Exopolysaccharide biosynthesis genes required for social motility in Myxococcus xanthus
Summary
Social (S)-motility in Myxococcus xanthus is a flagellum-independent gliding motility system that allows bacteria to move in groups on solid surfaces. S-motility has been shown to require type IV pili (TFP), exopolysaccharide (EPS; a component of fibrils) and lipopolysaccharide (LPS). Previously, information concerning EPS biogenesis in M. xanthus was lacking. In this study, we screened 5000 randomly mutagenized colonies for defects in S-motility and EPS and identified two genetic regions essential for EPS biogenesis: the EPS synthesis (eps) region and the EPS-associated (eas) region. Mutants with insertions in the eps and eas regions were defective in S-motility and fruiting body formation. These mutants failed to bind the dye calcofluor white, indicating that they lacked EPS; however, they retained normal TFP and LPS. Analysis of the eps locus showed several open reading frames (ORFs) that encode homologues to glycosyltransferases, glucanases and EPS transporters as well as regulatory proteins; the eas locus contains two ORFs: one exhibits homology to hypothetical proteins with a conserved domain of unknown function and the other displays no apparent homology to other proteins in the database. Further genetic mutagenesis analysis indicates that the whole eps region is involved in the biosynthesis of fibrils and fibril EPS. The operon at the proximal end of the eps region was analysed by generating in-frame deletion mutations. These mutants showed varying degrees of defects in the bacterium's ability to produce EPS or perform EPS-related functions, confirming the involvement of these genes in M. xanthus EPS biogenesis.
Introduction
Myxococcus xanthus is a Gram-negative proteobacterium of the delta subdivision with a life cycle involving social interactions during both the vegetative and developmental stages. This bacterium possesses two gliding motility systems, adventurous (A)- and social (S)-motility (Hodgkin and Kaiser, 1979), which allow the bacteria to translocate over solid surfaces. A-motility is used for the translocation of individual cells over relatively hard and dry surfaces (Shi and Zusman, 1993). Some of the genes required for A-motility have been identified (Stephens et al., 1989; Hartzell and Kaiser, 1991; Youderian et al., 2003); however, the cellular structures required for A-motility and the mechanism of propulsion are poorly understood. S-motility enables the translocation of groups of cells across relatively soft and wet surfaces (Shi and Zusman, 1993) or hard surfaces submerged in methylcellulose (Sun et al., 2000). S-motility is also required for the development of fruiting bodies during starvation (Shimkets, 1986a). The components known to be essential for S-motility are the lipopolysaccharide (LPS) O-antigen (Bowden and Kaplan, 1998) and two prominent surface structures: the polarly localized type IV pili (TFP) (Kaiser, 1979; Wu and Kaiser, 1995) and the peritrichous fibrils (Shimkets, 1986a,b). Equal amounts of protein and polysaccharide comprise the fibrils (Behmlander and Dworkin, 1994) with the polysaccharide portion being important for S-motility (Li et al., 2003).
In M. xanthus, the exopolysaccharide (EPS) has been shown to be essential for S-motility, cell–cell adhesion and fruiting body formation (Shimkets, 1986a,b; Li et al., 2003). TFP-mediated S-motility is achieved through pilus extension and attachment to an external substrate, followed by retraction. Pilus retraction provides the motor force to move the cells forward (Wall and Kaiser, 1999; Merz et al., 2000; Sun et al., 2000; Skerker and Berg, 2001). The EPS component of the fibrils constitutes the substrate which provides the anchor for the TFP (Li et al., 2003). The EPS is comprised of the monosaccharides mannose, galactosamine, galactose, glucosamine, N-acetylated-amine sugars, glucose, rhamnose and xylose (Sutherland and Thomson, 1975; Behmlander and Dworkin, 1994), but its macromolecular structure is unknown. Li et al. (2003) showed that polymerized amino sugars (e.g. chitin) that most probably exist in the EPS can function as the anchoring substrate required for TFP binding and retraction.
Before this study, no genes encoding specific biosynthetic functions such as EPS assembly or transport have been described. Based on previous studies, EPS-deficient mutants fail to bind calcofluor white (CFW) dye (Ramaswamy et al., 1997) and are defective in cellular agglutination, S-motility and fruiting body formation (Shimkets, 1986a,b). Previously isolated mutants defective in EPS were called dsp, resulting from their non-agglutinative (dispersed) phenotype. The dsp locus was later identified as identical to dif (Lancero et al., 2002), a chemotaxis-like operon (Yang et al., 1998a). sglK, a gene encoding an Hsp70 homologue, was also found to be required for EPS assembly (Weimer et al., 1998). Another group had recently found another locus with homologues to a transducer and a multidrug transporter apparently involved in EPS biogenesis (Kimura et al., 2004; Z. Yang, pers. comm.). The mechanisms of action for these genes are not known; however, strains with mutations in most of these genes show normal A-motility and possess the S-motility component TFP and LPS (Yang et al., 1998b; 2000; Li et al., 2003). This collection of characteristics is called ‘the Eps– phenotype’ in this study. All mutants defective in EPS biogenesis are expected to have the Eps– phenotype. By screening a random-insertion library of M. xanthus for mutants with these characteristics and excluding the known dif/sglK/pil genes, we have identified and characterized two new genetic regions that are required for M. xanthus EPS biogenesis: the EPS-synthesis (eps) region and the EPS-associated (eas) region.
Results
Identification and phenotypic characterization of genetic loci involved in EPS biogenesis
Random mutagenesis with the transposon Tn5 has been commonly used in M. xanthus to generate polar insertion mutants (Kuner and Kaiser, 1981; Torti and Zusman, 1981; Zusman, 1982; Chang and Dworkin, 1996; Rodriguez-Soto and Kaiser, 1997; Bowden and Kaplan, 1998; Yang et al., 1998b; Sun and Shi, 2001; Cusick et al., 2002). As Tn5 transposons have ‘hot spots’ and ‘cold spots’ for insertion, we screened a library previously created using another method for random mutagenesis developed based on recombination and integration of gene fragments cloned in a plasmid library (Cho and Zusman, 1999). Specifically, sheared genomic DNA fragments of ≈ 500 base pairs in length were cloned into a plasmid that encodes kanamycin resistance, as described (Cho and Zusman, 1999). The plasmids, which cannot replicate in M. xanthus, were then electroporated into wild-type cells. The KanR electroporants that resulted from homologous recombination between the fragments in the plasmids and chromosomal DNA were then screened for potential indicators of mutations in EPS biogenesis, specifically, defects in S-motility and fruiting body formation. Among the 5000 colonies screened, 68 showed phenotypes suggesting altered motility and fruiting body formation. These 68 mutants were further tested for phenotypes indicative of the loss of EPS, such as defects in cellular agglutination and CFW binding while retaining functioning A-motility and normal LPS. The sites of plasmid integration were then mapped and four previously unknown loci were identified for further study.
