Exploring prokaryotic diversity: there are other molecular worlds
Summary
Prokaryotes are the major source of biological diversity on earth. This is not simply because of the large number of species present, or because of their diverse growth conditions and environmental niches populated by them, but because of the wealth of genes, metabolic pathways and molecular processes that are only found in prokaryotic cells. Therefore, Bacteria and Archaea (and their phages) cannot be considered any longer as miniaturized models of Eukaryotes, but as a genuine source of unique biological processes that are mediated by unique sets of genes and molecular devices. A true understanding of complex biological phenomena will require a deeper knowledge of this vast prokaryotic world. The second European Molecular Biology Organization (EMBO) conference on Molecular Microbiology entitled ‘Exploring Prokaryotic Diversity’ explored many aspects of this newly emerging interest in the prokaryotic world.
The European Molecular Biology Organization (EMBO) hosted the conference on Molecular Microbiology ‘Exploring Prokaryotic Diversity’ organized by Víctor de Lorenzo (CNB-CSIC, Madrid, Spain), Ariane Toussaint (Free University of Brussels, Belgium) and Rolf Bernander (Uppsala University, Sweden), from 22 to 26 of April 2004, at its headquarters of the EMBL in Heidelberg. The meeting was devoted to the exploration of prokaryotic diversity stretching from molecular, structural, genetic, environmental aspects through to fundamental biological questions, such as the evolution of genomes, taking advantage of the large number of fully sequenced genomes available to date (>190; http://www.genomesonline.org/). Here, I can only summarize some of the highlights of the meeting and I apologize to participants whose excellent work is not mentioned here.
New tricks from old friends
An issue that was apparent throughout the meeting was the astonishing amount of new molecular information that is still being obtained from classical prokaryotic model organisms such as Escherichia coli or Bacillus subtilis. Richard Losick (Harvard University, Boston, USA) presented a beautiful example of this in his inaugural lecture. He showed that most B. subtilis‘wild’ strains found in nature have biological behaviours that are not present in the reference ‘wild-type’ strain of B. subtilis that has been maintained for decades in research laboratories. For instance, wild strains of B. subtilis have the ability to swarm in solid media and behave as multicellular communities able to generate robust biofilms with well-organized aerial structures similar to ‘fruiting bodies’ where sporulation preferentially takes place. A genetic analysis of these newly discovered biological behaviours identified some of the genes involved (Branda et al., 2004; Kearns et al., 2004). Among them were genes for exopolysaccharide, surfactant and nucleotide-sugar biosynthesis, an ABC transporter, phosphatases, and transcriptional regulators. In addition, many of the identified genes had been annotated in the B. subtilis genome as of ‘unknown function’, clearly emphasizing the need of a deeper understanding of the biology and physiology of the microorganisms in nature (and not only in the laboratory) to assign a biological function to genes.
Another example of exciting new information coming from a classical system was provided by the talk of Agneta Richter-Dahlfors (Karolinska Institute, Stockholm, Sweden). Her studies on mucosal inflammation (an essential process for clearing infectious agents) in the urinary tract revealed that low, sublytic concentrations of the classical pore-forming α-haemolysin toxin secreted by uropathogenic E. coli strains induce constant low-frequency Ca2+ oscillations in renal epithelial cells. These Ca2+ oscillations have a characteristic 12 min periodicity and alter gene transcription in renal tubule cells to induce proinflammatory cytokines interleukin-6 (IL-6) and IL-8 (Laestadius et al., 2002) that attract neutrophils. Other bacterial protein toxins have also been found to induce similar Ca2+ oscillations in the cytoplasm of their target cells (Soderblom et al., 2003).
Prokaryotic chromosomes encode a surprising number of toxin-antitoxin (TA) loci. TA loci have been classically viewed as plasmid maintenance and stability systems in which the toxin eliminates plasmid-free cells appearing as a result of a defect in plasmid segregation. Kenn Gerdes (University of Southern Denmark, Odense M, Denmark) presented a systematic survey of TA loci in 129 prokaryotic genomes (113 Bacteria and 16 Archeae). Six hundred TA loci were found in this search. All Archeal genomes analysed contained at least one TA locus, and over 20 could be found in Sulfolubus and Archaeoglobus fulgidus. Other microorganisms also contain surprisingly many TA loci. For instance, the chromosomes of Nitrosomonas europeaus, Mycobacterium tuberculosis and Vibrio cholerae contain at least 47, 37 and 13 TA loci respectively. Although the function of the chromosomal TA genes remains unclear, they seem to be related to regulation of translation in response to nutritional starvation or other unfavourable stress conditions (Aizenman et al., 1996; Gerdes, 2000; Hayes, 2003). In the case of relBE locus of E. coli, the RelE toxin is induced during nutritional stress and inhibits protein translation by cleavage of the mRNA positioned at the ribosomal A site (Christensen and Gerdes, 2003; Pedersen et al., 2003). In addition, TA loci are not randomly distributed along the chromosomes and are frequently within integron elements (e.g. the 13 TA loci of V. cholerae are located in a VCR megaintegron). This fact raises the possibility that TA loci might also be involved in the stabilization of integron elements in genomes.
