TUP1 disruption reveals biological differences between MATa and MATα strains of Cryptococcus neoformans
Summary
Cryptococcus neoformans exists in two mating types MATa and MATα. Although the morphology, growth characteristics and genetic segregation patterns among MATa and MATα strains are indistinguishable in the laboratory, the predominance of MATα strains in nature suggests that MATα strains are better suited for survival in nature. We disrupted the TUP1 gene, a global repressor, to find the possible biological differences in congenic MATα and MATa cells of C. neoformans. Disruption of TUP1 affected neither the yeast nor the hyphal cell morphology but resulted in a similar reduction of mating frequencies in both MATα and MATa cells. Disruption of TUP1, however, functionally manifested itself in several mating type-dependent phenotypes: (i) MATα cells became more sensitive to 0.8 M KCl while MATa cells showed no change in sensitivity, (ii) a temperature-dependent growth reduction was exhibited at both 30°C and 25°C in MATa but a similar growth reduction was not observed in MATα cells until the temperature was lowered to 25°C and (iii) the transcriptional level of genes in several different biological pathways was markedly altered in a mating type-dependent manner. This work is the first case in which non-mating-related biological differences are observed between two congenic mating partners in yeast.
Introduction
Yeasts are unicellular fungi that reproduce asexually and/or sexually. Sexual reproduction is initiated by fusion of mating partners that are morphologically and genetically indistinguishable except for the mating type locus and hence are commonly designated as MATα and MATa rather than male and female.
Cryptococcus neoformans, the aetiologic agent of cryptococcal meningoencephalitis, is a heterothallic yeast in which mating is controlled by one sex locus harbouring one of the two mating type alleles, MATα or MATa (Kwon-Chung, 1976). Although MATα and MATa cells are morphologically indistinguishable, have similar growth characteristics under laboratory conditions and mate readily to produce equal ratios of MATa and MATα progeny (Kwon-Chung and Hill, 1981), MATα strains predominate in nature (Kwon-Chung and Bennett, 1978). Because C. neoformans is an environmental pathogen and MATα strains are exceedingly prevalent in nature, cryptococcosis is mostly caused by the MATα strains worldwide (Kwon-Chung and Bennett, 1992). MATα strains of serotype D have also been shown to be more virulent in mice than the congenic MATa strains (Kwon-Chung et al., 1992). The mating type locus of C. neoformans is exceptionally large (>100 kb) relative to other fungi and contains mating type-specific pheromone response MAP kinase cascade genes, several novel genes, and many genes that are functionally unrelated to the pheromone response pathway (Karos et al., 2000; Lengeler et al., 2002). Genetic organization of the MAT locus in C. neoformans therefore shares characteristics of both fungal MAT alleles and the complexity of sex chromosomes from higher eukaryotes (Lengeler et al., 2002; Fraser and Heitman, 2004). The biological differences between MATα and MATa strains of C. neoformans, aside from the mating system-associated phenomenon, however, have largely gone unnoticed.
Yeast cells of the two mating partners are indistinguishable with respect to morphology (isogamous) and the sexual process. Nuclear exchange between the partners, which is characteristic of dioecism, has thus far not been reported. In Saccharomyces cerevisiae, both MATa and MATα cells initiate morphological change to form ‘shmoos’ when co-cultured and subsequently fuse to form a zygote (Byer, 1981). In C. neoformans, however, only MATα cells produce conjugation tubes that reach out to and fuse with MATa cells during mating (Chang et al., 2000; Wang et al., 2000). Dikaryotic hyphae are subsequently produced normally by the MATa cells and meiotic products (basidiospores) are borne on the resulting basidia (Kwon-Chung, 1980; McClelland et al., 2004) which mimics the situation in dioecious plants and animals. In support of this concept, mitochondrial inheritance in C. neoformans has been shown to be uniparental: meiotic products carry only MATa type mitochondria (Xu et al., 2000).
We hypothesize that MATα and MATa cells have different biological circuitry that renders the MATα cells more fit for survival, as evidenced by the skewed frequency of the two mating types in nature. One of the ways to expose the possible differences in the regulatory circuit would be to determine the phenotypic differences resulting from the disruption of a global regulator such as TUP1 in the two mating types. TUP1 is a global repressor which suppresses about 3% of the genes in S. cerevisiae and tup1 mutants display numerous phenotypes such as flocculation, temperature-sensitive growth, non-sporulation of homozygous diploids, and reduced pseudohyphal and invasive growth (Williams and Trumbly, 1990; Keleher et al., 1992; DeRisi et al., 1997). In Candida albicans, TUP1 regulates many genes involved in both filamentous growth and virulence (Braun et al., 2000; Murad et al., 2001). Disruption of TUP1 results in constitutive filamentous growth and loss of virulence in C. albicans (Braun and Johnson, 1997). In Penicillium marneffei, a dimorphic fungus, tupA is the homologue of TUP1 and appears to be an important factor in regulating the switch between yeast and filamentous growth (Todd et al., 2003). In the filamentous fungi Aspergillus nidulans and Neurospora crassa, disruption of TUP1 homologues rcoA and rco-1, respectively, severely affect growth and development (Yamashiro et al., 1996; Hicks et al., 2001). Disruption of TUP1 in C. neoformans therefore is likely to exhibit mating type-dependent phenotypes should disparity in the biological circuitry exist between the two sexes of this heterothallic organism.