The four strains containing insertions, SW801–SW804 (Tables 1 and 2; Fig. 1), exhibited the defects expected of EPS biogenesis mutants. They were unable to form fruiting bodies, bind CFW or spread on soft agar (Table 3). These insertions mapped to four genes in two regions, eps and eas, which had not been previously characterized (Fig. 1; Table 2).
| Strain or plasmid | Relevant feature | Source or reference |
|---|---|---|
| Strain | ||
| M. xanthus | ||
| DZ2 | Wild type | |
| DK1622 | Wild type | |
| SW501 | DK1622, difE::kan | |
| SW504 | DK1622, ΔdifA | Yang et al. (1998a) |
| DK1217 | DK1622, aglB1 (A–S+) | Hodgkin and Kaiser (1979) |
| DK1218 | DK1622, cglB2 (A–S+) | Hodgkin and Kaiser (1979) |
| DK1253 | DK1622, tgl-1 (A+S–) | Hodgkin and Kaiser (1979) |
| DK1300 | DK1622, sglG1 (A+S–) | Hodgkin and Kaiser (1979) |
| SW801 | DZ2::pKY301-224 | This study |
| SW802 | DZ2::pKY301-137 | This study |
| SW803 | DZ2::pKY301-192 | This study |
| SW804 | DZ2::pKY301-108 | This study |
| SW810 | DK1622, ΔepsA | This study |
| SW811 | DK1622, ΔepsB | This study |
| SW812 | DK1622, ΔepsC | This study |
| SW813 | DK1622, ΔepsD | This study |
| SW820 | DK1622 epsE::pAL101 | This study |
| DK1622::epsI kan | Caberoy et al. (2003) | |
| SW824 | DK1622::pAL102 | This study |
| SW840-46 | DK1622::pCR2.1 carrying internal fragments of respective genes (epsF, H, K, N, O, U, V) | This study |
| SW830 | DK1622::pAL103 | This study |
| E. coli | ||
| TOP10 | Host for cloning | Invitrogen |
| Plasmid | ||
| pKY481 | Used to create a lacZ translational fusion, Kmr | Cho and Zusman (1999) |
| pBJ113 | Cloning vector, Kmr, galK | |
| pZErO-2 | Cloning vector, Kmr | Invitrogen |
| pCR2.1 | Cloning vector, Kmr | Invitrogen |
| pKY301 | A library of plasmids containing ≈ 500 bp DNA fragments of DZ2 | Cho and Zusman (1999) |
| pKY301-224 | pZEro-2 carrying 5′ fragment of epsZ | Cho and Zusman (1999) |
| pKY301-137 | pZEro-2 carrying internal fragment of epsZ | Cho and Zusman (1999) |
| pKY301-192 | pZEro-2 carrying internal fragment of epsA | Cho and Zusman (1999) |
| pKY301-108 | pZEro-2 carrying 3′ fragment of easAB | Cho and Zusman (1999) |
| pAL101 | pCR2.1 carrying internal fragment of epsE | This study |
| pAL102 | pCR2.1 carrying fragment downstream of epsA | This study |
| pAL103 | pKY481 epsDΦlacZ fusion | This study |
| ORFb,c | TIGR ORF number | Position in locus sequence | Homology (% identity/similarity) | Extent of homologya/total number of amino acids | Putative function | Organism (name of protein) | GenBank Accession No. for homologues |
|---|---|---|---|---|---|---|---|
| epsA | ORFA00949 | 816-1 | 39/59 | 2-264/271 | UDP-N-acetyl-mannosamine transferase (EpsP) | Methylobacillus sp. 12S (EpsP) | BAC55144.1 |
| epsB | ORF06774 | 3008-813 | 29/45 | 199-720/731 | Endo-1,4-β-glucanase precursor | Paenibacillus lautus (Cellulase A) | P29719 |
| epsC | ORF06776 | 3668-3111 | 46/68 | 61-183/185 | Serine acetyltransferase | Clostridium acetobutylicum | NP_347324 |
| epsD | ORF06778 | 4691-3678 | 35/50 | 1-320/337 | Glycosyltransferase, family 2 | Chloroflexus aurantiacus | ZP_00018612 |
| epsE | ORF06781 | 5469-6707 | 50/69 | 6-216/412 | Glycosyltransferase, family 1 | Crocosphaera watsonii WH 8501 | ZP_00006724 |
| epsF | ORF06782 | 6957-8522 | 40/57 | 12-514/521 | PAS/PAC domain | Nostoc punctiforme | ZP_00106968 |
| 39/55 | Response regulator/sensor histidine kinase | Nostoc sp.PCC7120 | NP_485886 | ||||
| epsG | ORF06783 | 8568-9887 | 56/76 | 13-439/439 | Mg2+ transporter | Bdellovibrio bacteriovorus HD100 (MgtE) | NP_969349 |
| epsH | ORF06785 | 10284-12845 | 28/46 | 645-846/853 | Glycosyltransferase, family 1 | Pyrococcus abyssi | NP_126928 |
| 25/39 | 426-839/853 | Cytophaga hutchinsonii | ZP_00117032.1 | ||||
| epsI | ORF06787 | 14210-12855 | 100/100 | 23-451/451 | nla24 | Myxococcus xanthus | AAQ63914 |
| 48/66 | 7-451/451 | nla7 | Myxococcus xanthus | AAQ63900 | |||
| 39/61 | 5-438/451 | sigma-54-dependent DNA-binding response regulator | Geobacter sulfurreducens | NP_953957 | |||
| epsJ | ORF06789 | 15712-14207 | 26/47 | 19-501/501 | Sensory box histidine kinase | Geobacter sulfurreducens | NP_953958 |
| epsK | ORF06791 | 15842-16945 | 28/49 | 38-360/367 | Membrane fusion protein | Ralstonia metallidurans | ZP_00023921 |
| epsL | ORF06792 | 16967-20140 | 43/64 | 1-1013/1057 | Putative silver efflux pump | Dechloromonas aromatica RCB | ZP_00152199.2 |
| 40/61 | 5-1016/1057 | cation efflux system protein CzcA | Bacteroides thetaiotaomicron VPI-5482 | NP_809593 | |||
| epsM | ORF06793 | 20150-21928 | 26/44 | 153-592/592 | Outer membrane protein cobalt/zinc/cadmium efflux RND transporter, outer membrane protein, CzcC family | Ralstonia metallidurans | ZP_00022271 |
| 29/44 | 202-582/592 | Pseudomonas putida KT2440 | NP_742215 | ||||
| epsN | ORFA00953 | 23067-22372 | 50/68 | 20-218/232 | Hypothetical protein pc0846 | Parachlamydia sp. UWE25 | YP_007845.1 |
| 28/45 | 2-227/232 | COG0596: hydrolase alpha/beta fold family | Burkholderia fungorum LB400 | ZP_00277209.1 | |||
| epsO | ORF06796 | 25440-23338 | 40/56 | 178-632/700 | COG2304: von | Pseudomonas fluorescens PfO-1 and Shewanellaoneidensis MR-1 | ZP_00086384 |
