The contribution of MvfR to Pseudomonas aeruginosa pathogenesis and quorum sensing circuitry regulation: multiple quorum sensing-regulated genes are modulated without affecting lasRI, rhlRI or the production of N-acyl- l-homoserine lactones
Summary
The transcriptional regulator MvfR is required for full Pseudomonas aeruginosa virulence, the function of multiple quorum sensing (QS)-regulated virulence factors and the synthesis of 4-hydroxy-2-alkylquinolines (HAQs), including the Pseudomonas quinolone signal (PQS). Here we investigate the role of MvfR in the QS circuitry and P. aeruginosa pathogenesis. We demonstrate using a combination of biochemical and molecular approaches, including transcription profiling, that MvfR is involved in the regulation of multiple P. aeruginosa QS-controlled genes without altering the expression of lasRI/rhlRI or the production of N-acyl- l-homoserine lactone (AHL) signals. Dissection of how mvfR is interwoven into the P. aeruginosa QS circuitry reveals that the MvfR system, through the essential contribution of PqsE, positively regulates a subset of genes dependant on both LasR and RhlR. Animal studies show that MvfR contributes to P. aeruginosa virulence by controlling the transcription of genes not under RhlR regulation, and that reduced virulence of a mvfR mutant is caused by the loss of pqsE expression and not only a deficiency in HAQs/PQS production. This study provides novel insights into the unique role of the MvfR system in AHL-mediated QS and further supports its importance in P. aeruginosa pathogenesis.
Introduction
The versatile and ubiquitous opportunistic pathogen Pseudomonas aeruginosa is the leading cause of Gram-negative infections in immunocompromised individuals and those suffering from cystic fibrosis. As in many eubacteria, the expression of numerous virulence factors produced by this bacterium is controlled in a cell density-dependent manner via small diffusible signalling molecules (Van Delden and Iglewski, 1998; de Kievit and Iglewski, 2000), a process known as ‘quorum sensing’ (QS) (Fuqua et al., 2001). QS relies on the activation of specific transcriptional regulators by their corresponding autoinducers, such as N-acyl- l-homoserine lactones (AHLs) (Pesci and Iglewski, 1999), which function as intercellular signals. In P. aeruginosa, at least two separate QS systems (termed las and rhl), each comprised of an AHL synthase (LasI or RhlI) and its cognate transcriptional regulator (LasR or RhlR), modulate gene transcription in response to increasing AHL concentrations (Passador et al., 1993; Brint and Ohman, 1995; Latifi et al., 1995; Ochsner and Reiser, 1995; Pearson et al., 1995; Pesci et al., 1997). The major products of LasI and RhlI are N-(3-oxododecanoyl)- l-homoserine lactone (oxo-C12-HSL) and N-butanoyl- l-homoserine lactone (C4-HSL) respectively (Passador et al., 1993; Pearson et al., 1995). These two systems together comprise a hierarchical cascade (where las regulates rhl) that co-ordinates the expression of numerous genes, many of which encode virulence and survival factors, as well as AHL production (Latifi et al., 1996; Pesci et al., 1997). A third intercellular signalling molecule has been reported, the Pseudomonas quinolone signal (PQS) (Pesci et al., 1999). While PQS seems involved in the QS hierarchy and required for the expression of RhlR-dependent genes at the onset of the stationary phase, its function is still undetermined (Pesci et al., 1999; McKnight et al., 2000; Diggle et al., 2003).
Using the multihost pathogenesis system that we developed to find new P. aeruginosa virulence factors (Rahme et al., 2000), we have identified the QS-associated transcriptional regulator MvfR. This regulator is required for full P. aeruginosa virulence in multiple hosts, and MvfR inactivation affects the production of a number of QS-regulated virulence factors (e.g. pyocyanin, elastolytic proteases) and unidentified secreted compounds (Rahme et al., 1997; Cao et al., 2001). We have found that a mvfR mutant does not produce PQS and that mvfR controls the synthesis of 4-hydroxy-2-alkylquinolines (HAQs), a family of compounds released by P. aeruginosa starting at the end of exponential growth (Lépine et al., 2003; Déziel et al., 2004). HAQs are synthesized via the action of products from the co-regulated mvfR-controlled operons pqsABCD and phnAB (Cao et al., 2001; Gallagher et al., 2002; Déziel et al., 2004; McGrath et al., 2004). The pqs operon also includes pqsE whose function is unknown as a strain with a mutation in this gene is not defective in the production of HAQs (Gallagher et al., 2002; Déziel et al., 2004). While one of the HAQs, 4-hydroxy-2-heptylquinoline (HHQ), is the direct precursor of PQS (Déziel et al., 2004), the conversion of HHQ into PQS is regulated by LasR instead of MvfR, probably through the activity of the LasR-controlled enzyme PqsH (Gallagher et al., 2002; Déziel et al., 2004). Both HHQ and PQS mediate a cell-to-cell communication process distinct from AHL-mediated QS (Déziel et al., 2004).
In this study, we unveil the relationship between the MvfR and the AHL-mediated QS regulatory systems in P. aeruginosa. Our data show that mvfR plays a role in the regulation of multiple QS-controlled genes without affecting the expression of lasRI/rhlRI, and AHL production, supporting a model where the regulatory activity of the MvfR system is dependent on both PqsE and HAQs. Using a mice infection model, we further show that the MvfR system is important in P. aeruginosa pathogenesis independently of the rhl QS system.
Results
Production of AHLs is not affected in the mvfR mutant
Although the MvfR transcriptional regulator influences the expression of a number of QS-controlled genes and phenotypes in P. aeruginosa, the transcription of lasR and rhlR is not affected in a mvfR mutant (Cao et al., 2001). To better understand how MvfR modulates the expression of QS-controlled genes and possibly determine its mode of action, we first assessed whether the regulatory activity of MvfR might occur at a post-transcriptional level. As presented in Fig. 1A, the expression of lasI′–lacZ and rhlI′–lacZ translational fusions is the same in PA14 wild-type and mvfR mutant backgrounds, suggesting that AHL synthesis is normal in the mvfR mutant. To further confirm that mvfR does not modulate QS-regulated genes by affecting lasRI and rhlRI gene expression and resulting activity, we performed LC/MS analysis for oxo-C12-HSL and C4-HSL in culture supernatants. As shown in Fig. 1B, the total normalized concentrations of these AHLs are not significantly different between cultures of PA14 and the mvfR mutant. Similar results are obtained using the Chromobacterium violaceum CV26 bioassay (data not shown).
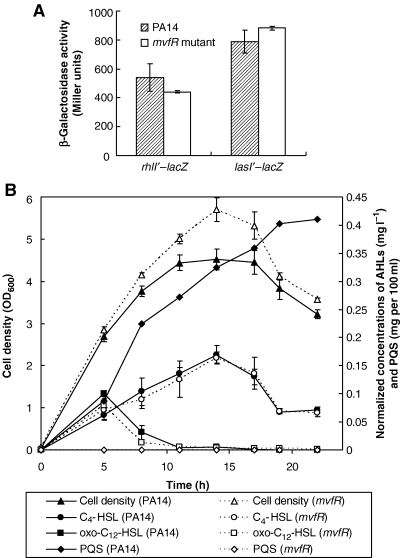
Expression of autoinducer synthases, and production of AHLs and PQS by P. aeruginosa PA14 and its mvfR mutant. A. Specific β-galactosidase activity (Miller units/OD600) of PA14 and the mvfR mutant carrying lasI′–lacZ and rhlI′–lacZ translational fusions assayed in LB at 37°C. Shown is a sampling time at 5 h. B. The concentrations of oxo-C12-HSL, C4-HSL and PQS determined in LB cultures by LC/MS analysis. Values are means of triplicates normalized over the sample OD600 ± SD, except PQS production shown as a control.
