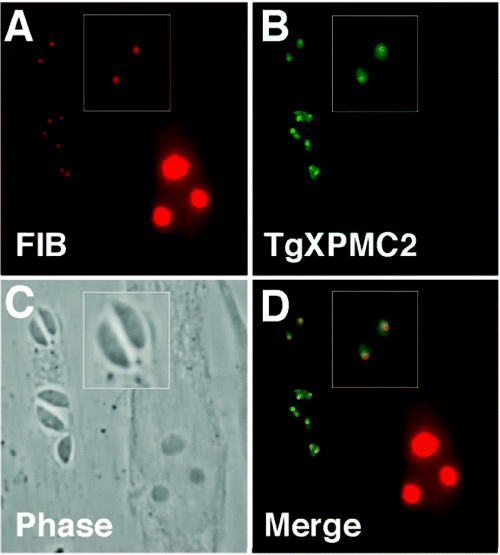
TgXPMC2–YFP is concentrated in the tachyzoite nucleolus. TgXPMC2–YFP transgenic parasites were co-stained with (A) anti-fibrillarin antibodies (FIB, nucleolus-specific protein) and (B) anti-GFP antibodies to establish the identity of the subnuclear body containing concentrated fusion protein. The co-localization of both proteins in (D) demonstrates that fibrillarin and TgXPMC2–YFP reside in the same structure within the parasite nucleus (see magnified image in A, B, which is merged in D). The two parasite vacuoles (containing single or double parasites) on the bottom of these images shows that nucleoli containing TgXPMC2–YFP and fibrillarin have duplicated while each parasite in the top vacuole possess a single nucleolus. Note that the large host cell nucleus (C) to the right of all three parasite vacuoles contains three distinct nucleoli that are stained with the anti-fibrillarin antibody (D).
Discussion
Toxoplasma gondii tachyzoites sharing the same parasitophorous vacuole divide synchronously and frequently remain connected posteriorly through several divisions (rosette). Notwithstanding the intimacy of these connections, sharing cytoplasm is not needed for synchrony and occupation of the same vacuole appears sufficient for synchrony as evident when observing transgenic parasites in real time (Radke et al., 2001). These observations suggest a hierarchical set of cell cycle controls are present that ensure the co-ordination of events inside parasites and also between parasites, although the molecular features of this hierarchy are undefined. Cell cycle checkpoints have been described in a wide variety of eukaryotic cell types (both unicellular and multicellular organisms) (Hartwell, 1991) and encompass a series of cyclin/cyclin-dependent kinase interactions that precisely regulate the staging of cell cycle events. There are checkpoints that control S phase entry (START), DNA replication and the timing of mitosis with respect to DNA synthesis, chromosome segregation and cytokinesis (Hartwell, 1991). Given the conservation of these mechanisms in eukaryotes, it is not surprising that T. gondii (Kvaal et al., 2002), as well as other Apicomplexans, possess and express cyclins and cyclin-dependent kinases (Le Roch et al., 2000; Waters et al., 2000; Merckx et al., 2003). Supporting the view that cyclins are essential to all eukaryotes, we have found that T. gondii cyclin 1 (TgCYC1) can functionally substitute for cyclin, CLN2, in yeast strain Dl-1 (Kvaal et al., 2002). While it is clear that cyclin mechanisms are present in T. gondii, the specific operation and number of cyclin checkpoints have yet to be resolved. Previous studies, which are supported by results presented here, document the cell cycle control of DNA replication in RHTK+-tachyzoites exposed to exogenous thymidine (Radke and White, 1998). Allosteric regulation of ribonucleotide reductase by high levels of dTTP (Eriksson et al., 1982) causes selective dNTP pool depletion (Terasima and Tolmach, 1963) which is a well-established trigger of the DNA replication checkpoint (Hung et al., 1996). A similar mechanism is probably active in T. gondii as thymidine-block of RHTK+-tachyzoites results in a reversible arrest of parasites at the G1/S boundary (Fig. 1) (Radke and White, 1998; Radke et al., 2001). The ability to synchronize the tachyzoite cell cycle by thymidine inhibition of DNA synthesis stands in contrast to the drug aphidicolin which in animal cells is also a tool for growth synchronization (Fox et al., 1987; Hung et al., 1996) but in tachyzoites disrupts the cell cycle (Shaw et al., 2001). This discrepancy is probably explained by differences in the mode of aphidicolin action. In animal cells aphidicolin reversibly inhibits DNA polymerase (Fox et al., 1987), while the drug is cytotoxic in T. gondii (Shaw et al., 2001) and does not inhibit DNA polymerase activity in parasite lysates (Makioka et al., 1993). It is of interest to note that preliminary studies from our laboratory indicate that tachyzoites exposed to a variety of DNA damage agents do not slow or alter their cell cycle (M. Behnke and M.W. White, unpublished results). Thus, the apparent absence of a DNA damage checkpoint in these parasites may be related to the loss of cell cycle control under aphidicolin treatment.
