Pharmacokinetic properties of toceranib phosphate (Palladia™, SU11654), a novel tyrosine kinase inhibitor, in laboratory dogs and dogs with mast cell tumors
Abstract
Yancey, M. F., Merritt, D. A., Lesman, S. P., Boucher, J. F., Michels, G. M. Pharmacokinetic properties of toceranib phosphate (Palladia™, SU11654), a novel tyrosine kinase inhibitor, in laboratory dogs and dogs with mast cell tumors. J. vet. Pharmacol. Therap.33, 162–171.
Toceranib phosphate (Palladia™, SU11654), an oral tyrosine-kinase inhibitor, is under investigation for the treatment of mast cell tumors in dogs. The pharmacokinetics of toceranib phosphate has been characterized in dogs. Means of the following pharmacokinetic parameters were estimated following a 1.0 mg/kg i.v. dose to laboratory beagles: plasma clearance of 1.45 L/kg/h, volume of distribution of 29.7 L/kg, and terminal half-life of 17.7 h. Following single oral doses of 3.25 mg/kg administered to laboratory beagles, mean Cmax estimates ranged from 68.6 ng/mL to 112 ng/mL with tmax ranging from 5.3 h and 9.3 h postdose. Terminal half-life was estimated at 31 h. Oral bioavailability was 76.9%. There were no statistically significant (P > 0.05) differences with any pharmacokinetic parameter due to fed/fasted state or with time during 13 weeks of every-other-day dosing at 3.25 mg/kg. Toceranib concentrations were proportional with dose over the range of 2.0 to 6.0 mg/kg. The pharmacokinetics of toceranib in client-owned dogs of a variety of pure and mixed breeds with mast cell tumors was similar to that in healthy laboratory dogs. In summary, toceranib phosphate exhibited moderate clearance, a high volume of distribution, and a moderate elimination half-life. After a single oral dose at 3.25 mg/kg, the concentration vs. time curve showed broad, sustained exposure with measurable concentrations for more than 48 h. These pharmacokinetic parameters support every-other-day administration of toceranib phosphate at an initial dose of 3.25 mg/kg for the treatment of mast cell tumors in dogs.
Introduction
Many of the significant advances in cancer management in human medicine in recent years have centered on the development of molecularly targeted therapies such as tyrosine kinase inhibitors (TKIs). Tyrosine kinase receptors are transmembrane proteins involved in signal transduction that are expressed in many different cells and regulate cellular growth, differentiation and angiogenesis. Tyrosine kinases are perhaps one of the most critical groups of signaling molecules involved in normal and abnormal cellular regulation. They are frequently mutated or otherwise dysregulated in malignancies. Abnormal activation of receptor tyrosine kinase (RTK)-mediated signal transduction pathways results in cancer growth and metastasis.
Toceranib phosphate (Palladia™, Pfizer Animal Health, New York, NY, USA; SU11654) (Fig. 1) is an orally administered TKI with both direct anti-tumor and anti-angiogenic activity through inhibition of the tyrosine kinases KIT, vascular endothelial growth factor receptor 2 (VEGFR2) and platelet-derived growth factor receptor 2 (PDGFR2) (Liao et al., 2002; London et al., 2003; Pryer et al., 2003). An in-vivo target modulation study in dogs with naturally occurring mast cell tumors demonstrated reduced levels of phosphorylated KIT relative to total KIT in tumor samples obtained after administration of Palladia to dogs at a dose of 3.25 mg/kg compared to pretreatment biopsies (Pryer et al., 2003). A clinical field study confirmed the safety and efficacy of this dose when administered every other day for the treatment of dogs with Patnaik grade II or III, recurrent, cutaneous mast cell tumors with or without regional lymph node involvement. Treatment with toceranib phosphate resulted in significantly greater response rates and a significantly longer time to tumor progression compared to placebo treatment (London et al., 2009). An initial dose of 3.25 mg/kg (as free base equivalents, fbe) administered orally every other day with dose reductions to as low as 2.2 mg/kg fbe every other day or dose interruptions (cessation of drug for up to 2 weeks) to manage adverse events, if needed, yielded plasma drug concentrations that were both effective and well tolerated (London et al., 2009).
Structure of Toceranib Phosphate.
Based on preclinical work in rodent models, it has been proposed that the therapeutic range of toceranib for target (e.g., KIT, VEGFR2, PDGFR2) inhibition is 50 to 100 ng/mL for at least 12 h of a 24-h dosing period (London et al., 2003). A phase I study in tumor-bearing dogs demonstrated that daily administration of toceranib phosphate at a dose of 3.25 mg/kg resulted in trough plasma concentrations ranging from 40 to 60 ng/mL. Hence, dogs on the daily dosing regimen in the phase I study maintained plasma concentrations within the purported therapeutic range throughout the entire dosing interval. However, due to the severity and frequency of adverse events observed in dogs at all daily dosage regimens, daily dosing was not considered tolerable. Dogs administered 3.25 mg/kg on an every-other-day schedule had trough plasma concentrations that ranged from 3 to 34 ng/mL (London et al., 2003). Plasma concentrations in dogs on the every-other-day dosing schedule presumably cycled in and out of the therapeutic range and all trough levels were below the therapeutic range. This dosage regimen was well tolerated and efficacious in dogs. Hence, it appears that achieving toceranib plasma concentrations above a certain threshold (e.g., 50 ng/mL in a rodent model) is important for clinical efficacy and allowing plasma concentrations to fall below this range is important to ensure clinical tolerability in dogs.