A group of presentations focused on various aspects of the bacterial physiology during nutritional starvation (i.e. stationary phase of growth). Maia Kivisaar (Estonian Biocentre and Tartu University, Estonia) showed that specific mutational mechanisms are induced under nutrient starvation in Pseudomonas putida. This stationary phase-dependent mutation might allow rapid evolution and adaptation of bacteria under environmental stresses (Kivisaar, 2003). By studying base substitution mutations and deletions occurring in growing and starving cells, Kivisaar found that different spectra of mutations accumulate under prolonged starvation. This suggests that different DNA replication and repair mechanisms might act on growing and starving cells. Stationary-phase mutation in long-term starved cells does not depend on the activity of methyl-directed mismatch repair systems, which plays a major role in suppressing mutations in growing cells and at the beginning of starvation. Furthermore, induction of error-prone DNA polymerases underlies the mechanisms of stationary-phase mutation. Kivisaar and co-workers have shown the error-prone DNA polymerase IV (pol IV) is essential for 1 bp deletions occurring in P. putida populations starved for more than 1 week (Tegova et al., 2004). Importantly, DNA-damaging agents (e.g. mitomycin C) do not cause induction of pol IV, which is RecA independent. Several important questions arise form this study, including the degree of specificity of the induction mechanism, whether other error-prone DNA polymerases are also induced, and the existence of similar mutational responses in other bacteria.
Thomas Nystrom (Goteborg University, Sweden) concentrated on the oxidative protein damage that occurs on E. coli cells at the stationary phase, a process that has striking similarities with ageing in Eukaryotic organisms. Protein oxidation occurs preferentially in subpopulations of cells that become viable but non-culturable after prolonged maintenance in stationary phase (e.g. about 60% of the E. coli cells in a culture become non-culturable after 48 h, although their membranes are still intact) (Desnues et al., 2003). Carbonylation is the one of the most common irreversible protein modifications that occur during E. coli senescence (loss of culturability and membrane integrity). Like other forms of protein oxidative damage, carbonylation is caused by the action of free radicals of reactive oxygen species (ROS) generated from respiratory activity (Berlett and Stadtman, 1997). However, the accumulation of carbonylated proteins in the stationary phase mostly results from diminished accuracy of translation (i.e. missense errors, frameshifting and stop codon readthrough) that generates aberrant protein forms that are more susceptible to oxidation by ROS (Ballesteros et al., 2001).
A long-recognized intracellular signalling nucleotide, guanosine 3′,5′-bispyrophosphate (ppGpp) (Ryals et al., 1982) appears to have a major role in the senescence of E. coli cells. Mutants deficient in ppGpp synthesis (relA) showed an accelerated senescence phenotype, although the molecular details of this genetic link appear to be complex (Nystrom, 2003). The lower translation fidelity reported in relA mutants (O’Farrell, 1978) can lead to an enhanced carbonylation of the mistranslated proteins, and could partially explain the accelerated senescence of these cells. However, ppGpp is directly responsible of the induction of some genes involved in oxidative and nutrient stress defences, which are essential for survival in stationary phase (e.g. rpoS encoding the E. coli stationary-phase sigma factor) (Hengge-Aronis, 2002). In addition, ppGpp favours the binding of alternative sigma factors like σs to the core of RNA polymerase (RNAP) (Jishage et al., 2002), thus allowing the activation of specific promoters important for survival under nutrient stress conditions. The precise contribution of ppGpp to the survival of E. coli in stationary phase and its relationship with the regulation of chromosomal TA loci (Pedersen et al., 2002) remain to be clarified.
Victoria Shingler (Umea University, Sweden) discussed the role of ppGpp on sigma factor competition during the activation of σ54-dependent promoters from operons involved in the degradation of phenol and other aromatic compounds in Pseudomonas (Laurie et al., 2003). These promoters are only active in stationary phase, apparently because the in vivo levels of the RNAP-σ54 holoenzyme are limiting during exponential growth (Cases et al., 1996). Interestingly, σ54 levels do not vary in vivo between the exponential and the stationary phase (Jurado et al., 2003). Increased levels of ppGpp at the onset of nutrient starvation permit effective competition of σ54 for the core of RNAP and, as a result, RNAP-σ54 holoenzyme reaches higher intracellular level. Therefore, in the absence of other mechanisms for recruitment of the holoenzyme (Bertoni et al., 1998), ppGpp is critical for the activation of some σ54-dependent promoters in stationary phase.