| 40/58 | 249-645/700 | Willebrand factor type A domain protein | NP_719099 | ||||
| epsP | ORF06797 | 25682-26101 | 47/63 | 1-120/139 | Transposase | Nitrosomonas europaea ATCC 19718 | NP_841625 |
| epsQ | ORF06800 | 27057-26641 | Hypothetical protein | ||||
| epsR | ORF06801 | 27265-27549 | Hypothetical protein | ||||
| epsS | ORF06802 | 28013-27696 | 41/51 | 13-51/106 | Hypothetical protein MG02468.4 | Magnaporthe grisea 70-15 | |
| epsT | ORF06803 | 28553-28137 | 38/54 | 3-134/138 | Conserved hypothetical protein Rv1044 | Mycobacterium tuberculosis H37Rv | NP_215560 |
| epsU | ORF06804 | 30036-28801 | 36/53 | 4-406/411 | Hypothetical protein | Chloroflexus aurantiacus | ZP_00018916 |
| 35/48 | 12-406/411 | Glycosyltransferase, family 2 | Desulfovibrio desulfuricans | ZP_00129998 | |||
| epsV | ORF06805 | 31584-30061 | 22/40 | 161-502/507 | Uncharacterized protein involved in exopolysaccharide biosynthesis | Nostoc punctiforme | ZP_00108474 |
| epsW | ORF06806 | 31985-31581 | 34/59 | 4-133/134 | COG0642: signal transduction histidine kinase | Trichodesmium erythraeum | ZP_00071530 |
| 37/60 | 8-122/134 | regulatory components of sensory transduction system | Synechocystis sp.PCC 6803 | NP_440125 | |||
| epsX | ORF06808 | 33411-32200 | Hypothetical protein | ||||
| epsY | ORF06810/ORF06812 | 35127-33451 | 37/51 | 386-557/558 | COG1596: polysaccharide biosynthesis/export protein OR conserved hypothetical protein/heteropolysaccha ride repeat unit export protein | Geobacter metallireducens OR ChloroflexusaurantiacusMethanosarcinamazei Goe1 | ZP_00079636 |
| OR | OR | OR | ZP_00018497 | ||||
| 35579-34107 | 26/47 | 5-316/490 | NP_634125 | ||||
| 22/46 | 15-373/490 | ||||||
| epsZ | ORF06813 | 37229-35754 | 57/73 | 294-490/491 | Glycosyltransferase domain protein | Geobacter sulfurreducens PCA | NP_952896 |
| 57/70 | 298-491/491 | capsular polysaccharide biosynthesis protein | Clostridium perfringens | NP_561409 | |||
| easA | ORF04096 | 1-809 | 59/74 | 17-268/269 | Hypothetical protein with DUF574 | Chloroflexus aurantiacus | ZP_00018698 |
| easB | ORF04097 | 963-1679 | Hypothetical protein |
- a . Amino acids over which homology is found.
- b . Underlined ORFs had been disrupted via insertional mutagenesis.
- c . Bold lines separate most probable transcriptional units.
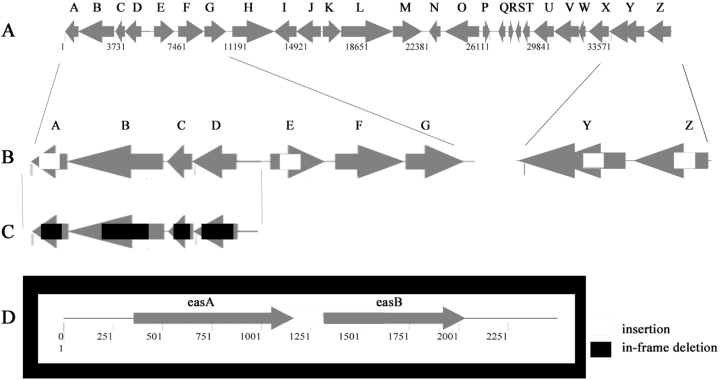
Maps of genes and mutations in the eps and eas loci. A. The eps locus showing the 26 most likely ORFs. B and C. The three eps operons analysed in present study showing locations of individual insertions. D. The eas locus showing the location of the insertion. White boxes show regions of insertions (insertions in epsA, Y and Z are from original random mutagenesis), black boxes show regions deleted by in-frame deletion mutations and grey arrows indicate ORFs.
 |
||||
| Agglutination | CFW binding | S-motility | Fruiting body formation | |
|---|---|---|---|---|
| IGS | + | + | + | + |
| epsA | – | – | – | – |
| epsE | +/– | + | – | – |
| epsF | + | + | +/– | + |
| epsH | – | – | – | – |
| epsI | +/– | – | – | – |
| epsK | + | + | + | – |
| epsN | + | + | + | + |
| epsO | + | + | + | – |
| epsU | – | – | – | – |
| epsV | – | – | – | – |
| epsY | – | – | – | – |
| epsZ | – | – | – | – |
None of these four mutations was located in any of the known pil genes, which encode proteins required for TFP biosynthesis, assembly and function. Furthermore, Western immunoblot analysis, using anti-PilA serum, confirmed that PilA was present in whole-cell lysates of the mutants and on their cell surfaces (data not shown). The phenotypes of the mutants were similar to the dif mutants, known to lack EPS (Bellenger et al., 2002; Li et al., 2003; Yang et al., 1998a): mutant strains SW801–SW804 spread poorly in soft agar (Table 3) and show elevated levels of sheared surface pilin (data not shown). It should be noted that the SW801–SW804 strains exhibited normal A-motility, as observed by examining the movement of individual cells near the colony edges (data not shown). To confirm these motility phenotypes, the mutations were transduced into strains containing only S-motility (A–S+), and resulted in non-motile transductants. These mutations were also transduced into A-motility (A+S–) strains resulting in cells with a phenotype similar to the parent strains. The results, listed in Table 4, indicate that these eps and eas mutations localize to genes specifically required for S-motility, but not A-motility.
| Recipient strains | Donor strains | ||||
|---|---|---|---|---|---|
| None | SW801 | SW802 | SW803 | SW804 | |
| DK1217, A–S+ | + | – | – | – | –/+ |
| DK1218, A–S+ | + | – | – | – | –/+ |
| DK1253, A+S– | + | + | + | + | + |
| DK1300, A+S– | + | + | + | + | + |
- Mx4 phage mediated generalized transduction of insertional mutations in SW801–SW804 into strains defective in either A-motility or S-motility. More than 20 transductants with consistent results were observed for each cross. (+), the resulting colonies have rough colony edges, indicating motile cells; (–), the resulting colonies have smooth colony edges, indicating non-motile cells; (–/+), the resulting colonies have slightly rough colony edges, indicating cells with limited motility.
