Functional interplay between the Bacillus subtilis DnaD and DnaB proteins essential for initiation and re-initiation of DNA replication
Present addresses: LIPM, INRA-CNRS, BP 27, 31326 Castanet Tolosan cedex, France;
Summary
Initiation and re-initiation of chromosomal DNA replication in bacteria rely on divergent multiprotein assemblies, which direct the functional delivery of the replicative helicase on single-stranded DNA (ssDNA) at specific sites. These two processes are triggered either at the single chromosomal origin oriC or at arrested forks by the conserved DnaA and PriA proteins respectively. In Bacillus subtilis, these two pathways further require the three essential proteins DnaB, DnaD and DnaI, restrictively encoded in Gram positive bacteria of low GC content. We have recently shown that DnaI and DnaB act as a pair of loaders of the DnaC replicative helicase. The role of DnaD appeared more enigmatic. It was previously shown to interact with DnaA and to display weak ssDNA binding activity. Here, we report that purified DnaD can interact physically with PriA and with DnaB. We show that the lethality of the temperature-sensitive dnaD23 mutant can be suppressed by different DnaB point mutants, which were found to be identical to the suppressors of priA null mutants. The DnaD23 protein displays lower ssDNA binding activity than DnaD. Conversely, the DnaB75 protein, the main dnaD23 suppressor, has gained affinity for ssDNA. Finally, we observed that this interplay between DnaD and DnaB is crucial for their concerted interaction with SSB-coated ssDNA, which is the expected substrate for the loading of the replicative helicase in vivo. Altogether, these results highlight the need for both DnaD and DnaB to interact individually and together with ssDNA during the early stages of initiation and re-initiation of chromosomal DNA replication. They also point at a main structural role of DnaD in the multiprotein assemblies built during these two essential processes.
Introduction
Initiation of DNA replication is a central process in the life cycle of all organisms, proceeding through a remarkably conserved general mechanism. Invariably, it relies on the controlled recruitment and loading of the replicative helicase at specified origins onto the chromosomal DNA. Next, the other proteins of the replication apparatus are recruited to build the replisome, which primes and extends DNA synthesis. This general scheme of initiation can be carried out by different sets of initiation proteins, defining distinct strategies of helicase recruitment and loading. Considering the number of proteins involved, the simplest initiation models have been found for extrachromosomal elements (plasmids, phages and viruses), while the cellular systems appear generally much more complex. Furthermore, several initiation strategies have evolved between and within each branch of life, as it emerges from the comparison of the initiation protein repertoires of the prokaryote, archae and eukaryote models characterized so far (Lemon et al., 2002; Grabowski and Kelman, 2003; Méndez and Stillman, 2003 and references therein).
Molecular studies of the mechanisms of initiation of DNA replication in bacteria have mainly been directed at the Gram-negative Escherichia coli and, to a lesser extent, at the Gram-positive Bacillus subtilis (Marians, 2000; Lemon et al., 2002). Duplication of their unique circular chromosome is initiated at a single origin, oriC, where two divergent replication forks are activated. In the case of accidental arrest of the fork leading to replisome collapse, replication can be re-initiated via re-assembly of the replisome onto the arrested forks. This replication restart process is essential to ensure complete replication of the bacterial chromosome during the cell cycle (Sandler and Marians, 2000). Both initiation and re-initiation mechanisms aim at recruiting and loading the hexameric replicative helicase on ssDNA before the other replisome components. The chromosomal initiation and re-initiation sites of DNA replication, i.e. oriC and randomly arrested forks, are specified by the DnaA and PriA proteins respectively (Marians, 2000). DnaA, PriA and the replicative helicase are widely conserved in bacteria and their characterization in E. coli and in B. subtilis has demonstrated their functional identity (Moriya et al., 1999; Marians, 2000; Polard et al., 2002). In both bacteria, DnaA and PriA are assisted by accessory proteins to recruit and load their cognate replicative helicases. Nevertheless, these intermediate initiation proteins are not conserved between E. coli and B. subtilis and appear functionally different.
The E. coli DnaA and PriA pathways share one primosomal protein, DnaCEc, which physically associates with the DnaBEc replicative helicase to form a heterododecameric complex [DnaBEc-DnaCEc]6−6 (Wahle et al., 1989). DnaCEc is referred to as the helicase loader, as it controls the loading of the ring-shaped DnaBEc hexamer around ssDNA by a ring-opening mechanism (Davey and O’Donnell, 2003). In contrast to DnaA, PriA is assisted by three additional proteins, namely PriB, PriC and DnaT, to recruit the [DnaBEc-DnaCEc]6−6 complex at the arrested fork (Marians, 2000). Interestingly, defects because of the lack of PriA can be suppressed by point mutations in DnaCEc, which can also bypass the need for PriB, PriC and DnaT (Sandler et al., 1996; 1999; Xu and Marians, 2000).
The B. subtilis DnaA and PriA pathways require the same set of three accessory proteins to recruit and load the DnaC replicative helicase (be aware of the confusing nomenclature between the E. coli and B. subtilis replication proteins). These are the DnaD, DnaB and DnaI primosomal effectors, which are essential for cell viability. Recently, we have shown that DnaB and DnaI cooperate to direct the loading of the hexameric DnaC replicative helicase around ssDNA by a ring-assembly mechanism in vitro (Velten et al., 2003). DnaB and DnaI interact physically and independently with the DnaC helicase. DnaI is essential for the functional loading of DnaC monomers on forked DNA and DnaB provides an additional interaction with the DNA to stimulate this loading step. We have previously mapped suppressors of the lack of PriA in the coding sequence of the dnaB gene, the most frequently obtained being the dnaB75 allele (Bruand et al., 2001). Interestingly, we observed that plasmidic circular ssDNA molecules produced by rolling-circle replication become more efficiently converted to dsDNA in the dnaB75 mutant than in wild-type cells (Bruand et al., 2001). This suggested that ssDNA becomes an efficient substrate to initiate DNA replication in vivo in the presence of the DnaB75 protein. The much higher stimulation of the DnaI-dependent helicase activity by DnaB75 than by DnaB in vitro supports this hypothesis, as it reflects a much more efficient loading of DnaC helicase onto ssDNA (Velten et al., 2003).
The role of DnaD is less well defined. This dimeric protein was reported to interact with DnaA in a two-hybrid assay (Ishigo-oka et al., 2001). We showed that purified DnaD displayed ssDNA binding activity (Marsin et al., 2001). This activity appeared to be higher on PriA-bound forked DNA, pointing at a functional interplay between DnaD and PriA. Furthermore, the affinity of DnaB for ssDNA appeared increased in the presence of DnaD, indicating that DnaD could also interact with DnaB.