Martin Buck (Imperial College, London, UK) discussed the mechanism of transcriptional activation at σ54-dependent promoters. This class of bacterial promoters use an ‘enhancer-like’ activation mechanism in which an special family of mechanochemical transcriptional activators, which have ATPase activity and bind DNA at a distance from the mRNA start site, contact σ54 to allow transcription initiation to occur. Well-characterized examples of σ54-dependent transciptional activators are the nitrogen-regulated proteins NtrC and NifA, the XylR/DmpR-like regulators of operons for the degradation of aromatic compounds, and the phage shock protein F (PspF). Compelling evidence indicates that these transciptional activators belong to a larger family of cellular ATPases called AAA+ that typically form ring-shaped oligomeric complexes that convert the energy of ATP hydrolysis into mechanical force to remodel their target substrates, including protein and nucleoprotein complexes, and nucleic acids (Vale, 2000). In contrast to the activation of the σ70 class of bacterial promoters, the binding of σ54-RNAP holoenzyme to the promoter is not in itself sufficient for transcription initiation. Rather, isomerization to an open complex requires the hydrolysis of ATP by the AAA+ activator. In the presence of ADP-AlFx, a non-hydrolysable transition state analogue of ATP, AAA+ activators form a stable complex with σ54 (Chaney et al., 2001). However, a small fragment of PspF, comprising residues 69–93 and having no ATPase activity, was shown to interact with the N-terminal 56 amino acid region of σ54 bound to an early melted promoter probe (Bordes et al., 2003). The PspF 69–93 fragment contains a conserved amino acid motif (GAFTGA) that is essential for transcriptional activation in vitro and in vivo but not for the ATPase activity of PspF (Bordes et al., 2003). A low-resolution structure of PspF bound to ADP-AlFx, obtained from cryo-electron microscopy images, indicates that it forms a hexameric ring characteristic of AAA+ proteins (Schumacher et al., 2004). The formation of this hexamer depends on binding of ADP-AlFx, as PspF is a dimer in the absence of the nucleotide (Schumacher et al., 2004). The PspF hexamer contrasts with the heptameric structure reported for the ATPase domain of an NtrC homologue from the extreme thermophile Aquifex aeolicus (Lee et al., 2003). A modelling of PspF 3D structure based on the AAA+ enzyme RuvB (Putnam et al., 2001) placed the GAFTGA motif in a loop close to the centre of the hexamer, at the opposite side to the DNA-binding domains (Schumacher et al., 2004). The GAFTGA loop can be relocated upon ATP hydrolysis so that it becomes accessible to σ54. Interestingly, M. Buck presented work from Xiaodong Zhang and Mathieu Rappas (Imperial College, London, UK) demonstrating low-resolution cryo-electron microscopy images of one molecule of σ54 in close contact with a hexameric form of the PspF AAA+ domain in the ADP-AlFx bound state (Fig. 1). Contact of σ54 appears to be restricted to some of the subunits of PspF and can be accounted for by a nucleotide-dependent repositioning of some GAFTGA loops to engage with σ54. Further work is needed to solve the conformational changes taking place in σ54 and AAA+ proteins during ATP hydrolysis and transcriptional activation at the atomic level, although details of the conformational signalling pathway are now emerging.
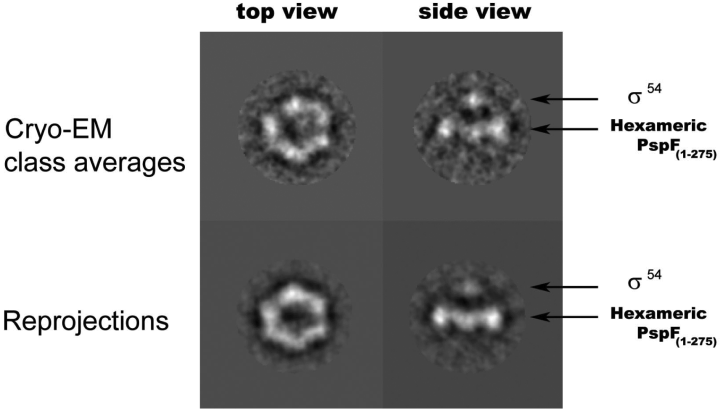
Image reconstruction of the σ54-PspF AAA domain complex forming with ADP-AlFx. Sigma 54 appears connected to the hexameric PspF by GAFTGA loops rising 12 Å above the ring. Protein density appears in white. EM, electron microscopy. (This image was a kind gift from Mathieu Rappas and Martin Buck, Imperial College, London, UK.)
The mechanism of bacterial conjugation is also closer to being defined at a molecular level thanks to the work of Matxalen Llosa and Fernando de la Cruz (Universty of Cantabria, Santander, Spain). Using the conjugative plasmid R388 as a model system, they have demonstrated a clear mechanistic link between bacterial conjugation and protein secretion. Although a close phylogenetic relationship has been long recognized between bacterial conjugation systems (DNA transfer) and type IV secretion (T4S) systems (secretion of proteins and nucleoprotein complexes) (Cascales and Christie, 2003), it has remained obscure how such similar machineries would modify their function to adapt to a DNA or protein substrate. The ‘shoot and pump’ model (Llosa et al., 2002) solved this paradox by suggesting a common molecular mechanism for protein secretion and DNA transfer in bacterial conjugation and T4S (e.g. T-DNA transfer in Agrobacterium tumefaciens). In this model, the DNA to be transferred (in a single stranded form) is covalently bound to a protein that is the actual substrate of the secretion machinery. ATP hydrolysis allows the substrate protein to be shot out of the cell along with the bound single-stranded DNA (ssDNA) that trails behind the secreted protein. Further ATP hydrolysis will pump the rest of the long ssDNA molecule completely out of donor and into to the recipient cell.