Presence of HAQs, including PQS, does not overcome the absence of mvfR or pqsE
We have recently shown that MvfR controls the synthesis of HAQs, a large family of extracellular compounds that includes HHQ, the precursor of PQS (Déziel et al., 2004). To assess whether any of these compounds, which comprise signalling molecules, actually mediate the regulatory activity of MvfR, we investigated the effect of the three principal members of the various congener series of HAQs on the transcription of the phz1 operon, a target of the MvfR regulatory system involved in the synthesis of pyocyanin (Déziel et al., 2004; and Table 1). HHQ, its hydroxylated product PQS or its N-oxide derivative HQNO all fail to activate transcription from the phzA1 promoter in mvfR and pqsE mutant backgrounds (Fig. 2). These results indicate that the function of MvfR is not limited to the control of HAQ synthesis and, furthermore, that pqsE is essential for the activity of the MvfR regulatory system.
| Identifierb | OD600a | Annotationd | Probable function/other features | Predicted operonse | K-means setf | ||
|---|---|---|---|---|---|---|---|
| 1.5c | 2.5 | 3.5 | |||||
| QS regulatedg | |||||||
| PA0122 | 1.9 | Conserved hypothetical protein with homology to haemolysin from Aspergillus fumigatus | Set 6 | ||||
| PA0355 | 2.6 | Intracellular protease PfpI | Set 5 | ||||
| PA0567 | 2.1 | Conserved hypothetical protein | Set 5 | ||||
| PA0996 | 4.9 | 16.1 | 25.2 | pqsA, probable coenzyme A ligase | HAQ biosynthesis | PA0996-PA1000 | Set 3 |
| PA0997 | 47.2 | 109.0 | 41.2 | pqsB, hypothetical protein | HAQ biosynthesis | Idem | Set 3 |
| PA0998 | 306.7 | 157.8 | 211.5 | pqsC, hypothetical protein/similarity to 3-oxoacyl-[acyl-carrier-protein] synthase III | HAQ biosynthesis | Idem | Set 3 |
| PA0999 | 33.9 | 27.8 | 27.4 | pqsD, 3-oxoacyl-[acyl-carrier-protein] synthase III | HAQ biosynthesis | Idem | Set 3 |
| PA1000 | 171.6 | 79.9 | 30.8 | pqsE, hypothetical protein | Quinolone signal response | Idem | Set 3 |
| PA1001 | 54.7 | 44.6 | 17.0 | phnA, anthranilate synthase component I | Anthranilic acid and HAQ biosynthesis | PA1001-PA1002 | Set 3 |
| PA1002 | 29.4 | 13.0 | 14.0 | phnB, anthranilate synthase component II | Anthranilic acid and HAQ biosynthesis | Idem | Set 3 |
| PA1003 | 2.0 | 2.2 | 2.7 | mvfR, transcriptional regulator/LysR family | Regulation of pqsA–E and phnAB operons/HAQ biosynthesis/virulence | Set 3 | |
| PA1216 | 4.1 | Hypothetical protein | Similarity to glycine methyl transferase | PA1221-PA1216* | Set 2 | ||
| PA1431 | 1.3 | rsaL | Repressor of lasI AHL synthase (de Kievit et al., 1999) | Set 6 | |||
| PA1656 | 3.6 | Hypothetical protein | PA1656-PA1659* | Set 3 | |||
| PA1914 | 3.4 | Homology to halovibrin HvnA NAD+-glycohydrolase from Vibrio fischeri (Stabb et al., 2001) | Toxin? | Set 6 | |||
| PA1984 | −1.9 | Probable aldehyde dehydrogenase | Set 10 | ||||
| PA2031 | 2.6 | Hypothetical protein | PA2030-PA2031* | Set 6 | |||
| PA2067 | 1.8 | Probable hydrolase | PA2069-PA2066* | Set 6 | |||
| PA2069 | 3.2 | Probable carbamoyl transferase | Idem | Set 6 | |||
| PA2134 | 1.9 | Hypothetical protein | PA2134-PA2135* | Set 5 | |||
| PA2146 | 13.2 | 2.9 | Conserved hypothetical protein | Set 5 | |||
| PA2159 | 2.0 | Conserved hypothetical protein | Set 5 | ||||
| PA2172 | 7.0 | Hypothetical protein/similarity to glucanase | PA2171-PA2173* | Set 5 | |||
| PA2173 | 4.4 | Hypothetical protein | Idem | Set 5 | |||
| PA2193 | 2.6 | hcnA, hydrogen cyanide synthase HcnA | Toxin | PA2193-PA2195 | Set 4 | ||
| PA2194 | 2.2 | 2.9 | hcnB, hydrogen cyanide synthase HcnB | Same | Idem | Set 4 | |
| PA2195 | 2.5 | hcnC, hydrogen cyanide synthase HcnC | Same | Idem | Set 4 | ||
| PA2274 | 2.9 | 5.0 | 16.4 | Probable monoxygenase (Sciara et al., 2003) | Polyketide antibiotic synthesis | Set 4 | |
| PA2300 | 3.8 | 5.7 | chiC, chitinase | Carbon compound catabolism, virulence | Set 6 | ||
| PA2327 | −1.6 | Probable permease of ABC transporter | PA2331-PA2327* | Set 2 | |||
| PA2329 | −1.6 | 2.7 | Probable ATP-binding component of ABC transporter | Idem | Set 6 | ||
| PA2331 | −2.1 | 2.5 | Hypothetical membrane protein/similarity to macrophage infectivity potentiator of L. pneumophila | Idem | Set 6 | ||
| PA2570 | 9.4 | lecA, galactose-specific PA-I lectin | Adhesin and cytotoxin | Set 6 | |||
| PA2747 | 2.1 | Hypothetical protein | Set 5 | ||||
| PA2753 | 1.5 | Hypothetical protein | Set 8 | ||||
| PA3186 | −2.1 | oprB, glucose/carbohydrate outer membrane porin OprB precursor | PA3189-PA3186* | Set 1 | |||
| PA3189 | −4.6 | Probable permease of ABC sugar transporter | Idem | Set 10 | |||
| PA3195 | −2.3 | −2.4 | Glyceraldehyde 3-phosphate dehydrogenase | Set 1 | |||
| PA3335 | −1.9 | Hypothetical protein | Set 1 | ||||
| PA3361 | 6.8 | lecB, fucose-binding lectin PA-IIL | Adhesin and cytotoxin | Set 4 | |||
| PA3369 | 2.4 | Hypothetical protein | Set 5 | ||||
| PA3370 | 2.7 | Hypothetical protein | PA3370-PA3371 | Set 5 | |||
| PA3371 | 2.6 | Hypothetical protein | Idem | Set 5 | |||
| PA3392 | −3.8 | nosZ, nitrous-oxide reductase precursor | PA3391-PA3396* | Set 10 | |||
| PA3478 | 2.1 | 2.8 | rhlB, rhamnosyltransferase 1 | Rhamnolipid biosynthesis | PA3479-PA3478* | Set 6 | |
| PA3520 | 5.1 | copP, heavy-metal-associated-containing periplasmic metal-binding protein/copper chaperone (Adaikkalam and Swarup, 2002) | Set 6 | ||||
| PA3678 | 4.0 | 4.1 | mexL, transcriptional regulator of TetR family (Chuanchuen et al., 2002) | Repressor of the mexJK RND efflux pump (PA3676-77) | Set 3 | ||
| PA3691 | 2.7 | Hypothetical protein | PA3691-PA3692 | Set 5 | |||
| PA3692 | 2.5 | Probable outer membrane protein precursor | Similarity to porin F precursor | Idem | Set 5 | ||
| PA3721 | 4.7 | Transcriptional regulator nalC (Cao et al., 2004) | Repressor of PA3719-20/inactivation results in overexpression of MexAB-OprM efflux pump | Set 3 | |||
| PA4078 | 3.2 | Probable non-ribosomal peptide synthetase with AMP binding domain | PA4078 | Set 6 | |||
| PA4141 | 2.1 | 2.6 | Hypothetical protein | Set 6 | |||
| PA4205 | 16.4 | 29.0 | 85.2 | mexG, hypothetical protein/similarity to quinoline oxidase complex protein DoxD | RND family efflux pump (Aendekerk et al., 2002) | PA4205-PA4208 | Set 4 |
| PA4206 | 7.8 | 23.1 | mexH, probable RND efflux membrane fusion protein precursor | Same | Idem | Set 4 | |
| PA4207 | 11.7 | mexI, probable RND efflux transporter | Same | Idem | Set 4 | ||
| PA4208 | 20.2 | opmD, probable outer membrane protein precursor | Same | Idem | Set 4 | ||
| PA4209 | 7.1 | 3.9 | phzM, probable O-methyltransferase | Phenazine biosynthesis | Set 2 | ||
| ig(4713795−4713098)h | 14.1 | Intergenic region between phzA1 (PA4210) and phzM (PA4209) (phzM promoter?) | Set 2 | ||||