Mutations in LPS O-antigen were previously shown to result in defective S-motility and fruiting body formation (Bowden and Kaplan, 1998), but EPS from these cells was normal. To rule out the possibility of an LPS mutation, the LPS of mutant strains SW801–SW804 was therefore analysed by silver staining of a deoxycholate (DOC)-gel that separated the LPS prepared from the mutant strains using the hot phenol–water method (Apicella et al., 1994). In all four mutants, the LPS appeared indistinguishable from the wild-type strain DK1622 and the dif mutant SW504 (data not shown). Thus, the eps and eas mutations appear to be unrelated to LPS biogenesis.
Sequence analysis of mutations
The sites of the SW801–SW804 mutations were determined by sequencing. The sequences were analysed using blast (Altschul et al., 1990; 1997; Gish and States, 1993) and the nearly completed TIGR genome sequence of M. xanthus (http://www.tigr.org/tdb/mdb/mdbinprogress.html). The possible open reading frames (ORFs) were then aligned against the NCBI database, yielding the following results: strains SW801 (epsZ) and SW802 (epsY) have insertions in two different genes at the distal end of the eps locus (Fig. 1; Table 2). EpsZ is a homologue of a Clostridium perfringens capsular polysaccharide biosynthesis protein with glycosyltransferase activity. There are two overlapping ORFs in the location of the epsY gene, both of which are disrupted by the insertion. One ORF is 37% identical and 51% similar to a Geobacter metallireducens periplasmic protein involved in polysaccharide export. The other ORF is 26% identical and 47% similar to a Chloroflexus aurantiacus heteropolysaccharide repeat unit export protein. The insertion in strain SW803 is located in epsA, at the proximal end of the eps locus. EpsA is 39% identical and 59% similar to EpsP from Methylobacillus sp. 12S containing a conserved domain with UDP-N-acetyl-mannosaminuronic acid transferase/UDP-N-acetyl- d-mannosamine transferase activity. According to their presumed functions, the genes found in the eps region are likely to be involved in EPS biosynthesis, assembly or transport.
The insertion in strain SW804 contains partial sequences of two ORFs, easA and easB. EasA is similar to conserved hypothetical proteins and EasB has no significant similarity to anything in the databases. The conserved hypothetical proteins similar to EasA contain a conserved domain of unknown function (DUF574) and this domain is found in Chloroflexus aurantiacus, Streptomyces coelicolor, Thermobifida fusca, Streptomyces collinus and Streptomyces avermitilis MA-4680. Sequence analysis of the eas region shows that the insertion could have been incorporated into either one of two hypothetical proteins. It is likely that this insertion had a polar effect on the second gene of the operon, effectively inactivating it. This analysis did not yield any information regarding the potential function of these genes in EPS biogenesis.
Genomic analysis of the eps region
The eps genes disrupted in strains SW801–SW803 were further analysed with respect to their relative positions within the M. xanthus genome and were found to localize to either end of a 37 kb region containing 26 putative ORFs, which we call the eps region (Fig. 1). This region contains genes similar to those involved in EPS biogenesis in other bacteria, and many genes of unknown function (Table 2). Including the genes disrupted in strains SW801–SW803, the eps region appears to encode at least six glycosyltransferase homologues, four homologues to regulatory proteins, one polysaccharide transporter homologue, one (endo)glucanase homologue and two ORFs that encode homologues to other proteins known to be involved in EPS biogenesis.
The original insertions were found in the following ORFs: MXORF06813 (epsZ), MXORF06810/12 (epsY ) and MXORFA00949 (epsA). epsZ appears to be a single gene operon with a strong hairpin loop terminator sequence between it and epsY. epsY, on the other hand, appears to be the first gene in five-gene operon suggesting that the downstream operon is involved in EPS biogenesis. epsZ and epsY are located at the distal end of this 37 kb region whereas epsA appears to be the last gene of a four-gene operon at the proximal end of this locus (Fig. 1). Analysis of the regions downstream of epsA and upstream of epsZ shows no ORFs. These regions appear to be large intergenic spaces with the nearby genes having no suspected EPS-related function. An insertion in the intergenic region downstream of epsA appeared to be a normal, EPS+ strain (Table 3). Insertional mutations introduced throughout the region into the first ORF of each operon supports the hypothesis that the whole region is involved in the biosynthesis of fibrils and fibril EPS (Table 3).
Sequence analysis of the operon containing epsA revealed three upstream genes corresponding to homologues of a glycosyltransferase (ORF06778, epsD), a serine acetyltransferase (ORF06776, epsC) and an endoglucanase (ORF06774, epsB) (Fig. 1). As insertion mutations have the possibility of introducing polar effects, this operon was analysed further using in-frame deletion analysis to determine the role of each individual ORF in EPS biogenesis, as described below.