In plasmid R388, the two key players for DNA transfer have been well characterized. TrwB is an inner membrane hexameric F1-like ATPase (Gomis-Ruth et al., 2001) that acts as a motor to move the DNA in the conjugative pilus. TrwC is a DNA relaxase-helicase (Guasch et al., 2003) that covalently binds to oriT and is responsible for initiation and termination of DNA processing (nicking of the conjugative plasmid to initiate conjugation and religation of the ssDNA intermediate when transfer to the recipient cell is completed). TrwC bound to ssDNA is recruited to the T4S machine using TrwB as a coupling factor. Taking advantage of the ability of TrwC to excise intervening DNA inserted in a lacZ reporter gene between two oriT sequences, Llosa and de la Cruz obtained the first genetic evidence that TrwC is transferred to the recipient cell along with the ssDNA, thus strongly supporting the ‘shoot and pump’ model for bacterial conjugation and T4S.
Biofilms go cell signalling
Biofilms (bacterial communities that grow on surfaces and are embedded in an extracellular matrix, often composed of polysaccharides) have a major impact in human health and on environmental processes, as clearly exemplified by Ken Timmis (GBF, Braunschweig, Germany) in his analysis of two biofilms found in two distinct ecological niches, a contaminated soil and a medical catheter. Biofilms from polychlorobiphenyl (PCB)- contaminated soils (Lunsdorf et al., 2000) developed in a complex manner and contained various bacterial species organized around clay leaflets and mineral grains to obtain limiting minerals as well as a carbon source from bound PCBs. This novel microbe mineral biofilm represents a soil microhabitat in which diverse bacteria can access and degrade toxic pollutants efficiently.
Another interesting example is found in biofilms that develop in biliary stents, a medical catheter inserted into the bile duct between the gall bladder and the small intestine. These biliary stents usually become contaminated and occluded by bacterial biofilms, and thereby become life-threatening. Analysis of the bacterial community in biliary stent biofilms revealed the presence of Pseudomonas aeruginosa, Klebsiella pneumoniae, Enterococcus faecalis, Enterobacter aerogenes and two species of unculturable bacteria (Wenderoth et al., 2003). Interestingly, P. aeruginosa, a well known opportunistic pathogen that serves as a model for biofilm research, was shown to be the pioneer microbe colonizing biliary stents, and might thus serve as a target for future studies to find ways to prevent formation of biliary stent biofilms.
Work on P. aeruginosa has revealed a number of genes important for biofilm formation in this organism, including those coding for extracellular organelles such as type IV pili and flagella (O’Toole and Kolter; 1998Klausen et al., 2003). Alain Filloux (CNRS, Marseille, France) focused on the genes involved at the early stages of biofilm formation in P. aeruginosa. Using a Tn5 mutagenesis strategy in a non-piliated strain he previously identified a fimbrial system that participates in the early cellular adherence to abiotic surfaces that leads to biofilm formation (Vallet et al., 2001). This fimbrial operon was named cupA for its relatedness to other fimbria belonging to the chaperone-usher pathway (Sauer et al., 2000). Two homologous systems (cupB and cupC) exist in the P. aeruginosa genome, but their participation in biofilm formation have not been proven. Importantly, Filloux's group found that all P. aeruginosa cup fimbrial systems are controlled by common negative regulator (mvaT) (Vallet et al., 2004). Mutants lacking mvaT showed enhanced biofilm formation and upregulation of fimbrial (cupA, cupB and cupC) and lectin (lecA) genes. On the other hand, A. Filloux presented the identification of histidine kinase sensor protein (LadS) that acts as a positive regulator of biofilm formation in P. aeruginosa, apparently by inducing multiple adhesins, a glycosyl transferase, as well as other genes whose functions remain unknown. Therefore, the antagonist functions of MvaT and LadS provide a balanced way of regulating biofilm formation in P. aeruginosa.
The challenge of investigating gene expression in biofilms by genome-wide approaches was discussed by Jean Marc Ghigo (Institute Pasteur, Paris, France) using E. coli K-12 as a model (Beloin et al., 2004). His work, along with that from other laboratories (Schembri et al., 2003), demonstrated that a large number of E. coli genes (≈2%) are differentially expressed in biofilms compared with planktonic growth. Some of the genes that become upregulated in biofilms include those encoding fimbria (e.g. fim operon), genes induced under nitrogen and oxygen limitation, sensor systems of various cell envelope stresses (e.g. Psp-, Cpx- and σ54-pathways) (Raivio and Silhavy, 1999), autotransporter proteins that play a role in adhesion and cellular autoaggregation (e.g. Ag43), and multiple genes of unknown function. By disrupting 54 of the genes that were most strongly induced in biofilms, Ghigo and co-workers showed that 20 are required for biofilm formation (Beloin et al., 2004). Interestingly, 11 of these genes belonging an assigned function.
