Identification and characterization of the Pasteurella multocida toxin translocation domain
Summary
The Pasteurella multocida toxin (PMT) is a potent mitogen which enters the cytosol of eukaryotic cells via a low pH membrane translocation event. In common with the Escherichia coli cytotoxic necrotizing factor 1 (CNF1), the core of the PMT translocation domain is composed of two predicted hydrophobic helices (H1 – residues 402–423, H2 – 437–457) linked by a hydrophilic loop (PMT-TL – 424–436). The peptide loop contains three acidic residues (D425, D431 and E434), which may play a role equivalent to D373, D379 and E382/383 in CNF1. To test this hypothesis, a series of point mutants was generated in which acidic residues were mutated into the permanently charged positive residue lysine. Individual mutation of D425, D431 and E434 each caused a four- to sixfold reduction in toxin activity. Interestingly, mutation of D401 located immediately outside the predicted helix–loop–helix motif completely abolished toxin activity. Individual mutations did not affect cell binding nor greatly altered toxin structure, but did prevent translocation of the surface-bound proteins into the cytosol after a low pH pulse. Moreover, we demonstrate using an in vitro assay that PMT undergoes a pH-dependent membrane insertion.
Introduction
An increasing number of bacterial protein toxins are being identified which exert their effects through covalent modification of key cytoplasmic targets. The consequences for the cell vary from changes in cell physiology and proliferation to necrosis or apoptosis. These toxins are opportunistic molecules that have evolved to utilize various steps along the membrane trafficking pathways in mammalian cells to gain access to the cytosol where they exert their function (Falnes and Sandvig, 2000; Schiavo and van der Goot, 2001). While the exact mechanisms used by toxins to gain cellular entry are still poorly characterized, two broad classes of toxins have been identified. The first class of toxins possess the ability to translocate directly from endosomes to the cytosol. This translocation event is thought to be driven by the low pH of the endosomal compartments. The second class of toxin requires the retrograde transport of toxin molecules to the endoplasmic reticulum where they enter the cytosol via the secretory protein apparatus.
The mechanism of membrane translocation is best understood for diphtheria toxin (DT), produced by certain strains of Corynebacterium diphtheriae (Roux and Yersin, 1888). It is a medium-sized protein (Mr 58348) that is secreted by the bacterium as a single polypeptide chain, but in its functional form consists of two distinct chains linked by a single disulphide bond. Upon entry into mammalian cells, DT is cleaved by enzymes belonging to the Furin class of proteases (Gordon et al., 1995). Furin cleavage generates a 21 kDa N-terminal fragment A and a 37 kDa C-terminal fragment B.
Diphtheria toxin initially binds to its cellular receptor, the precursor of a heparin-binding EGF-like growth factor (Naglich et al., 1992), enters the cell via clathrin mediated endocytosis and then, in early endosomes, the catalytic fragment is released into the cytoplasm (Papini et al., 1993; Lemichez et al., 1997a). The low-pH environment encountered in early endosomes is known to be required for the penetration of DT into the cytoplasm. It has been shown for DT that a low extracellular pH, mimicking the conditions of endosomes, is sufficient to trigger entry of receptor-bound toxin into the cytosol (Draper and Simon, 1980; Sandvig and Olsnes, 1980).
The crystal structure of DT reveals it to be a Y-shaped molecule organized into three independently folded domains (Choe et al., 1992). Fragment B consists of two domains: the receptor binding domain composed of a flattened β-barrel and a ‘membrane translocation’ domain (T-domain) consisting of nine α-helices. As in the pore forming domain of the colicin A toxin (Parker et al., 1989), these are arranged in three layers with two unusually hydrophobic ones forming the central layer. Upon exposure to low pH within the endosomes, DT undergoes a major structural change. The three domains unfold to form a molten globule (MG) structure leading to exposure of hydrophobic regions and insertion of the T-domain (Sandvig and Olsnes, 1981; Tortorella et al., 1995). Thus, the low pH encountered by DT in the endosome triggers the translocation of the A-domain to the cytosol.
The Pasteurella multocida toxin (PMT) is produced by certain strains of P. multocida associated with porcine atrophic rhinitis (Hunt et al., 2000). PMT has been shown to be a potent mitogen for mesenchymal cells in culture (reviewed by Lax and Grigoriadis, 2001). The catalytic mechanism of the toxin is not currently known, but a prominent role for the heterotrimeric protein Gq has been identified (Wilson et al., 1997; Zywietz et al., 2001; Baldwin et al., 2003). The toxin affects several signal transduction pathways, resulting in increased inositol phosphate production, stimulation of protein kinase C activity, Ca2+ mobilization, actin rearrangements and increased protein tyrosine phosphorylation (Lax and Grigoriadis, 2001).
There is a substantial body of evidence to suggest that PMT is an intracellularly acting bacterial toxin. First, there is a notable lag period between the addition of toxin and any observed effects. The activity of PMT can be inhibited by early, but not late addition of a polyclonal anti-serum to PMT, suggesting the toxin has become internalized and is refractory to inactivation (Rozengurt et al., 1990). A similar time-dependent action is also observed upon incubation of cells with membrane permeable agents such as methylamine. These compounds act by inhibiting the acidification of newly formed vesicles and thus block the action of PMT, presumably in a manner analogous to the inhibition observed with other toxins. Furthermore, transient exposure of cells to PMT at 37°C, but not at 4°C, results in stimulation of DNA synthesis (Rozengurt et al., 1990). Finally, PMT is known to undergo structural changes at acidic pH (< pH 5.0), where it becomes sensitive to protease action and urea denaturation (Smyth et al., 1995; 1999). These observations suggest that PMT requires cellular entry and processing via an acidic compartment before stimulating DNA synthesis. Binding and internalization studies utilizing Monkey Kidney (Vero) cells revealed that, in these cells, the toxin interacts with a ganglioside-type receptor and is transported to the cytosol in endocytotic vesicles (Pettit et al., 1993).