Mitotic checkpoints ensure co-ordination of DNA replication with respect to balanced segregation of chromosomes into viable daughter cells. When mitotic checkpoints are disrupted, loss of control over chromosome copy number is a principle consequence. The distinctive phenotype of ts11C9 parasites demonstrates that mitotic checkpoints are active in Toxoplasma as arrest of this mutant at high temperature results in a rapid (within one cell cycle) and co-ordinate cessation of mitosis and daughter budding (cytokinesis). Inhibition of mitosis in ts11C9 parasites does not lead to polyploidy (>2 N), the formation of multiple daughters (Radke et al., 2000) or nuclear missegregation demonstrating there are mechanisms in T. gondii that regulate chromosome copy number with respect to the accurate completion of mitosis and cytokinesis. Unlike oryzalin-treated tachyzoites (Morrissette and Sibley, 2002) where disruption of microtubule formation appears to uncouple budding and nuclear division, the cytoskeleton of temperature-shifted ts11C9 parasites is intact and plastid division and centrosome duplication and the migration of these organelles with respect to the nucleus remains active (Fig. 1). While it was not demonstrated that control over chromosome copy number is lost in oryzalin-treated parasites, the observed disruption of cytokinesis with respect to nuclear division caused by this drug suggests that some mitotic controls in Toxoplasma must require the parasite cytoskeleton to function.
The cell cycle arrest of mutant parasite ts11C9 is characterized by a bipartite distribution into subpopulations containing either 1 N or 2 N DNA contents (Fig. 4) (Radke et al., 2000). There is no evidence of active S phase in these populations by either flow cytometry (Radke et al., 2000) or analysis of the formation of TgPCNA1 replication foci (Radke et al., 2001; M.E. Jerome and M.W. White, unpubl.), which is consistent with the rapid growth arrest at the non-permissive temperature. These results suggest that the defect in mutant ts11C9 effects two different control points in the tachyzoite cell cycle. The list of proteins that could be responsible for this phenotype is not exhaustive, and could be limited to a factor involved in the Toxoplasma mitotic checkpoint. Yeast strains deficient in the cyclinB/cdk pathway have also been shown to arrest in G1 and mitosis (van der Velden and Lohka, 1993; Clute and Pines, 1999; D’Angiolella et al., 2001; O’Farrell, 2001) which reflects the dual action of this cyclin class on START and mitotic checkpoints (Hartwell, 1991; O’Farrell, 2001). Rescue of the defect in ts11C9 with a homologue of eukaryotic XPCM2 is also consistent with a defect involving the mitotic cyclin pathway. X. laevis XPMC2 was previously shown to act as a suppressor of a mitotic catastrophe event by complementing both the wee1-50/mik1 and wee1-50/cdc2-3w yeast mutants (Su and Maller, 1994; 1995). Thus, pathways analogous to the yeast cyclinB/cdk are probably active in tachyzoites. Moreover, pathways involving TgXPMC2 are probably conserved throughout the Apicomplexa as homologues of this gene are found in all of the members of the phylum for which genome sequence is available (Fig. 3 and data not shown).
It is not generally understood how XPMC2 acts to suppress mitotic catastrophe (Su and Maller, 1995). XPMC2 may divert inappropriate cdc2 kinase activity by serving as an alternative phosphorylation substrate, although proteins with similar activity do not complement mitotic mutants (Su and Maller, 1995). Colocalization of TgXPMC2–YFP and fibrillarin (Yang et al., 2001) in transgenic parasites (Fig. 7) clearly demonstrates that TgXPMC2 is concentrated in the nucleolus throughout the cell cycle and maintains that sequestration during duplication of this nuclear structure (Fig. 7). The nucleolar localization of TgXPMC2 may be relevant to the ability of TgXMPC2-tr11C9 transgenic parasites to complete mitosis as the factors known to regulate the end of mitosis including factors such as cdc14, which critically activate the anaphase-promoting complex (APC) to degrade mitotic cyclins, reside in the nucleolus (Visintin et al., 1999; Cerutti and Simanis, 2000). Surveys of the Toxoplasma genome provide evidence for homologues of APC components and cdc14 and their presence suggests that there is probably a form of APC checkpoint in this parasite. The relationship between cyclins and the nucleolus is also well documented. Mitotic cyclins are known to concentrate on the nucleolus during specific stages of the cell cycle (e.g. Ookata et al., 1992; Juan and Cordon-Cardo, 2001; Barbier et al., 2003) and are responsible for the regulation of important nucleolar functions (reviewed in Hernandez-Verdun and Roussel, 2003). Further studies of TgXMPC2 and its potential interaction with Toxoplasma cyclins should shed light on which of these mechanisms may be active in this parasite.