Genetic analysis of the epsD-A operon
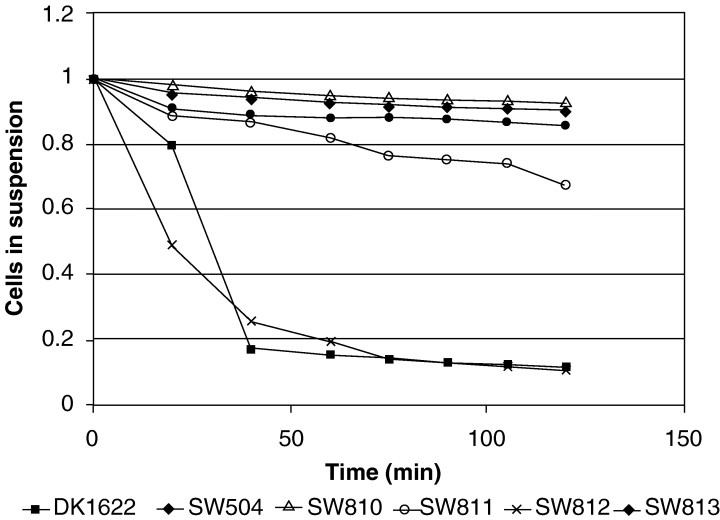
After reverse transcription polymerase chain reaction (RT-PCR) analysis confirmed that epsD-A constitutes a single transcriptional unit (data not shown), we generated in-frame mutations in each of the genes in the epsD-A operon to determine their effect on the production of M. xanthus EPS. Mutants SW810 (ΔepsA) and SW813 (ΔepsD), which carry deletions in genes that encode glycosyltransferase homologues, were found to have Eps– phenotypes, including deficiencies in CFW binding, agglutination, S-motility and fruiting body formation 2-4). They also react poorly with Mab2105, a monoclonal antibody generated against FibA protease, a major protein component of the fibril material (Fig. 6). SW812 (ΔepsC), which has an in-frame deletion in the gene encoding a serine acetyltransferase homologue, exhibits a less severe EPS– phenotype. This mutant binds CFW (Fig. 3D), but has a delay in developmental aggregation (data not shown) and is unable to form mature wild-type fruiting bodies (Fig. 2D). SW811 (ΔepsB), which contains an in-frame deletion in a gene encoding an endoglucanase homologue, did not show a clear EPS– phenotype, but still presented an overall colony morphology different from wild type 2-45, 6). A modified trypan blue assay was used to determine relative amounts of EPS in each of these mutant strains (Black and Yang, 2004). The results show a significant different in the binding of trypan blue to DK1622 cells versus the mutants (Fig. 7). Notably, SW812 (ΔepsB) shows less than 40% of the binding seen in wild-type cells, even though the other assays show no difference between the two strains. The gene identified in strain SW811 was independently discovered by the Hartzell/Youdarian labs (NCBI Accession No. AAF00919) to have a possible role in S-motility; however, they performed no further analysis of the region. In summary, this operon appears to function in M. xanthus EPS biogenesis and mutations in these genes have noticeable effects on the bacterium's ability to produce wild-type EPS or perform EPS-related functions, including fruiting body formation and S-motility.
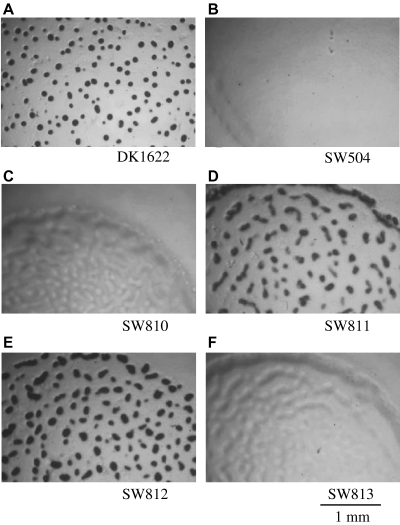
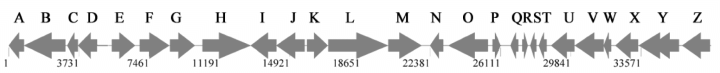
Fruiting body formation of wild-type and mutant M. xanthus strains, performed as described in Experimental procedures. The pictures were taken after 48 h of incubation on starvation MOPS plates. DK1622 (A) and SW812 (E) show fruiting body formation. All others are defective at different stages of fruiting body development. SW811 (D) shows translucent mounds.
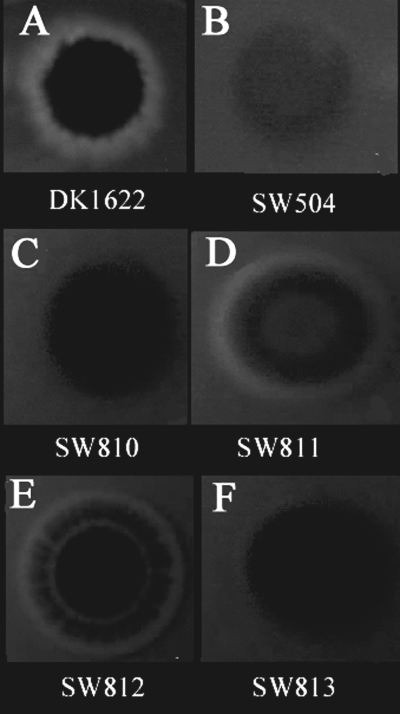
Calcofluor white binding of M. xanthus colonies, as described in Experimental procedures. Binding of calcofluor white to EPS results in a blue fluorescent ring seen in wild-type DK1622 (A), the in-frame deletion mutants SW811 (D) and SW812 (E). The other mutants do not bind the calcofluor white dye. The pictures were taken after 6 days of incubation, using a hand-held digital camera under a long-wavelength UV light source. The pictures are not to scale.
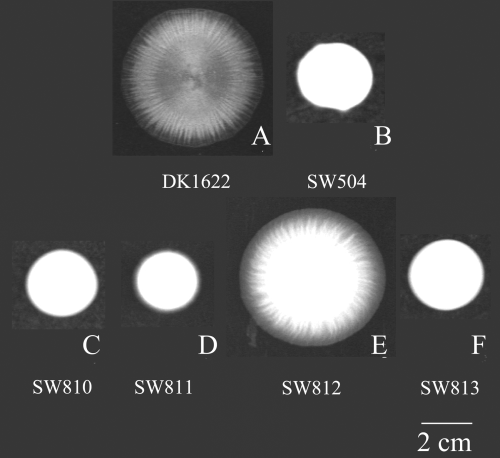
Colony spreading on soft agar, as described in Experimental procedures. The pictures were taken after 3 days of incubation on 0.3% CYE agar. DK1622 (A) and SW812 (E) show normal swarming and social motility. All other mutants are defective in colony spreading on soft agar.
Agglutination defects of esp/eas mutants. Cells were grown overnight in CYE medium and the OD600 was adjusted to about 1.0. The assay was conducted as described in Experimental procedures.
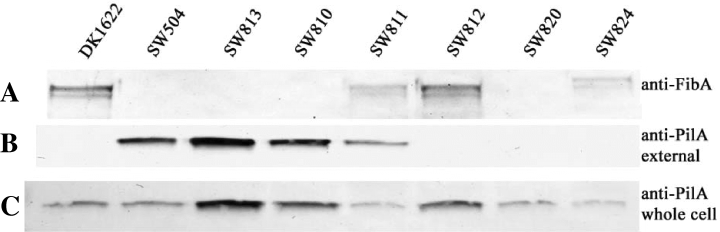
Western blotting analyses of FibA and PilA in whole-cell lysate or on the cell surface. See text and Experimental procedures for details. A. Monoclonal antibody, MAb2105, was used to probe the amount of FibA in whole-cell lysates. B. External pili were collected using a procedure that included vortexing, precipitation and purification. The anti-PilA antibody was then used to assay the amount of external pili. C. The anti-PilA antibody was used to probe the amount of PilA in whole-cell lysates.