This manuscript describes for the first time the pharmacokinetic (PK) properties of toceranib phosphate in dogs following oral and intravenous (i.v.) administration. Toceranib phosphate has recently been approved by both US and European regulatory authorities for the treatment of mast cell tumors in dogs.
Materials and methods
Pharmacokinetic studies
All references to doses are as toceranib phosphate, the salt form of the drug, and are given as free base equivalents. All references to plasma concentrations and pharmacokinetic estimates are to toceranib, the free base.
Five PK studies were conducted with toceranib phosphate. Study 1 examined the single dose oral and i.v. PK and oral bioavailability of toceranib phosphate in beagle dogs. Study 2 determined the effect of food on systemic exposure in beagle dogs. Study 3 examined the changes in PK after 7 every-other-day oral doses, the intended dosing frequency in dogs. Study 4 evaluated the systemic exposure to mast cell tumor-bearing dogs that had been treated with toceranib phosphate for at least 6 weeks in a field efficacy study. Study 5 was designed to evaluate the margin-of-safety after dosing every-other-day for 13 weeks.
The initial target therapeutic dose of toceranib phosphate is 3.25 mg/kg; however, using tablets, the actual delivered dose ranged from 2.8 to 3.7 mg/kg, depending on body weight and the strength of the tablet. Oral doses in Studies 1, 4, and 5 were administered as the commercial tablets. Tablets used for Studies 2 and 3 were the final formulation that differed from the commercial tablets only in the tablet strength (20 mg compared to 10, 15, and 50 mg), scoring vs. no scoring, and a change in film-coating from Opadry to Opadry II (superior coating properties but retaining immediate release of drug). All other excipients remained the same and the two formulations showed no differences with in vitro dissolution.
These studies were performed over the period of late discovery through development and, thus, employ different analytical methods and software for PK calculations and are not presented in the order they were conducted. All experiments were carried out in compliance with national legislation and subject to local ethical review. All studies were either carried out in compliance with Good Laboratory Practices (GLP) standards (Studies 1 and 5), Good Clinical Practices (GCP) standards (Study 4), or were well documented consistent with internationally recognized standards (Studies 2 and 3).
Study 1 (i.v./oral PK and oral bioavailability)
Eight clinically healthy beagle dogs (four male and four female, weighing between 5.9 kg and 7.9 kg at the time of randomization) were included in this development stage study. Dogs were individually housed in stainless-steel cages. The study was designed as a two-treatment, two-period cross-over study with a 14-day washout period between treatments. Subjects were randomized into two groups of four dogs, each with two males and two females, and were administered one of two treatments. On study day 0, one group received a single oral administration of toceranib phosphate at a target dose of 3.25 mg/kg using a combination of commercial tablets containing toceranib phosphate at 10, 15, or 50 mg/tablet. The remaining dogs received a single bolus i.v. administration of toceranib phosphate as a sulfobutyl ether β-cyclodextrin-based solution via the cephalic vein at a dose of 1.0 mg/kg. The oral dose of 3.25 mg/kg was selected as this is the target therapeutic dose. The i.v. dose of 1.0 mg/kg was selected as it provided adequate concentrations to allow for PK parameter estimation and to minimize the possibility of toxicity at extreme concentrations. After a 2-week washout period (study day 14), the dogs received the other treatment. All dogs were fed a diet of Harlan Teklad dog diet 2021 (Harlan Teklad, Madison, WI, USA), provided once daily in the morning in amounts appropriate for the size and age of the dogs. Feed was provided for approximately 1 h each day. Dogs were fasted for 22 to 23 h prior to dosing and feed was withheld until approximately 4 h after dosing. Blood samples were collected from all dogs at 0 (predose), 0.5, 1, 2, 4, 8, 12, 24, 32, 48, 56, 72, 96, and 120 h after each dose. Blood samples were collected from the jugular vein into tubes containing K2EDTA. The samples were mixed immediately by inversion, chilled on ice and protected from light, and centrifuged within 30 min of collection. Plasma from each sample was transferred to a resealable tube, and stored at <−60 °C.
Study 2 (fed/fasted effects)
The late discovery phase study was designed as a two-treatment, two-period crossover study with a washout period of 7 days. Twelve clinically healthy beagle dogs (six males and six females, weighing between 7.7 kg and 14.0 kg at the time of randomization) were randomized into two groups of six dogs each (three males and three females). On study day 0, one group was fed prior to dose administration and the other group was fasted as described. Dogs in the fed group were given a portion of their daily ration one h prior to dosing by administering 100 to 150 mL of food/water slurry (one part food homogenized with four parts water) using a feeding tube. These dogs had immediate ad libitum access to the remainder of their daily ration. Dogs in the fasted group were fed approximately 24 h prior to dosing and not again until approximately 8 h postdose. At all other times prior to and during the study, dogs had ad libitum access to their daily ration of up to 300 g per day of Certified Canine Diet #5007 (PMI® Feeds, Inc., St. Louis, MO, USA). Following a 7-day washout period, the feeding state of each animal was reversed in period 2. In each period, all dogs received a single dose of toceranib phosphate at a target dose of 3.25 mg/kg using scored 20 mg prototype tablets. The number of tablets and half tablets was adjusted so that dogs received as close to the target dose rate as possible. Potable rechlorinated deionized water was offered ad libitum throughout the study. Dogs were individually housed in stainless-steel cages. Blood samples were collected from all dogs at 0 (predose), 0.5, 1, 2, 4, 6, 8, 12, 16, 24, 28, 32, 48, 56, and 72 h after each dose and processed for plasma analysis as described for Study 1.