We cloned and disrupted the TUP1 homologue in congenic MATα and MATa C. neoformans strains and characterized the mutant phenotypes. We have determined the mating type-dependent effects of the TUP1 disruption in C. neoformans. These include growth characteristics at different temperatures, sensitivity towards high concentrations of KCl and transcriptional profiles. Cell morphology, hyphal development, or mating efficiency, however, were not differentially affected. The phenotypic differences between MATa- and MATα tup1 strains suggest the existence of disparity in biological pathways outside of the mating cascade.
Results
Identification of TUP1 homologue
To identify homologues of TUP1 in C. neoformans, a blast search was launched against the Cryptococcus genomic sequence database at Stanford DNA sequencing and technology centre (http://www-sequence.stanford.edu/group/C.neoformans/). A genomic DNA sequence containing the homologue of TUP1 was retrieved. An open reading frame (1695 bp) containing nine introns was identified by comparing the genomic sequence with the putative cDNA sequence at TIGR (The Institute for Genomic Research at http://www.tigr.org/tdb/e2k1/cna1/index.shtml, locus name CNF03830). The predicted amino acid sequence from the putative C. neoformans TUP1 nucleotide sequence showed homology with N. crassa RCO-1 (60% similarity/52% identity), A. nidulans RcoA (61%/52%), P. marneffei TupA (62%/53%) and S. cerevisiae Tup1p (49%/41%). Furthermore, the predicted seven WD40 repeats and their intervening sequences in C. neoformans Tup1p are highly conserved with the corresponding regions of other Tup1p sequences. The sequence similarity with other Tup1p homologues strongly suggests the possible functional conservation of C. neoformans Tup1p.
Disruption and complementation of TUP1 gene
To study the function of TUP1, we disrupted TUP1 from both the MATα strain, LP1, and the MATa strain, LP2. A major portion of the TUP1-coding region was replaced with the ADE2 gene sequence and the resulting disruption construct, pHL3, was introduced to the ade2 ura5 mutant strains, LP1 as well as LP2, by the biolistic transformation method. Several polymerase chain reaction (PCR)-positive clones of the putative tup1 strain were identified and subjected to Southern blot analysis. Genomic DNA was extracted from the putative tup1 strains, transferred to nitrocellulose filters and hybridized with the 1.5 kb XbaI/BglII 5′ flanking DNA fragment which had been used in the disruption construct pHL3 (Fig. 1B). Figure 1C and D show results of the Southern blot analysis in the MATα and MATa background respectively. The signals detected in the putative disruptants corresponded to fragments of the predicted size. In HindIII-digested genomic DNA, the 7.8 kb fragment in the wild-type strain, LP1, resulted in a 4.7 kb fragment in the putative tup1 clones (Fig. 1C and D). When EcoRI-digested genomic DNA was probed with the 1 kb EcoRI 3′ flanking fragment of pHL3, the > 9.0 kb fragment in the wild-type strain resulted in a 1.7 kb signal in the tup1 clones (data not shown). These results indicated that TUP1 locus had been disrupted in these strains. The MATα tup1 strain ♯5 and MATα tup1 strain ♯23 were designated as HL14 and HL40, respectively, and were used for further study.
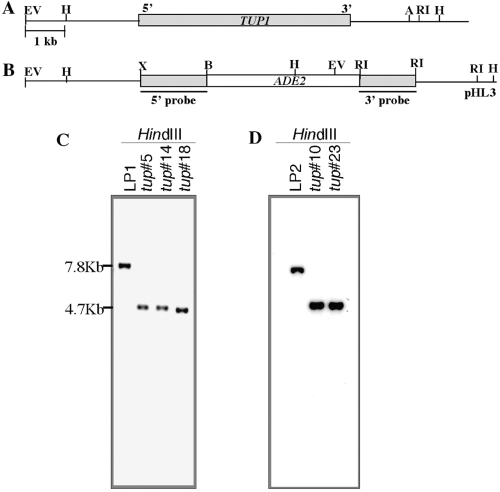
Disruption of TUP1. A and B. (A) Genomic arrangement of TUP1. Shaded and open boxes represent TUP1 and ADE2 genes respectively. (B) Diagram for the disruption plasmid construct, pHL3. C and D. Southern blot analysis. Genomic DNA was prepared from the wild-type (LP1) and putative tup1 strains (♯5, 14 and 18) (C) and wild-type (LP2) and tup1 strains (♯10, 23) (D), digested with HindIII, fractionated on an agarose gel and transferred to a nylon membrane. The resulting blots were hybridized with 5′ 1.5 kb fragment of TUP1.