| PA4211i | 11.2 | 8.2 | phzB1, probable phenazine biosynthesis protein | Same | PA4210-PA4216* | Set 2 | |
| PA4212 | 8.6 | 8.1 | phzC1, phenazine biosynthesis protein PhzC | Same | Idem | Set 2 | |
| PA4213 | 6.2 | 7.4 | phzD1, phenazine biosynthesis protein PhzD | Same | Idem | Set 2 | |
| PA4214 | 11.6 | 7.0 | phzE1, phenazine biosynthesis protein PhzE | Same | Idem | Set 2 | |
| PA4215 | 9.5 | 15.0 | phzF1, probable phenazine biosynthesis protein | Same | Idem | Set 2 | |
| PA4216 | 16.9 | 12.0 | phzG1, probable pyridoxamine 5′-phosphate oxidase | Same | Idem | Set 2 | |
| PA4217 | 10.6 | 8.7 | phzS, FAD-dependent monooxygenase | Same | Set 2 | ||
| PA4587 | 1.8 | ccpR, cytochrome c551 peroxidase precursor | Set 10 | ||||
| PA4739 | 4.2 | Conserved hypothetical protein | PA4739-PA4738* | Set 9 | |||
| PA4876 | 2.6 | Osmotically inducible lipoprotein OsmE | Set 5 | ||||
| PA4880 | 2.9 | Probable bacterioferritin | Set 5 | ||||
| PA4917 | 1.7 | Hypothetical protein | PA4917-PA4916* | Set 3 | |||
| PA5027 | 1.8 | Hypothetical protein | Universal stress protein signature | Set 3 | |||
| PA5481 | 4.6 | Hypothetical protein | PA5482-PA5481 | Set 9 | |||
| PA5482 | 4.6 | Hypothetical protein | Idem | Set 9 | |||
| Not among published QS-regulated genes | |||||||
| PA0038 | 1.8 | Hypothetical protein | Set 5 | ||||
| PA0048 | −2.5 | Probable transcriptional regulator (MerR family) | Set 1 | ||||
| PA0049 | −2.8 | Hypothetical protein | Set 1 | ||||
| PA0200 | 1.8 | 11.1 | Hypothetical protein | Set 7 | |||
| PA0283 | 2.9 | Sulphate-binding protein precursor/transport of small molecules | Set 6 | ||||
| PA0284 | 8.5 | Hypothetical protein | Set 7 | ||||
| PA0336 | 1.5 | Nudix hydrolase YgdP | PA0336-PA0338* | Set 8 | |||
| PA0387 | 1.3 | Hypothetical protein | PA0388-PA0387* | Set 2 | |||
| PA0456 | 1.8 | Probable cold-shock protein | Set 8 | ||||
| PA0589 | 1.8 | Conserved hypothetical protein | PA0592-PA0589* | Set 8 | |||
| PA0590 | 1.4 | apaH, bis(5′-nucleosyl)-tetraphosphatase | Idem | Set 8 | |||
| PA0591 | 2.0 | Conserved hypothetical protein | Idem | Set 3 | |||
| Ig(727608−721556_s) | −3.4 | Ribosomal RNA cluster between PA0669 and PA0668 (opposite strand) | Set 1 | ||||
| PA0762 | 1.8 | algU, ECF sigma factor AlgU | PA0762-PA0766* | Set 9 | |||
| PA0807 | 2.0 | Conserved hypothetical protein | Set 9 | ||||
| PA0905 | 1.6 | rsmA, global post-transcriptional regulator | Modulation of virulence factors and AHL synthesis | Set 9 | |||
| PA1024 | −4.3 | Homology to 2-nitropropane dioxygenase | Probable electron transfer flavoprotein | PA1020-PA1024* | Set 1 | ||
| PA1333 | 1.9 | Hypothetical protein | Set 3 | ||||
| PA1430 | 2.2 | Transcriptional regulator LasR | Quorum sensing regulator | Set 2 | |||
| PA1456 | 1.6 | 1.6 | cheY, two-component response regulator CheY | PA1456-PA1457* | Set 3 | ||
| PA1556 | 2.1 | Probable cytochrome c oxidase subunit | PA1557-PA1556* | Set 10 | |||
| PA1673 | 1.5 | Hypothetical protein/similarity to oxygen transporter protein hemerythrin | Iron-binding protein | Set 8 | |||
| PA1776 | 1.4 | Probable sigma-70 factor, ECF subfamily | Set 9 | ||||
| tRNA-His | −2.3 | Set 1 | |||||
| PA1852 | −2.4 | Hypothetical protein | Set 6 | ||||
| PA2204 | 10.4 | Probable binding protein component of ABC transporter/extracellular solute binding protein signature | Set 7 | ||||
| PA2299 | 2.5 | Probable transcriptional regulator | GntR family signature | PA2299-PA2298* | Set 4 | ||
| PA2486 | 2.3 | Hypothetical protein | PA2485-PA2486* | Set 5 | |||
| PA2501 | 1.7 | Hypothetical protein | Set 3 | ||||
| tRNA-Cys | 1.6 | 3.4 | Set 3 | ||||
| PA2620 | 1.6 | clpA, ATP-binding protease component ClpA | Stress response | PA2621-PA2620* | Set 5 | ||
| PA2662 | −4.1 | Conserved hypothetical protein | PA2664-PA2662* | Set 10 | |||
| PA2663 | −3.6 | Hypothetical protein | Idem | Set 10 | |||
| PA2754 | 1.7 | Conserved hypothetical protein | Set 9 | ||||
| tRNA-Ser | 2.2 | 4.0 | Set 4 | ||||
| PA2885 | 1.8 | Probable transcriptional regulator/TetR family | Set 8 | ||||
| PA3031 | 1.7 | Hypothetical protein | Set 5 | ||||
| PA3126 | 1.4 | Heat-shock protein IbpA | Set 8 | ||||
| PA3351 | 1.3 | 1.4 | Hypothetical protein | Similarity to FlgM anti-sigma factor | PA3351-PA3353* | Set 2 | |
| PA3403 | −2.0 | Hypothetical membrane protein | Set 1 | ||||
| PA3531 | 1.8 | Bacterioferritin | Set 8 | ||||
| PA3569 | −2.2 | mmsB, 3-hydroxyisobutyrate dehydrogenase (NAD+) | Valine metabolism | PA3570-PA3569 | Set 1 | ||
| PA3812 | 4.8 | iscA, probable iron-binding protein IscA | Iron-sulphur cluster assembly and heat-shock chaperones (Zheng et al., 1998) | PA3815-PA3808* | Set 7 | ||
| PA3813 | 4.6 | iscU, probable iron-binding protein IscU | Same | Idem | Set 7 | ||
| PA3973 | 1.7 | Probable transcriptional regulator/TetR family | PA3973-PA3970* | Set 8 | |||
| PA4079 | 3.7 | Probable secreted dehydrogenase | Set 6 | ||||
| tRNA-Trp | −2.0 | Set 1 | |||||
| tRNA-Tyr | 1.9 | Set 4 | |||||
| PA4352 | 1.7 | Conserved hypothetical protein | Universal stress protein family | Set 4 | |||
| PA4387 | 1.5 | Conserved hypothetical protein | Set 8 | ||||
| ig(4956028−4956733) | −4.0 | Intergenic region between PA4421 and PA4422 | Set 1 | ||||
| PA4441 | 1.2 | Hypothetical protein | Set 8 | ||||
| PA4500 | 1.6 | Probable binding protein component of ABC transporter | Set 8 | ||||
| PA4542 | 1.5 | ClpB heat-shock chaperone | Set 8 | ||||
| PA4793 | 1.5 | Hypothetical protein | PA4793-PA4796* | Set 8 | |||
| PA4877 | 3.0 | Hypothetical protein | Set 5 | ||||
| PA4919 | 1.7 | pncB1, nicotinate phosphoribosyltransferase | PA4918-PA4920* | Set 8 | |||
| PA4940 | 1.5 | Conserved hypothetical protein | PA4940-PA4938* | Set 3 | |||
| PA5015 | 1.2 | aceE, pyruvate dehydrogenase | Set 8 | ||||
| PA5053 | 1.8 | hslV, heat-shock protein HslV | PA5053-PA5054 | Set 8 | |||
| PA5054 | 1.6 | hslU, heat-shock protein HslU | Idem | Set 8 | |||
| PA5170 | 1.7 | arcD, arginine/ornithine anti-porter | PA5170-PA5171 | Set 8 | |||
| PA5171 | 1.4 | arcA, arginine deiminase | Idem | Set 3 | |||
| PA5212 | 2.3 | Hypothetical protein | Set 9 | ||||
| PA5303 | 2.4 | Conserved hypothetical protein | Amino acid catabolism | PA5304-PA5303* | Set 8 | ||
| PA5436 | −2.1 | Probable biotin carboxylase subunit of a transcarboxylase | PA5436-PA5435* | Set 10 | |||
| PA5461 | 1.2 | Hypothetical protein | Set 9 | ||||
| PA5495 | 1.6 | thrB, homoserine kinase | PA5494-PA5495* | Set 8 | |||
- a . The ratios represent expression levels in PA14 relative to that of the mvfR mutant at specific growth phase time points, and correspond to averages of the four signal log ratios calculated by MAS change call algorithm after inverse log transformation. Minus (−) sign indicates the value is higher in mvfR mutant.