Study 3 (2 week repeat dosing)
Eleven clinically healthy adult beagle dogs (six male and five female weighing between 7.0 kg and 12.9 kg) were included in this late discovery stage study. Dogs received food and water ad libitum. Dogs were individually housed in stainless-steel cages. Toceranib phosphate was administered orally via a prototype tablet at a target dose rate of 3.25 mg/kg per dose using scored 20 mg prototype tablets. The number of whole tablets was adjusted so that dogs received as close to the target dose rate as possible. Each dog received seven doses on an every-other-day schedule over 2 weeks. Blood samples were collected from all dogs at 0 (predose), 1, 2, 4, 6, 8, 12, 24, 28, 32, and 48 h after dose 1; at 0 and 8 h after doses 3 and 5; and at 0, 1, 2, 4, 6, 8, 12, 24, 28, 32, 48, 56, 72, 96, 120, 144, 168, 192, 216, and 240 h after dose 7. Blood samples, approximately 3 mL/sample, were collected from the jugular vein into tubes containing K2EDTA and processed for plasma as described for Study 1.
Study 4 (field efficacy)
Adult dogs with naturally occurring recurrent cutaneous mast cell tumors were enrolled in a GCP field efficacy study in which they were treated with toceranib phosphate. The nominal dose of toceranib phosphate was 3.25 mg/kg administered every other day with or without food; however, dose reductions to a minimum dose of 2.2 mg/kg every other day and dose interruptions for up to 2 weeks were permitted, if needed, to manage adverse events. Any dog participating in the PK analysis portion of this study was required to be on toceranib phosphate treatment for at least 6 weeks without missing any of the three doses immediately prior to sample collection. In addition, the owners were required to provide written informed consent for collection of blood samples for PK analysis. Eight (three male and five female) of 151 dogs enrolled in the clinical field study had owners willing to have their dog participate and met the above requirements. These eight dogs represented a variety of breeds and mixed breeds (beagle, Labrador retriever, Rhodesian ridgeback, and mixed breed) from 6 to 10 years old and weights ranging from 8.2 to 39.4 kg. Each of the eight dogs also received from 1 to 6 noninvestigational concomitant medications. Six dogs received anti-infective and antiparasitic agents (metronidazole, milbemycin oxime, imidacloprid/permethrin, or fipronil). Three dogs received an antihistamine (diphenhydramine), three received H2-antagonists (cimetidine or ranitidine), and three received nonsteroidal antiinflammatory drugs (carprofen, etodolac, or salicylic acid.) Other concomitant drugs used by individual dogs included amitryptyline, acepromazine, levothyroxine, glucosamine and diethylstilbestrerol. Each dog was fed according to the normal feeding pattern for that animal. Potable water was offered ad libitum. Dogs were housed at the clinical trial sites during the period of sample collection but were otherwise housed with their owners. Blood samples were collected into tubes containing EDTA at target times of 0 (predose), 1, 2, 4, 6, 8, 12, 24, 28 and 32 to 36 h after dose administration with samples taken at both 32 and 36 h, if possible. Flexibility in sampling times was permitted, with up to a 0.5 h difference allowed in samples taken between 1 and 12 h and 2 h difference allowed in later sampling times. Blood samples were processed for plasma as described for Study 1. Exact sampling times were used for the PK analysis.
Study 5 (13-week margin of safety)
This development stage study included three treatment groups plus a placebo-treated control group. The treatment groups received 2, 4, or 6 mg/kg of toceranib phosphate every-other-day for 13 weeks. There were 4 males and 4 females for each of the 2 and 6 mg/kg dose groups and six males and six females for the 4 mg/kg dose group (body weight ranged from 6.2 to 15.1 kg at the beginning of the study). Blood samples were collected into tubes containing EDTA at 0 (predose), 1, 3, 6, 24, and 48 h after dosing on days 0, 28, and 86. Blood samples were processed for plasma as described for Study 1.
Analytical methods
Toceranib was quantitated in plasma samples by high-performance liquid chromatography (HPLC) with tandem mass spectrometry detection (LC-MS/MS). Aliquots (50 μL) of control plasma, calibration standards, QC samples, and study samples were fortified with an internal standard (IS), either a deuterated toceranib analog or a chlorine-substituted analog, prior to extraction. Toceranib and the IS were extracted from the plasma using either liquid/liquid extraction with ethyl acetate or by protein precipitation using acetonitrile. The analyte and IS are light sensitive and will undergo photoisomerization when exposed to light. Therefore, the 96-well plates were protected from light by wrapping the bottom and sides with foil and securing with adhesive tape. Calibration standards (1 to 1000 ng/mL) and quality control samples were prepared by fortifying control canine plasma with toceranib solution. Study samples were analyzed as single replicates.
Prepared samples were analyzed by LC-MS/MS using either a Sciex 4000 or Sciex 3000 triple-quadrupole mass spectrometer with Turboionspray ionization source (MDS Sciex, Toronto, Canada), or a ThermoFinnigan TSQ Quantum mass spectrometer (Thermo Fisher Scientific, Inc., Waltham, MA, USA). HPLC was performed using a Betasil C18 column (100 × 2.1 mm, 5 μm, Thermo Fisher Scientific, Inc., Waltham, MA, USA) or Zorbax StableBond CN column (50 × 2.1 mm, 5 μm, Agilent) with a mobile phase of acetonitrile: 15 mm ammonium formate solution (pH 3.25) 30:70 (v:v) at a flow rate of 0.40 mL/min (isocratic elution of analyte and IS). After 5 min, the column was flushed with acetonitrile:15 mm ammonium formate solution (pH 3.25) 80:20 (v:v) at a flow rate of 0.40 mL/min to remove late-eluting endogenous materials. Both toceranib and its IS eluted from the column at about 2.2 min. Transition ions of m/z 397.2 → 283.2 for toceranib and 401.3 → 283.2 for the IS were used.