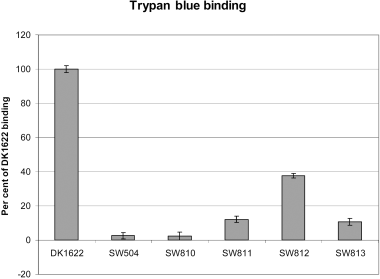
Trypan blue binding by various M. xanthus strains. This assay was conducted as described in Experimental procedures. The error bars show the standard deviation of three samples.
Transcriptional regulation of epsD-A during development
It has been demonstrated by several laboratories that EPS production in M. xanthus increases under high cell density and during starvation-induced development (Bacon et al., 1975; Sutherland and Thomson, 1975; Kim et al., 1999). The discovery of EPS biogenesis genes permitted us to test whether these conditions would increase transcription of the EPS biosynthesis genes. We constructed an epsD-A–lacZ transcriptional fusion strain (SW830) and assessed the expression patterns of epsD-A under conditions known to effect EPS production. As shown in Fig. 8, the β-galactosidase activities of the epsD-A–lacZ fusion strain did not show statistically significant increases under these conditions. In a similar manner, the β-galactosidase activities of the epsD-A–lacZ transcriptional fusion were indistinguishable between cells in high density (5 × 109 cells per ml) and low density (1 × 108 cells per ml) (data not shown). These data suggest that this operon is not regulated at the level of transcription under the conditions tested.
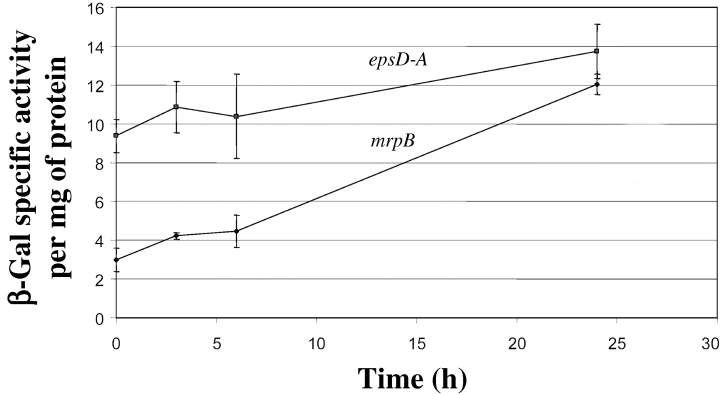
Expression of the epsD-A operon during fruiting body formation. β-Galactosidase (β-gal) activity from an epsD-A–lacZ fusion was determined. mrpB–lacZ, which showed at least fourfold increased expression over the course of development (Sun and Shi, 2001), is included as a developmentally regulated positive control. The data shown are averages of duplicate samples.
Do the dif genes control eps expression?
Strains with mutations in the dif genes, which are defective in a set of chemotaxis-like proteins (Yang et al., 1998a), display the Eps– phenotype (Yang et al., 2000; Bellenger et al., 2002). As chemotaxis-like pathways generally perform regulatory functions and the dif operon does not encode enzymes likely to be involved in EPS biogenesis, we wondered whether the dif genes play a direct or indirect role in the regulation of the eps genes. We used real-time RT-PCR to examine such a possibility. As shown in Fig. 9, expression levels of various eps genes are similar in dif mutant strains and wild-type DK1622, indicating that the transcription of these eps genes is not dependent on dif.
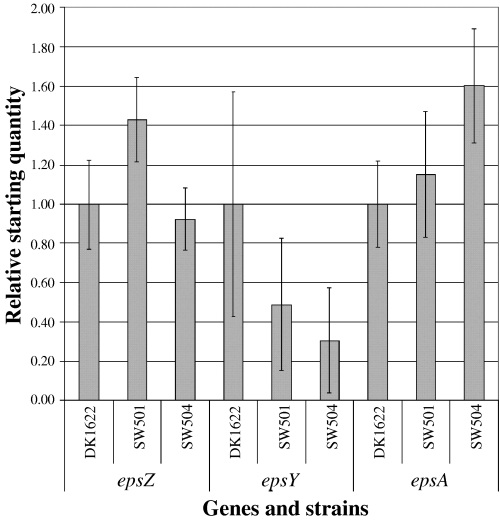
Expression of the epsA, epsY and epsZ in wild-type and dif mutants assayed by real-time RT-PCR, as described in Experimental procedures. The data shown are averages of duplicate samples. Statistical analysis (anova) does not show significant difference of the expression levels of eps genes in wild-type and dif mutant backgrounds.
Discussion
TFP and EPS are cell surface elements essential for S-motility and fruiting body formation in M. xanthus. Although much research has focused on the function and biogenesis of TFP, little information was available regarding EPS biogenesis. By analysing randomly generated mutants that do not map to the known dsp, dif, sglK or pil regions, four mutants were isolated that identify two new regions essential for wild-type EPS biogenesis.
The eps region contains genes involved in M. xanthus EPS production. Involvement of the entire eps region in EPS-related functions was demonstrated through a comprehensive screen revealing that inactivation of the majority of the putative operons resulted in the loss of EPS-dependent features (e.g. agglutination, CFW binding, fruiting body formation and/or S-motility) to various degrees. Not surprisingly, the most drastic effects were observed for operons containing ORFs likely to encode key proteins for EPS biogenesis, identified through homology to key EPS biogenesis proteins in other bacteria. Mutations in the eps operons with at least one ORF with homology to a glycosyltransferase resulted in strains defective in CFW binding, agglutination, S-motility and fruiting body formation, whereas other surface structures (TFP and LPS) required for these functions in M. xanthus were not affected, in tested strains. One operon in this region encodes the epsD-A genes, which was shown to play a role in EPS biogenesis. The exo gene cluster that mediates EPS biogenesis in Rhizobium meliloti appears to contain many of the same genes found in this M. xanthus eps region. As is the case for R. meliloti, Xylella fastidiosa and Xanthomonas campestris (Koplin et al., 1992; Katzen et al., 1998; da Silva et al., 2001), the M. xanthus genes required for the synthesis of the monosaccharides that compose the EPS do not appear to colocalized with the genes required for EPS assembly and transport.
The genes of the first operon in the eps locus are all related to EPS assembly. Specifically, the gene products are homologous to glycosyltransferases (epsD), endoglucanases (epsB), serine acetyltransferases (epsC) and UDP-N-acetyl-mannosamine transferases (epsA). Although the assays for Eps– phenotypes showed no significant difference between the wild-type strain DK1622 and the epsB mutant, their colony morphologies were clearly different. The trypan blue binding assay clearly shows a significant difference in the ability of the EPS in the mutant to bind the dye. As epsB is an endoglucanase homologue, it may be required to shed the EPS. If the epsB mutant does not shed its EPS, the cells may sense an increase in local concentrations of EPS, therefore slowing down production. New assays to test these hypotheses are being developed.