Pasteurella multocida toxin is a large monomeric protein (relative molecular mass 143.6 kDa) which has been purified, cloned and sequenced (Lax et al., 1990). PMT shares significant homology with the cytotoxic necrotizing factors (CNF1 and CNF2) of Escherichia coli. The homology is highest in an N-terminal region of PMT (residues 250–530). In CNF1, this region functions as a T-domain (Pei et al., 2001) and is flanked by an N-terminal receptor binding domain and a C-terminal catalytic domain (Lemichez et al., 1997b) (see Fig. 1). Similarly, it has been shown that the receptor binding and catalytic domains of PMT are also located at the N- and C-termini, respectively, of the toxin (Busch et al., 2001; Pullinger et al., 2001).
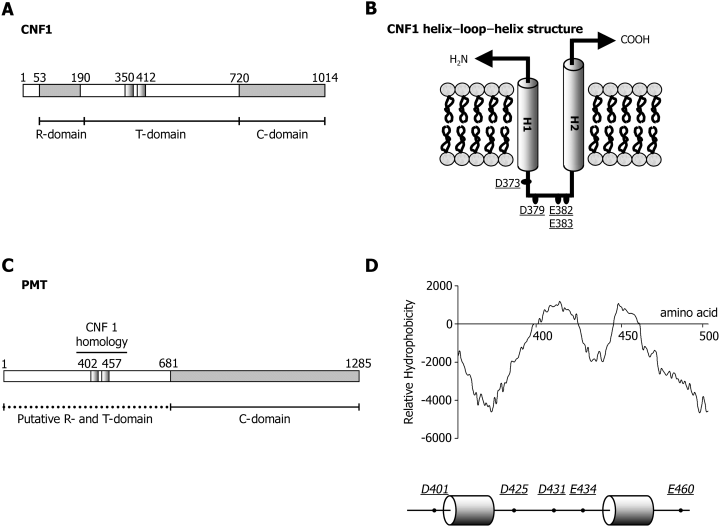
Diagram of primary and predicted secondary structures. A. Cytotoxic necrotizing factor 1 (CNF1). B. CNF1 helix–loop–helix structure. C. Pasteurella multocida toxin (PMT). D. PMT hydrophobicity plot (top) and predicted PMT helix–loop–helix structure (bottom). Numbered amino acids delineate the domains of the toxin and the location of the predicted helices. B and D. Residues in CNF and PMT which have been substituted for lysine are underlined (D).
The aim of this study was to determine whether the central region of PMT encodes a T-domain. We demonstrate that PMT, like several other intracellularly acting toxins encodes a functional T-domain required for translocation of the toxin.
Results
Identification of the potential PMT transmembrane domain
Previous studies had identified a hydrophobic region of the toxin (residues 370–470) as being a potential membrane spanning region (Lax et al., 1990). Moreover, this region of PMT displays significant sequence homology to a corresponding region of the E. coli CNF1 protein (Fig. 1A) (Pullinger et al., 2001). It has recently been shown that this region functions as a T-domain in CNF1 and thus could perform a similar function in PMT (Pei et al., 2001). To address this possibility, the sequence of PMT was analysed using the tmpred program (available at http://www.ch.embnet.org/software/TMPRED_form.html) which identifies potential transmembrane regions. The output (Fig. 1D) predicted that this region is organized in the same manner as the helix–loop–helix motif found in E. coli CNF1 (Fig. 1B). Helix 1 (H1) is predicted to extend from amino acid 402–423, with helix 2 (H2) extending from 437 to 457 and being linked by a hydrophilic loop from residues 424–436. Interestingly, this peptide loop – denoted PMT-TL, contains three acidic residues (D425, D431 and E434), which may play a role equivalent to D373, D379 and E382/383 in CNF1. To test this hypothesis, a series of point mutants were generated (Fig. 1D) in which acidic residues were mutated into the permanently positively charged residue lysine. Residues were replaced individually or in pairs using site-directed mutagenesis and recombinant proteins were purified using standard protocols as detailed. Purified wild-type or mutant PMT (1 µg) was separated on 10% (w/v) acrylamide gels and either stained with colloidal coomassie blue (Fig. 2A, top) or immunoblotted with an anti-PMT anti-serum (Fig. 2A, bottom). The toxins were all found to be highly expressed and did not appear to be degraded.
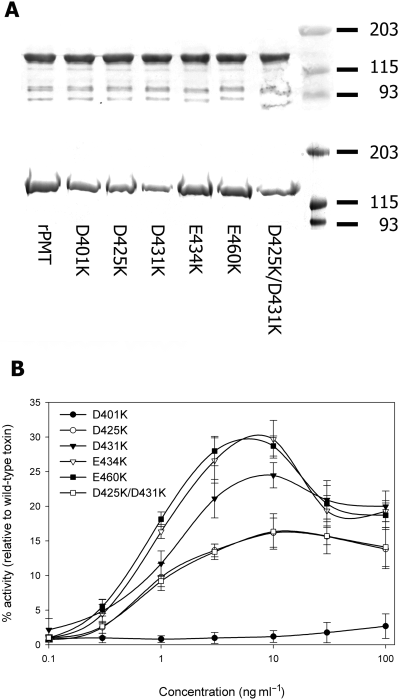
Mutation of acidic residues impairs toxin function. Acidic residues were replaced individually or in pairs with lysine using site-directed mutagenesis and recombinant proteins purified using standard protocols as described. A. Purified wild-type or mutant PMT (1 µg of protein) was separated on 10% (w/v) acrylamide gels and either stained with colloidal coomassie blue (top) or immunoblotted with an anti-PMT anti-serum (bottom). B. The ability of mutant toxins to stimulate DNA synthesis in quiescent Swiss 3T3 fibroblasts was measured over a concentration range of 0.1–100 ng ml−1 and compared with that of wild-type PMT. Each result is expressed as a percentage of the level of [H3]-thymidine incorporation stimulated by wild-type PMT and is representative of three independent determinations.