- b . PA numbers are from http://www.pseudomonas.com (Stover et al., 2000).
- c . The numbers indicate the optical density (600 nm) of the cultures at time of sampling.
- d . Annotations are from http://www.pseudomonas.com (Stover et al., 2000) and PseuReCa (http://maine.ebi.ac.uk:8000/services/pseureca) (Weinel et al., 2003).
- e . Shown are predicted ORFs from http://www.cifn.unam.mx/moreno/pub/TUpredictions/Predictions (Moreno-Hagelsieb and Collado-Vides, 2002; Salgado et al., 2000), with minor modifications as indicated in materials and methods.
- * *Indicate at least one gene of that operon is missing from the list.
- f . The K-means set represent the grouping of 10 cluster K-means (Figure 3 and data not shown).
- g . These genes were identified as QS-regulated in previous transcriptome studies: (Hentzer et al., 2003; Schuster et al., 2003; Wagner et al., 2003– supplementary information not included).
- h . Represent Affymetrix probe set identification.
- i . The Affymetrix P. aeruginosa microarray contains probe sets for only one copy of each phzA-G gene, labeled ‘PA4210’ (phzA1), ‘PA4211’ (phzB1) and ‘PA1901’ through ‘PA1905’ (phzC2D2E2F2G2). Because only phzA and phzB have sequence differences between the two operons (Mavrodi et al., 2001), we can assume these two probe sets are accurately identified. Since the phzM and phzS genes border the phz1 operon and all nine phz genes necessary for pyocyanin synthesis show very low expression in the mvfR mutant, we have reassigned the PA1901-PA1905 labels to their corresponding phz1 names, since the probes can correspond to either of the two operons. Schuster et al. reached a similar conclusion, noting the absence of a las box in the promoter region of the phz2 operon (Schuster et al., 2003).
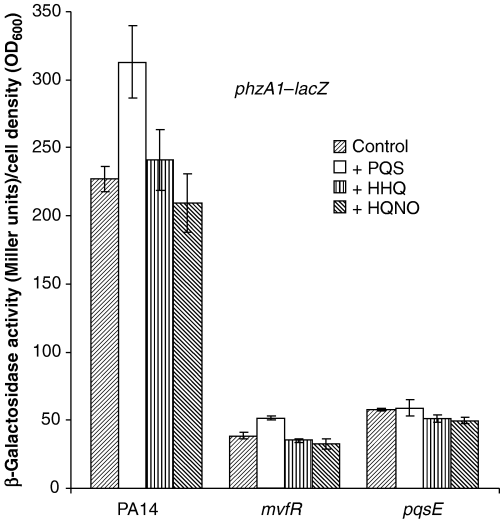
Addition of HAQs do not rescue mvfR and pqsE mutants. PQS, HHQ or HQNO were added from methanol stock solutions to a final concentration of 60 µM to LB cultures of PA14, mvfR and pqsE mutants carrying the phzABC1–lacZ reporter. An equal amount of methanol was added to the control cultures. Shown is a sampling time at 5 h. Data are shown as mean from triplicate experiments ± SD.
Identification of genes expressed differently in an mvfR mutant versus wild-type PA14
Transcriptome studies were therefore carried out to identify genes controlled by the transcriptional regulator MvfR, in order to gain insight into the function of this regulator and its role in the QS circuitry and P. aeruginosa virulence. Using the P. aeruginosa GeneChip®, whole-genome expression profiles were obtained for the wild-type PA14 strain and the isogenic mvfR mutant, at three time points corresponding to different bacterial growth stages from two independent experiments. Using the criteria outlined in Experimental procedures, we identified 141 genes as being differentially regulated, with 121 showing higher expression in wild-type PA14 compared with mvfR mutant, and 22 showing higher expression in the mvfR mutant relative to PA14 at least in one time point (Table 1). The signal values for these genes and the whole data set can be viewed using a GuiGraph template (see Experimental procedures) available as supplementary material (TableS1).
The K-means and GeneTree methods were used to cluster the differentially transcribed genes. In general, both clustering methods give results that agree well with each other, and most known co-regulated genes and transcriptional units cluster together. These analyses reveal the sequential activation of genes involved in the production of HAQs, phenazines, and other toxic and virulence-related factors, most of which are also known to be regulated by QS (Fig. 3). The salient features of these clustering analyses are summarized below.

GeneTree based on hierarchical clustering of genes differentially induced between P. aeruginosa PA14 and mvfR mutant of PA14. Numbers 1–3 represent samples at OD600 = 1.5, 2.5 and 3.5 respectively. Shown are median normalized values. The tree branches are coloured by the 10 classes identified in a K-means clustering analysis. The colouring scheme for the K-means sets and expression values are shown below the tree.
- (i)
Expression of the co-regulated pqs/phn operons is completely abolished in the mvfR mutant, as reported (Déziel et al., 2004). In addition, the mexGHI-opmD operon, which encodes a recently identified putative efflux pump of the Resistance/Nodulation/cell Division (RND) family (Aendekerk et al., 2002), maintains only background expression levels (Table 1 and Fig. 4A).
- (ii)
A second prominent differential response is exhibited by the phz1 phenazine biosynthetic gene cluster, which shows higher expression in PA14 cells peaking at late logarithmic/early stationary phase of growth (Fig. 4B). Two independent and highly homologous phzABCDEFG operons (98% identical at the DNA level) are present in P. aeruginosa (Mavrodi et al., 2001), and each of these biosynthetic operons is sufficient for phenazine-1-carboxylic acid (PCA) production for subsequent conversion to pyocyanin. This conversion requires phzM (PA4209) and phzS (PA4217), which are both single-copy genes (Mavrodi et al., 2001) and apparently co-regulated with the phz1 operon as they closely cluster (Fig. 4B and K-means set 2 in Fig. 3).
- (iii)
Many genes involved in the synthesis of virulence-related factors and toxic products, such as hydrogen cyanide (hcnABC), chitinase (chiC), lectins (lecA and lecB) and putative secondary metabolites (PA2274, PA4078), exhibit reduced activation (or transcriptional repression) in the mvfR mutant, at the stationary growth phases (Table 1 and Fig. 4C).