The regulation of biofilm formation in Salmonella and other Enterobacteriacea was addressed by Ute Römling (Karolinska Institute, Stockholm, Sweden) and Iñigo Lasa (Public University of Navarra, Pamplona, Spain). Salmonella and other enteric bacteria isolated from human gut (e.g. Escherichia, Citrobacter, Enterobacter, Klebsiella) form various types of biofilms characterized by the production of a strong extracellular matrix that is highly resistant to hydrolytic enzymes and disinfectants (e.g. ethanol, chlorine) (Solano et al., 2002; Zogaj et al., 2003). The formation and composition of these biofilms are highly regulated in response to environmental conditions (e.g. temperature, oxygen tension, nutrient availability, etc.). For instance, Salmonella enterica forms biofilms composed of thin aggregative fimbria called curli and cellulose when grown in rich medium of low osmolarity [e.g. Luria–Bertani (LB) broth without salts] (Romling et al., 1998; Zogaj et al., 2001) whereas the main polymer in biofilms grown in certain nutrient-deficient medium [e.g. adherence test medium (ATM)] is cellulose (Solano et al., 2002).
Ute Römling's work (Romling et al., 2000) revealed that biofilm formation by Salmonella in LB media is controlled by a ‘master’ regulator, CgsD, which induces transcription of the curli operon and adrA, a gene encoding an inner membrane protein with diguanylate cyclase activity (Simm et al., 2004). The diguanylate cyclase activity of AdrA is responsible for the biosynthesis of bis-(3′−5′) cyclic GMP (c-di-GMP) (see below). Transcription of cgsD is itself subjected to a complex regulation by specific (MlrA) and global (RpoS, OmpR and CpxR) transcriptional regulatory proteins, as well as by architectural proteins such as IHF and H-NS (Brown et al., 2001; Prigent-Combaret et al., 2001; Gerstel et al., 2003). Notably, the operons for biosynthesis of cellulose (bcs) are constitutively expressed and their transcription is not induced by CgsD or AdrA (Zogaj et al., 2001). In addition, CgsD, OmpR or RpoS do not play any role in biofilm formation in nutrient-deficient media (Solano et al., 2002). Taken together, these observations strongly indicated the existence of a post-transcriptional regulation of cellulose biosynthesis in Salmonella.
Cyclic di-GMP is the key mediator of the post-translational control of cellulose biosynthesis in Salmonella. c-di-GMP was formerly known to regulate cellulose biosynthesis in Gluconacetobacter xylinus (Ross et al., 1987) by binding to the regulatory subunit of a membrane-bound cellulose synthase complex (Weinhouse et al., 1997). In bacteria, c-di-GMP levels are controlled by the opposing actions of diguanylate cyclase and c-di-GMP diesterase. These enzymes share two conserved motifs (Tal et al., 1998; Ausmees et al., 2001). One domain, named GGDEF, has guanylate cyclase activity (Paul et al., 2004) and a second conserved motif, EAL, has phosphodiesterase activity (Simm et al., 2004; Tischler and Camilli, 2004). The results presented by U. Römling showed that the GGDEF domain of AdrA is required for the production of c-di-GMP (Simm et al., 2004).
Iñigo Lasa presented a thorough study demonstrating the major role of GGDEF proteins in cellulose biosynthesis and biofilm formation in Salmonella. By using a systematic deletion and complementation approach, Lasa and co-workers investigated the role that the eight GGDEF-containing proteins (Gcp) of S. enterica sv. Typhimurium have in biofilm formation in both rich (LB) and nutrient-deficient (ATM) media (García et al., 2004). Apart from AdrA, which is essential for cellulose synthesis in LB media, none of the other proteins (named GcpA-G) had been previously characterized. Their results clearly show that Gcp proteins have distinct but overlapping functions in cellulose synthesis in vivo. GcpA was identified as essential for cellulose synthesis and biofilm formation in ATM media, whereas GcpE appeared to act as a negative regulator of cellulose biosynthesis in LB media. It is likely that GcpA functions in vivo as a diguanylate cyclase and GcpE might be a c-di-GMP diesterase. Furthermore, overproduction of GcpA, GcpB, GcpC or GcpF was shown to complement the phenotype of an adrA mutation in LB medium. Similarly, the phenotype of a gcpA mutant in ATM medium could be complemented by overproduction of GcpB, GcpC, GcpD and GcpF. Interestingly, none of these Gcp proteins (except AdrA) is under the transcriptional control of MlrA and CgsD (García et al., 2004). Taken as a whole, these data suggest a model for cellulose biosynthesis in Salmonella(Fig. 2) in which the concerted activity of GGDEF proteins, differentially controlled by environmental conditions, modulate the levels of c-di-GMP, which in turn elicits an allosteric regulation over the membrane-bound cellulose synthase.