To confirm whether tup1 phenotypes were caused by disruption of the TUP1 gene, two sets of control strains were generated (Table 1). First, tup1 was separately transformed with two URA5-containing plasmids, the vector (pCIP3) and a pCIP3-based plasmid containing the wild-type TUP1 gene (pHL45). Second, F1 progeny carrying either TUP1 or tup1 in both MATa and MATα backgrounds was obtained from a cross between HL14 (tup1, MATα) and the wild-type MATa strain, JEC32.
| Strain | Genotype/comment | Source or reference |
|---|---|---|
| LP1 | MATα ura5 ade2 | This study |
| LP2 | MATa ura5 ade2 | This study |
| JEC32 | MATa lys2 | J. Edman |
| JEC31 | MATα lys1 | J. Edman |
| HL14 | MATα ura5 ade2 tup1::ADE2 | This study |
| HL40 | MATa ura5 ade2 tup1::ADE2 | This study |
| HL17 | MATa, F1 of HL14 | This study |
| HL18 | MATα, F1 of HL14 | This study |
| HL19 | MATa tup1::ADE2, F1 of HL14 | This study |
| HL20 | MATα tup1::ADE2, F1 of HL14 | This study |
| HL27 | MATα ura5 ade2 tup1::ADE2 pCIP3(URA5) | This study |
| HL32 | MATα ura5 ade2 tup1::ADE2 pHL45(URA5 TUP1) | This study |
TUP1 disruption affects the growth but not morphology of the yeast
We first examined the effect of TUP1 disruption on growth and morphology of the yeast. Disruption of TUP1 affected growth in both MATα and MATa strains (HL14 and HL40 respectively) at both 25°C (Fig. 2B) and 30°C (Fig. 2C) but the difference was more noticeable at 25°C than at 30°C. At 37°C, however, growth of the tup1 strains was similar to that of the wild-type strains (Fig. 2D). Retarded growth at 30°C or 25°C was also observed for the F1 progeny pairs of tup1 genotype (Fig. 2, right). tup1 strains complemented with the intact TUP1 gene grew at rates comparable to that of the wild-type strains at all temperatures tested while the growth of vector-transformed tup1 strains remained retarded (data not shown). Interestingly, the growth differences at 25°C between wild-type and tup1 strains were more pronounced in the MATa pair than in the MATα pair. This was clearly evident in the F1 progeny (Fig. 2, right). However, this growth difference between the MATa pair and the MATα pair was not as clear in the original disruptants as in the F1 progeny (Fig. 2, left). These differences were more evident when the cells were plated at lower densities (Fig. 2E). The tup1 strains of either mating type failed to produce sizable colonies with the same inoculum at 25°C for 4 days (data not shown).

Cryptococcus neoformans TUP1 is required for growth at temperatures lower than 37°C. Wild-type LP1 (MATα), HL14 (tup1 MATα), LP2 (MATa), HL40 (tup1 MATa) and HL17-HL20 (F1 strains) were plated as indicated in (A) on YEPD media and grown for 2–4 days at 25°C (B), 30°C (C) and 37°C (D). (E) About 200–400 cells of LP1, HL14, LP2 and HL40 were spread on YEPD plates and incubated at 30°C for 3 days.
Although TUP1 is known to play an important role in the morphology of vegetative cells in other fungi (Yamashiro et al., 1996; Braun and Johnson, 1997; Hicks et al., 2001; Todd et al., 2003), disruption of TUP1 in C. neoformans showed no effect on yeast morphology and did not cause the flocculation phenotype in either mating type. Furthermore, the ability of tup1 strains to form filaments in haploid fruiting or in confrontation assays was indistinguishable from that of the wild-type strains regardless of mating type (data not shown).
TUP1 disruption affects mating
As disruption of TUP1 significantly affects mating in S. cerevisiae and N. crassa (Williams and Trumbly, 1990; Yamashiro et al., 1996), we tested the effect of TUP1 disruption on mating in C. neoformans. A wild-type MATα strain crossed with the tester strain JEC32 produced extensive mating hyphae after 2 days of incubation on V-8 juice agar (Fig. 3A). In contrast, the production of hyphae in the cross between MATα tup1 and JEC32 was significantly reduced. However, production of typical basidiospore chains, although at a much lower frequency, was readily observed in the cross involving the tup1 disruptant and the resulting basidiospores were viable. Similar phenotype was observed for tup1 strain in MATa background (data not shown). When the mating frequency was determined by a quantitative mating assay, tup1 MATα and tup1 MATa strains expressed mating frequencies that were 7% and 15% of the wild-type strains LP1 and LP2 respectively. Furthermore, tup1 strains when complemented with an intact TUP1 gene restored the mating frequency while the vector-transformed tup1 strains did not (data not shown). The mating was also tested with F1 progeny by crossing the opposite mating type strains in four combinations (Fig. 3B and C). Wild-type strains mated efficiently and produced abundant hyphae. In contrast, mating of the tup1 MATa (HL19) strain with the tup1 MATα (HL20) strain rarely produced any hyphae. Furthermore, mating of the tup1 strains with the wild-type strains (HL19 × HL18, HL20 × HL17) produced intermediate amounts of hyphae relative to crosses between the wild type and that of the tup1 pairs. Although mating efficacy of the F1 prototrophs could not be determined by our standard quantitative assay method, we observed an additive effect of the TUP1 mutation in the cross. These data suggested that a mutation in TUP1 is the cause of reduced mating efficiency. Taken together, the role of TUP1 in fungal sexual reproduction appears to have been conserved in C. neoformans.