The standard curves were generated using peak area ratios of toceranib to the IS and a 1/concentration2 weighted quadratic regression. Toceranib concentrations were determined by interpolation over the quantitation range of 1.00 ng/mL to 1000 ng/mL. Back calculated calibration standards from seven analytical runs from the validation showed a mean bias ranging from −2.8% to 3.6% with CVs of 6% or less. Six sets of control samples were analyzed at concentrations of 1.00 (lower limit of quantitation), 3.00, 450, and 800 ng/mL during each of the validation runs. Intrarun bias ranges from −6.4% to 15% at the lower limit of quantitation and −6.0% to 8.2% for the remaining control samples. CVs were 5.3% or less. Inter-run bias ranged from 0.3% to 5.1% with CVs of 10.5% or less. There were no interfering peaks at the retention time of toceranib greater than 4.4% of the lowest standard, and no measurable interference at the retention time of the IS.
Each analytical run performed using these methods met the acceptance criteria established in the Guidance for Industry, Bioanalytical Method Validation (FDA Guidance for Industry 2001).
Data analysis
Noncompartmental PK calculations were conducted using either Watson (Thermo Fisher Scientific, Inc., Waltham, MA, USA; ver. 7.2) for the development Studies 1 and 5 or WinNonlin (Pharsight Corporation, Mountain View, CA, USA; ver 03.1A) for the late-discovery Studies 2, 3, and 4. Both calculation methods employed the trapezoidal rule for estimation of AUC values. The portion of AUC extrapolated from the last measurable concentration to infinity was calculated as the last measurable concentration divided by the elimination rate constant, k. Mean residence time (MRT) was calculated as AUMC0-∞ (area under the first moment curve extrapolated to infinity) divided by AUC0-∞. Clearance (CL) was determined following i.v. administration by the dose divided by AUC0-∞. Volume of distribution at steady state (Vss) was calculated following i.v. administration as CL times MRT. Dose proportionality was determined by evaluating the linear regression slope of log-transformed Cmax or AUC vs. log Dose. If the 90% confidence interval for slope included 1.0, then toceranib was considered dose proportional in the dose range tested.
Because oral doses were administered as tablets, the actual dose varied from animal to animal. Therefore, the PK parameters of Cmax, Cmin, and AUC in all studies were normalized to the nominal dose prior to statistical analysis. Differences between fasted and fed feeding states were tested using the paired t-test. The SAS MIXED procedure was used for the analysis of study 1 (SAS Institute, Inc., Cary, NC, USA ver 9.1). Means and standard deviations (SD) were calculated using Microsoft Excel.
Results
Single-dose – i.v. and oral administration
Plasma concentrations of toceranib following an i.v. bolus dose of 1.0 mg/kg to fasted beagles (Study 1) are shown in Fig. 2 and mean PK parameters are shown in Table 1. Concentrations of toceranib were above the lower limit of quantitation of 1.0 ng/mL out to 56 to 72 h postdose. The mean CL was 1.45 L/kg/h with individual animals ranging from 1.29 to 1.66 L/kg/h. Mean Vss was 29.7 L/kg and ranged from 25.2 to 34.8 L/kg. Mean terminal phase half-life (t1/2) was 17.5 h, and ranged from 15.0 to 19.1 h. The extrapolated portion of the AUC was less than 6.3% of AUC0-∞. There were no adverse events observed with the single i.v. dose of toceranib phosphate at 1.0 mg/kg in this study.
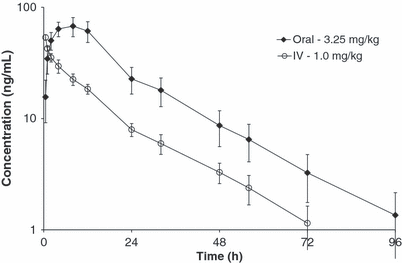
Mean Toceranib concentrations (±1 Standard Deviation) after Single Oral and i.v. Doses from Day 0 of the i.v./Oral Crossover PK/Bioavailability Study (Study 1). Concentrations from oral dose normalized to dose of 3.25 mg/kg.
| Route | Oral | i.v. | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| AUC0-t(last)(ng·h/mL) | 1730* | 368* | 662 | 71.1 |
| AUC0-∞(ng·h/mL) | 1770* | 368* | 698 | 77.4 |
| F (%) | 76.9 | 16.2 | NC | NC |
| C max (ng/mL) | 68.6* | 12.9* | 53.6 | 2.92 |
| t max (h) | 7.0 | 2.0 | NC | NC |
| t 1/2 (h) | 17.1 | 2.33 | 17.7 | 1.82 |
| CL/F(L/kg/h) | 1.89 | 0.377 | 1.45 | 0.165 |
| MRT (h) | 22.4 | 2.83 | 20.7 | 2.67 |
| V ss/F(L/kg) | 42.0 | 7.45 | 29.7 | 3.97 |
- n = 4 dogs per dose group (two males and two females). NC, not calculated; LS-Mean, least squares mean; SD, standard deviation.
- *Concentration-based parameters were normalized for the oral dose to a dose of 3.25 mg/kg to account for tablet dosing.