Here, we have further investigated the role of DnaD by combining a genetic and biochemical characterization of the temperature sensitive mutant dnaD23. The incapability of growing at high temperature of the dnaD23 strain could be suppressed by several distinct point mutations in dnaB. Interestingly, we had previously characterized the same DnaB derivatives as suppressors of priA null mutants (Bruand et al., 2001). In both cases, the dnaB75 allele was the most frequently arising mutant. In parallel, we showed that DnaD physically interacts with PriA and DnaB in solution. We demonstrated that the dnaD gene cannot be disrupted in the dnaB75 mutant, but that the amount of DnaD molecules needed for growth is lower in dnaB75 cells than in wild-type cells. In parallel, we observed in vitro that a main defect of DnaD23 is a decreased affinity for ssDNA, while the DnaB75 protein has gained affinity for ssDNA. Finally, we showed that these individual interactions with ssDNA are impeded and/or unstable when the substrate is prebound by the single-stranded DNA-binding protein SSB. However, a mixture of DnaD and DnaB75 was able to interact with SSB-coated ssDNA. The efficiency of this reaction gradually decreased by exchanging DnaD for DnaD23 or DnaB75 for DnaB. This points at the importance of the ssDNA binding activity exhibited by each of these two essential initiation proteins. Altogether, these results highlight the need for DnaD and DnaB to be able to interact with ssDNA in order to bind on SSB-coated ssDNA. We discuss this functional interplay between DnaD and DnaB as required for structurally orchestrating on ssDNA the early steps of initiation and re-initiation of chromosomal DNA replication.
Results
The amount of DnaD molecules is diminished in dnaD23 cells
DnaD has been characterized as an essential protein involved in initiation and re-initiation of DNA replication through the study of the temperature sensitive dnaD23 mutant strain (Gross et al., 1968; Bruand et al., 1995). The dnaD23 allele carries a single mutation changing a highly conserved alanine residue to a threonine (A166T). In this study, we have firstly analysed the consequence of this mutation on the quantity of the DnaD protein present in dnaD23 cells, grown at 30°C and 50°C (permissive and non-permissive temperatures for the dnaD23 strain respectively). For these experiments, antibodies to DnaD were used in Western blot analysis of total protein extracts prepared from wild-type and dnaD23 cells (see Experimental procedures). DnaD can be estimated at ∼3000–5000 molecules per wild-type cell grown at either 30°C or 50°C (Fig. 1A and B). In dnaD23 cells grown at 30°C, the DnaD23 protein was about fivefold less abundant (Fig. 1A). At 50°C, the same amount of DnaD23 was still detected, but its concentration could not be reliably estimated because of the cell growth arrest (not shown).
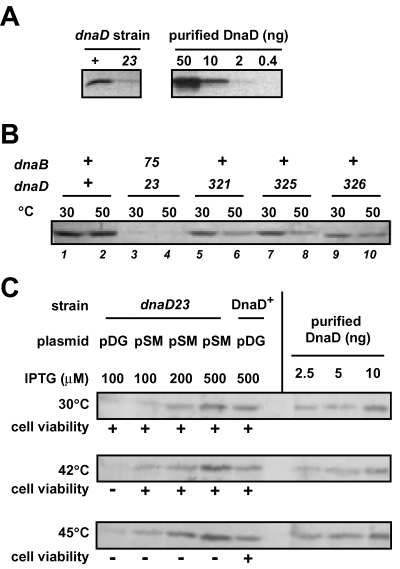
The DnaD23 mutant protein is a loss-of-function derivative of DnaD. A. Immunodetection of DnaD protein in wild-type and dnaD23 cells. Equivalent amounts of total protein extracts of strains 168 (wt) and PPBJ128 (dnaD23) grown at 30°C, together with a range of purified DnaD, were analysed by Western blot using antibodies raised against DnaD. B. Immunodetection of DnaD in intragenic and extragenic suppressors of the temperature sensitivity of dnaD23 cells. Equivalent amounts of total protein extracts of strains L1430 (wt), CBB320 (dnaD23 dnaB75), CBB321 (dnaD321), CBB325 (dnaD325), CBB326 (dnaD326) grown at 30°C or 50°C were analysed by Western blot with antibodies to DnaD. C. Effect of DnaD23 overexpression on the temperature sensitivity of the dnaD23 strain. The dnaD23 strains (PPBJ128) harbouring either the pDG148 vector (pDG) or its DnaD23-overexpressing derivative pSMG39 (pSM) were grown at 30, 42 or 45°C with various IPTG concentrations (indicated above the immunoblot). Total protein extracts were prepared and analysed by Western blot with antibodies to DnaD. The wild-type strain (168) harbouring the vector alone was similarly analysed. Cell viability at the different temperatures is reported as + (∼2.108 cfu ml−1/A600nm) or – (<10 cfu ml−1/A600nm) under the immunoblots.
Therefore, one of the consequences of the dnaD23 mutation is a nearly fivefold decrease of the amount of DnaD in the cell, even when growth is performed at the permissive temperature.
dnaD23 is a ‘loss-of-function’ mutation
To understand the defects caused by the dnaD23 mutation, we undertook the isolation of suppressor mutations of the temperature sensitivity of the dnaD23 strain. We isolated nine independent spontaneously arising derivatives of the dnaD23 strain able to grow at 51°C. Four were intragenic suppressors, four were extragenic suppressors and the last one was a revertant (see Experimental procedures). All suppressors restored a wild-type viability to the dnaD23 strain at 51°C, as measured by colony-forming ability, although the growth rate of extragenic suppressors was slightly lower than that of the wild-type strain (doubling time increased less than twofold; not shown).
Sequence analysis of the four intragenic suppressors of dnaD showed that they contained another point mutation in addition to the A166T change of the DnaD23 protein. Three different mutations were identified (Table 1): dnaD321 and dnaD325 are point mutations of the same codon resulting in different amino-acid substitutions, and dnaD326 is an in-frame deletion of two codons. Immunodetetection of the mutated DnaD derivatives encoded by the dnaD321, dnaD325 and dnaD326 alleles showed that, in all cases, a nearly wild-type concentration of DnaD was present at 30°C (Fig. 1B, lanes 5–10). The amount of DnaD molecules in the dnaD321, dnaD325 and dnaD326 strains grown at 50°C was lower than at 30°C, but still higher than in the dnaD23 strain grown at 30°C (Fig. 1A and B).
| Allele | Codon | Change | Independent clones |
|---|---|---|---|
| dnaD321 | 193 | Leu (CTT) to Val (GTT) | 1 |
| dnaD325 | 193 | Leu (CTT) to Ile (ATT) | 2 |
| dnaD326 | 155–156 | ΔGln-Asp (CCAGGA deletion) | 1 |
| dnaB75 | 371 | Ser (TCG) to Pro (CCG) | 4 |
Because a consequence of these intragenic suppressive mutations is an increase of DnaD concentration, we wondered whether the unique consequence of dnaD23 would be a quantitative defect because of a progressive decrease of the protein concentration with increasing temperature. Therefore, we tested whether overexpressing the DnaD23 protein could cure the temperature sensitivity of the dnaD23 strain. For this, the dnaD23 open reading frame and its translational signals were cloned under the control of the IPTG-inducible Pspac promoter in the rolling-circle plasmid pDG148 (giving plasmid pSMG39).