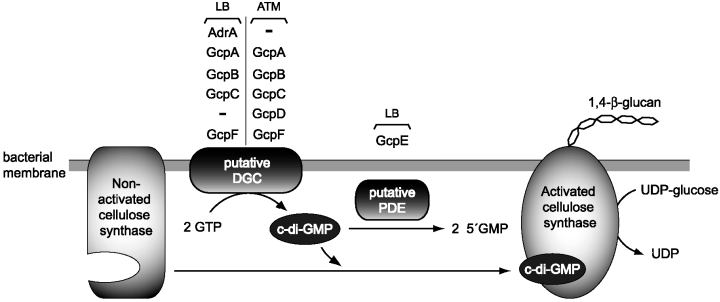
Hypothetical model illustrating the role of GGDEF containing proteins (Gcp) – AdrA, GcpA (STM1987), GcpB (STM4551), GcpC (YegE), GcpD (YeaJ), GcpE (YciR), GcpF (STM3388) – in cellulose production in Salmonella typhimurium under different environmental conditions. LB, biofilm formation in standing LB broth at 25°C temperature; ATM, biofilm formation in nutrient deficient (ATM) medium at 37°C under strong shaking; c-di-GMP, cyclic diguanylic acid; DGC, diguanylate cyclase; PDE, phosphodiesterase; UDP-glucose, uridin-5′-diphosphoglucose. (This figure was kindly provided by Iñigo Lasa, Public University of Navarra, Pamplona, Spain.)
In addition to cellulose synthesis, other important biological functions are being uncovered for c-di-GMP. Urs Jenal (University of Basel, Switzerland) presented the role of c-di-GMP in polar development and differentiation in Caulobacter cercentus. This bacterium has a complex life cycle that oscillates between a sessile form with a stalk and a free swarming flagellated form (Jenal and Stephens, 2002). The synthesis of the flagellum and the stalk is temporally controlled at opposite cell poles. The concerted action of two-component signal transduction systems is responsible for this tight control: PleC and DivJ (two polar-localized histidine sensor kinases) and DivK and PleD (the response regulators). PleD, which is phosphoryated by DivJ, contains a C-terminal GGDEF diguanylate cyclase domain (Aldridge et al., 2003). PleD is required during the swarmer-to-stalked cell transition for flagellar ejection and stalk biogenesis. Jenal's research has revealed that PleD is sequestered to the cell pole only in its phosphorylated form, and that this cell targeting activates its guanylate cyclase domain (Paul et al., 2004). Thus, localization of PleD in one cell pole leads to the synthesis of c-di-GMP, which might act as a local signalling molecule to promote differentiation of the stalk. Given these facts, Jenal proposed that the general function for c-di-GMP might be to control the synthesis of certain surface structures in bacteria (Jenal, 2004).
Archaeal surprises and uncultivable bacteria
Studies focusing on the Archaeal domain of life provide a continuous wealth of biological information that challenges old dogmas and preconceived ideas. Archaea have distinct molecular characteristics, some resembling Bacteria (e.g. genome organization, cellular structure) and others Eukarya (e.g. transcriptional machinery). Genomics revealed that the archaeal DNA replication machinery is a simplified version of the eukaryotic DNA replication apparatus. Stephen Bell (MRC Cambridge, UK) and Magnus Lundgren (Uppsala University, Sweden) provided an elegant example of the degree of similarity in DNA replication between archaeal and eukaryotic cells. They described independent studies revealing that Sulfolobus possesses multiple replication origins (Lundgren et al., 2004; Robinson et al., 2004). Thus, these findings abolish the dogma that all prokaryotic chromosomes have a single origin of replication.
By using microarray-based marker frequency analysis, Lundgren showed that bidirectional replication is initiated in the Sulfolobus chromosome from three separate origins in near synchrony (Lundgren et al., 2004). Furthermore, he showed that the replication forks of Sulfolobus advance at rates similar to those of eukaryotic replication forks (≈100 bp min−1) and much lower than E. coli elongation rates (≈1000 bp min−1). M. Lundgren also reported that, in contrast to initiation, replication termination in Sulfolobus occurred asynchronously with certain replication forks still progressing over 40 min after the others had terminated.
Bell described the high resolution in vitro and in vivo molecular characterization of two replication origins in Sulfolobus solfataricus using 2D gel analysis and replication initiation point mapping to reveal the precise initiation sites of bidirectional replication. He demonstrated that the three homologues in Sulfolobus of the eukaryotic initiator proteins Orc1 and Cdc6 have different specialized functions in vivo. DNA binding sites for Cdc6-like proteins exist in at least two of the replication origins of Sulfolobus. The cdc6-1 gene is located close to one origin of replication (oriC1), whereas cdc6-3 is located close to a second origin (oriC2). Bell demonstrated that different subsets of Cdc6 proteins bind to oriC1 and oriC2 in the G1 to S growth-phase transition. Cdc6-1 binds to oriC1, whereas both Cdc6-1 and Cdc6-3 bind to oriC2. Interestingly, Cdc6-2 binds to the origins of replication in G2, preventing Cdc6-1 and Cdc6-3 from binding and therefore providing a model for the regulation of origin activity (Dionne et al., 2003; Robinson et al., 2004).