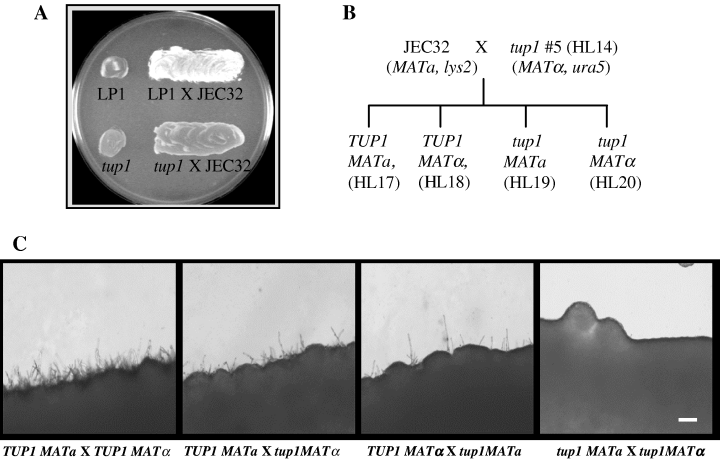
TUP1 disruption affects mating. A. LP1 and tup1 (HL14) were each mixed with JEC32 (MATa) on the V-8 juice agar, incubated for 48 h and observed for hyphal formation. B. Diagram showing generation of the F1 strains. HL14 (tup1, MATα) and a MATa strain JEC32 were mated and four prototrophic F1 progeny were obtained as indicated. C. Wild-type (TUP1) and tup1 F1 strains with opposite mating type were mixed and observed for the mating under the microscope. Bar = 60 µm.
TUP1 influences growth in the presence of high KCl concentrations
The possible role of TUP1 in cellular responses towards stress-related culture conditions was investigated by exposing the tup1 strains to high concentrations of KCl. We observed that tup1 MATα (HL14) exhibited increased sensitivity to 0.8 M KCl compared with the wild-type strain, LP1 (Fig. 4, top). Surprisingly, sensitivity of the tup1 MATa strain (HL40) towards this cation was indistinguishable from that of the wild-type MATa strain (LP2) (Fig. 4, middle). The same phenomenon was observed in the F1 progeny with the tup1 genotype. The tup1 MATα (HL20) progeny was clearly more sensitive to KCl than the wild-type MATα (HL18) strain while the tup1 MATa (HL19) progeny was resistant to the same concentrations of KCl as the wild-type MATa (HL17) (Fig. 4, bottom). The observed phenotype is probably a salt-induced effect, not an osmotic effect, as tup1 disruptants showed wild-type level of growth on media containing 0.8 M NaCl and 1.0 M sorbitol (data not shown). TUP1 therefore appears to exert a differential response in MATa and MATα strains towards harsh environmental stresses such as high concentrations of KCl.
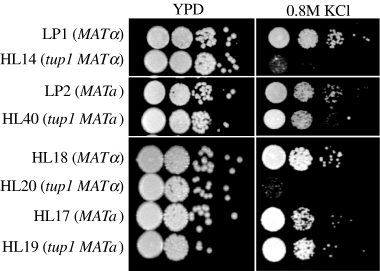
Stress-related phenotype of TUP1-disrupted strains. Exponentially growing cultures were washed, diluted serially, spotted onto YEPD agar and incubated for 3 days at 30°C or spotted onto YEPD agar supplemented with 0.8 M KCl and incubated for 4 days at 30°C.
Disruption of TUP1 affects the expression of several genes in different biological pathways in a mating type-dependent manner
We considered the possibility that phenotypic differences between tup1 MATa and tup1 MATα strains described above may reflect the differential regulation of genes by TUP1 in the two mating types. To study the genes regulated by TUP1, we created a small-scale microarray using 222 genes (see Experimental procedures). The transcription profile of numerous genes (about 35 of the 222 genes) showed significant differences between the tup1 and the wild-type strains of the corresponding mating type (data not shown). Furthermore, expression of several genes in various biological pathways was altered differently in the two mating types (data not shown).
To confirm that the differentially expressed genes identified in the microarray analysis were indeed regulated by TUP1 in a mating type-dependent fashion, five genes, CTR1, MFa/α, CEL1, FDH1 and MP88, that represent different biological pathways were chosen for further study by Northern blot analysis. CTR1 (Cryptococcal TUP1Regulated) was one of the genes whose expression was most significantly altered as the result of the TUP1 disruption. The transcriptional levels of CTR1 were markedly elevated in tup1 strains of both mating types implying that expression is under the control of TUP1 repression. No sequences homologous to CTR1 were found in blast searches performed against the NCBI nr database (http://www.ncbi.nlm.nih.gov/Database/index.html). MFa and MFα are the MATa- and MATα-specific genes, respectively, that encode pheromone precursors. The pheromone functions in the signalling pathway that leads to sexual morphogenesis (Moore and Edman, 1993; Davidson et al., 2000; McClelland et al., 2002; Shen et al., 2002). CEL1 is a gene closely linked to CAP60 and encodes a putative protein with an architecture that resembles the multidomain fungal cellulase (Armesilla et al., 1994; Chang and Kwon-Chung, 1998). FDH1 encodes a putative formate dehydrogenase that plays an important role in detoxification of formate in the methanol dissimilation pathway reported in the methylotrophic yeast Candida boidinii (Sakai et al., 1997). MP88 encodes a mannoprotein that can stimulate a T-cell response which is critical for effective host defences against cryptococcosis (Huang et al., 2002). Figure 5A shows that the expression of all five genes was altered differently in tup1 strains of the two mating types compared with the wild type. Quantitative data of the Northern blot analysis are presented in Fig. 5B. Expression of CTR1 and MF was significantly higher in both tup1 MATa and tup1 MATα strains compared with that in corresponding wild-type strains. While CTR1 was slightly more derepressed in tup1 MATa than in tup1 MATα strain, the extent of MF derepression was drastically higher in tup1 MATa than in tup1 MATα strain. In contrast, transcription of FDH1 and MP88 was derepressed in tup1 MATa but repressed in tup1 MATα strain. CEL1, on the other hand, was slightly repressed in tup1 MATa but derepressed in tup1 MATα. These data suggested that TUP1 plays a different regulatory role for genes in several different biological pathways in two mating types.