Surprisingly, dnaD23 cells harbouring the pDG148 vector alone presented an exacerbated temperature sensitivity, as they could not grow at 42°C (dnaD23 cells can sustain growth up to 45°C). We interpret this defect as a titration of DnaD by the plasmid, because its rolling-circle replication is known to depend on DnaD (Viret and Alonso, 1988). In contrast, when DnaD23 was overexpressed in dnaD23 cells from pSMG39, cells were viable at 42°C. Growth of this strain at 42°C was IPTG-dependent, 100 µM IPTG being the minimal amount required (not shown). At this IPTG concentration, DnaD23 amount was higher than in dnaD23 cells, and a wild-type level was reached at 200 µM IPTG, as shown by Western blot analysis (Fig. 1C). These observations show that at 42°C the activity of DnaD23 is limiting in dnaD23 cells, and can be compensated for by increasing the concentration of DnaD23. However, this did not hold true at 45°C and above whatever the IPTG concentration used. This growth failure was neither resulting from a reduction of stability nor from a defect of synthesis, of the DnaD23 protein at high temperature, as the protein concentration gradually increased in response to IPTG concentration, in a similar manner at all temperatures (Fig. 1C). Synthetizing even threefold more DnaD23 in dnaD23 cells than the natural level of DnaD in wild-type cells did not alleviate the temperature sensitivity at 45°C. Temperature sensitivity was not resulting from an excess of DnaD23 either, as the same overexpression of DnaD23 in wild-type cells was not accompanied by temperature sensitivity (not shown).
These results show that intragenic suppression of dnaD23 temperature sensitivity is not simply because of an increased amount of the mutated DnaD23 protein. Furthermore, this DnaD derivative is immunodetected at a similar amount at all temperatures tested. This indicates that DnaD23 is a ‘loss-of-function’ mutant of DnaD, one of its functions being sensitive to the temperature.
Extragenic suppressors of dnaD23 temperature sensitivity are dnaB mutants which bypass the need for PriA
The four extragenic suppressor mutations of dnaD23 temperature sensitivity were all mapped in the essential dnaB gene (Table 1 and Experimental procedures). All of them were found to carry the same point mutation in the coding sequence of DnaB, a serine to proline substitution (S371P). Interestingly, we had previously isolated the same dnaB mutation as the most frequently arising suppressor of priA mutants, namely the dnaB75 allele (Bruand et al., 2001). We demonstrated that the dnaB75 mutation is sufficient to cure dnaD23 temperature sensitivity (see Supplementary material, Fig.S1). We also demonstrated that the other dnaB alleles that we had previously isolated as suppressors of the defects of the priA null mutant, were suppressors of dnaD23 thermosensitivity as well (Fig.S1). Western blot analysis revealed that DnaD23 concentration in the dnaD23 dnaB75 double mutant, grown at 30°C or 50°C, was similar to the concentration of DnaD23 in the dnaD23 DnaB + strain grown at 30°C (i.e. fivefold lower than in the wild-type strain; Fig. 1B). Therefore, in contrast to the intragenic suppressors of dnaD23 temperature sensitivity, the dnaB75 mutation did not increase the concentration of DnaD23. Following the lead that DnaD23 is a ‘loss-of-function’ derivative of DnaD, it is tempting to speculate that in the dnaD23 dnaB75 cells, DnaD23 deficiency is compensated for by DnaB75, a ‘gain-of-function’ derivative of DnaB.
DnaD is essential for the growth of the dnaB75 strain
Suppression of dnaD23 temperature sensitivity by dnaB75 suggested the possibility that DnaD could be dispensable in the dnaB75 background. To test this hypothesis, we attempted to disrupt the dnaD gene using the non-replicative plasmid pCB321, which carries an erythromycine resistance marker (EmR) and an ∼1.5 kb DNA fragment encompassing the dnaD sequence disrupted by a spectinomycine resistance marker (SpcR). Transformation of dnaB75 cells to SpcR with pCB321 plasmid gave transformants with similar efficiencies as for DnaB + cells. In both cases all transformants were EmR, indicating that pCB321 never integrated into the chromosome by double crossing-over. This result strongly suggests that dnaD cannot be disrupted in the dnaB75 strain. To make sure that this failure was not a result of a polar effect of the disrupting insertion on the expression of the genes located downstream from dnaD, we constructed a strain carrying an ectopic copy of dnaD (at the amyE locus; strain CBB488). Transformation of this strain with pCB321 to SpcR gave 98% of EmS transformants (i.e. double crossing-over integration events). Six clones were further analysed, showing that the allelic copy of dnaD was disrupted in four of them and the ectopic copy twice. Thus, dnaD disruption has no polar effect on the dowstream genes.
These results show that DnaD exerts an essential function for viability in dnaB75 cells. This essential function is conserved in DnaD23 as proven by the growth of dnaB75 dnaD23 cells at high temperature.
Lower amount of wild-type DnaD is required for cell viability and initiation of extrachromosomal DNA replication in dnaB75 cells
Because DnaD is still essential in the dnaB75 strain, and because the dnaB75 mutation does not increase the cellular concentration of the DnaD23 protein, we tested whether less wild-type DnaD is required in a dnaB75 mutant.
To control DnaD expression from the allelic dnaD gene, its promoter was replaced by the IPTG-inducible Pspac promoter, giving the dnaDind gene (CBB462). Growth of this strain was IPTG-dependent, while that of a control strain (CBB482), in which dnaD remained under the control of its own promoter but in which the Pspac promoter was inserted immediately downstream from dnaD, was not (not shown). This showed that the IPTG dependence of CCB462 is only because of IPTG-dependent transcription of dnaD, not of the gene(s) located downstream from it. The lowest IPTG concentration giving fully viable dnaDind cells was ∼25 µM (Fig. 2A). However, when the dnaDind gene was introduced into the dnaB75 strain, the resulting strain (CBB464) was still fully viable in the presence of 12 µM IPTG and could even form small, slowly growing colonies in the complete absence of IPTG (Fig. 2A). This difference between DnaB+ and dnaB75 strains containing the dnaDind gene did not result from a different expression of the DnaD protein in the two backgrounds, as shown by Western blot analysis (Fig. 2B). Therefore, DnaD is needed in lower amount for the viability of dnaB75 cells than for DnaB + cells, providing another evidence of the functional interplay between DnaD and DnaB.