Forward genetics is a classical strategy to identifying genes associated with a variety of biochemical events, which until recently was not available for the study of essential genes in T. gondii. We have combined features of phage recombination (Hartley et al., 2000) with the high frequency of integration in the Toxoplasma genome (Donald and Roos, 1993) in order to develop a robust method to complement T. gondii tachyzoite mutants (Striepen et al., 2002). This method was used to isolate potential drug targets in heterologous apicomplexan models (Striepen et al., 2002) and recently to isolate genes based on their development-specific expression or subcellular localization in Toxoplasma (Gubbels et al., 2004; Radke et al., 2004). The results here demonstrate an exciting and much needed advance in the studies of Toxoplasma. Given that genetic deletion or disruption of essential genes is not possible in haploid cells, this new application offers an avenue of access to the study of essential genes by chemical mutagenesis. Thus, the door is now open to generating and complementing conditional T. gondii mutants defective in critical functions involved in cell cycle, parasite invasion and egress, and parasite development.
Experimental procedures
Cell culture and parasite growth
The RHΔhxgprt strain used in these studies contains a deleted hypoxanthine-guanine-phosphoribosyl-transferase (HXGPRT) gene which allows for the selection of transfected tachyzoites using mycophenolic acid (Donald et al., 1996; Donald and Roos, 1998). The temperature-sensitive mutant ts11C9 was produced by chemical mutagenesis of the RHΔhxgprt tachyzoite strain (Radke et al., 2000) and is maintained by serial passage in human foreskin fibroblasts (HFF) at 35°C. Complete growth arrest of this mutant occurs within one to two divisions at 40°C with reversion to a temperature-resistant phenotype occurring at a frequency of <1 in 106 parasites (Radke et al., 2000). RHTK+ is a transgenic isolate (from RH parental strain) expressing active thymidine kinase (TK+) from a fusion of the chloramphenicol acetyltransferase and herpes simplex virus thymidine kinase coding sequences (Radke and White, 1998). Tachyzoite strains were maintained and purified according to standard protocols (Roos et al., 1994). HFF cells were cultured in Dulbecco's modified Eagle medium (DMEM, Gibco BRL, Grand Island, NY) supplemented with 10% (v/v) fetal bovine serum (Atlanta Biologicals, Atlanta, GA).
Immunofluorescence and microscopy
Centrin staining was evaluated in RHTK+ parasites synchronized by a 4 h thymidine treatment (1-[2-deoxy-β- d-ribofuranosyl]-5-methyluracil, Sigma, St Louis, MO) and released to grow for 2, 4, 6 h (Radke and White, 1998) and in ts11C9 and RHΔhxgprt parasites grown for 24 h at 37°C or 40°C. Parasites were fixed for 20 min using warmed (37°C) 3% paraformaldehyde and then permeabilized for 20 min in 0.25% Triton X-100 in 1× PBS at room temperature. Parasites were incubated with anti-centrin monoclonal antibody 26-14.1 (provided by Dr Salisbury, Mayo Clinic, Rochester, MN) (Striepen et al., 2000) diluted 1:2000 in 1× PBS containing 3% bovine serum albumin for 1 h in a humid chamber and the slides were washed three times in 1× PBS. The slides were then treated with secondary antibody (FITC conjugated, goat anti-mouse IgG diluted 1:100, Sigma-Aldrich, St. Louis, MO) for 1 h in the dark, washed three times in 1× PBS, and the slides were mounted using Gel-mount aqueous mounting media. Simultaneous with secondary staining, parasite nuclei were stained using 4′,6′-diamidino-2-phenylindole (DAPI, 0.16 g l−1).
ACP–GFP transgenic parasites were evaluated in eight-well chamber slides and inoculated with 2.5 × 104 parasites per well. Parasites were allowed to invade for 1 h at 35°C then shifted to 40°C. After 24 h, slides were fixed for 10 min in 3.7% formyl saline (pH 7.4) and permeabilized for 20 min in 0.25% Triton X-100. Monolayers were incubated for 30 min with rabbit anti-GFP antibody (1:150) (Chemicon, Temecula, CA) and DAPI (0.16 g l−1). After treatment with primary antibody, monolayers were washed three times with PBS and then incubated with goat anti-rabbit secondary at 1:250 (Jackson Immunoresearch, West Grove, PA) for 30 min and evaluated as described above.