Mutation of putative T-domain residues reduces toxin activity
The abilities of the mutant toxins to stimulate DNA synthesis in quiescent Swiss 3T3 fibroblasts was measured over a concentration range of 0.1–100 ng ml−1 and compared with that of wild-type PMT. Mutation of any one of five acidic residues within the predicted T-domain caused marked attenuation of PMT activity (Fig. 2B). The dose response curves for mutants D425K, D431K, E434K, E460K and D425K/D431K were similar to those of the wild-type toxin except that maximal DNA synthesis was not achieved. Addition of higher doses of these mutants did not increase the levels of DNA synthesis (data not shown). Mutation of D401K resulted in complete inactivation of the toxin over the concentration range tested. This mutant did not display any mitogenic activity at concentrations up to 1000 ng ml−1, i.e. three orders of magnitude greater than that required to achieve half maximal stimulation by wild-type PMT. Repetition of the experiment using mutant toxin re-purified from either the same clone or an independent clone produced identical results (data not shown).
Mutation of putative T-domain residues does not block cell binding
It was important to ensure that mutations within the potential T-domain were acting to block translocation and not receptor binding or catalytic activity. The latter possibility was unlikely because the defined catalytic region begins at residue 681. However, the generated mutants do fall within the region known to mediate receptor binding (Pullinger et al., 2001). Introduction of the point mutations into PMT did not decrease their ability to compete with 125I-PMT (Fig. 3). Both wild-type and mutant toxins blocked binding of labelled PMT to cells when added at a 50 000-fold molar excess. The relatively high level of toxin molecules required to block binding was in agreement with previously determined values (Pullinger et al., 2001). These results indicate that the decreased mitogenic activity of the mutants did not result from their cell binding properties.
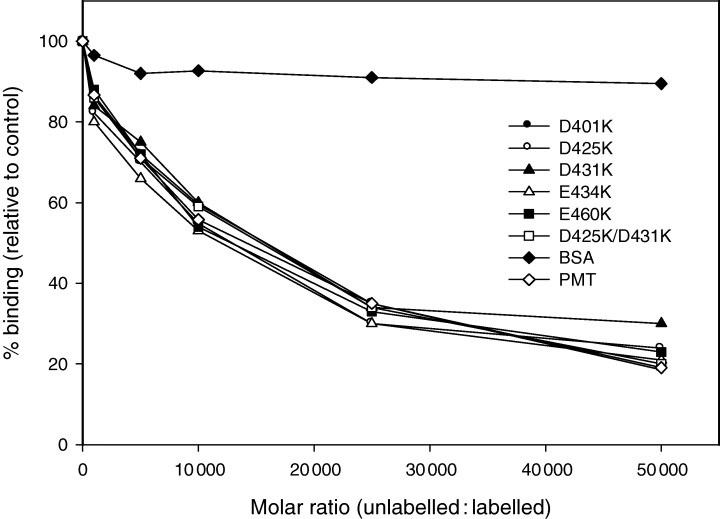
Cell binding activity of PMT mutants. 125I-PMT (1 ng ml−1) was added to cells in quadruplicate wells at 4°C in DMEM medium containing 25 mM Hepes buffer (pH 7.4) and 0.1% BSA in the presence of the indicated concentration of unlabelled PMT or the different unlabelled PMT mutants. Cells were incubated for 90 min at 4°C and then washed and counted for radioactivity as described. As a control, an equivalent concentration of BSA was added. Each result is expressed as a percentage of the level of 125I-PMT bound to cells in the absence of any additions. The experiment was repeated once with similar results.
PMT entry is inhibited by bafilomycin A1
The activity of PMT on Swiss 3T3 cells can be inhibited by the addition of membrane-permeable weak bases such as methylamine (Rozengurt et al., 1990). This suggests PMT is trafficked through a low-pH environment during the intoxication process. To investigate further this effect, cells were treated with bafilomycin A1 which is a well-known inhibitor of vacuolar ATPases, including those involved in trafficking (Yoshimori et al., 1991). Swiss 3T3 cells were pre-treated either with DMSO alone or with increasing concentrations of bafilomycin A1 for 1 h before stimulation with PMT. Bafilomycin A1 inhibits the activity of PMT in a dose-dependent fashion (Fig. 4A). This result supports the concept that PMT is trafficked via an acidic compartment during the intoxication process.
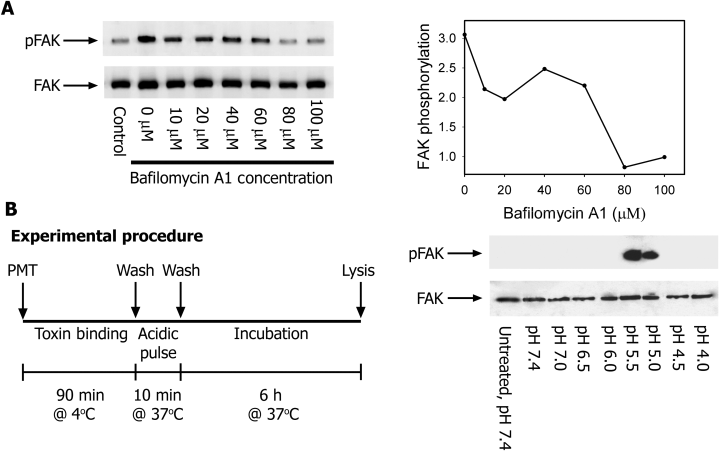
Low extracellular pH promotes direct transfer of PMT across the plasma membrane. A. Quiescent Swiss 3T3 cells were incubated for 1 h with increasing concentrations of bafilomycin A1 and then either left unstimulated or treated with 1 ng ml−1 PMT for 6 h. Focal adhesion kinase (FAK) was immunoprecipitated from RIPA extracts with anti-FAK antibody and Western blotted with either anti-Tyr(P) (top) or anti-FAK antibody (bottom). The right panel shows fold stimulation relative to the untreated control. B. Quiescent Swiss 3T3 cells were incubated at 4°C with 1 ng ml−1 PMT for 90 min to allow toxin binding. Cells were then acid-pulsed for 10 min at 37°C in various pH buffers in the presence of 100 nM bafilomycin A1. After pH shock, cells were incubated for a further 6 h in DMEM + 100 nM bafilomycin. FAK was immunoprecipitated from RIPA extracts with anti-FAK antibody and Western blotted with either anti-Tyr(P) (top) or anti-FAK antibody (bottom).