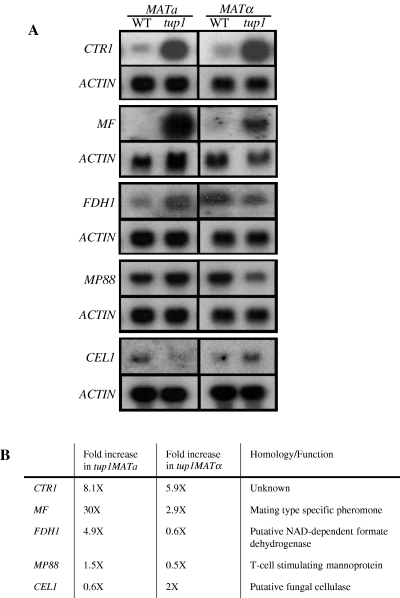
Disruption of TUP1 affects gene expression differently between MATa and MATα strains. A. RNA was extracted from 16 h culture of the wild type and tup1 of the MATa and MATα strains and hybridized with CTR1-, MF-, FDH1-, MP88-, CEL1- and ACTIN-specific DNA fragments as specified. B. Comparison of the results from quantitative Northern blot analysis. Each gene-specific signal was normalized to that of the ACTIN gene. The relative expression levels of each gene were compared and expressed by multiples relative to the corresponding wild-type levels.
In order to determine whether regulation by TUP1 is influenced by the environmental conditions, a MATα wild-type and a MATα tup1 strain were analysed further. RNA was extracted from cell cultures at different stages of growth in YEPD broth with either 2% or 0.2% glucose concentration. As CTR1 was one of the genes whose expression was altered markedly in both mating types as a result of the TUP1 disruption, it was chosen for Northern blot analysis. Expression of CTR1 was not detectable in log phase cells of the wild-type strain grown in YEPD but was detectable in a 10 h culture. The expression level increased gradually as the culture aged (Fig. 6A). In the tup1 strain, however, CTR1 was expressed in log-phase cells under the same growth condition and the age-dependent increase in expression was significantly more pronounced than in the wild-type strain (Fig. 6A). These data indicated that TUP1 regulation of the CTR1 gene expression is age dependent and disruption of TUP1 results in derepression of CTR1.
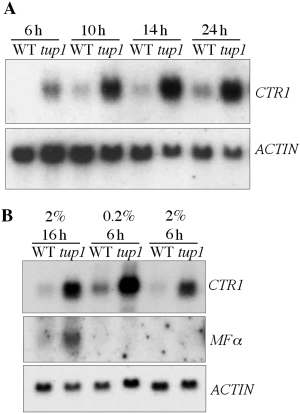
Disruption of TUP1 affects CTR1 expression. A. RNA was isolated from cells of wild-type (WT) and HL14 (tup1) strains, which were grown in YEPD and harvested at different time points (6, 10, 14 and 24 h). The probes used in each hybridization were as specified. B. RNA was extracted from wild-type (WT) and HL14 (tup1) strains grown in YEPD with different concentration of glucose (0.2% versus 2%) for 6 h versus 16 h.
The expression of CTR1 was higher in the log-phase cultures under low-glucose condition (0.2%) than under the standard condition (2% glucose) and the expression levels were much higher in the tup1 disruptant than in the wild-type strain (Fig. 6B, 6 h). The same Northern blot was probed with the MFα gene sequence to determine whether the pattern of TUP1 regulation varied depending on the target gene. In contrast to CTR1, the expression of MFα was affected neither by the growth phase of the culture nor by the glucose concentrations. Derepression of MFα as a result of the TUP1 disruption was evident only in stationary phase (Fig. 6B). These data suggested that the extent of repression by TUP1 also varies depending on the growth conditions as well as the target genes.
Discussion
We hypothesized that over-representation of the C. neoformans MATα strains in nature relative to MATa strains is attributable to the biological differences between the two mating partners. One approach to revealing such differences would be to examine the results of disrupting a global regulator. TUP1 was chosen to test this hypothesis as it is one of the most pervasive transcriptional repressors in S. cerevisiae (Williams and Trumbly, 1990; Keleher et al., 1992; DeRisi et al., 1997) as well as in several other fungi (Yamashiro et al., 1996; Hicks et al., 2001). Interestingly, the sequence of CnTUP1 is closer to the TUP1 homologues of filamentous ascomycetes such as RCO-1 of N. crassa, RcoA of A. nidulans and TupA of P. marneffei than to those of ascomycetous yeasts such as TUP1 of S. cerevisiae and TUP11 or TUP12 of Schizosaccharomyces pombe.