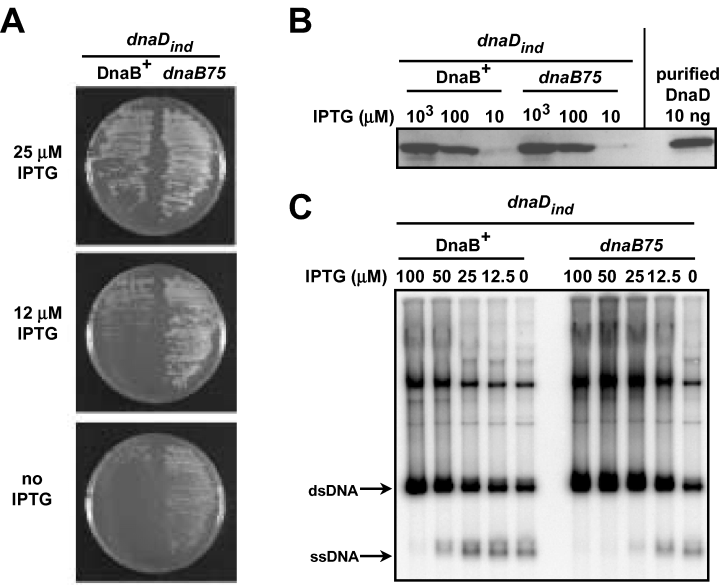
Lower amount of wild-type DnaD is required for cell viability and initiation of extrachromosomal DNA replication in dnaB75 cells. A. Amount of DnaD required for viability of dnaB75 compared to DnaB+ cells. The strains CBB462 (dnaDind DnaB+) and CBB464 (dnaDinddnaB75) grown at 37°C in the presence of 200 µM IPTG were streaked on agar plates of the same medium supplemented with the indicated IPTG concentrations, and incubated for 16 h at 37°C. B. Immunodetection of DnaD in dnaDind strains. Equivalent amounts of protein extracts (corresponding to ∼108 cells) of strains CBB462 (dnaDind DnaB+) and CBB464 (dnaDinddnaB75) grown at 37°C in the presence of 1000, 100, or 10 µM IPTG as indicated, were analysed by Western blotting with antibodies to DnaD. Purified DnaD (10 ng) was loaded as a control. C. Amount of DnaD required for initiation of DNA replication in dnaB75 cells compared to DnaB+ cells. Overnight cultures at 37°C of cells CBB462 (dnaDind DnaB+) and CBB464 (dnaDinddnaB75) carrying pVA798ΔRCR in the presence of 200 µM IPTG were diluted 103-fold in the same fresh medium supplemented with 100, 50, 25, 12.5 or 0 µM IPTG as indicated, and grown up to A600nm∼1. Total DNA was prepared and analysed by Southern blot using 32P-labelled pVA798ΔRCR as a probe.
Next, we wondered whether initiation of DNA replication requires less DnaD in dnaB75 than in DnaB+ cells. To answer to this question we used the pVA798ΔRCR plasmid, a member of the pAMβ1 family, whose dsDNA is replicated co-ordinately by the cellular replisome, as is chromosomal DNA. The replisome is assembled on an early D-loop intermediate of the plasmid in a PriA-dependent and PriA-independent way in wild-type and dnaB75 cells respectively (Bruand et al., 1995; 2001; Polard et al., 2002). A deficiency in PriA, DnaB, DnaD or DnaI, as well as in a protein of the replisome, reduces the plasmid copy number and causes the appearance of ssDNA corresponding to the lagging strand template (Ceglowski et al., 1993; Bruand et al., 1995; Dervyn et al., 2001; Polard et al., 2002). Here, we modulated cellular DnaD synthesis with the use of the IPTG-inducible dnaDind gene (see above) to estimate the minimal amount of DnaD required for pVA798ΔRCR replication in DnaB+ and dnaB75 cells. We observed that plasmid ssDNA appears in both strains upon DnaD depletion, concomitant with a reduction of the copy number of the plasmid (Fig. 2C). However, these two defects appeared at a lower IPTG concentration in dnaB75 cells than in DnaB+ cells (Fig. 2C). This shows that pVA798ΔRCR replication requires lower amounts of DnaD in a dnaB75 strain than in a wild-type strain, as does presumably chromosomal replication. This would explain why a lower amount of DnaD is required for the growth of the dnaB75 cells.
The DnaD protein interacts physically with PriA and DnaB in vitro
The observation that the same DnaB mutations can suppress the temperature sensitivity of the dnaD23 mutant and bypass the need for PriA led us to hypothesize that PriA, DnaD and DnaB might interact with each other. We had previously tested this hypothesis by gel filtration analysis using purified proteins, but failed to detect any interaction (Marsin et al., 2001). Here, using higher initial protein concentrations, we observed that DnaD and DnaB interact in solution (Fig. 3A). Their rather weak physical association was demonstrated by the shift towards higher molecular weight of their individual elution profile from the sizing column when mixed together (Fig. 3A, compare fractions 8, 9 and 10). We also observed an interaction between PriA and DnaD, their individual elution profile being clearly modified upon mixing (Fig. 3B, compare fractions 11, 12 and 13). Finally, we did not detect any interaction between DnaB and PriA by gel filtration (Fig. 3C). Therefore, these data indicate that DnaD interact physically with both PriA and DnaB. In addition, it appears that the amount of DnaD interacting with DnaB is lower than with PriA, indicating that DnaD–PriA interaction is tighter than the DnaB–DnaD interaction in those conditions.
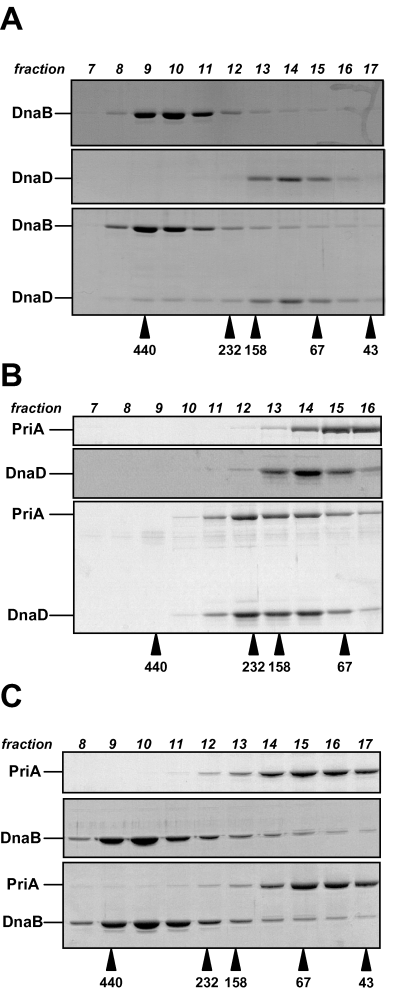
DnaD protein interacts physically with DnaB and PriA in solution. Gel filtration analysis of physical interactions between DnaD, DnaB and PriA proteins. Proteins were incubated, either separately or in combination for 1 h at 4°C, and then loaded onto a Superdex 200 HR10/30 column. Protein fractions of 0.5 ml were collected and analysed by SDS-PAGE. Gels stained with Coomassie blue are presented. A. 20 µM of DnaD and 10 µM of DnaB. B. 20 µM of DnaD and 10 µM of PriA. C. 10 µM of DnaB and 10 µM of PriA. The protein concentrations are expressed in monomers. Arrows beneath the gels denote the elution position and the molecular masses (in kDa) of the protein standards used for the column calibration.
The DnaD23 protein displays diminished and temperature sensitive ssDNA binding activity
Genetic analysis led to the hypothesis that the DnaD23 protein was a ‘loss-of-function’ derivative of DnaD compensated for by the DnaB75 protein. Therefore, we undertook the in vitro study of the DnaD23 and DnaB75 proteins in comparison with their wild-type counterparts.