Expression levels of some prominent responses of P. aeruginosa PA14 dependent on MvfR function. mvfR (left) and PA14 (right) signal intensity values calculated by MAS 5.0 software (average of two independent experiments). A. mexGHI-opmD operon. B. Phenazine biosynthetic cluster. C. Virulence-related genes.
Interestingly, rsmA, which encodes a QS system regulator, and lasR, which encodes a QS regulator function, are differentially transcribed at one of the time points. Also, mvfR itself exhibits lower transcription in the mvfR mutant background (Table 1).
Within this analysis, no further generalization can be made regarding the remaining genes that are not present in previously reported QS transcriptome lists (results described below). Most of them display a differential expression at only one of the sampling times and/or encode hypothetical/unknown functions. Some of these genes may be related to QS or represent secondary effect derived from the differential expression of primary responsive genes.
Relationship between genes affected by MvfR and P. aeruginosa genes identified in previous genome-scale studies
We compared our list of differentially expressed genes with several previously reported large-scale studies of P. aeruginosa: a genome-wide transposon mutagenesis to identify genes involved in QS by reporter gene construct activation (Whiteley et al., 1999); a microarray analysis of genes differentially expressed in biofilm (Whiteley et al., 2001); GeneChip® analyses of genes differentially expressed in mucoid strains (Firoved and Deretic, 2003), under iron starvation (Ochsner et al., 2002), in las/rhl QS regulatory mutants (Hentzer et al., 2003; Schuster et al., 2003; Wagner et al., 2003), in a vqsR mutant (Juhas et al., 2004), under conditions that induce type III secretion systems (Wolfgang et al., 2003); and proteome analyses of magnesium starvation (Guina et al., 2003) and QS (Arevalo-Ferro et al., 2003).
As seen in Table 1 and Fig.S1, a large overlap exists between the genes differentially regulated by MvfR and previously reported QS transcriptomes (Hentzer et al., 2003; Schuster et al., 2003; Wagner et al., 2003). While this overlap is incomplete, the data sets in these three studies also show very significant areas of disagreement between each other (Fig.S1) (Hentzer et al., 2003; Vasil, 2003). Comparison of transcription profiling with the recently reported virulence and QS regulator VqsR (Juhas et al., 2004) shows a limited number of genes shared with the MvfR-modulated genes that are also QS regulated (22 in common out of 66 for mvfR and 57 for vqsR). As the inactivation of vqsR inhibits the production of AHLs (Juhas et al., 2004), it appears to modulate QS-regulated genes through a different route from the MvfR system. Beyond the differences in experimental designs and data analysis methodologies, use of a different strain from PAO1 in the latter study and our own might also have contributed to the observed differences. Nevertheless, we find a strong correlation between QS-controlled genes displaying reduced expression in the mvfR mutant and rhl-dependent genes requiring both autoinducers for full induction. These genes include the hcnABC and phzA1-G1 operons, and phzS, which were originally described as class III/IV QS-controlled transcriptional units (Whiteley et al., 1999). Other previously reported rhl-dependent QS-controlled genes/operons include lecA and lecB, rhlAB, chiC, PA2066-PA2069 and phzM (Winzer et al., 2000; Hentzer et al., 2003; Medina et al., 2003a), all of which belong to the mvfR transcriptome. Many of these QS-controlled genes encode virulence factors whose production had previously been shown to be reduced in mvfR mutants, such as pyocyanin, hydrogen cyanide (HCN) and LecA lectin (Rahme et al., 1997; Gallagher et al., 2002; Diggle et al., 2003). As MvfR controls the synthesis of PQS (Cao et al., 2001; Gallagher et al., 2002), this corroborates the recent observation that PQS is involved for the expression of primarily rhl-regulated genes (Diggle et al., 2003).
Although we had previously reported that lasR and rhlR mutations do not affect the activity of a mvfR–lacZ reporter (Cao et al., 2001), recent transcriptome studies have shown that LasR actually induces the transcription of mvfR in very early growth stages (Hentzer et al., 2003; Schuster et al., 2003). We recently resolved this inconsistency by the absence in our original lacZ construct of a critical las box found unusually high upstream in the mvfR promoter region (G. Xiao and L. G. Rahme, unpubl. data). Accordingly, the production of HAQs/PQS is reduced in lasR, but not rhlR, mutants (Pesci et al., 1999; Gallagher et al., 2002; Déziel et al., 2004). These observations collectively indicate that the MvfR system upregulates a subset of QS genes requiring both LasR and RhlR, while the las system is required for the expression of mvfR.
Finally, no significant overlaps with genes induced by mucoidy, genes preferentially upregulated in cells growing as biofilms, or genes differentially expressed during conditions inducing the type III secretion system, are observed. As discussed by the authors, there are major differences when comparing QS proteome with microarray data (Arevalo-Ferro et al., 2003). Therefore, it is not surprising that we see very limited correlation with our list, as only five QS-regulated proteins correspond to MvfR-modulated genes.
Gene fusion reporter validation of GeneChip® data
To confirm the transcriptomic results for genes not previously reported to be under MvfR (or PQS) regulation, we performed reporter expression assays. Using lacZ fusions, we show that transcription from the promoter region of mexGHI-opmD is almost abolished in the mvfR mutant (Fig. 5), in agreement with the microarray results (Fig. 4A). Furthermore, there is no activity in rhlR and pqsE mutant backgrounds, and delayed expression in a lasR mutant. Among the primary extracellular products whose synthesis depends on the rhl QS system are rhamnolipids (Ochsner et al., 1994; Pearson et al., 1997). A rhlA′–lacZ fusion reveals a delayed expression in the mvfR mutant (Fig. 6A), also manifested by a slightly postponed halo formation on blue rhamnolipid indicator plates (data not shown), in agreement with the presence of rhlB in Table 1 and previous observations (Diggle et al., 2003). We also investigated the expression of two regulators found in Table 1. Expression of the rsmA′–lacZ translational reporter is strongly reduced in both mvfR and pqsE mutant backgrounds (Fig. 6B), while mvfR seems to slightly autoregulate its own transcription (Fig. 6C). That the activity of the rsmA′–lacZ translational reporter is nearly abolished suggests an additional post-transcriptional level of regulation of this gene, as the transcription of rsmA is only slightly attenuated (Table 1). RsmA, an RNA-binding protein, acts as a post-transcriptional negative regulator of extracellular products by effecting message decay (Pessi et al., 2001). Whereas rsmA inactivation had only minor effects on elastolytic and staphylolytic activities as well as HCN, lectin and pyocyanin production, overexpression of this gene resulted in severe downregulation of these QS-regulated factors (Pessi et al., 2001). Interestingly, it has not been reported to be QS-regulated in any of the previous whole-genome profiling studies. More work is required to elucidate how the MvfR system might regulate RsmA activity and to which extent the mvfR regulon is affected by this regulator.
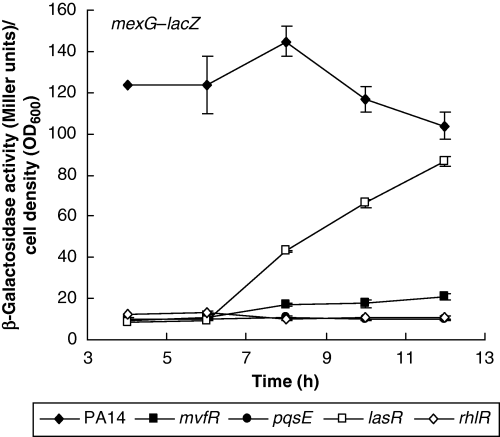
Transcription from the promoter of the mexGHI-opmD operon in P. aeruginosa PA14 and various mutants. Bacteria were grown in LB at 37°C and β-galactosidase activity was assayed at regular time intervals. Data are shown as mean ± SD from triplicate experiments.