While the phenotypes manifested by tup1 strains of C. neoformans suggested some similarities in the role of CnTUP1 to TUP1 homologues of other fungi in cell growth and sexual reproduction, no gross defects were found in the cell morphologies of either mating types. In other fungi, TUP1 disruption clearly affected the haploid cell morphology. Disruption of the TUP1 homologue in C. neoformans only affected growth rates at certain temperatures. Temperature sensitivity of tup1 mutants has been reported in other organisms. The tup1 mutants of S. cerevisiae are sensitive to 37°C and growth is arrested in C. albicans tup1 mutants at 42°C (Manney et al., 1983; Braun and Johnson, 1997). Interestingly, disruption of TUP1 had no effect on the growth of C. neoformans at 37°C while it had a drastic effect on growth at 25°C as well as a modest effect at 30°C. Importantly, the degree of effect on growth at temperatures 30°C or lower was asymmetric in the two mating types; MATa strains were more severely affected than MATα strains. Flocculations of yeast cells reported in the tup1 strains of ascomycetous yeasts such as S. cerevisiae or S. pombe (Lipke and Hull-Pillsbury, 1984; Mukai et al., 1999) were not found in our tup1 strains of either mating type. As TUP1 disruption does not affect capsule formation in C. neoformans, lack of flocculation is not surprising. Unlike in some dimorphic fungi or filamentous fungi (Yamashiro et al., 1996; Braun and Johnson, 1997; Hicks et al., 2001), there were no defects in filament formation or in the formation of typical basidial structure.
TUP1 homologues are known to be important for mating and are involved in repression of pheromone precursor genes in N. crassa and S. cerevisiae (Lemontt et al., 1980; Fujita et al., 1992; Bobrowicz et al., 2002). A tup1 null mutation in α and diploid S. cerevisiae cells results in an inappropriate expression of a type-specific genes, including the pheromones. In N. crassa, mutations in rco-1, a TUP1 homologue, resulted in an elevated expression of the pheromone precursor genes, mfa-1 and ccg-4, compared with the wild-type strain. However, in contrast to S. cerevisiae, the expression of pheromone precursor genes in N. crassa remained strictly mating type specific in rco-1 mutant (Lemontt et al., 1980; Fujita et al., 1992; Bobrowicz et al., 2002). Several lines of evidence strongly support that TUP1 plays an important role in mating in C. neoformans as well. First, the original tup1 strains in both MATa and MATα backgrounds showed significantly reduced mating ability. Second, reintroduction of the wild-type TUP1 gene into tup1 strains by complementation restored the mating efficiency. Third, the cross between F1 strains of tup1 MATa (HL19) and tup1 MATα (HL20) was almost sterile (Fig. 3C). Furthermore, our Northern blot data indicated that the MFa and MFα genes were significantly upregulated in tup1 strains grown on YEPD, whereas the wild-type strains do not express the gene. More interestingly, the degree of upregulation was 10-fold higher in MATa tup1 strains relative to MATα tup1 strains. C. neoformans TUP1 therefore has a conserved role in the mating process but with different regulatory efficiency within each of the two mating types.
Microarrays have been used extensively in several organisms to elucidate the link between genes and their phenotypes based on the alterations in the transcriptional profile. To determine whether CnTUP1 is a global regulator and also to determine whether it regulates genes in the two mating types differently, we performed a small-scale microarray study. Our microarray experiments had limitations with respect to the number and the type of genes represented. We had 222 cDNAs on each microarray slide, which covers <3% of the estimated total number of genes in C. neoformans. Of these, 158 cDNAs were highly biased towards mating-related or STE12-regulated genes as the cDNAs were isolated from a subtraction library constructed from either the mated or the STE12-deleted cells. Furthermore, a majority of the genes on the slides do not have good overall homology to known genes, which makes the interpretation of the data challenging. Nevertheless, the array data did corroborate the quantitative Northern blot analysis, confirming its reproducibility and validity, and served the initial purpose of our attempted analysis. With the small-scale microarray, we have obtained preliminary but interesting information. About 35 (approximately 15%) of the 222 genes were found to be either up- or downregulated by the TUP1 disruption in both mating types (data not shown). Alteration in the expression of such a high percentage of genes was predictable as the genes in the array were highly biased towards the mating cascade. Furthermore, many genes were affected differently in the two mating types by the TUP1 disruption. Although the mechanism of Tup1p function and its possible interactions with other DNA-binding proteins in C. neoformans has yet to be demonstrated, our microarray and Northern analysis, coupled with phenotypic observations, indicated that C. neoformans TUP1 is probably a global transcriptional regulator as in S. cerevisiae. It is plausible to suppose that C. neoformans TUP1 regulates the genes in many different biological pathways, and hence, results in pleiotrophic phenotypes upon disruption.
The most intriguing aspect of our study is that several phenotypes and the gene expression pattern of tup1 disruptants are mating type dependent. Mating type-dependent regulation by TUP1 has been studied in S. cerevisiae, where α cell-specific genes are repressed in α cells by α2 with the help of the global repressor complex Tup1p–Ssn6p (Johnson and Herskowitz, 1985; Keleher et al., 1992). As a2 is a gene in the mating type locus, the phenotypic differences between MATa tup1 and MATa tup1 in S. cerevisiae are mostly related to the mating cascades. The observed phenotypes in C. neoformans such as sensitivity to KCl and growth rate as well as the several genes affected by TUP1 disruption are unlikely to be directly involved in the pheromone response pathway.