We had previously reported that the wild-type DnaD protein is a dimer able to bind onto a ssDNA oligonucleotide in a band-shift assay (Marsin et al., 2001; this study, Fig. 4A). Here, the ssDNA binding activity of DnaD was further supported by a gel filtration assay, showing DnaD interaction on large circular ssDNA molecules (see Supplementary material, Fig.S2). We purified DnaD23 and showed by gel filtration analysis that it was also a homogeneous dimer (not shown), indicating that the A166T mutation in the protein did not dramatically modify its structure. Next, we tested by band-shift assay the ability of purified DnaD23 to bind ssDNA, an appropriate method for quantifying its ssDNA binding activity by measuring the remaining unbound ssDNA probe over an increased range of protein. The ssDNA substrate used throughout this study was a 90 nts long polydT oligonucleotide. We found that DnaD23 was able to bind ssDNA but with lower affinity than wild-type DnaD (Fig. 4A; see Fig. 4C for quantification). The band-shift patterns obtained with the two proteins were different: the ssDNA probe was trapped by wild-type DnaD in high molecular weight species (HMW) barely entering the gel; HMW were also observed with DnaD23, but at higher protein concentration and together with the formation of discrete intermediate shifted bands (Fig. 4A). These binding experiments were performed at 25°C. Knowing the temperature sensitivity of the dnaD23 strain, we reproduced the band-shift assay at 42°C. Remarkably, the ssDNA binding activity of DnaD23 appeared to be temperature sensitive (Fig. 4A and B; see Fig. 4C for quantification). Indeed, while the overall band-shift pattern observed with DnaD23 was similar at 42°C and 25°C (as judged by the typical intermediate shifted bands in addition to the HMW species), a higher protein concentration was required to shift the ssDNA at 42°C than at 25°C (Fig. 4B and C for quantification). The band-shift assays performed with the wild-type DnaD protein at 25°C and at 42°C gave identical results (Fig. 4A–C). The lack of intermediate shifted bands with wild-type DnaD in comparison to DnaD23 could be explained by a cooperative binding of DnaD onto ssDNA to produce efficiently the HMW species. If true, DnaD23 would be defective and temperature sensitive for this activity. The main conclusions coming out from these band-shift assays are that DnaD23 can still bind ssDNA but that its ssDNA binding activity is diminished and temperature sensitive compared to that of wild-type DnaD.
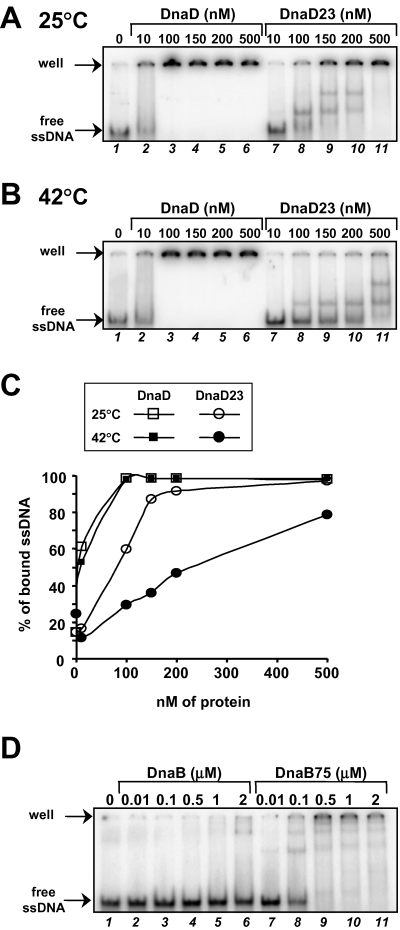
ssDNA binding activities of the DnaD23 and DnaB75 proteins. A and B. Band-shift analysis of ssDNA binding activity of DnaD and DnaD23 at 25°C and 42°C respectively. The proteins were mixed at the concentrations indicated above the wells (in nM of monomers) with 0.1 nM of radiolabelled ssDNA (a 90 nts polydT oligonucleotide), incubated for 10 min at the indicated temperature, and then the mixtures were submitted to native 5% PAGE at 4°C. Autoradiography of dried gels are presented. C. Quantification of the band-shift experiments presented in (A) and (B): the percentage of bound ssDNA was plotted as a function of protein concentration. D. Band-shift analysis of ssDNA binding activity of DnaB and DnaB75. The experiment was performed as above, except that the incubation of the proteins with ssDNA was carried out at 30°C.
Because temperature sensitivity of the dnaD23 strain can be cured by the dnaB75 mutation, we wondered what was the ssDNA binding activity of the DnaB75 protein compared with DnaB. We tested this by a band-shift assay similar to that used for DnaD/D23 proteins. Remarkably, we found that DnaB75 was more than 50-fold more efficient than DnaB to bind ssDNA (Fig. 4D).
These results provide a simple explanation for the defects observed in the temperature sensitive dnaD23 strain and their suppression by the dnaB75 allele: the decreased ssDNA-binding activity of DnaD23 compared to wild-type DnaD could be compensated for by an increased ssDNA-binding activity of its DnaB partner in the DnaB75 derivative.
DnaD and DnaB75 proteins cooperate to bind onto SSB-coated ssDNA
The respective ‘loss-’ and ‘gain-of-function’ in DnaD23 and DnaB75 proteins for interaction with ssDNA point to their ssDNA binding activities as being key functional features for their essential role in DNA replication. In the cell, ssDNA is covered by the single stranded DNA binding protein (SSB), which creates a barrier against the binding of other proteins. Therefore, we tested whether SSB impeded the binding of DnaB/B75 and DnaD/D23 proteins to ssDNA by band-shift analysis.
First of all, we analysed binding of B. subtilis SSB onto the 90 nts long ssDNA probe, in order to determine the saturating protein concentration and, thereby, to obtain the SSB-coated ssDNA substrate. As shown in Fig. 5A, increasing SSB concentration was accompanied by the successive formation of two bands, designated SSB1 and SSB2, that we interpret as the result of binding one and two SSB tetramers respectively. Next, we tested the binding of DnaB/DnaB75 and DnaD/DnaD23 proteins onto the SSB-coated ssDNA substrate SSB2 [prepared by preincubation of ssDNA with a saturating concentration of SSB (10 nM); Fig. 5B, lane 2]. We found that DnaB75, used at concentrations supporting an efficient binding onto the free ssDNA probe (4, 5, lane 12), did not modify the band-shift pattern obtained with SSB alone (Fig. 5B, compare lane 2 with lanes 6–8). The same result was obtained with DnaB (Fig. 5B, lanes 3–5). Similarly, DnaD (and DnaD23) did not produce the typical HMW species with the ssDNA when coated by SSB (Fig. 5C and D, lanes 2–4). Therefore, SSB appears to impede the binding of DnaB/B75 proteins as well as that of DnaD/DnaD23 proteins onto ssDNA.