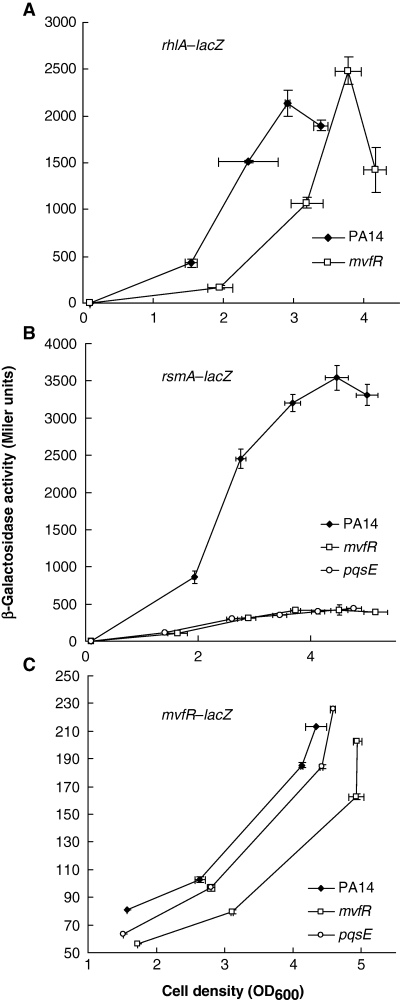
Effect of mvfR inactivation on the transcription/translation of some microarray-determined MvfR-dependent genes in P. aeruginosa PA14. Bacteria were grown in LB at 37°C and β-galactosidase activity was assayed at time intervals. A. rhlA′−lacZ. B. rsmA′−lacZ. C. mvfR−lacZ. Data are shown as mean ± SD from triplicate experiments.
Reduced pathogenicity of pqs operon mutants, but not of a rhlR mutant
lasR, mvfR and pqsB mutants display reduced pathogenicity in plants, nematodes, insects and mice (Rahme et al., 1997; Mahajan-Miklos et al., 1999; Tan et al., 1999; Jander et al., 2000; Cao et al., 2001). Accordingly, we find that pqsA and pqsE mutants also display attenuated virulence in mice with mortality rates similar to the mvfR mutant (Fig. 7), thus confirming the importance of these mvfR-dependant genes in mammalian pathogenesis. Importantly and unexpectedly, an isogenic rhlR mutant does not display a reduced virulence phenotype under the same experimental conditions (Fig. 7). These data indicate that the impaired virulence of the mvfR mutant in this model is mediated by factors regulated through the MvfR system and not overlapping with the rhl QS regulon.
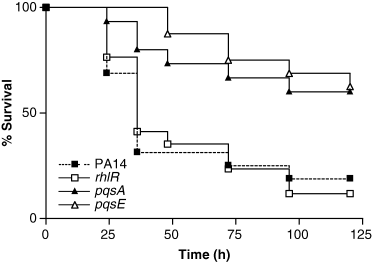
Five-day survival curves for mice after burn injury and infection. Inoculation with 105 cells of P. aeruginosa PA14 and pqsA, pqsE and rhlR mutants. n = 15–17 for each P. aeruginosa strain from two independent experiments.
Discussion
This study provides novel insights into the role of MvfR in AHL-mediated QS and pathogenesis of P. aeruginosa, and demonstrates that the MvfR system does not mediate its regulatory activity through lasRI, rhlRI or the production of AHLs. Furthermore, our results indicate that the MvfR system is important in P. aeruginosa pathogenesis independently of the rhl QS regulon and modulates additional genes whose precise combined function, or relevance to the QS circuitry, is still undefined. As presented below, several lines of evidence support these conclusions, and lead to a proposed integrative model.
MvfR is part of the QS system but is not involved in AHL-mediated gene regulation
The most striking feature of the mvfR transcriptome is the over-representation of QS-controlled genes (Table 1). Collectively, about 55% of the MvfR-dependent genes are regulated by QS in PAO1, whereas QS-controlled genes represent no more than 6–7% of the PAO1 genome (Schuster et al., 2003; Wagner et al., 2003), supporting our assertion that the MvfR system serves as a modulator of QS-regulated genes in P. aeruginosa (Cao et al., 2001). The moderate differences on transcription levels we see for a number of these QS-influenced genes possibly reflect the fact that these genes are under the control of more than one system, i.e. mvfR system and QS.
While the maximal expression of mvfR requires LasR, and also itself (Fig. 6C), several arguments indicate that the mechanism used by the MvfR system to regulate many QS-controlled genes does not operate via lasRI and rhlRI. First, while MvfR regulates the expression of genes requiring both LasR and RhlR, it does not alter the expression of these regulators (Cao et al., 2001) or, as we show in this study using a very precise LC/MS-based method of analysis, the extracellular levels of the C4-HSL and oxo-C12-HSL autoinducers. Our results agree with recent observations that mvfR inactivation and loss of PQS production actually do not alter rhlI transcription (Gallagher et al., 2002) and C4-HSL production (Diggle et al., 2003). Second, the mvfR mutation nearly abolishes the expression of many RhlR-dependent genes (e.g. phz1), suggesting that they require both rhlR and mvfR, while other RhlR-dependant genes seem less or not dependant on MvfR activity. For instance, we see a limited effect of MvfR on rhlAB expression (Table 1 and Fig. 6A) and rhamnolipid production, while the expression of this operon and rhamnolipid production are nearly abolished in a rhl mutant background (Ochsner et al., 1994; Pearson et al., 1997).
Results from this study and earlier reports (Pesci et al., 1999; McKnight et al., 2000) indicate that LasR controls HAQ and PQS synthesis on at least two separate levels. First, mvfR is only maximally transcribed in the presence of LasR and second, LasR controls pqsH (Whiteley et al., 1999), which is required for PQS synthesis (Gallagher et al., 2002; Déziel et al., 2004). Nevertheless, that the MvfR system does not operate through modulation of the las and rhl AHL-based QS systems implies an additional, independent level of regulation for many QS-controlled genes.
Reduced expression of mexGHI-opmD and other efflux pump-related genes
Besides the co-regulated operons involved in HAQ synthesis and response (pqsABCDE, phnAB) (Déziel et al., 2004), the other group of early differentially expressed genes in the mvfR mutant consist of the PA4205-PA4208 transcriptional unit, which corresponds to the mexGHI-opmD operon (Aendekerk et al., 2002) of unknown function. The expression of this operon is upregulated by both oxo-C12-HSL and C4-HSL in lasI/rhlI mutants (Whiteley et al., 1999; Schuster et al., 2003; Wagner et al., 2003). Inactivation of this putative efflux pump in PAO1 was reported to result in the decreased production of multiple virulence factors regulated by QS (Aendekerk et al., 2002). We show here that this operon is completely dependent on pqsE and rhlR, while its expression is only delayed in a lasR mutant (Fig. 5). Such a postponed response of lasR mutants has been observed previously for the production of pyocyanin (Diggle et al., 2002) and PQS (Diggle et al., 2003). As suggested by the microarray and lacZ reporter results, some delayed and very low transcription seems to occur in the mvfR mutant (Fig. 5). This might explain why we fail to observe any difference in extracellular AHL concentrations between PA14 and the mvfR mutant, whereas mexGHI-opmD was previously postulated to be involved in AHL homeostasis (Aendekerk et al., 2002). Further studies should be designed to address the role of this operon in the MvfR system.
Other interesting observations that enhance the findings on the mexGHI-opmD operon is that four putative transcriptional regulators of the TetR (tetracycline repressor) family, PA2885, PA3678, PA3721 and PA3973, display lower expression levels in the mvfR mutant. TetR family members are typically autoregulated repressors that bind specific ligand molecules. PA3678 encodes MexL, a transcriptional repressor of the adjacent mexJK-encoded efflux pump (Chuanchuen et al., 2002), and PA3721 is disrupted in nalC-type mutants of P. aeruginosa that hyperexpress the MexAB-OprM efflux pump (Cao et al., 2004). At least seven efflux pumps have been identified in P. aeruginosa, MexAB-OprM, MexCD-OprJ, MexEF-OprN, MexGHI-OpmD, MexJK-OprM, MexVW-OprM and MexXY-OprM (Poole and Srikumar, 2001; Li et al., 2003; Schweizer, 2003). The intended natural substrates for these pumps are unlikely to be only anti-microbial/toxic compounds as they are expressed by both antibiotic-susceptible and -resistant bacteria (Poole and Srikumar, 2001; Schweizer, 2003). The other two putative TetR regulators exhibiting lower expression in the mvfR mutant have no known function and are not located close to efflux pump genes. Although we might expect that a number of efflux pumps would be derepressed in the mvfR mutant, this is not reflected in the transcriptome data, highlighting the complexity of the global regulatory circuits of efflux pumps in P. aeruginosa.