Plasma concentrations of toceranib following mean actual oral doses of 3.32 mg/kg administered to fasted beagle dogs (Study 1) are shown in Fig. 2. Concentrations of toceranib were above the lower limit of quantitation of 1.0 ng/mL out to 72 to 96 h postdose. The mean Cmax (normalized to a dose of 3.25 mg/kg) was 67.8 ng/mL with individual animals ranging from 55.4 to 86.4 ng/mL. Mean tmax was 7.0 h, ranging from 4.0 to 8.0 h. Mean t1/2 was 16.8 h and ranged from 13.7 to 19.1 h. Absolute oral bioavailability was estimated to be 76.9%. The extrapolated portion of AUC was less than 2.7% of AUC0-∞. There were no adverse events observed with the single oral dose of toceranib phosphate at 3.25 mg/kg.
In Study 1, there was a statistically significant period effect between Days 0 and 14 (Periods 1 and 2) for plasma concentration (P = 0.02), AUC0-t(last) (P = 0.02), AUC0-∞ (P = 0.02), Cmax (P = 0.02), t1/2 (P < 0.01), F (P = 0.01), CL (P < 0.01), and Vss (P = 0.02). However, the sequence effect was not significant (P ≥ 0.07). A washout period of 14-days was considered adequate since Study 2, a late discovery study, showed no period effect with only a 7-day washout period. Therefore, summaries of the PK parameter estimates above are given only for Period 1.
To assess the effect of food upon toceranib exposure, PK parameter estimates were compared using data from Study 2. Dose-normalized plasma concentrations of toceranib following mean actual oral doses of 3.29 and 3.32 mg/kg to fed and fasted beagle dogs, respectively, are shown in Fig. 3. Mean PK parameters are shown in Table 2. Concentrations of toceranib were above the lower limit of quantitation of 1.0 ng/mL for the entire 72 h period of sample collection. Mean ± SD dose-normalized Cmax of toceranib was 102 ± 30 ng/mL and 112 ± 28 ng/mL for the fasted and fed groups, respectively. Cmax was observed at approximately 6 h after dosing for both dose groups. Mean ± SD t1/2 were 17.2 ± 2.5 h and 18.4 ± 3.6 h for the fasted and fed states, respectively. There was about a two-fold range in values for Cmax, AUC, and t1/2, in both the fed and fasted treatment groups, but the ranges of values between the two treatment groups were nearly identical. There were no statistically significant differences in Cmax (P = 0.06), t1/2 (P = 0.10), tmax (P = 0.62), or AUC0-∞ (P = 0.14) between the fed and fasted states.
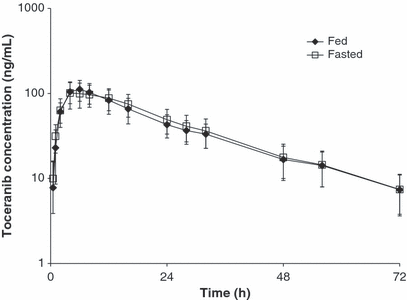
Mean Toceranib Plasma Concentration Profiles (±1 Standard Deviation) for Fed and Fasted Beagle Dogs Receiving Target Doses of 3.25 mg/kg by Tablet (Study 2). Concentrations normalized to dose of 3.25 mg/kg.
| Fasted | Fed | |||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| AUC0-∞*(ng·h/mL) | 3010 | 803 | 2870 | 705 |
| C max*(ng/mL) | 102 | 30 | 112 | 28 |
| t max (h) | 6.2 | 2.5 | 5.5 | 0.9 |
| t 1/2 (h) | 17.2 | 2.5 | 18.4 | 3.6 |
| MRT (h) | 26.3 | 4.1 | 26.8 | 5.1 |
- n = 12 for each dose group (six males and six females). SD, standard deviation. *Concentration-based parameters were normalized to a dose of 3.25 mg/kg.
Multiple-dose – oral administration
Seven every-other-day doses of toceranib phosphate were administered to beagles at mean actual doses of 2.89 mg/kg (dose 1) and 2.86 mg/kg (dose 7) (Study 3). Doses were more variable and lower for Study 3 because only whole tablets of 20 mg/tablet were used rather than the smaller tablets available in Study 1 or the use of whole and half tablets in Study 2. Doses were kept below the threshold of 3.25 mg/kg due to potential adverse effects of the higher doses. Dose-normalized plasma concentration data are shown in Fig. 4. PK data from doses 1 and 7 are shown in Table 3.
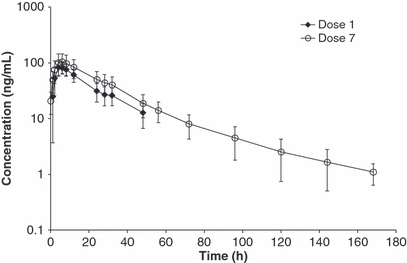
Mean Plasma Concentration Profiles (±1 Standard Deviation) of Toceranib in Beagle Dogs Receiving Seven Every-Other-Day Doses of Toceranib Phosphate by Tablet (Study 3). Concentrations normalized to dose of 3.25 mg/kg.
| Dose 1 | Dose 7 | |||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| AUC0-48*(ng·h/mL) | 1830 | 510 | 2640 | 940 |
| AUC0-∞*(ng·h/mL) | 2160 | 670 | 3270 | 1120 |
| C max*(ng/mL) | 86.1 | 21.5 | 108 | 41 |
| C min*(ng/mL) | 12.7 | 6.0 | 18.7 | 8.3 |
| t max (h) | 5.3 | 1.6 | 6.2 | 2.6 |
| t 1/2 † (h) | 16.4 | 3.6 | 17.2 | 3.9 |
- n = 11 dogs in each dose group (six males and five females). However, data from one male dog was not included for dose 7 due to the dog being mis-dosed. SD, standard deviation. *Concentration-based parameters were normalized to a dose of 3.25 mg/kg. †t1/2 was calculated using the terminal slope from 8 to 48 h postdose.