Low extracellular pH promotes direct transfer of PMT across the plasma membrane
To investigate further whether PMT utilizes a low-pH environment to mediate translocation, endocytic conditions were simulated by exposing cells with toxin bound to their surface to acidic medium (Fig. 4B). The binding of the toxin at 4°C coupled with the presence of inhibitory concentrations of bafilomycin A1 prevents endocytosis via the normal cellular route. Thus, any activity detected after the acid pulse would indicate that the toxin had translocated directly across the plasma membrane. No activity was detected at physiological pH (Fig. 4C). However, an increase in toxin activity was detected when the pH of the medium was lowered to between pH 5.5 and pH 5.0. The lack of activity below pH 5.0 probably results from the unfolding of PMT at low pH (Smyth et al., 1999).
Mutation of putative T-domain residues impairs translocation
The data presented in Fig. 4C indicate that PMT can be induced to translocate across the plasma membrane by brief exposure to low pH. Using this assay, we assessed what effect, if any, mutation of acidic residues within the T-domain region would have. As illustrated in Fig. 5A, when toxins were pulsed with medium at pH 7.4, no activity could be detected with either the wild-type or mutant toxins. However, when the cells were pulsed with medium at pH 5.4, an increase in wild-type toxin activity was detected (Fig. 5B). By comparison, no increase in activity could be observed with any of the mutant toxins. This suggests that they are defective in their ability to translocate across the plasma membrane.
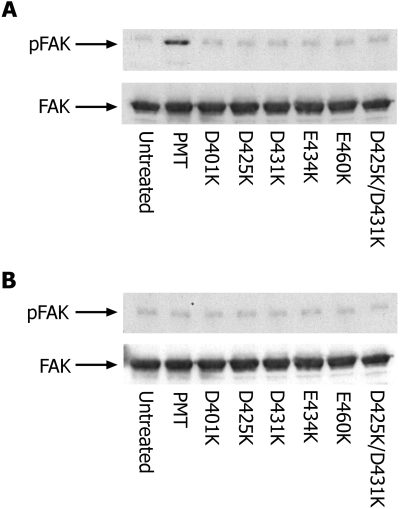
Mutation of acidic residues impairs translocation. Quiescent Swiss 3T3 cells were incubated at 4°C with 1 ng ml−1 PMT for 90 min to allow toxin binding. Cells were then acid-pulsed for 10 min at 37°C in buffer adjusted to pH 5.4 (A) or pH 7.4 (B) in the presence of 100 nM bafilomycin A1. After pH shock, cells were incubated for a further 6 h in DMEM + 100 nM bafilomycin. Focal adhesion kinase (FAK) was immunoprecipitated from RIPA extracts with anti-FAK antibody and Western blotted with either anti-Tyr(P) (top) or anti-FAK antibody (bottom).
Comparison of proteolytic susceptibility of wild-type and mutant toxins
It was important to determine whether the mutant toxins with altered biological activity had localized or major structural changes. Altered biological activity in the absence of significant structural change would indicate that the mutations had affected the ability of the toxin to undergo membrane translocation. To assess whether toxins had undergone major structural changes, the proteolytic susceptibility of the proteins was investigated. As previously reported (Smyth et al., 1995; 1999), PMT is highly resistant to proteolysis by endoproteinase Glu-C (Fig. 6A). However, there is a marked change in the susceptibility of PMT to proteolysis when SDS is present. PMT was completely digested by Glu-C at an SDS concentration of 0.0125% (w/v) (Fig. 6A). Mutants D401K, D431K, E434K, E460K and D425K/D431K were all resistant to Glu-C when SDS was not present, but were digested at slightly lower concentrations of SDS than wild-type toxin, indicating they could adopt a more dynamic structure than the wild-type protein. The D425K mutant was completely digested by Glu-C at the lowest concentration [0.005% (w/v)] of SDS. This suggests that its structure is much more open than that of the wild-type or other mutant proteins. Interestingly, however, this protein is not completely inactive.
Comparison of proteolytic susceptibility of wild-type and mutant toxins. A. Wild-type or mutant PMT (1 µg) was incubated with endoproteinase Glu-C (1 : 1 molar ratio) for 2 h at 37°C in 50 mM Tris/Acetate buffer, pH 7.4 containing increasing concentrations of SDS. Proteins were resolved by SDS-PAGE and visualized by staining with silver. B. Wild-type or mutant PMT (1 µg) was incubated with endoproteinase Glu-C (1 : 1 molar ratio) for 2 h at 37°C in 50 mM Tris/Acetate buffer, pH 7.4 or pH 4.5. Proteins were resolved by SDS-PAGE and visualized by staining with silver.
Pasteurella multocida toxin is also known to become sensitive to proteolysis by Glu-C at pH 5.3 or below (Smyth et al., 1999). Experiments were set up at different pH values to confirm these findings. As previously reported, PMT was degraded by Glu-C at pH 5.3 or below (data not shown). Further experiments were performed using wild-type and mutant toxins at either pH 7.4 or pH 5.0. Under these conditions, all proteins were resistant at pH 7.4, but became susceptible at pH 5.0 (Fig. 6B).