In C. neoformans, it has been proposed that MATα and MATa cells, despite their identical morphology, function distinctly in the mating process partly because some of the mating type-specific genes expressed differently in MATα and MATa cells (McClelland et al., 2002; 2004; Chang et al., 2003). The demonstration of different effects of the TUP1 disruption in MATa versus MATα cells supports the recent notion regarding inherent biological disparity between MATa and MATα strains besides the genetic make-up of the mating type locus. One can speculate as to the mechanism of mating type-dependent regulation of TUP1. There are unique mating type locus genes in each mating type strain, such as SXI1α (Hull et al., 2002; Lengeler et al., 2002) and SXI2a (J. Heitman, pers. comm.), transcriptional regulators in MATα and MATa strains respectively. TUP1 may interact with these unique gene(s) or those under their control. Such interactions may be mediated by a co-repressor(s) or other genes encoding DNA-binding proteins that are not in the mating type locus. In such a case, tup1 may manifest itself in non-mating-related phenotypes in a mating type-dependent manner. Alternatively, as the mating type loci contain homologues with significantly diverged sequences, such as STE12α versus STE12a, it is possible that TUP1 may interact with them differently. Differential regulation of these genes in MATa tup1 and MATαtup1 may affect not only mating-related phenotypes but also non-mating-related phenotypes in a mating type-dependent manner. As pheromone genes were more severely derepressed in MATa tup1 than in MATα tup1 (Fig. 5), it is possible that differential expression of such genes in the two mating type cells may have a different effect on phenotypes not necessarily related to mating or cell morphology, e.g. growth rate or the response towards high cation levels. Examples of the mating type-specific genes that influence biological processes not directly related to mating have been documented in S. cerevisiae: a1 and α2 primarily function in mating type identity and sporulation but also function in the maintenance of cell wall integrity and responses to heat shock (Verna and Ballester, 1999). It is possible that some mating type-specific genes in C. neoformans may play different roles in the two mating types under the control of Tup1p.
Cryptococcus neoformans may be a species of functionally dioecious fungi in which two mating partners are isogamous but functionally different in the mating process. Unilateral production of conjugation tubes by MATα, unidirectional transfer of nuclei from MATα to MATa (McClelland et al., 2004), uniparental (MATa specific) inheritance of mitochondria (Xu et al., 2000), presence of mating type-specific pheromone response MAP kinase cascade genes, and the phenotypic differences revealed between tup1 strains of the two mating types point to the dioecism in C. neoformans. Dioecism by definition denotes the separation of sexes: presence of male and female sex organs in two different individuals and sexual reproduction requiring nuclear transfer from male to female (Alexopoulos et al., 1996). The majority of fungal species that require cross-mating are monoecious in which each strain functions as a nuclear donor as well as a nuclear acceptor. In these fungi, incompatibility factors prevent self-fertility and allow cross-mating. Morphologically dioecious fungi are known only in a few genera of filamentous Zygomycota (e.g. Ancylistis) and Ascomycota (e.g. Ascosphaera, Laboulbenia) but not in Basidiomycota (Alexopoulos et al., 1996). As yeasts are isogamous, morphological dioecism has not been known. C. neoformans may represent the early step of evolution towards true dioecism in Basidiomycota. With the Cryptococcus sequencing project in the finishing stages, genomic approaches can be undertaken to determine the regulatory circuitry of TUP1 and other global regulators. Such studies will facilitate further understanding on the functional dioecism in C. neoformans and may shed light on the genetic bases that contribute to the prevalence of MATα strains in nature.
Experimental procedures
Strains and media
Table 1 summarizes the strains used in this study. LP1 and LP2 were constructed as following. An adenine mutant, red13B (Chang et al., 1996), was backcrossed four times with strain B-4476 and two adenine F4 prototrophs, one MATa and one MATα, were obtained. These two F4 strains were plated on 5′-fluoroorotic acid to obtain the adenine and uracil double mutants and were designated as LP1 and LP2 respectively. As the strain red13B was derived from B-4500, a congenic strain of B-4476, and LP1 and LP2 were the siblings derived from the fourth backcrossing using B-4476 as a foundational strain, LP1 and LP2 are believed to be congenic. Strains HL17 to HL20 are F1 progeny obtained from a cross between a MATα tup1 disruptant (HL14) and a MATa strain JEC32. All strains were maintained on YEPD (1% yeast extract, 2% Bacto peptone and 2% dextrose). Minimal medium (YNB) contains 6.7 g of yeast nitrogen base (Difco) without amino acids and 20 g of glucose per litre. V-8 juice agar for mating was prepared as described previously (Kwon-Chung et al., 1982). Synthetic low ammonia dextrose (SLAD) medium and filament agar were prepared as described previously (Chang et al., 2000).
Construction of plasmids
To generate TUP1 disruption construct, 1.5 kb XbaI/BgIII 5′ and 1 kb EcoRI 3′ flanking fragments were amplified by PCR and subcloned into pYCC76 (Chang and Kwon-Chung, 1998). The resulting plasmid, pHL3, contained the disrupted TUP1 gene in which 1.5 Kb of the TUP1-coding region was removed and replaced by the selectable marker ADE2 gene (Fig. 1B). Wild-type TUP1 gene was isolated from lambda phage genomic DNA library using 5′ and 3′ flanking fragments as a probe (Fig. 1B). The 5.8 kb EcoRI/ApaI TUP1 fragment was excised from the resulting phage and subcloned into the EcoRI/SmaI site in pCIP3 to obtain pHL45, which was transformed into HL14 to complement TUP1 disruption.