The MvfR system controls the virulence of P. aeruginosa independently of the rhl QS system
Besides the predicted involvement of QS in pathogenesis via the regulation of virulence factors (Van Delden and Iglewski, 1998; de Kievit and Iglewski, 2000), experimental evidences obtained in various animal models demonstrate the implication of the QS system in the pathogenesis of P. aeruginosa (Tang et al., 1996; Rumbaugh et al., 1999; Tan et al., 1999; Pearson et al., 2000; Wu et al., 2001; Smith et al., 2002; Lesprit et al. 2003). Remarkably, however, our studies show that a rhlR mutant is not attenuated in the burn mouse model whereas mvfR (Cao et al., 2001), pqsA and pqsE mutants all display a similarly reduced virulence phenotype (Fig. 7). Earlier studies reporting decreased virulence were conducted with lasR, lasI, rhlI or lasIrhlI mutants, and there are no reports using a rhlR mutant. Although one might expect rhlR and rhlI mutants to display similar behaviours, a recent study has proposed that RhlR might actually act as a repressor in the absence of its cognate autoinducer C4-HSL (Medina et al., 2003b). Hence, it is likely the expression of genes co-regulated by the rhl system and the MvfR system would be inhibited in a rhlI mutant but would simply fail to be upregulated in a rhlR mutant. Alternative explanations include compensatory secondary mutations that could arise in rhlR mutant in vivo as has been reported for rhlI and lasI mutants in vitro (Beatson et al., 2002).
Our results support a model where a subset of genes regulated by the MvfR system but not overlapping with the rhl regulon is important in P. aeruginosa pathogenesis.
pqsE is essential for the regulatory activity of the MvfR system and the full virulence of P. aeruginosa
MvfR controls the transcription of two co-regulated operons, pqsABCDE and phnAB, the products of which, with the exception of pqsE, mediate the synthesis of a large array of HAQs, including HHQ, the direct precursor of PQS (Déziel et al., 2004). Intriguingly, a pqsE mutant is not defective in the production of HAQs or PQS (Gallagher et al., 2002; Déziel et al., 2004), but still is deficient in production of pyocyanin, lectin and HCN, and expression of the corresponding synthetic operons phz1, lecA and hcnABC (Gallagher et al., 2002; Diggle et al., 2003; and data not shown). We are now adding mexGHI-opmD to the list of pqsE-dependent genes (Fig. 5). Although the above-mentioned factors are also controlled by the rhl system, production of C4-HSL and expression of rhlI and rhlR are not affected in a pqsE mutant, as it is for a mvfR mutant (Gallagher et al., 2002; Diggle et al., 2003; and data not shown). Basically, apart from the production of HAQs, a pqsE mutant seems to display the same phenotypes as a mvfR mutant.
Pseudomonas quinolone signal or HQNO addition to cultures of any pqs or mvfR mutants, or induction of PQS synthesis by HHQ addition, all fail to complement the absence of pqsE. Although the production of HAQs (including PQS) is unaffected in the pqsE mutant, the pathogenic fitness of this mutant is as reduced as the mvfR and pqsA/pqsB mutants (Fig. 7). Genomic context and blast analysis suggest that pqsE probably encodes an hydrolase. Further investigations to elucidate how pqsE mediates the function of the MvfR system are warranted.
While PQS and other HAQs appear to act as intercellular signals (Déziel et al., 2004), and might be involved in the activity of PqsE (Diggle et al., 2003), their ultimate function is still unresolved. Nevertheless, here we demonstrate that the loss of PqsE function, and not only PQS production, is responsible for the phenotypes of lasR or mvfR mutants previously solely attributed to PQS production deficiency.
Conclusion
Based on the results from this study and from previous work, we propose a regulatory model of how mvfR is interwoven into the P. aeruginosa QS circuitry, shown in Fig. 8. MvfR exerts its function primarily through the regulation of pqsA–E and phnAB which, with the exception of pqsE, are involved in the synthesis of HAQs and their PQS analogue derivatives. The MvfR system, through the essential contribution of PqsE, positively regulates a subset of genes dependant on both LasR and RhlR.
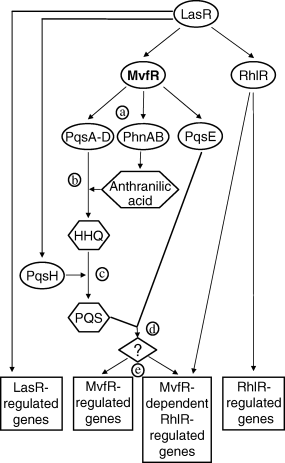
Model describing the position of mvfR in the QS cascade of P. aeruginosa. (a) MvfR controls the transcription of pqsA–E and phnAB, (b) which, with exception of the pqsE, direct the synthesis of HAQs, including HHQ, the precursor of PQS, (c) the conversion of HHQ into PQS is catalysed by PqsH, which is LasR-controlled, as is MvfR, (d) through an unknown mechanism, apparently requiring PQS, PqsE mediates the regulatory activity of the MvfR system, (e) this system controls the expression of two subsets of genes, one also dependent on the rhlR QS system.
We conclude that (i) reduced virulence of an mvfR mutant is caused by the loss of pqsE expression and not only a deficiency in HAQs/PQS production and (ii) the contribution of the MvfR system to the virulence of P. aeruginosa is most probably via its transcriptional control over genes not under RhlR regulation. Elucidation of the mechanism through which the MvfR system exerts its regulation aids in dissecting the complex QS circuitry and will provide new therapeutic targets for P. aeruginosa infections.
Experimental procedures
Strains, plasmids and growth conditions
Table 2 lists strains and plasmids. Bacteria were routinely grown in Luria–Bertani (LB) broth at 37°C in a roller drum or on a gyratory shaker at 250 r.p.m. LB plates contained 1.5% agar.
| Strains/plasmids | Characteristics | Source or reference |
|---|---|---|
| P. aeruginosa | ||
| PA14 | Clinical isolate UCBPP-PA14 | Rahme et al. (1995) |
| PA14 mvfR– | PA14 containing a nonsense point mutation in mvfR | Cao et al. (2001) |
| PA14 pqsE– | pqsE non-polar deletion mutant | Déziel et al. (2004) |
| PA14 lasR– | lasR::Gm derivative of PA14 | Déziel et al. (2004) |
| PA14 rhlR– | rhlR::Tc derivative of PA14 | This study |
| Plasmids | ||
| pQF50 | lacZ transcriptional fusion vector, Cbr | Farinha and Kropinski (1990) |
| pSB224.10 A | pRIC380 suicide vector carrying rhlR::Tc | Beatson et al. (2002) |
| pED1 | mexG–lacZ transcriptional fusion, Cbr | This study |
| pGX1 | mvfR–lacZ transcriptional fusion, Cbr | This study |
| pECP60 | rhlA′–lacZ translational fusion, Cbr | Pesci et al. (1997) |
| pMW303 | phzA1–lacZ transcriptional, Cbr | Whiteley et al. (2000) |
| pME3846 | rhlI′–′lacZ translational fusion, Tcr | Pessi et al. (2001) |
| pME3853 | lasI′–′lacZ translational fusion, Tcr | Pessi et al. (2001) |
| pME3859 | rsmA′–′lacZ translational fusion, Tcr | Pessi et al. (2001) |
- Cb, carbenicillin; Gm, gentamicin; Tc, tetracycline.