EPS biogenesis involves at least three steps: synthesis of monosaccharides, assembly of the monosaccharides into polysaccharides and transport of the polysaccharides to the cell surface. In R. meliloti, the glycosyltransferases in the exo region catalyse the addition of monosaccharides to the growing polysaccharide chain of succinoglycan whereas other genes in the region are involved in the addition of the first sugar to the lipid carrier, the polymerization of the octasaccharide subunit and the transport of the completed polymer (Glucksmann et al., 1993; Reuber and Walker, 1993). Some hints to the structure of the M. xanthus EPS may be found by analysing of the genes in the EPS region. As CFW is able to bind the M. xanthus EPS, its polysaccharide assembly must utilize at least some β-1,(3)4-linkages (Maeda and Ishida, 1967). An additional indication of the usage of β-1,(3)4-linkages is that epsB appears to be a β-1,4-endoglucanase precursor homologue. The epsY gene product is expected to be involved in EPS assembly, because it is a homologue of a bacterial EPS transporter (Stevenson et al., 1996).
Recently, one of our laboratories (HBK) screened a library of 20 000 mariner transposon insertion mutants and identified 52 that were CFW non-reactive. Two mutations inserted into polysaccharide biogenesis genes, which mapped to the eps locus reported here. A thorough analysis of the genes in these loci is in progress. However, before we can propose a pathway for M. xanthus EPS biogenesis and possibly discover its mode of action in TFP-mediated motility, it will be necessary to determine the structure of the EPS.
Previous studies suggested that EPS production in M. xanthus is regulated by environmental factors such as cell density and nutrient availability (Bacon et al., 1975; Sutherland and Thomson, 1975; Kim et al., 1999). We found that transcription of epsA was not increased under these conditions. However, there are a couple of different possibilities. First, the activity of these genes may not be limiting for EPS biosynthesis and other genes, not analysed in this study, may increase under developmental conditions. One ORF in the eps region, epsI (nla24), encodes an NtrC-like protein which has been shown to be involved in EPS biogenesis, S-motility and fruiting body formation (Caberoy et al., 2003; Lancero et al., 2003). Preliminary analysis of the eps region using the online analysis program, promscan (Studholme and Dixon, 2003), revealed nine putative sigma-54 promoters located in the region, one of which is downregulated in the nla24 mutant (data not shown). Interestingly, a mutation in the NtrC homologue, nla24 (also known as epsI), has been shown to result in strains with EPS biogenesis and A- and S-motility defects (H. Lancero, pers. comm.). This sigma-54-dependent regulator is located within the eps locus (Table 2, epsI) and may function as a global regulator of EPS production. Interactions may also occur between the dif proteins or other regulatory proteins and the eps gene products. There are response regulator homologues within the locus (Table 2, epsF, epsJ, epsW) that may regulate EPS assembly and/or transport. These response regulators are located within operons that also contain glycosyltransferases and, in one case, a polysaccharide chain length determinant protein family homologue. Studies on transcriptional regulation of the eps region by these regulators as well as others are currently underway.
The discovery of the EPS genes described in this article is significant for the understanding of motility in M. xanthus as EPS plays a critical role for S-motility. With the identification of these genes, we can begin to study the biogenesis and assembly of M. xanthus EPS and its role in co-ordinating social behaviours.
Experimental procedures
Bacterial strains, plasmids, phages and media
Bacterial strains used in this study are listed in Table 1. Escherichia coli TOP10 (Invitrogen) was used for in vitro DNA cloning. M. xanthus strains were grown and maintained at 32°C in CYE (Casitone-yeast extract medium) culture with appropriate antibiotics. Liquid cultures were grown to OD600 = 0.6–1 (3–5 × 108 cells per ml). MCM buffer [10 mM MOPS (pH 6.8), 10 mM MgCl2, 1 mM CaCl2] and MOPS (10 mM, pH 7.0) medium were used for fruiting body formation assays. Mutant strains were confirmed using Southern blots. E. coli cells were grown in Luria–Bertani (LB) with appropriate antibiotics at 37°C (Sambrook et al., 1989).
Generation of random insertional mutants
Mutants were generated as described previously (Cho and Zusman, 1999). Briefly, random pieces of genomic DNA from wild-type M. xanthus DZ2, ≈500 base pairs each, were cloned into plasmid pZErO-2 (Invitrogen), which carries a kanamycin resistance gene (Cho and Zusman, 1999). The resulting plasmid library was then electroporated into M. xanthus DZ2 (Kashefi and Hartzell, 1995), selected for KanR on CYE plates containing 100 µg ml−1 kanamycin and further screened for the phenotype of interest.
Generation of insertional mutants
Insertional mutants were created by electroporating vector pCR2.1 (Invitrogen) carrying internal fragments of the genes of interest into DK1622 (Table 5).