Disruption and complementation of TUP1 in C. neoformans
Biolistic method (Toffaletti et al., 1993) was used to transform both LP1 and LP2 with the disruption construct, and the transformants were selected on adenine-free YNB media supplemented with uracil and 1 M sorbitol. Prototrophic transformants were screened to identify the tup1 disruptants by colony PCR. Disruption of TUP1 was confirmed by Southern blot analysis.
To complement the TUP1 disruption, pHL45 was linearized by ApaI and electroporated into HL14 as described by Edman and Kwon-Chung (1990). Stable transformants were selected after repeated transfer on YEPD agar. PCR was used to identify integrative transformants containing an intact TUP1 gene and Southern blot analysis was used to confirm the transformation.
Preparation and analysis of nucleic acid
Isolation and analysis of genomic DNA was carried out as described previously (Chang and Kwon-Chung, 1994). Radioactive probes were prepared using StripEZ kit (Ambion, Austin, TX) according to manufacturer's manual. Total RNA was extracted by using FastRNA kit (Bio101, La Jolla, CA). Northern blot analysis was performed as described previously (Chang et al., 1995). For quantitative Northern analysis, after each hybridization, the blot was exposed to Phosphoimager Screen and quantified with ImageQuant 1.1 (Molecular Dynamics). Each gene-specific signal was normalized to that of the ACTIN gene. The relative expression levels of each gene were compared and expressed as multiples of the wild-type levels. To obtain total RNA from cells at different stages of growth under regular or low-glucose conditions, each strain was grown in YEPD with 2% or 0.2% glucose and harvested at different time points (6, 10, 14 and 24 h).
Mating, confrontation, filamentation and quantitative mating assay
For mating assay, strains were grown on YEPD slants for 2 days. The cells of MATa and MATα strains were mixed on V-8 juice agar medium, incubated and monitored for the evidence of mating. In confrontation assays, two strains pre-grown on YEPD medium for 48 h were streaked in parallel onto SLAD in a close proximity (100–500 µm distance) (Chang et al., 2000) and hyphal formation was monitored. The assay was performed between wild-type and tup1 disruptant as well as between tup1 disruptants. For the haploid filamentation assay, cells grown on YEPD slants for 2 days were patched on YEPD agar plate and incubated at 40°C for 3 days. Cells were then streaked on a filament agar plate (Wickes et al., 1996). Incubation was at 30°C for all the above tests and cells were monitored daily using a low power microscopy.
The assay for the quantitative mating frequency was performed as previously described (Chang et al., 2000). In brief, auxotrophic MATa or MATα strains (JEC32 and JEC31 respectively) were used as tester strains to determine the mating frequency of any given MATα or MATa strains carrying different auxotrophic markers. The relative frequency was expressed as a percentage of the mating frequency of LP1 or LP2. Experiments were repeated at least twice to confirm reproducibility. All crosses were performed on V-8 juice agar for 6 h. This assay was designed to measure the frequency of MATa and MATα fusion product which forms filaments. Quantitative mating is a more objective (unbiased) way to compare the mating ability of strains than visual observation. However, it can be tricky to do quantitative mating for strains with different growth rate and viability. Because both HL14 and HL40 grew much slower than wild type especially when they were grown on agar media and have low viability at 30°C, our standard quantitative mating method underestimated the actual input cell numbers for those strains. To overcome such a problem, we used more cells (5 × 107 cells for each strain) and longer incubations at 30°C or 37°C.
Mini-microarray experiment
We chose 222 cDNA sequences either from two subtraction libraries constructed in this lab (our unpublished data) or from published sequences. A mini-microarray was constructed by synthesizing short 70mer oligonucleotides based on the sequences of the 222 cDNAs (Illumina, San Diego, CA) and printed on a glass slide (microarray research facility at NIH). Each 70mer was spotted four times on an array slide to increase reliability of the measurements. Positive and negative internal control spots were included to help ensure the quality of data and calibration of the signal. RNA was extracted from yeast cells grown in YEPD for 20 h using FastRNA kit (Bio101, La Jolla, CA) and treated with RNAse-free DNase (Ambion, Austin, TX) for the removal of genomic DNA. Cyanine 3 (Cy3) or Cy5 (Amersham, Piscataway, NJ) labelled cDNA was synthesized with Fairplay Microarray Labeling Kit (Stratagene, La Jolla, CA) according to manufacturer's manual and subjected to competitive hybridization on the microarray. cDNAs from wild-type and tup1 strains were labelled with Cy3 and Cy5, respectively, and dyes were reversed for the reverse fluor control. Cy3-cDNA and Cy5-cDNA samples were mixed and heated for 3 min at 98°C before the samples were applied to the microarray slide. Samples were hybridized for 18–20 h at 42°C, and the slides were washed and dried before the analysis. Arrays were scanned on a GenePix 4000B scanner and analysed using GENEPIX PRO 4.0 (Axon Instruments, Foster City, CA). Data were further analysed in mAdb database at http://www.nciarray.nci.nih.gov (NCI).
Spot assay
Exponentially growing cultures (OD600 = 0.5–1.0) were washed, resuspended in 0.9% NaCl and adjusted to OD600 = 0.1 for wild type or 0.2 for tup1 disruptant. Adjusted cell suspensions were serially diluted, spotted onto YEPD media supplemented with either KCl or NaCl, at the indicated concentration and incubated for 3–4 days at 30°C.
Acknowledgements
We thank Ashok Varma for critical reading of the manuscript.