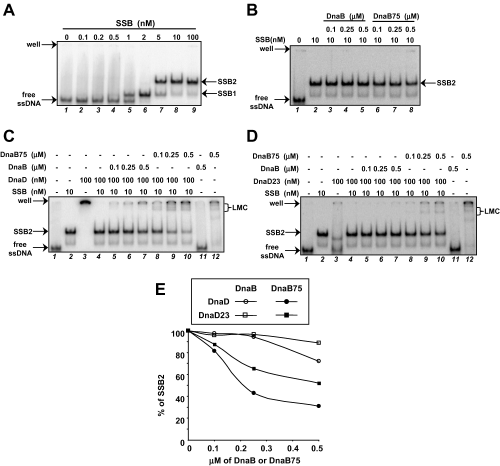
DnaD and DnaB cooperate to bind SSB-coated ssDNA.Band-shift assays were performed as in Fig. 4D. A. Preparation of the SSB-coated ssDNA substrate. Increasing amounts of SSB were incubated with the probe, and the SSB-coated ssDNA substrate was then defined as being ‘SSB2’, obtained by incubating of 0.1 nM of the probe with 10 nM of SSB (in monomer). B. SSB impedes interaction of DnaB and DnaB75 with ssDNA. ssDNA was incubated with saturating amount of SSB (10 nM of monomer) before the addition of either DnaB or DnaB75 as indicated at the top of the gel. C and D. DnaD and DnaB cooperate to bind onto the SSB-coated ssDNA. A range of DnaB/DnaB75, with or without DnaD (C) or DnaD23 (D), was mixed with the SSB-coated ssDNA substrate ‘SSB2’. Low Mobility Complexes, i.e. LMC, characteristic of the DnaB–DnaD interaction, are pointed in brackets on the right of the gels. E. Quantification of the free SSB-coated ssDNA ‘SSB2’ in the distinct DnaD/DnaD23-DnaB/DnaB75 mixtures assayed in (C) and (D). The percentage of ‘SSB2’ quantified in each experiment is reported as a function of the concentration of DnaB/DnaB75, the concentration in DnaD/DnaD23 being constant (100 nM).
Next, we tested whether mixtures of DnaB/B75 and DnaD/D23 proteins could modify the mobility of the SSB2 species in the band-shift assay. Remarkably, we observed that mixing DnaD with DnaB75 led to the formation of new shifted bands of low mobility (LMC) together with HMW. The efficiency of LMC formation was proportional to the DnaB75 concentration and at the expense of SSB2 (Fig. 5C, lanes 2 and 8–10 and Fig. 5E). A similar observation was made with the DnaB-DnaD pair, although LMC formation was less efficient than with the DnaB75-DnaD pair (Fig. 5C, lanes 5–7 and Fig. 5E). This difference between DnaB75 and DnaB for their DnaD-dependent binding onto SSB-coated ssDNA most probably reflected their different affinities for ssDNA. To evaluate the importance of the ssDNA binding activity of DnaD in this ‘DnaD-DnaB/B75′-cooperation to interact with SSB-coated ssDNA, we reproduced these band-shift assays with DnaD23 that presents a lower individual affinity for naked ssDNA than DnaD (compare lanes 3 in Fig. 5C and D). It appeared that DnaD23 was less efficient than DnaD to produce LMC in the presence of DnaB75 and was unable to produce LMC with DnaB (Fig. 5D, lanes 8–10 and 5–7 respectively). Finally, quantification of these band-shift experiments showed that the strongest decrease in the amount of SSB2 band (i.e. the most efficient LMC formation) was obtained with the DnaB75/DnaD combination (Fig. 5E). These results strongly support the conclusion that both DnaD and DnaB ssDNA binding activities are involved in the concerted interaction of DnaD and DnaB with SSB-coated ssDNA.
Altogether, these results lead to the conclusion that the physical and functional interactions between DnaD and DnaB are aimed at getting accessibility to and/or a better stability on ssDNA in the presence of SSB.
Discussion
DnaD is an essential B. subtilis protein, required for initiation and for re-initiation of chromosomal DNA replication (Gross et al., 1968; Bruand et al., 1995; 2001; this study). DnaD shares this dual role with two other essential B. subtilis proteins, DnaB and DnaI. Consequently, the three of them were proposed to act either with DnaA or with PriA to drive the DnaC replicative helicase at oriC or at arrested forks respectively (Moriya et al., 1999; Polard et al., 2002). Recently, we have described the functions of DnaI and DnaB in the mechanism of DnaC helicase delivery on ssDNA in vitro (Velten et al., 2003). DnaI is strictly essential to the functional loading of DnaC helicase on ssDNA, a loading step activated by DnaB. In the present study, we have investigated the enigmatic role of DnaD. We previously reported that, in vitro, DnaD displays ssDNA binding activity and seems to assist DnaB in interacting with ssDNA (Marsin et al., 2001). We show here, through the genetic and biochemical study of the DnaD23 mutant protein, that these two features of DnaD are centrally needed for its essential biological role. This role appears mainly structural and aimed at stabilizing and/or providing access at DnaB on SSB-coated ssDNA.
We show that the ssDNA binding activity of DnaD23 is diminished and temperature sensitive in comparison with DnaD. dnaD23 cells can neither grow, nor initiate and re-initiate chromosomal DNA replication at high temperature. These defects are suppressed by point mutations in the DnaB protein. Remarkably, we report here that the DnaB75 protein, one of these suppressors, has gained strong affinity for ssDNA in comparison with wild-type DnaB. This result leads to the proposal that DnaB carries a cryptic ssDNA binding activity. Therefore, temperature sensitivity of the dnaD23 strain could be explained by a decrease of ssDNA affinity of the DnaD23 protein, a failure which can be compensated for by the DnaB75 protein. This balance effect is most probably direct, because we show here that DnaD and DnaB interact physically in solution. A lower requirement of DnaD amount in dnaB75 cells in vivo further supports this balance effect between the ssDNA binding activities of DnaB and DnaD in vitro. Together, these results highlight the necessity for DnaD to interact with ssDNA as well as with DnaB to exert its essential role during initiation and re-initiation of DNA replication.
Interestingly, defects associated with the dnaD23 mutation are not fully bypassed by the dnaB75 mutation. Indeed, we have previously shown that the DnaB75-mediated conversion of the ssDNA circular intermediate of rolling-circle plasmid into dsDNA is temperature sensitive in dnaD23 cells (Bruand et al., 2001). This contrasts with the suppression of cell viability and of initiation and re-initiation of chromosomal DNA replication at high temperature in the same dnaB75 dnaD23 cells (this study). Thus, the dnaD23 mutant could not support an elevated number of DnaD-dependent replication events at high temperature even with the dnaB75 suppressor. Consequently, DnaB75 can attenuate but not totally bypass all the defect(s) of the DnaD23 protein. In addition, we show that the amount of DnaD23 is diminished about fivefold in dnaD23 cells when compared to wild-type cells, even at a temperature permissive for growth. In dnaB75 dnaD23 cells, the amount of DnaD23 remains equally diminished at low and high temperature. This indicates that the suppression of dnaD23 defects by DnaB75 does not proceed by a stabilization of the DnaD23 protein. Furthermore, overexpression of DnaD23 in dnaD23 cells does not cure temperature sensitivity for the growth, implying that the defects at high temperature in dnaD23 are not resulting from the low amount of DnaD23 protein but from the loss of its essential function(s). Therefore, everything concurs to the proposal that the ssDNA binding activity of DnaD is centrally needed for a specific and discrete event in the initiation process, which is different from the event requiring the ssDNA binding activity of DnaB75 (cryptic within DnaB).