| Construct | Primer 1, 5′−3′ | Primer 2, 5′−3′ | Primer 3, 5′−3′ | Primer 4, 5′−3′ |
|---|---|---|---|---|
| Intergenic space downstream from epsA | GCGGTACATCCTGAATGACTC | GCGTACCACGAACTGGTCTT | ||
| epsE insert | CACGTCCACGTCCATTCAT | CTGAACGTCGTCTCCACCTT | ||
| EpsF insert | GTCCTGCTGGTGGACGAC | AGCAGCTCCCAGACGAACT | ||
| epsH insert | CGGTACTGAGGGAAGACGAA | AGTACACCACGTCCCACTCC | ||
| epsK insert | GCTCCGGTTTTTCTTCACC | CCTGGAGCTGGAGCATGT | ||
| epsN insert | GCAAGAACTGCACGACCAC | CGGTCTGGAGCTCTCTCGT | ||
| epsO insert | GAAGTCCATGGTGCTCACG | ATGCTCACCAAGGAGCAGTT | ||
| epsU insert | CACCTTCCACACCACGTAGA | GTGGTGGAGGACCTGGAGTA | ||
| epsV insert | CACGGGTAGCTTCAGAATCC | GAGCAGCGCGTCATCTATTC | ||
| ΔepsD | GAATTCCTCCGTCGTACAGCATCACC | TCTAGATGTACGGGATGGAGTATCTGC | TCTAGAGAGGCCAAAATCCAGGAGAC | AAGCTTCCAATCTTCTGCACACTGGA7 |
| ΔepsC | GAATTCCTCCATGGGGATGACAATCT | TCTAGAGCAAGGACACGGACGAAGT | TCTAGACACCTTGGCGATGGACTT | AAGCTTTTCGACAAGTCATTGGGACA |
| ΔepsB | GAATTCTTCATTAGCGGGAGGATGAG | TCTAGACGCATCTCCATTGGTGAGTA | TCTAGAGGAAGTCGTCGTAGCTCAGG | AAGCTTCCCATGGAGGATGAAGTGAT |
| ΔepsA | GAATTCCGGAGAACATCGTCCAGATT | TCTAGAGCGGTACATCCTGAATGACTC | TCTAGAGAGCTCATGGCTTCTTCACC | AAGCTTCCAGACGTACATCCTGGACA |
| epsD-A, lacZ | GGTACCATCTTCTGCACACTGGATGG | GGATCCTTGTACGTGGCGATGACG | ||
| epsD-epsC, real time (rt) | TGTACGGGATGGAGTATCTGC | CACCTTGGCGATGGACTT | ||
| epsC-epsB, rt | ACAGGCCTCCGTCGTACA | GTGGTGCTCCAGGACGTG | ||
| epsB-epsA, rt | GTTGGCGGTGAACACGTAG | AGGCGCAGATTCACCTCAAG | ||
| recA2, rt | ACTAAAAGCGCGTTCAGGTG | AGTTCGATCGCCTTTTCCTT | ||
| epsY, rt | TCAATGACTTGTCCCTGAGC | CTGCACGAAAGTGAGCAGAG | ||
| epsZ, rt | AGAACACCGATCAGGACCAC | TGGACATTGCTCACCATAGC | ||
| epsA, rt | TCCTCCCGCTAATGAAACTG | CGTACTGCTCGCTGACGAT |
Generation of in-frame deletion mutants
Genomic DNA was used as template for PCR amplification of fragments used in cloning for all mutants. The vector pBJ113 was used for cloning in-frame constructs.
Trypan blue binding assay
Cells (100 µl), resuspended in MCM to 5 × 108 cells per ml, were added to 900 µl of 0.005% trypan blue in MCM. Sample were vortexed, then allowed to incubate undisturbed at room temperature for 30 min. The samples were then spun down and the OD595 of each supernatant was recorded. Assays were performed in triplicate (Black and Yang, 2004).
Generation of a lacZ fusion mutant in the epsD-A operon
The following primers and constructs were used for creating lacZ fusion to the epsD-A operon: an insert that includes the region 600 bp upstream of the ATG (putative start codon) of epsD, as well as the codons for the first 17 amino acids of epsD, was generated using the oligonucleotides 5′-GGTACC ATCTTCTGCACACTGGATGG-3′ and 5′-GGATCCTTGTA CGTGGCGATGACG-3′ and cloned into pKY481, a plasmid that carries a promoterless lacZ.
Swarm plates
Myxococcus xanthus cells (2 µl) concentrated to OD600 = 10 (≈ 5 × 109 cells per ml) were spotted onto the centre of a CYE plate containing 0.3% agar (Shi and Zusman, 1993) and incubated for 3 days at 32°C. S-motility was analysed by observing the colonies for expansion. Colony morphology was documented using the GelDoc system (Bio-Rad).
Calcofluor white binding assay on plates
Myxococcus xanthus cells (10 µl) concentrated to OD600 = 10 were spotted onto CYE plates containing 1.5% agar and 5 µg ml−1 CFW. The plates were incubated for 6 days at 32°C. CFW binding was documented under exposure to long-wavelength UV light using a hand-held digital camera (Canon Powershot S200).
Generalized transduction
Generalized transduction was performed as previously described using phage Mx4 (Campos et al., 1978; Geisselsoder et al., 1978).
Western blots
Whole-cell lysates were made by pelleting cells and resuspending cells to 5 × 108 cells per ml in protein lysis buffer with SDS. These lysates were electrophoresed through SDS-PAGE gels and transferred to nitrocellulose membrane. MAb2105 (Behmlander and Dworkin, 1991) against FibA, an EPS-associated protein, and rabbit polyclonal anti-PilA antibody (Wu and Kaiser, 1997) against pilin subunits were used as primary antibodies. Cell surface pilin was isolated and assayed as described previously (Wu and Kaiser, 1997).
Agglutination assay
The cohesion of M. xanthus cells was measured with an agglutination assay described by Shimkets (1986a,b).
Fruiting body formation on MOPS plates
Cells (5 µl) harvested by centrifugation (3000 g for 6 min at 4°C) and resuspended in MOPS buffer to 5 × 109 cells per ml were spotted on MOPS/1.5% agar. Fruiting body development was documented after 48 h.
LPS analysis
LPS was isolated from 10 ml of logarithmically growing liquid cultures of M. xanthus cells using the modified hot phenol-water method (Apicella et al., 1994). Approximately 1–5 µg of each preparation was separated by electrophoresis through a DOC-15% polyacrylamide gel (Laemmli, 1970), and visualized by silver staining (Tsai and Frasch, 1982).
β-Galactosidase assay
Samples were collected at different time points during fruiting body formation in submerged culture. Specifically, the cells, incubated at 2.5 × 108 cells per well in 24-well culture plates with 400 µl of MCM buffer, were harvested and 40 µl of 500 mM phosphate buffer (pH 7.2) was added to bring the final concentration to 50 mM phosphate buffer. The cells were frozen at −20°C for at least 12 h. Cells were thawed, 500 µl of each of the cultures was combined with 10 µl of toluene, vortexed for 30 s and incubated at 37°C for 5 min. β-ONPG (20 mM; 50 µl) was added and samples were incubated until yellow colour develops, at which time 700 µl of NaCO3 was added to stop the reaction. The samples were spun down to remove the debris and they were read on the spectrophotometer at a wavelength of 450 nm. β-Galactosidase assays were presented in specific activity per mg of protein.
Real-time RT-PCR
RNA was isolated from vegetative cells using Trizol (Invitrogen) extraction. cDNA was made using Stratascript reverse transcriptase (Stratagene) and primed with random hexamers. The real-time PCR was performed with the iCycler from Bio-Rad using the primer pairs listed in Table 5. SYBR Green I (Molecular Probes) was used for detection of the amplified products. For each gene being measured, a sequence-specific standard curve was generated and expression of each gene in the dif genetic background was compared with wild-type expression.
Acknowledgements
We would like to thank Dr Hope Lancero, Yinuo Li and Justin Merritt for helpful discussion and experimental assistance. This work was supported by NIH Grant GM54666 to W. Shi, NIH Grant GM47444 to H.B. Kaplan, NIH Grant GM20509 to D.R. Zusman and NSF Grant MCB-0135434 to Z. Yang.