The most extremophilic cells on earth, from hyperthermophiles growing above 80°C, to acidophiles growing below pH 3, or halophiles growing at 3 M KCl are all Archaea. David Prangishvili (University of Regensburg, Germany) showed that phages from Archaea also rival common prokaryotic viruses in terms of resistance to harsh environments, diverse morphologies and extraordinary genome composition (Rachel et al., 2002; Prangishvili and Garrett, 2004). In a systematic search for viruses in hot terrestrial environments of North America and Europe, Prangishvili and co-workers found 16 novel double-stranded DNA viruses from hyperthermophilic archaeal hosts (e.g. Sulfolobus, Acidianus, Thermoproteus, Pyrobaculum). Electron microscopic studies revealed particles with different morphotypes, from filamentous, rod-shaped and head-and-tail viruses to novel morphotypes previously not observed in nature, like balloon-shaped, ampulla-shaped and lemon-shaped viral particles. To classify these bizarre phages, three novel virus families have been introduced: Globuloviridae, Ampullaviridae and Bicaudaviridae. Surprisingly, some bicaudavirus particles are capable of undergoing a dramatic extracellular morphogenesis by extending two tails at the tips of their lemon-like capsids at temperatures above 75°C.
The morphology of these hyperthermophilic viruses is not their only astonishing feature. The sequencing of their genomes unveiled that most (>90%) of their putative genes, and sometimes all their open reading frames (ORFs), have no significant matches in current DNA databases, and are therefore considered as of unknown function. This fact suggests that these hyperthermophilic viruses have found completely novel molecular solutions for their biological functions. It is tempting to speculate whether these ‘novel’ (unknown for us) molecular apparatuses are kept in these viruses as molecular fossils from primitive cells of thermophilic character.
Equally astonishing is the identification of Nanoarchaeum equitans reported by Harald Huber (University of Regensburg, Germany) (Huber et al., 2002). This extremely small microorganism (400 nm in diameter, representing 1% of the cell volume of E. coli) was isolated as an obligate symbiont/parasite from new species of the hyperthermophilic chemolithoautotrophic Archaea Ignicoccus (a organism with an inner and outer membrane and a large periplasmic-like space). Cells of N. equitans are spherical and grow attached to the surface of Ignicoccus cells growing under anaerobic conditions at temperatures between 75 and 98°C (Fig. 3). The genome of N. equitans is only 0.49 Mb and lacks most biosynthetic and metabolic pathways. For instance, lipids from its cell membrane derived from the Ignicoccus inner membrane. However, N. equitans has a hexagonal S-layer. N. equitans represents a novel phylum of Archaea, the Nanoarchaeota, with unique 16S RNA sequences. Using specific primers for the signature sequences found in the 16S RNA genes of N. equitans, Huber and co-workers found evidence of a worldwide distribution of Nanoarchaeota (Hohn et al., 2002; Huber et al., 2003).
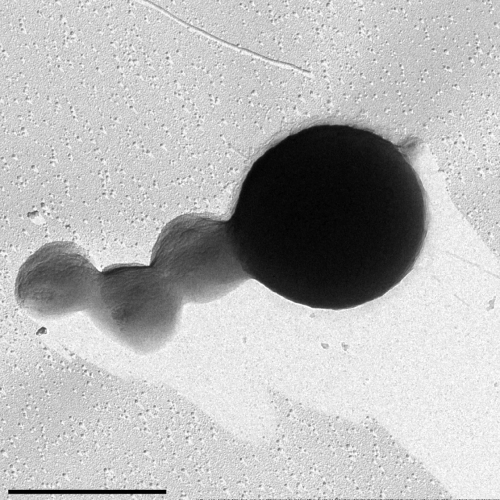
Transmission electron micrograph of three cells of Nanoarchaeum equitans attached on the surface of an Ignicoccus cell (right). Platinum shadowed. Bar: 1 µm. (This electron micrograph was kindly provided by Harald Huber, University of Regensburg, Germany.)
Apart from Nanoarchaeota, the two classical phyla of Archaea are the Crenarchaeota and Euryarchaeota (Forterre et al., 2002). Indications of the existence of unculturable Archaea belonging to a third phylum, the Korarchaeota, have been obtained from environmental DNA sequences (Barns et al., 1996). Using a similar approach, Christa Schleper (Darmstadt University of Technology, Germany) reported the detection of novel phylogenetic lineages of uncultivated Crenarchaeota in low to moderate temperature, marine and terrestrial environments. Using 16S rDNA-based phylogenetic surveys, Schleper and co-workers found one particular lineage of crenarchaeota in most soil and water samples (Ochsenreiter et al., 2003). The use of 16S rDNA surveys has revealed that microbial diversity in soil is extremely high and current estimates suggest that > 99% of existing microorganisms have not been cultured or characterized. The Schleper lab has constructed complex large-insert metagenomic libraries from soil DNA to access the genomes of these uncultivable microorganisms, including the Crenarchaeota and Acidobacteria, a novel phylum of uncultivable Bacteria that is detected (by 16S rDNA screening) in many different habitats around the globe (Quaiser et al., 2002; 2003).