Transgenic parasites expressing epitope-tagged TgXPMC2 were processed as described for centrin and reacted with anti-GFP 1:500 (rabbit polyclonal; Clontech, Palo Alto, CA), anti-myc 1:200 (mouse monoclonal, University of Pennsylvania Cell Facility) and anti-fibrillarin monoclonal antibody 17C12 diluted 1:1500 (Yang et al., 2001). Appropriate Alexa 488 (green) or Alexa 546 (red) conjugated goat anti-rabbit or goat anti-mouse secondary antibodies (Molecular Probes, Eugene, OR) were used. Slides were evaluated with an epifluorescence microscope (Eclipse TE300, Nikon, Melville, NY), and images were collected with a digital camera (SPOTTM, Dynamic Instruments, Sterling Heights, MI) or using a DMIRB inverted microscope (Leica Wetzlar, Germany) equipped with a 100 watt HBO lamp. Image processing and analysis was performed using Openlab 3.0.3 software (Improvision, Quincy, MA).
Complementation of cell cycle mutant ts11C9
Tachyzoite mutant ts11C9 was transformed with a T. gondii RH strain cDNA library where each cDNA insert is flanked by lambda recombination sites (att-sites) (Striepen et al., 2002). A total of 80 million tachyzoites (20 million parasites per cuvette per 50 µg of library DNA) were transfected using a modification of the restriction enzyme-mediated integration protocol as previously described (Black and Boothroyd, 1998). Briefly, library plasmid DNA was linearized with AscI (50 U per 50 µg for 16 h at 37°C), and after ethanol precipitation, air-drying and resuspension in cytomix (Roos et al., 1994), the DNA was added to 20 million ts11C9 parasites in a cytomix that contained 50 U of NotI (Striepen et al., 2002). After electroporation, the parasites were cultured without selection in 1% DMEM parasite medium (Roos et al., 1994) for 3 h at 35°C to allow invasion to occur and the cultures were shifted to 40°C and 1 µM pyrimethamine was added to the media to select for transgenic, temperature-resistant parasites.
The vector pDHFR*-Tsc3ABP-P30 (Striepen et al., 2002) was modified to construct a new destination vector (Hartley et al., 2000) by replacing the P30 coding region with an EcoRV restriction site into which the ccdB recombination cassette was inserted (reading frame A; Gateway Vector Conversion System, Invitrogen, Carlsbad, CA). Bacterial strain DB3.1 (Invitrogen) was used to propagate the vector under chloramphenicol (30 µg ml−1) selection. The resulting vector designated Tg-pgraDEST/DHFR+ is capable of LR recombination (Hartley et al., 2000) and is used to re-express recovered cDNA inserts from parasites selected under 1 µM pyrimethamine and high temperature (40°C).
Recovery of cDNA inserts from the T. gondii genome by recombination followed published protocols (Hartley et al., 2000; Striepen et al., 2002). Genomic DNA was purified from primary temperature-resistant parasites (polyclonal) and used directly to recover en mass all cDNA inserts in the pDONOR201 vector (Invitrogen) by BP in vitro recombination (designated 1°-BP library) (Hartley et al., 2000; Striepen et al., 2002). Plasmids were transformed into bacteria and the resulting colonies scraped, collected by centrifugation, and plasmids purified using standard methods. Inserts in the 1°-BP library were then shuttled into the Tg-pgraDEST/DHFR+ destination vector via a second recombination (LR) and the 1°-LR library plasmids recovered and purified as above. The 1°-LR plasmid library was transfected into ts11C9 as above and the parasites again double selected at 40°C and in 1 µM pyrimethamine which was added 24 h post electroporation. Genomic DNA from the secondary temperature-resistant parasites was then used to construct consecutive 2°-BP and -LR libraries and the complete process was then repeated once more in order to generate 3°-transformants before cDNA insert sequencing. At each round of electroporation, parasite viability and the rate of stable transformation was determined by plaquing parasites with or without selection. Enrichment of cDNA inserts was evaluated by colony PCR or Sfi-1 restriction enzyme digestion as described (Striepen et al., 2002). Vector primers proximal to the attL sites of pDONOR201 (sense 5′-TCGCGTT-AACGCTAGCA TGGATCTC-3′ and antisense 5′-GTAACATCAGAGATTTTG AGA-CAC-3′) were used for colony PCR.
Single-pass sequencing and blastx of selected cDNA inserts was performed to ascertain the identity of potential gene complements. The full coding region was determined for one cDNA insert, TgXPMC2, and primers were then designed to clone the allele from parental ts11C9 mRNA (sense 5′-TTATCCGACG-TTAGTACGGGGC-3′ and antisense 5′-CCGCTGTGGAAGCAA-CGATCG-3′) by RT-PCR using PFU polymerase (Stratagene, La Jolla, CA) to reduce PCR-induced base changes. The coding region of ts11C9 TgXPMC2 was completely sequenced from three independent isolates.
Acknowledgements
This work was supported in part by grants from USDA CSREES NRI and the NIH to M.W.W. and B.S. We thank Dr Salisburg and David Roos for kindly providing antibodies used in these studies. This manuscript is a contribution from the Montana State University Agricultural Experiment Station.