Quenching of the intrinsic fluorescence of PMT by Br-DOPG vesicles – dependence on pH
The intrinsic tryptophan fluorescence of proteins has been extensively exploited to study membrane insertion of proteins. One such approach utilizes brominated phospholipids (Gonzalez-Manas et al., 1992), which display characteristics very similar to the unsaturated precursors. Bromine atoms are known to quench the intrinsic tryptophan fluorescence of proteins. The effect decreases as the sixth power of the tryptophan–bromine separation (e.g. r6) and 50% quenching occurs when the bromine atom is 9 Å from the tryptophan. As the closest Van der Waals approach is about 6 Å, the quenching has been treated as being collision dependent (Bolen and Holloway, 1990; Abrams and London, 1992). Thus, quenching by lipids brominated at carbons 9–10 can only occur if the protein inserts tryptophans directly into the lipid bilayer.
The intrinsic fluorescence of tryptophan is pH dependent. It was therefore important to measure the emission spectrum of PMT over the range of pH to be tested. As expected, the intensity of fluorescence decreased with decreasing pH. Importantly, however, the wavelength at which the emission was maximum (337 nm) was not affected by pH (Fig. 7A).
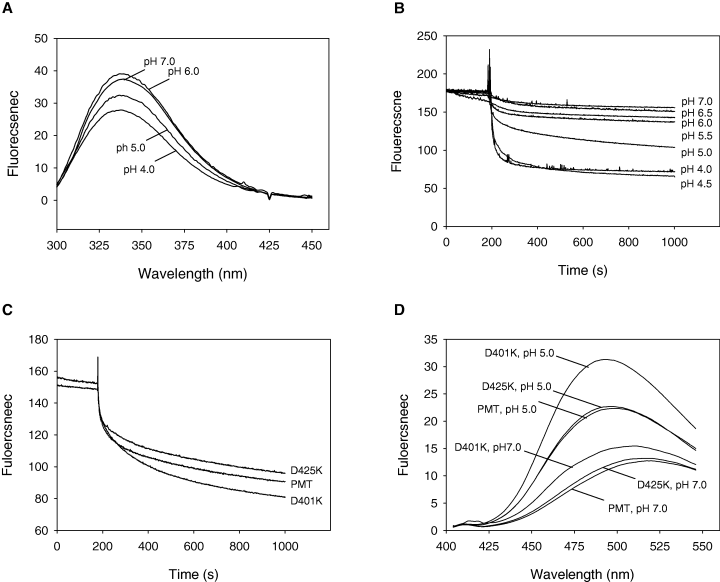
pH-dependent PMT membrane insertion and structural changes. A. PMT was diluted to 5 µg ml−1 in 2 ml of 50 mM Tris/Acetate buffer (various pH values), and the emission spectrum of PMT was recorded using an excitation wavelength of 290 nm. The Raman scatter contribution was removed by subtraction of Tris/Acetate buffer blanks. B. To 2 ml of the protein (5 µg ml−1), in 50 mM Tris/Acetate buffer (various pH values), was added 10 µl of a 10 mg ml−1 Br-DOPG vesicle suspension in the same buffer and the tryptophan fluorescence recorded as a function of time. The data are representative of three independent determinations. The data have been normalized based on the zero time value of the sample at pH 7.0. The zero time values of the samples were: 100.98 (pH 4.0), 104.46 (pH 4.5), 156.71 (pH 5.0), 156.32 (pH 5.5), 180.07 (pH 6.0), 178.54 (pH 6.5) and 179.20 (pH 7.0). C. Insertion of mutant PMTD401K and PMTD425K into Br-DOPG vesicles. D. ANS was diluted from stock to 0.1 mM in 400 µl of Tris/Acetate buffer, pH 7.0 or pH 5.0. Wild-type or mutant toxins were then added to a final concentration of 0.05 mg ml−1. The stable emission spectrum of ANS was then recorded using an excitation wavelength of 360 nm. Increases in fluorescent intensity and decreases in maximum emission wavelength compared with PMT at pH 7.0 indicate the existence of partially unfolded states.
Measurement of the kinetics of the intrinsic PMT fluorescence quenching allowed the insertion to be followed in real time. The addition of brominated-dioleoylphosphatidylglycerol (Br-DOPG) vesicles to a PMT suspension resulted in an exponential decrease in the fluorescence emission of the protein which displayed first-order kinetics. The data obtained from the membrane translocation experiments (4, 5) suggested that the insertion of PMT into lipids was pH dependent. Using the kinetic assay described, this was investigated further. The insertion of PMT into the Br-DOPG vesicles was shown to be pH dependent with a transition from a slow insertion to a fast insertion occurring at ≈pH 5.0 (Fig. 7B). The data have been normalized to the zero time value of the sample at pH 7.0.
Effect of mutations on the insertion rate
The insertion of the D401K and D425K mutants was assessed as described. The insertion was monitored at the apparent transition point of pH 5.0, because the assay sensitivity is maximal at this pH. While the effects were small, there were reproducible differences in the way each mutation altered the ability of the protein to insert (Fig. 7C). Mutation of D425 to K425 resulted in a protein with a slower rate of insertion (2.39 ± 0.05 × 10−3 s−1), while conversely the inactive D401K mutant had a higher rate of insertion (3.54 ± 0.03 × 10−3 s−1) relative to the wild-type toxin (2.62 ± 0.05 × 10−3 s−1). This can be compared with the pH-induced changes in PMT which lead to insertion rates of 11.8 ± 0.05 × 10−3 s−1 and 0.14 ± 0.05 × 10−3 s−1 at pH 4.0 and 7.0 respectively.
Binding of the fluorescent dye ANS by PMT
The ability of PMT to bind the fluorescent dye 1-anilino-8-naphthalenesulphonate (ANS) was determined in order to investigate these effects further. ANS is weakly fluorescent in aqueous solution; however, on addition of proteins containing hydrophobic pockets, its emission maximum shifts to shorter wavelengths, and its emission intensity is enhanced. Figure 7D shows the fluorescence of ANS-bound PMT at various pH values. The λmax,emission of ANS undergoes a blue shift in the presence of toxin as well as an increase in intensity.