Mean ± SD Cmax of toceranib was 86 ± 22 and 108 ± 41 ng/mL following doses 1 and 7, respectively, an increase of 25%. With the exception of one dog, the individual animal range was nearly the same between dose 1 and 7, 62.0 to 135 ng/mL after dose 1 and 62.7 to 125 ng/mL plus one dog at 203 ng/mL after dose 7. The tmax typically ranged from 4 to 8 h after dosing. Mean ± SD trough concentrations (Cmin) at 48 h after doses 1 and 7 were 12.7 ± 6.0 and 18.7 ± 8.3 ng/mL, respectively, an increase of 47%. Trough concentrations (48 h postdose) were variable, covering a 4- to 5-fold range of values. Mean AUC0-48 increased 44% between doses 1 and 7. Plots of mean plasma concentrations at 48 h after several discrete doses (Cmin, trough) are shown in Fig. 5 to illustrate changes in mean concentrations. Steady-state appears to have been achieved by dose 4.
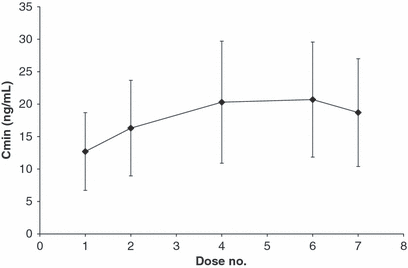
Mean Plasma Concentrations (±1 Standard Deviation) of Toceranib at 48 h PostDose (Cmin, trough concentrations) in Dogs Receiving Multiple Every-Other-Day Doses of Toceranib Phosphate by Tablet (Study 3) Concentrations normalized to dose of 3.25 mg/kg.
In Study 3, plasma samples were collected between 0 and 48 h after dose 1 and to 240 h after dose 7. Concentrations of toceranib were above the lower limit of quantitation of 1.0 ng/mL out to 96 to 216 h after dose 7. The t1/2 calculated using 0 to 48 h data ranged from 11.7 to 22.5 h after dose 1 and from 13.2 to 24.8 h after dose 7 with means ± SD of 16.4 ± 3.6 h and 17.2 ± 3.9 h, respectively. There was no appreciable change over the 2-week period of the study. However, by evaluating the data out to 240 h after dose 7, t1/2 ranged from 18.2 to 44.6 h (mean ± SD of 31 ± 8.9 h).
During the PK sample collection period of the field efficacy study (Study 4), doses ranged from 2.30 mg/kg to 2.99 mg/kg with the exception of one dog dosed at 0.93 mg/kg due to dose reductions due to adverse events (London et al., 2009). With the exception of the latter dog, individual observed Cmax (not dose-normalized) values ranged from 38.9 to 103 ng/mL. Dogs in this study had recurrent mast cell tumors and had been on drug treatment for at least 6 weeks without missing any of the three doses immediately prior to sample collection. PK parameters have been normalized to the target dose of 3.25 mg/kg to facilitate comparisons of results across studies (Table 4). Mean ± SD normalized maximum plasma concentrations of 79.0 ± 25.6 ng/mL were reached 6 to 12 h after dosing. Mean ± SD normalized trough concentrations, measured immediately prior to dosing, were 19.1 ± 11.8 ng/mL. The mean ± SD t1/2 was 18.2 ± 4.5 h.
| Mean | SD | |
|---|---|---|
| AUC0-t(last)*(ng·h/mL) | 1870 | 660 |
| C max*(ng/mL) | 79.0 | 25.6 |
| C min*(ng/mL) | 19.1 | 11.8 |
| t max (h) | 9.3 | 2.3 |
| t 1/2 (h) | 18.2 | 4.5 |
- n = 8 (three males and five females). SD, standard deviation. *Concentration-based parameters were normalized to a dose of 3.25 mg/kg.
Pharmacokinetic data are summarized in Table 5 for the margin-of-safety study (Study 5) in which healthy beagle dogs received every-other-day oral target doses of 0, 2, 4, or 6 mg/kg (mean ± SD actual doses of 2.28 ± 0.17, 4.25 ± 0.19, and 6.28 ± 0.20 mg/kg). Although a limited number of samples were collected from this study, comparisons across both doses and time could be made. The means for dose-normalized Cmax were 36.2, 78.8, and 120 ng/mL for the 2, 4, and 6 mg/kg dose groups, respectively, on Day 0. On study Day 86, the mean Cmax was 38.7, 62.7 and 103 ng/mL. Cmax increased at a rate proportional with dose in female dogs, but increased at a rate slightly greater than dose in male dogs (90% CI for slope was 1.07 to 1.60). Similarly, mean dose-normalized AUC0-48 was 799, 1780, and 2580 ng·h/mL for the 2, 4, and 6 mg/kg dose groups, respectively, on Day 0 and it was 859, 1420, and 2290 ng·h/mL, respectively, on day 86. AUC0-48 increased at a rate proportional with dose for both male and female dogs. There were no statistically significant differences in Cmax or AUC0-48 between study days 0 and 86 (P = 0.55 and 0.83, respectively).