What are the functions specifically associated with the ssDNA binding activities of DnaD and DnaB? Concerning DnaB, we have recently reported that DnaB75 better activates the DnaI-dependent loading of the DnaC helicase on ssDNA than the wild-type DnaB (Velten et al., 2003). This shows that the cryptic ssDNA binding activity of DnaB is central to its coloader function of the DnaC helicase. Concerning the role of the ssDNA binding activity carried by DnaD, we show here that it is necessary for DnaD to cooperate with DnaB75 in order to interact with SSB-coated ssDNA. The efficiency of this DnaD-DnaB75 cooperation is diminished with the DnaD23 protein and even more with the wild-type DnaB, correlating with the individual level of affinity for ssDNA of each of these proteins. Therefore, we suggest that a key role of DnaD, requiring its ssDNA binding activity, is to stabilize and/or to assist the interaction of its DnaB partner with SSB-coated ssDNA.
How to replace the functional DnaD-DnaB interplay in a biological frame? An important clue is given by the efficient conversion of the circular ssDNA intermediate of rolling-circle plasmid into dsDNA mediated by the dnaB75 mutant, which is dependent on DnaD, DnaI and DnaC helicase (Bruand et al., 2001). Molecularly, this reflects the loading of DnaC helicase mediated by DnaI and DnaB75 on ssDNA. Once loaded, the helicase recruits the primase to trigger DNA synthesis. Therefore, ssDNA appears to be an efficient and sufficient substrate for initiating DNA replication in dnaB75 cells. This phenomenon highlights a gain of function of the DnaB75 protein, which is to promote the initiation of DNA replication on any ssDNA. In the cell, this plasmidic ssDNA substrate is expected to be covered by SSB. We report here that SSB impedes the binding of DnaB75 on ssDNA, forming a protein barrier that appears overcome with the help of its DnaD partner. Consequently, DnaD would act in concert with DnaB75 in order to promote the DnaI-dependent loading of the DnaC helicase on ssDNA in the presence of SSB. This model further explains another property of the dnaB75 allele, which is to bypass the need of PriA in vivo (Bruand et al., 2001). PriA directs helicase loading onto three-stranded DNA molecules covered by SSB that mimick replication forks with the lagging strand template single-stranded (Marians, 2000). These forked substrates include the ssDNA template onto which DnaB75 promotes helicase loading with DnaI (Velten et al., 2003). We propose that this helicase delivery relies on a prior concerted binding of DnaD and DnaB75 on the SSB-coated ssDNA at the fork.
In wild-type cells, ssDNA is not a substrate for initiation of DNA replication, although it is the ultimate substrate for the loading of the helicase mediated by DnaI and DnaB. Helicase loading occurs at oriC and arrested forks and requires the action of DnaA and PriA respectively. We show here that DnaD and DnaB do cooperate, while less efficiently than DnaD and DnaB75, to interact with SSB-coated ssDNA. The efficiency of this reaction could be conditional on unmasking the cryptic ssDNA binding activity of DnaB, the apparent consequence of the mutation in the DnaB75 derivative. Therefore, PriA and DnaA could participate with DnaD in assisting the binding of wild-type DnaB on SSB-coated ssDNA, in order to restrict the loading of the replicative helicase at their binding sites. We show here that PriA interacts physically with DnaD but not with DnaB. It has been reported that DnaA can interact with DnaD (Ishigo-oka et al., 2001). Thus, a central issue of this work is to understand how is orchestrated the interaction of DnaD and DnaB on SSB-coated ssDNA at arrested forks with PriA, as well as at ori C with DnaA. Another important related perspective is to explain precisely how DnaD and DnaB75 cooperate to interact efficiently with SSB-coated ssDNA substrate. Precisely, it will be important to know (i) if DnaD and DnaB act sequentially or simultaneously in this interaction, (ii) if they both remain bound to the substrate and (iii) if they remove SSB from the subtrate upon their binding. This latter point is central to fully understand the process of helicase loading, which appears strictly dependent on DnaI and activated by DnaB (Velten et al., 2003). In E. coli, a point mutant of the helicase loader DnaCEc, DnaCEc810, characterized as a suppressor of priA null mutants, has been shown to promote helicase loading on SSB-coated ssDNA without the need of any other initiation proteins (Xu and Marians, 2000). This gain of function exhibited by DnaCEc810 has been explained by an increase of its cryptic ssDNA binding activity. We have come to the same proposal for the B. subtilis DnaB75 mutant protein, the main suppressor of priA null mutants, which has gained strong ssDNA-binding activity when assayed alone onto naked ssDNA. Its interaction with SSB-coated ssDNA is dependent upon the presence of the initiation protein DnaD. Distinctively, the binding of DnaCEc810 on SSB-coated ssDNA strictly depends on its interaction with the replicative helicase (Xu and Marians, 2000). Importantly, it has also been shown that SSB is displaced from ssDNA upon the cobinding of DnaCEc810 and the helicase. We are currently testing whether this is also the case for the B. subtilis proteins, by adding in the reaction the replicative helicase and the DnaI protein. In addition, it is worth mentioning a recent parallel and complementary study conducted on DnaB, DnaD, and the DnaD23 and DnaB75 mutants, which points at the control of DNA replication exercised through the timely regulated physical interaction between these two proteins at the cell membrane (Rokop et al., 2004). In conclusion, the interaction between DnaD and DnaB would underlie a key mechanistic step of the initiation and re-initiation of DNA replication and a way to appropriately control these two essential processes during the cell-cycle.
Experimental procedures
Strains and plasmids
The strains and plasmids used in this study are listed in Table 2. Strains and plasmids used for constructions are listed in TableS1 (see Supplementary material), together with the details of the constructions. All strains were cultivated in LB medium, supplemented when required with erythromycin (Em, 0.6 µg ml−1), chloramphenicol (Cm, 4 µg ml−1), spectinomycin (Spc, 60 µg ml−1), kanamycin (Km, 5 µg ml−1), ampicillin (Ap, 60 µg ml−1) and IPTG. DNA transformations were performed as described (Polard et al., 2002). To determine cell viability in Fig. 1C, the strains were grown at 30°C up to A600∼0.5–1, the cultures were then serial diluted and plated onto fresh medium supplemented with IPTG at the indicated concentrations, and plates were incubated ∼14 h at the indicated temperatures. Colonies were counted and results were expressed as colony-forming units (cfu) ml−1 A600−1.
| Strain/plasmid | Relevant features | Reference, source |
|---|---|---|
| B. subtilis | ||
| 168 | trpC2 | C. Anagnostopoulos |
| L1430 | ilvA metC lys21 | Maüel and Karamata (1984) |
| L1434 | metC lys21 dnaD23 | Maüel and Karamata (1984) |
| CBB319,320 322 324 | L1434 dnaD23 dnaB75 | This worka |
| CBB321 | L1434 dnaD321 | This worka |
| CBB323, 325 | L1434 dnaD325 | This worka |
| CBB326 | L1434 dnaD326 | This worka |
| CBB462 | 168 Pspac-dnaD + pMAP65 | This workb |
| CBB464 | 168 dnaB75 Pspac-dnaD + pMAP65 | This workb |
| CBB482 | 168 Pspac-nth + pMAP65 | This workb |
| CBB488 | 168 amyE::Pspac-dnaD | This workb |
| CBB671 | L1434 nth::pSG05(CmR) | This workb |
| PPBJ128 | 168 trpC + dnaD23 | This workb |
| Plasmids | ||
| pVA798ΔRCR | PstI-KpnI deletion derivative of pIP501 | Pujol et al. (1998) |
| pMAP65 | pUB110 penP-lacI | Petit et al. (1998) |
| pCB321 | pMTL20E-dnaD::SpcR | This workb |
| pDG148 | pBR322- and pC194-based hybrid vector | Joseph et al. (2001) |
| pSMG39 | pDG148::dnaD23 | This workb |
- a . See Table 1 and Experimental procedures.