These results indicate that PMT possesses a hydrophobic region(s) that is buried in the native state but is exposed at low pH. Mutant D425K displayed identical properties to the wild-type toxin. By contrast, mutant D401K bound the dye more efficiently at both neutral and low pH, but still showed a pH-sensitive difference.
Discussion
The work presented here has investigated whether PMT encodes a membrane translocation domain (T-domain). The existence of a T-domain was suggested by data showing that the activity of PMT could be inhibited by agents which interfere with endosomal trafficking (Rozengurt et al., 1990). Furthermore, sequence analysis indicated that the central region of the toxin was particularly rich in α-helical structures, a feature of membrane interacting proteins (Pullinger et al., 2001). We have shown here that PMT contains a T-domain, and, in common with several other bacterial toxins, there is a critical role for acidic residues in the translocation process ( Fridd and Lakey, 2002).
Based on sequence analysis, we predicted that the PMT T-domain was composed primarily of two hydrophobic helices (residues 402–423; 437–457) (H1–H2) linked by a short peptide loop (424–436). The predicted loop contains acidic residues (D425, D431 and E434), which could function in a mechanism similar to that used by DT and CNF1. Mutation of PMT residues D425, D431 and E434 to lysine caused a marked decrease in toxin activity (Fig. 2B). However, the mutant toxins retained some activity, suggesting that other amino acids were involved in the translocation process.
The function of this region was investigated further by mutation of D401 and E460, which lie immediately outside the predicted helix–loop–helix structure. Lysine substitution of E460 had a similar effect on toxin activity as lysine substitution of D425, D431 or E434. Mutation of D401 caused a complete loss of mitogenic activity at all concentrations tested without affecting cell binding. Mutation of the equivalent residue in CNF1 (D349K) also caused a significant decrease in toxin activity, but resulted from a defect in cell binding (Pei et al., 2001). These findings demonstrate that the mechanisms of translocation of PMT and CNF are not identical.
These data all support the concept of a low-pH-dependent translocation event. Membrane insertion was examined in vitro using small unilamellar vesicles (SUVs) based on Br-DOPG lipids. The resultant vesicles provide a good model system to investigate membrane insertion, although however, it should be noted that Br-DOPG carries a 100% negative charge, an artefact not found in nature. Using this system, we demonstrated that PMT can insert into lipid membranes (Fig. 7). PMT insertion was pH dependent, with the transition from a slow net insertion to a rapid insertion occurring at ≈pH 5.0. The rapid insertion of PMT at pH ≤ 5.0 agrees well with data indicating that PMT unfolds at similar pH values (Smyth et al., 1999). We also assessed the insertion of D401K and D425K. The D425K mutant was still able to insert into the vesicles at low pH, although at a reduced rate. Surprisingly the D401K mutant showed a faster rate of insertion at pH 5.0 than the wild-type toxin. The significance of this result is not clear, although it may be that the mutant protein interacts inappropriately with the plasma membrane. A similar result has been observed in a mutant of colicin A, which inserts into Br-DOPG membranes more rapidly but shows no toxicity (Baty et al., 1987). The observed differences in insertion rate between the wild-type and mutant proteins are low. However, the use of SUVs carrying a 100% negative charge may mask the true effect of the mutations.
Several bacterial toxins which encode translocation domains can translocate directly across the plasma membrane (Sandvig et al., 2002). When cells with PMT bound to the surface were exposed to a brief low-pH pulse (between pH 5.5 and pH 5.0), the toxin was able to translocate across the plasma membrane (Fig. 4). The translocation of PMT bound to cells thus appears to occur at a slightly higher pH than predicted from in vitro data. This is not entirely unexpected because cellular factors, including the toxin receptor, may facilitate the translocation process in vivo. Furthermore, DT is known to unfold in vitro at a higher pH in the presence of physiological concentrations of NaCl (Blewitt et al., 1984). Thus, the composition of the cell incubation medium may also contribute to translocation of PMT occurring at higher pH. Interestingly, no translocation was observed at pH values less than 5.0. It is possible that the toxin is completely unfolded at low pH and therefore may dissociate from its receptor, or be subject to proteolytic degradation. Urea denaturation experiments at different pH values support this explanation (Smyth et al., 1999). Finally, the point mutants were unable to translocate directly across the plasma membrane, supporting a role for these residues in the translocation process.
The ability of PMT to translocate across a lipid bilayer necessarily implies that it undergoes partial unfolding to form an MG state, with an increase in exposed hydrophobicity. The unfolding of PMT under various conditions has been extensively investigated (Smyth et al., 1995; 1999). PMT was found to unfold in the presence of denaturants such as SDS and urea and become sensitive to digestion by endoproteinase Glu-C. Removal of the urea allowed refolding of the protein without loss of activity. PMT also became sensitive to digestion by Glu-C at pH ≤ 5.3 (Smyth et al., 1999). We confirmed here that PMT undergoes a conformational change at pH 5.0 based on an increased susceptibility to proteolysis. Far-UV CD spectra for PMT revealed only a minor change in secondary structure at pH 5.0 (Smyth et al., 1999), an event that agrees with the formation of an MG state.
The formation of an MG state is associated with increases in hydrophobicity. As shown in Fig. 7D, PMT bound the fluorescent dye, ANS, only weakly at neutral pH. However, as pH decreased, the emission maximum of the dye shifted to shorter wavelengths, and its emission intensity was enhanced. These data clearly demonstrated that conformational changes at low pH exposed hydrophobic pockets within the molecule to which the dye could bind. Interestingly, the inactive D401K mutant protein showed higher levels of dye binding at both pH 7.0 and pH 5.0. The increased hydrophobicity of the protein implies it adopts a more open structure with increased exposure of hydrophobic patches. These data also correlate well with the increased insertion rate of this mutant protein.