| Study Day | Treatment | Mean (SD) | |||
|---|---|---|---|---|---|
| C max (ng/mL) | AUC0-48(ng·h/mL) | t max (h) | C min (ng/mL) | ||
| 0 | 2 mg/kg | 36.2(17.2) | 799(415) | 4.8(1.6) | 4.31(3.50) |
| 4 mg/kg | 78.8(38.0) | 1780(1120) | 5.5(1.2) | 9.60(11.7) | |
| 6 mg/kg | 120(18.4) | 2580(484) | 4.7(1.6) | 12.7(5.31) | |
| 28 | 2 mg/kg | 33.8(13.5) | 759(312) | 4.5(1.6) | 4.36(2.67) |
| 4 mg/kg | 79.3(27.5) | 1640(476) | 3.8(1.4) | 6.58(2.39) | |
| 6 mg/kg | 105(20.4) | 2530(837) | 5.0(1.6) | 13.9(11.1) | |
| 86 | 2 mg/kg | 38.7(14.3) | 859(287) | 6.4(7.3) | 4.58(1.96) |
| 4 mg/kg | 62.7(24.7) | 1420(452) | 5.5(1.2) | 6.72(2.02) | |
| 6 mg/kg | 103(16.2) | 2290(270) | 5.0(1.6) | 9.87(2.27) | |
- n = 8 for the 2 and 6 mg/kg dose groups (four males and four females) and 12 for the 4 mg/kg dose group (six males and six females).
- SD, standard deviation.
Discussion
This manuscript reports for the first time the PK properties of toceranib phosphate in both laboratory beagle dogs and in dogs with naturally occurring mast cell tumors from a field efficacy study.
There was notable animal-to-animal variability in toceranib concentrations after single oral doses with Cmax and AUC showing as much as a 2-fold range of values, both within and between studies. When evaluating the data from one study and comparing it to another, there are apparent differences. However, when looking at the individual animal data from all of these studies as a whole, these differences are not as obvious. There is significant overlap of the individual animal results between these studies. When viewed as a whole, the PK profiles for toceranib are consistent among the studies presented here.
The tmax varied from 4 to 12 h; however, the concentration vs. time profiles for toceranib were quite flat over that time interval with only slight differences in concentrations (Fig. 2). The t1/2 after single oral doses were similar to those after i.v. dosing at about 17 h suggesting that absorption was not impacting t1/2 for toceranib in the dog, i.e., there was no evidence of flip-flop kinetics for this drug in the target species. In Study 1, the absolute oral bioavailability of toceranib was 76.9% when administered as the commercial tablets to fasted dogs. Sex differences in toceranib PK parameters have not been significant in the laboratory studies.
As noted in the Results section, there was a statistically significant period effect between the two periods for most of the PK parameters in Study 1, even with the 14-day washout between periods. The reasons for this period effect are not clear. Mean toceranib concentrations were lower in the second period than in the first period. This difference in concentration was more notable after i.v. dosing than oral dosing. Clearance was much higher for period 2 than in period 1 resulting in shorter t1/2 in the second period. However, there was no statistically significant sequence effect (i.e., period by treatment interaction) observed. A significant period effect indicates something changed from period 1 to period 2. As the cause of this shift is unknown, it does appear some bias was introduced. Therefore, only estimates from period 1 are reported. Study 3 (seven every-other-day doses) showed no change in t1/2 between dose 1 and dose 7 based on plasma concentrations out to 48 h. Although t1/2 was not calculated for Study 5 (margin of safety study), there were no changes in plasma concentrations or PK parameters that would indicate the same effect in this study. The reason for the increased clearance for Period 2 in Study 1 is unexplained, but was considered to be unique to this study.
After i.v. dosing, toceranib exhibited a moderate plasma clearance in laboratory dogs of 1.45 L/kg/h (Table 1) and a high apparent volume of distribution at steady-state of 29.7 L/kg. This high volume indicated that the drug was well distributed in the body and probably sequestered in various tissues. The long t1/2 and the large Vss are consistent with a radiolabeled ADME study showing that there was significant drug-related material remaining in several tissues 7 days after a single dose of [14C]-toceranib (Yancey et al., in press).
Clearance of toceranib appears to be primarily hepatic. Only about 7% of drug related material was excreted in urine after a single dose of [14C]-toceranib to dogs, but 92% was collected in the feces (Yancey et al., in press). Based on canine hepatic blood flow of about 2.4 L/kg/h, and accounting for the 7% cleared by the kidneys, the remaining CL of toceranib is 56% of hepatic blood flow, assuming equal blood and plasma clearance.
With PK parameters independent of food intake, toceranib phosphate can be administered with or without a treat or meal with no impact on plasma drug concentrations. The absence of a significant food effect on efficacy is clinically relevant since cancer-bearing dogs may have variable appetites. However, while there were no statistically significant differences in PK parameters, there were minor differences in profiles for individual dogs between the fed and fasted states. There was a wider range in observed tmax in fasted dogs (4 to 8 h in fed vs. 4 to 12 h in fasted dogs); however, this difference was considered to be insignificant since there was often less than 10% difference in plasma concentrations between adjacent time points near the observed Cmax. Profiles in some fasted dogs had two maxima (the first at 4 or 6 h postdose and the second at 8 or 12 h postdose). The second maximum corresponds with the time food was restored to the fasted dogs (8 h) and may be explained by enterohepatic recirculation. This was substantiated by the presence of high concentrations of toceranib drug related material found in bile (Yancey et al., in press). Bile salts are concentrated in the gallbladder during fasting. In response to a meal, bile salts are released into the intestine. It has been reported that a Pavlovian-type effect can cause the release of bile salts prior to eating the meal (Polli et al., 1996) thus accounting for the second maximum concentration at 8 h. Thus, enterohepatic recycling may be contributing to the broad concentration profile observed.