Is the prokaryotic gene forest illuminating the tree of life?
The advent of genomics has provided access to massive amounts of genetic information that can be used for molecular phylogenetic studies to understand the history of life and the evolution of genomes. This is more so in Microbiology than in any other discipline because of the large number of microbial genomes sequenced and the recent advances in the metagenomics of uncultivable microorganisms. The basis of molecular phylogeny is that the evolutive relationship of (micro)organisms can be inferred from the relationships of their genes and their genomes. However, horizontal gene transfer (HGT) and extensive deletion and reorganization of genomes in closely related microorganisms raise serious concerns about the validity to the definition of phylogenetic relationships based on genomic information. Indeed the meaning of a tree of life, traditionally based on 16S rRNA sequences, has been questioned because of the high occurence of HGT in prokaryotes (Gogarten et al., 2002). Gary Olsen (University of Illinois, Urbana, USA) discussed these concerns and assessed how comparative genomics can help us understand the history of life. Olsen's arguments lead to the conclusion that, despite the existence of HGT, careful comparative microbial genomic studies that include rRNA sequences, selected genes and genomic information from characterized and uncultivated organisms, can provide a complete picture of the phylogenetic tree of life. On the other hand, he pointed out that the continuous finding of new genes with unknown functions might indicate the existence of a huge reservoir of genes in nature that has not been identified, and that will probably be found in uncultivable bacteria.
The final lecture of the meeting by Siv Andersson (Evolutionary Biology Center, Uppsala, Sweeden) provided strong support to the value of comparative genomics to understand microbial history. The work of Andersson and co-workers concentrates on the evolution of microbial genomes with a particular focus on α-proteobacteria. Within the α-proteobacteria, there is a 10-fold variation in genome size, therefore providing an excellent model system to study genome size evolution. They have recently completed the genome sequences of two α-proteobacterial species, Bartonella henselae, the agent of cat-scratch disease and Bartonella quintana, the agent of trench fever (Alsmark et al., 2004). Andersson found massive gene expansions at α-proteobacteria branches representing plant-associated microorganims (e.g. Sinrhizobium, Bradyrhizobium) and extreme losses at branches separating intracellular microbes infecting animals and humans (e.g. Rickettsia, Bartonella, Brucella) (Boussau et al., 2004). Indeed, she found that many (80%) of the ORFs with unknown functions identified in Rickettsia were short gene segments, containing stop codons and deletions, and rearrangements (gene fusions) of ancestor genes (Amiri et al., 2003). In some cases, they have been able to reconstruct the sequence of the ancestral gene and infer the function of the lost protein. From these studies, Andersson suggested that the common ancestor of α-proteobacteria had 3000–5000 genes and was a free-living, aerobic and motile bacterium. Andersoon also compared the flexibility of the genome of the α-proteobacterial group with the stability of the small genome of Buchnera (0.6 Mb), an endosymbiont belonging to γ-proteobacteria. Buchnera evolved from a common ancestor of E. coli, which was domesticated by aphids and eliminated about 75% of its original genome. Surprisingly, the resulting small genome of the endosymbionts has been stably maintained over the last 50 million years because of a nutrient-rich environment and a strong host-level selection (Canback et al., 2004; Klasson and Andersson, 2004).
Conclusion
The excellent level of top-quality science presented in the 2004 EMBO meeting reflects the excellent health condition of Molecular Microbiology around the globe. This still-emerging discipline is supported by enthusiastic scientists and by the introduction to microbiology of powerful methodologies (genomics) developed under ‘the human genome project’. However, this flourishing situation of is also very fragile. Often seen as a discipline from the past by other scientists, who employ ‘higher’ organisms as model systems, and facing severe funding problems (except for work on important human pathogens), microbiologists tackle the everyday challenge of persuading political authorities, colleagues and public opinion of the need to increase the funding for microbiological research in the 21st century. The major argument for this task is the extraordinary biological diversity that microbes maintain and of which we still know very little. We must investigate this rich molecular diversity and preserve it as a treasure in order to understand the evolution of life on earth and as the major source of new genes with amazing and unexpected properties.
Acknowledgements
I thank the EMBO for supporting and hosting these meetings focused on Molecular Microbiology. I also thank all the authors cited in the text for providing unpublished data and reviewing this manuscript. I am grateful to the Spanish Ministerio de Eduación y Ciencia and Fondo de Investigaciones Sanitarias for financial support (Ramón y Cajal contract and Grants BMC2002-03024 and COLIRED-O157 G03/025).