In summary, this study has revealed that PMT, like several other intracellularly acting toxins, contains the machinery necessary to mediate its translocation across cellular membranes. It was demonstrated for the first time that PMT could insert into membranes both in vitro and in vivo. The study identified a number of acidic residues involved in the translocation process. The data presented indicate that at low pH PMT is converted to a MG state, an event likely to be critical for the translocation of the protein (van der Goot et al., 1991).
Experimental procedures
All chemical reagents were from Sigma, Ltd, unless otherwise stated. Restriction enzymes and DNA-modifying enzymes were purchased from Promega Corporation or New England Biolabs and used according to the manufacturer's instructions. Oligonucleotides were synthesized by Sigma Genosys. Chromatography reagents, ECLTM reagents, [3H]-thymidine (code No. TRK296) and Na125I (code No. IMS-30) were purchased from Amersham Biosciences.
Bacterial strains, vectors and growth conditions
Escherichia coli strain XL-1 blue was used as a general purpose host for both cloning and expression of full-length toxins contained on plasmid pTox2 (Ward et al., 1998). E. coli strains were grown routinely in Luria–Bertani (LB) broth or on LB agar aerobically at 37°C. Medium was supplemented with tetracycline (20 µg ml−1) and ampicillin (100 µg ml−1).
DNA isolation and sequence analysis
Plasmid DNA for use as a polymerase chain reaction (PCR) template was prepared using Wizard kits (Promega). For sequence analysis, DNA was further purified using Qiaquick PCR purification columns (Qiagen). Sequencing was performed using a Beckman Coulter CEQ2000XL automated DNA sequencer in accordance with the manufacturer's protocol.
Site-directed mutagenesis
Mutagenesis was performed by using the Quikchange mutagenesis kit (Stratagene) in accordance with the manufacturer's instructions. For generation of individual or double mutants, the following primers were used: D401K FOR (5′-TTA TCT GCA ATA AAA ATG TTA GTA CCA GCA) and D401K REV (5′-TGC TGG TAC TAA CAT TTT TAT TGC AGA TAA); D425K FOR (5′-TTA GGA CTT AGC TCG AAA ATT GTA GTT AAT GGA) and D425K REV (5′-TCC ATT AAC TAC ATT TTT CGA GCT AAG TCC TAA); D431K FOR (5′-ATT GTA GTT AAT GGA AAA TCA TAT GAA AAG AGA) and D431K REV (5′-TCT CTT TTC ATA TGA TTT TCC ATT AAC TAC AAT); E434K FOR (5′-AAT GGA GAT TCA TAT AAA AAG AGA AAA TAT GGA) and E434K REV (5′-TCC ATA TTT TCT CTT TTT ATA TGA ATC TCC ATT); E460K FOR (5′-ATT CCA GTT ATT TCG AAA ACC GCA GAA ATT TTA) and E460K REV (5′-TAA ATT TTC TGC GGT TTT CGA ATT AAC TGG ATT).
Expression and purification of wild-type and mutant PMT
The untagged recombinant PMT and its mutants were purified as previously described (Ward et al., 1998). Glycerol was added to 50% (v/v), and preparations were stored at −20°C for up to 3 months.
Cell culture
Stock cultures of Swiss 3T3 fibroblasts were maintained in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% (v/v) fetal bovine serum (FBS), penicillin (100 U ml−1) and streptomycin (100 µg ml−1) in a humidified atmosphere containing 10% CO2 and 90% air at 37°C. Stock cultures were grown for a maximum of 6 weeks after which a new vial (passage number <150) was thawed.
For experimental purposes, cells were routinely cultured in six-well plates at 105 cells per well or in 100 mm dishes at 6 × 105 cells per dish in DMEM supplemented with 10% (v/v) FBS and antibiotics, and used after 6–8 days when the cells were confluent and quiescent.
Thymidine incorporation assay
DNA synthesis was measured using the method of Dicker and Rozengurt (1980). Briefly, Swiss 3T3 cells were cultured in 96-well plates at 2.5 × 103 cells per well and used after 6 days. Cells were rinsed with PBS and then incubated for 40 h in DMEM/Waymouth's medium 1 : 1 (v/v) containing 0.0075 MBq of [3H]thymidine and various concentrations of wild-type or mutant toxins. DNA synthesis was determined by quantifying the radioactivity in the acid insoluble material.
Cell lysis and immunoprecipitation
After treatment of cell monolayers as appropriate, cultures were washed three times with ice-cold PBS and lysed in 1 ml of lysis solution [50 mM NaHepes, pH 7.4, 150 mM NaCl, 30 mM sodium pyrophosphate, 10 mM sodium fluoride, 5 mM EDTA, 1 mM sodium vanadate, 1% (w/v) sodium cholate, 0.1% (w/v) sodium dodecyl sulphate (SDS) and 1% (w/v) Triton X-100]. Cell lysates were clarified by centrifugation (13 000 r.p.m. for 10 min at 4°C) and supernatants transferred to fresh tubes.
The cell lysate was immunoprecipitated for 16 h at 4°C with rabbit polyclonal antibodies (2 µg) coupled to protein G-sepharose (50 µl drained gel). Immunoprecipitates were recovered by centrifugation, washed three times with fresh lysis buffer and extracted for 10 min at 100°C in 2× sample loading buffer [200 mM Tris/HCl, 4% (w/v) SDS, 10% (w/v) 2-mercaptoethanol, 20% (v/v) glycerol, 0.01% (w/v) bromophenol blue, pH 6.8]. The denatured proteins were then resolved by gel electrophoresis.