- b . See Supplementary material.
DNA Techniques
Total DNA preparations from B. subtilis and Southern blot analysis were performed as described (Bruand et al., 1995). DNA sequencing was performed on PCR products or plasmid templates using the PRISM sequencing kit and an automated DNA sequencing apparatus (Applied Biosystems).
Isolation and mapping of mutations suppressing dnaD23 temperature sensitivity
Twelve clones of strain L1434 (dnaD23) were cultivated at 37°C overnight and 100 µl of the resulting culture was plated and incubated overnight at 51°C. One to 150 thermoresistant clones were obtained for nine of the cultures. One clone was retained from each plate and tested as revertants of the dnaD23 mutation, or as intra- or extragenic suppressor mutations. For this, we measured the genetic linkage between the dnaD23 mutation and the allele conferring thermoresistance: chromosomal DNA of these strains was used to transform to thermoresistance a dnaD23 mutant that carries a CmR marker ∼0.3 kb downstream of the dnaD23 gene (strain CBB671), and the thermoresistant transformants were tested for resistance to Cm. In five cases 27–50% of the transformants were CmS, indicating that the mutation conferring thermoresistance was close to or into the dnaD23 gene itself. The presence of the mutations in the gene coding sequence was shown by DNA sequencing: four intragenic mutations (strains CBB321, 323, 325, 326) and one revertant were found; Tables 1 and 2. However, in four cases (strains CBB319, 320, 322, 324), 96–100% of the transformants were CmR, indicating that the mutation conferring thermoresistance in these clones was not linked to dnaD23, and was an extragenic suppressor. To map these extragenic suppressor mutations, we asked whether they were located in genes encoding other initiation proteins. We first tested the ability of DNA segments corresponding to the dnaB gene of these strains, generated by PCR, to transform a dnaD23 mutant to thermoresistance. Transformants were obtained in every case, suggesting that the mutation was located in the dnaB gene. This was confirmed by sequence determination of the dnaB gene in these strains, which was proved to be the dnaB75 allele in all cases (Tables 1 and 2).
Immunodetection of DnaD
Rabbits antisera directed against the purified DnaD protein were prepared by Eurogentec. For the Western blot analysis presented in Fig. 1. Bacillus subtilis strains were grown overnight at 30°C, then diluted 102-fold in fresh medium and grown up to A600∼0.5–1 at the indicated temperature. In Fig. 2, strains were grown overnight at 37°C in the presence of 1 mM IPTG, then diluted 103-fold in fresh medium supplemented with IPTG at the indicated concentration, and grown up to A600∼0.5–1 at 37°C. In all cases, total protein extracts were fractionated by 12.5% SDS-PAGE, and transferred onto PVDF membranes (Hybond P, Amersham) by semidry electroblotting. Membranes were incubated with antibodies to DnaD and immunodetection was performed using the ECL+ kit (Amersham) with HRP-coupled protein G (Bio-Rad). Images of the blots were obtained with a StormTM apparatus (Molecular Dynamics) and quantification was performed with the ImageQuant software. Intracellular amount of DnaD was estimated by comparing the signal obtained with cell lysates with the signals obtained with known amounts of purified DnaD protein. The protein concentration per cell is expressed as the amount of protein per living cell estimated by plating and colonies formation. Antibodies to DnaD were shown to react identically against purified DnaD and DnaD23 proteins.
Protein purification
DnaD, DnaB, DnaB75 and SSB were purified as previously described (Marsin et al., 2001) using the IMPACT system (NEB). DnaD23 was purified as DnaD (with the expression plasmid pSMG22*D23), except that the expression step was performed at 16°C. The yield of protein was found to be threefold lower for DnaD23 than for DnaD. DnaB and SSB proteins were also expressed as wild-type proteins (i.e. without any added tag) in E. coli and purified as detailed in Supplementary materials.
Protein concentrations were determined by the Bradford method (Bio-Rad) as recommended by the supplier.
Gel filtration chromatography
Gel filtration chromatography experiments were carried out at 4°C using an FPLC system (APB). Samples were incubated for 1 h at 4°C, in a final volume of 600 µl, centrifuged for 15 min at 10 000 g at 4°C and then 500 µl were loaded onto a Superdex 200 HR 10/30 column (APB) pre-equilibrated in buffer 50 mM Tris-Cl [pH 8], 100 mM NaCl, 1 mM DTT (Fig. 3B and C) or in 50 mM Tris-Cl [pH 7.5], 50 mM NaCl, 2 mM MgCl2, 1 mM DTT (Fig. 3A). Fractions of 0.5 ml were collected at a flow rate of 0.4 ml min−1. Twenty microlitre of each fraction was analysed by 12.5% SDS-PAGE and Coomassie blue staining. The column was calibrated using proteins with known molecular masses and Stokes radii, respectively (APB): ferritin (440 kDa; 61 Å), catalase (232 kDa; 52.2 Å), aldolase (158 kDa; 48.1 Å), albumin (67 kDa; 35.5 Å), ovalbumin (43 kDa; 30.5 Å).
Band-shift assays
The same 32P-radiolabelled ssDNA–probe was used all along this study, i.e. a 90 nts long polydT oligonucleotide. Proteins were mixed on ice in 50 mM Tris pH 7.8, 10 mM NaOAc, 10 mM MgOAc, 1 mM DTT, 200 µg ml−1 BSA, in a final volume of 20 µl. Next, 0.1 nM of the 32P-ssDNA probe [preincubated with SSB (10 nM) 10 min at 30°C in experiments of Fig. 5B–D] was added and the samples were incubated at 30°C (unless otherwise stated) for 10 min. After cooling on ice, each sample (13 µl) was loaded directly on a native 5% acrylamide gel and run at 4°C for 3 h. Gels were dried under vacuum and revealed with a Storm apparatus (Molecular Dynamics). For the quantification, we measured the ratio of the radioactivity contained in the band of interest (either the free probe or the ‘SSB2’ species) and the radioactivity contained in the whole lane. These ratio are reported as percentage, as a function of initial protein concentration.
Acknowledgements
We thank Bénédicte Michel, David R. F. Leach and Philippe Noirot for critical reading of the manuscript and M.-A. Petit for helpful discussions. P.P. is on the CNRS staff.
Supplementary material
The following material is available from http://www.blackwellpublishing.com/products/journals/suppmat/mmi/mmi4451/mmi4451sm.htm
Fig. S1. Temperature sensitivity of dnaD23 cells can be cured by dnaB suppressors of priA null mutants.
Fig. S2. Interaction of DnaD with M13 ssDNA.
Table S1. Strains, plasmids and phages used in this study.
Appendix S1. Experimental procedures.