Isogenic knockout mutants were generated by allelic exchange using sucrose counterselection (Schweizer, 1992; Alm and Mattick, 1996), using described constructs (Beatson et al., 2002).
Standard methods were used to manipulate DNA. The parent plasmid pQF50 was used to construct the mvfR–lacZ and mexG–lacZ transcriptional fusion reporters pGX1 and pED1 respectively. To ensure that the entire regulatory region is included, 746 bp upstream to the 160 bp downstream of the mvfR translational initiation site and 586 bp upstream to the 119 bp downstream of the mexG translational initiation site were amplified. The promoter fragments were generated from genomic DNA using polymerase chain reaction with primers 5′-GGATCGGTACCAGTCGCTACACCTGAAGGC-3′ and 5′-CTAGGAAGCTTCCCGACGGACCAGCTCCAC-3′ for mvfR and with 5′-GAAGATCTTCGCACTACGGAGCCAGA GC-3′ and 5′-GGGGTACCCCTGGCCTGATAGTCGAACA-3′ for mexG. The resulting fragments were digested with KpnI and HindIII for mvfR and with BglII and KpnI for mexG, then inserted into pQF50 digested with the corresponding enzymes. The intended subcloning event was confirmed by DNA sequencing.
Plasmids were introduced by electroporation (Smith and Iglewski, 1989). For P. aeruginosa, carbenicillin (300 µg ml−1), tetracycline (100 µg ml−1), gentamicin (100 µg ml−1) and X-Gal (40 g ml−1) were included when required.
β-Galactosidase activity assays
Pseudomonas aeruginosa strains harbouring lacZ fusions were typically grown overnight with the appropriate antibiotic, then subcultured without antibiotic in LB to a starting OD600 of 0.05. Culture samples were obtained at regular intervals for determination of growth (OD600) and β-galactosidase-specific activity (Miller, 1972). Experiments were performed in triplicate.
For the HAQ addition experiment, PQS and HHQ were synthesized as described (Lépine et al., 2003) and HQNO was obtained from Sigma-Aldrich (Oakville, Canada). Stocks were prepared in methanol.
RNA isolation and generation of transcriptomic data
Pseudomonas aeruginosa PA14 and the isogenic mvfR mutant were grown in 1 l Erlenmeyer flasks containing 100 ml of LB incubated at 37°C and 200 r.p.m. Culture samples were harvested at three growth stages (OD600 = 1.5, 2.5 and 3.5) and the RNA was immediately stabilized with RNAprotect Bacteria Reagent (Qiagen, Valencia, CA). Samples were stored at −80°C. Total RNA was isolated with the RNeasy spin column (including an on-column DNase digestion step) according to the manufacturer (Qiagen), treated with RQ1 DNase I (Promega, Madison, WI) for 1 h at 37°C, and repurified through an RNeasy column. Samples were labelled according to the manufacturer (Affymetrix, CA) and hybridized to the Affymetrix GeneChip®P. aeruginosa Genome array for 24 h at 50°C using the GeneChip® hybridization oven at 60 r.p.m. Chip washing, staining and scanning was performed according to instructions from Affymetrix. Experiments were performed in duplicate for independently performed experiments.
Analysis of GeneChip® expression data
The data files of scans of the PAO1 GeneChip® arrays hybridized with different probes were converted to cell intensity files (.CEL files) with the Microarray suite 5.0 (MAS, Affymetrix, CA). The data were scaled to target intensity of 500, and for each time point all possible pair-wise array comparisons of the replicates were performed (i.e. four combinations) with mutant array as baseline, using a change call algorithm of MAS 5.0. Genes that had (i) change calls in the same direction in each experiments and (ii) change calls of same direction in three of four comparisons, with additional constraints that in each comparison a signal value difference greater than 100, non-zero signal log ratio and one of the two samples being compared was not called ‘Absent’, were scored as differentially modulated between the mutant and wild-type bacteria. The ratio of differential expression is the average of all four ratios. For genes showing higher expression in mvfR mutant (i.e. decreased in the wild type), the average of the inverse of the ratios was calculated, and scored as the negative change value.
GeneTree analysis (Eisen et al., 1998) of signal values (averages of duplicate experiments) normalized to median values per gene across all time points in log ratio mode using Pearson correlation was performed using Gene Spring (version 5.1, Silicon Genetics, CA). Ten clusters K-means clustering (Hartigan and Wong, 1979) was also performed on the log ratio of signal values normalized to median for each gene across all time points using Pearson correlation, and they were used to colour tree branches.
Transcriptional units in Table 1 were based on predicted open reading frames (ORFs) from http://www.cifn.unam.mx/moreno/pub/TUpredictions/Predictions/ (Salgado et al., 2000; Moreno-Hagelsieb and Collado-Vides, 2002) as of 20 May 2003. One operon, PA3478-PA3479, was added to the list and minor errors were corrected. The list of transcriptional units used and the template for selecting the genes satisfying the above-mentioned rules are available at http://genetics.mgh.harvard.edu/RahmeWeb/home.htm
GuiGraph: a tool for visualization of gene expression data
In many instances gene expression data can be better perceived by looking at both the signal values and the fold change. In order to facilitate visualization for large data sets, we have developed GuiGraph, a tool to aid interpretation of transcriptome data. The GuiGraph version adapted for the Affymetrix P. aeruginosa GeneChip is available at http://www.genetics.mgh.harvard.edu/RahmeWeb/
AHL and HAQ quantification
An estimate of AHL production was obtained using the C. violaceum CV26 bioassay (McClean et al., 1997). Quantification was performed by LC/MS analysis as follows. Aliquots of culture samples were taken at regular intervals, and diluted with an equivalent volume of methanol containing 2% of acetic acid and 1000 mg l−1 tetradeutero-PQS, as internal standard. After centrifugation, 20 µl of the supernatants were injected for LC separation onto an Agilent HP1100 (Agilent Canada, Montreal, Canada) equipped with a 150 × 3 mm C8 Luna (Phenomenex, Torrance, CA) reverse phase column, using a water (A)/acetonitrile (B) gradient both containing 1% acetic acid. A 30% to 80% B gradient over 20 min was followed by 100% B for two additional minute. The HPLC flow rate was 400 µl, split to 10% with a Valco Tee. The mass spectrometer was a Micromass Quattro II (Waters Canada, Montreal, Canada) triple quadrupole operating in positive electrospray ionization mode. Data acquisition was performed in full scan mode with a scanning range of 130–350 Da. Precise quantification of C4-HSL (Sigma-Aldrich Canada, Oakville, Canada) and oxo-C12-HSL (Quorum Science, Coralville, IA) was performed in MS/MS mode by monitoring the intensity of daughter ions produced by collisional activation of their pseudomolecular ions at m/z 172 and 298 respectively. Argon was the collision gas at 2 × 10−3 mTorr, and the collision energy was 13 eV. HAQs, including PQS, were also measured using LC/MS (Lépine et al., 2003).
Mice mortality studies
Animal pathogenicity was assessed using the thermal injury mouse model (Stevens et al., 1994) as described (Rahme et al., 1995). Animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the Massachusetts General Hospital.
Acknowledgements
We thank D. Haas, S. Beatson, M. Whiteley and B. Iglewski for gifts of plasmids, A. Remick and S. Milot for technical assistance, M. Mindrinos from Department of Biochemistry, Stanford University for array processing, G. Coelho for the development of the GuiGraph tool and S. Stachel for comments and editing. We acknowledge Cystic Fibrosis Foundation (CFF) Therapeutics for subsidizing the P. aeruginosa Affymetrix microarrays. The CFF ♯02G0 and Shriners ♯8800 Awards (to L.G.R.) and Aventis S.A. supported this work. E.D. is recipient of a Canadian Institutes of Health Research (CIHR) postdoctoral fellowship.
Supplementary material
The following material is available from http://www.blackwellpublishing.com/products/journals/suppmat/mmi/mmi4448/mmi4448sm.htm
Fig. S1. Overlap (55%) between MvfR system-induced and QS-induced genes.
Table S1. Complete transcriptome data of PA14 versus mvfR mutant.