Facilitation of PMT entry into cells by exposure to low pH
The assay was performed essentially as described by Sandvig and Olsnes (1980). Briefly, quiescent Swiss 3T3 cells were incubated for 45 min in DMEM containing 25 mM NaHepes, pH 7.2 at 4°C to block all endocytic processes. Wild-type or mutant toxins were then added to a final concentration of 1 ng ml−1 for 90 min at 4°C to allow cell binding. After toxin binding, medium was aspirated and cultures rinsed three times in warmed shock buffer, pH 7.2 (0.5 mM MgCl2, 0.9 mM CaCl2, 2.7 mM KCl, 1.5 mM KH2PO4, 3.2 mM Na2HPO4, 137 mM NaCl). Cells were then incubated for 10 min at 37°C in shock buffer (pH adjusted with concentrated phosphoric acid as indicated) containing 100 nM bafilomycin A1. After low-pH exposure, cells were rinsed three times in shock buffer, pH 7.2 and then incubated for a further 6 h in DMEM supplemented with 25 mM Hepes, 0.1% (w/v) BSA and 100 nM bafilomycin A1. Cells were extracted as outlined above and subjected to immunoprecipitation using the indicated antiserum.
Iodination of wild-type PMT
Iodination of PMT was carried out using the Iodobead reagent (Perbio Science UK Ltd) in accordance with the manufacturer's guidelines. Briefly, a single Iodobead was transferred to a reaction vessel containing 9.25 MBq Na125I in PBS (total volume 0.4 ml). The reaction was allowed to proceed for 5 min before the addition of 20 µg of PMT in 0.1 ml of PBS. The incubation was continued for a further 20 min with mixing until the reaction was complete. Protein was separated from unincorporated label by gel filtration using a Nap5 column (Amersham Biosciences). Protein was eluted using PBS containing 0.1% (w/v) BSA and 0.1 ml fractions were collected and stored at 4°C. Fractions were analysed by SDS-PAGE and autoradiography using Kodak BioMax film. We have previously examined the biological activity of PMT iodinated in the manner reported here and found that its mitogenic activity was reduced threefold (G.D. Pullinger and A.J. Lax, unpubl. obs.).
Competition assay for cell binding
Quiescent Swiss 3T3 cells were washed twice with binding medium [DMEM/Waymouth's medium 1 : 1 (v/v) supplemented with 25 mM NaHepes and 0.1% (w/v) BSA] and incubated in the same medium at 4°C for 45 min to block all endocytic processes. Unlabelled wild-type or mutant PMT was then added at various dilutions and incubation continued for a further 30 min at 4°C. 125I-labelled PMT was then added to a final concentration of 1 ng ml−1 and incubation continued for a further 90 min at 4°C. Medium was aspirated and cell layers washed five times with ice-cold PBS. Plates were left to dry at room temperature and cultures extracted with 1 M NaOH for 1 h at 37°C. A fraction of the sample was counted for 30 min in 4 ml of Sigma-Fluor liquid scintillant cocktail (Sigma-Aldrich Ltd).
Proteolysis of PMT
Experiments to determine the proteolytic susceptibility of PMT and its mutants to endoproteinase Glu-C were carried out as described previously (Smyth et al., 1995; 1999).
Western blotting
Proteins separated by SDS-PAGE were transferred to Hybond ECLTM membranes for 2 h at 350 mA. Membranes were blocked in PBS – 0.5% (w/v) milk powder for 1 h and then placed in 0.1% (v/v) Tween 20 – phosphate-buffered saline (PBS-T) containing primary antibody at a dilution of 1:1000. After three washes in PBS, membranes were incubated in horseradish peroxidase-conjugated secondary antibody in PBS-T, followed by three more washes. Blots were developed by using the ECLTM kit (Amersham Biosciences).
Preparation of small unilamellar vesicles
Brominated-Dioleoylphosphatidylglycerol (Br-DOPG) was taken from a stock solution in chloroform (1 mg ml−1) and evaporated in a rotary evaporator. To the film of lipid was added a buffer solution (50 mM Tris/Acetate, pH 7.0) to yield a final lipid concentration of 10 mg ml−1, and the milky suspension thoroughly mixed by probe sonication on ice. The sonicated vesicles were centrifuged for 10 min at 4°C and the supernatant containing the SUVs was removed for use. The vesicles were stored at 4°C at all times.
Measuring insertion of PMT into Br-DOPG vesicles
Fluorescence spectra were recorded at 30°C using a Varian Instruments Cary Eclipse fluorescence spectrophotometer. The sample was excited with horizontally polarized light at 280 nm and vertically polarized emission recorded at 340 nM with spectral bandwidths of 5 and 10 nm for excitation and emission respectively.
Pasteurella multocida toxin (2.5 mg ml−1) was diluted to 10 µg ml−1 in 2 ml of 50 mM Tris/Acetate buffer at various pH values and incubated at 30°C with stirring for 5 min. After stabilization, 20 µl of vesicle suspension (10 mg ml−1) was added and the intrinsic protein fluorescence was recorded as a function of time.
Dye-binding assay
A 10 mM stock of ANS was prepared in MilliQ water. ANS was diluted from stock to 0.1 mM in 400 µl of Tris/Acetate buffer, pH 7.0 or pH 5.0 and the emission spectrum recorded using an excitation wavelength of 360 nm to establish a baseline. Wild-type or mutant toxins were then added to a final concentration of 0.05 mg ml−1 and a new emission spectrum was determined. When the signal had stabilized the changes in fluorescent intensity and maximum emission wavelength indicated the amount of ANS bound and the hydrophobicity of the binding site. Both fully folded and fully unfolded proteins show low ANS binding and it is therefore an indicator of partially folded states in which the protein hydrophobic core provides pockets for ANS binding.
Acknowledgements
The authors thank Dr Mark Munson and Dr Ana de Lillo of the Dental Institute for their assistance with DNA sequencing. This work was supported by a Medical Research Council Studentship (M.R.B.), Wellcome Trust Project Grant 62877 (A.J.L.) and Wellcome Trust Equipment Grant 56232 (J.H.L.). J.H.L. is a BBSRC Research Development Fellow.