Further explanation of the broad toceranib concentration profile may be found in the dependence of solubility of toceranib on pH and salt formation (data not shown). In aqueous HCl at pH ≤ 3, the solubility of toceranib is at least 5 mg/mL. As solution pH and phosphate concentrations increase, as would be expected across the stomach-duodenum interface, toceranib solubility drops to <0.2 mg/mL at pH 6 and <0.01 mg/mL at pH 7, with solids precipitating as a mix of toceranib phosphate salt and free base. It is likely that toceranib is soluble in the acidic environment of the stomach, but precipitates upon entering the higher pH of the duodenum. Thus, due to the low solubility in the duodenum, the absorption of toceranib is likely prolonged, contributing to the broad plasma concentration vs. time profile.
Toceranib has been shown to have good permeability (data not shown). Based on the poor solubility at high pH described above, the Biopharmaceutics Classification System (BCS) classification should be considered to be class II (Martinez et al., 2002). However, tablets disintegrate and dissolve quickly under the acidic conditions of the stomach and formulation changes are not likely to improve overall solubility or absorption of toceranib. A conclusion from the PK work is that the formulation goal of developing a rapidly disintegrating tablet was successful, presenting the drug for dissolution where it is highly soluble in the stomach. Thus, the commercial tablet formulation has minimum impact on the PK profile. Overall, the tablets do not exhibit formulation-controlled dissolution rate-limited absorption.
Changes in PK parameters after multiple doses were evaluated in Studies 3 and 5. As described in the Results section, the mean ± SD t1/2 calculated in Study 3 using 0 to 48 h data was 16.4 ± 3.6 h after dose 1 and 17.2 ± 3.9 h after dose 7. There was no appreciable change over the 2-week period of the study. However, by evaluating the data out to 240 h after dose 7, t1/2 increased to 31 ± 8.9 h. The calculated accumulation index for a compound dosed every 48 h with a t1/2 of 31 h was 1.52, reasonably close to the increase seen for Cmin, although the increases for Cmax and AUC0-48 were less than that. The longer t1/2 is also inconsistent with the lack of accumulation seen in the 90-day margin of safety study (Study 5) where there were no significant differences in Cmax, Cmin, or AUC between study days 0 and 86 (P > 0.05). Although not statistically significant, all three of these parameters showed a small absolute increase between days 0 and 86 (10 to 24%) at a dose of 2 mg/kg every other day. However, at doses of 4 and 6 mg/kg, these values decreased over the same time period by 3 to 19%. It is possible that the long t1/2 may be associated with a deep compartment that contributes little to the overall accumulation of drug. However, since there was no significant accumulation of toceranib after 90 days of every-other-day dosing at doses as high as 6 mg/kg, it is unlikely that there will be any significant changes in the PK parameters for toceranib when administered long term.
Dose proportionality was evaluated in the 13-week margin-of-safety study (Study 5) with doses ranging from 2 mg/kg to 6 mg/kg. AUC0-t(last) was proportional with dose in both male and female dogs over the dose range of 2 to 6 mg/kg. Cmax was proportional with dose in female dogs, but increased at a rate slightly greater than proportional with dose in male dogs. Although this increase was statistically significant, the magnitude was small and probably not of physiological importance. Therefore, if dose reductions are made (e.g., to manage adverse events), proportional changes in toceranib concentrations are expected. This proportionality also supports the dose normalization to the target dose of 3.25 mg/kg as was described in this paper for study-to-study comparisons.
Administration of toceranib phosphate to dogs with naturally occurring mast cell tumors in Study 4 resulted in dose-normalized plasma concentrations, Cmax, and AUC similar to those from laboratory beagles. Differences in mean tmax were not considered to be clinically important due to the nearly constant concentration near tmax. The t1/2 observed in mast cell tumor-bearing dogs was consistent with the other four studies presented here when based on concentrations measured for the comparable time periods. Therefore, both the absorption and elimination of toceranib does not appear to be dependent on breed, age, or disease state and not appreciably influenced by the concomitant medications used in these animals.
Each of the 8 dogs in the efficacy study was also receiving from 1 to 6 noninvestigational concomitant medications. Of these, two dogs received cimetidine, identified as a weak inhibitor of various cytochrome P450 isoforms (Flockhart, 2007). Although there were only a limited number of dogs receiving both drugs, there was no evidence that cimetidine had any effect on the clearance of toceranib.
A dose of 3.25 mg/kg was associated with target modulation (Pryer et al., 2003) and clinical efficacy (London et al., 2003, 2009) in dogs with naturally occurring mast cell tumors. With the exception of one dog dosed at 0.93 mg/kg, the actual observed maximum plasma concentrations among the eight dogs in Study 4 ranged from 38.9 to 103 ng/mL; all eight dogs had a successful biological response of their tumors at the time of PK sample collection (London et al., 2009). These data suggest that plasma concentrations of approximately 40 ng/mL or greater for a portion of a 48 h dosing interval are associated with clinical efficacy for naturally occurring mast cell tumors in dogs. The data from the healthy laboratory dogs presented here demonstrated that administration of a target dose of 3.25 mg toceranib phosphate/kg every other day resulted in actual plasma concentrations of 40 ng/mL for 6 to 33 h of a 48 h dosing interval. Thus, it appears that the previously cited preclinical work in rodent models that indicated target inhibition occurred at concentrations of 50–100 ng/mL for half of a 24-h dosing interval in rodents with human xenografted tumors (London et al., 2003) provided a good approximation of therapeutic concentrations in dogs with naturally occurring mast cell tumors. The recommended 48-h dosing interval allows trough concentrations to fall below the therapeutic concentration and this is important for acceptable tolerability in dogs (London et al., 2003, 2009).
In summary, toceranib phosphate had good oral bioavailability when dosed as the commercial tablets. A target dose of 3.25 mg/kg administered orally every other day is a dose known to be clinically efficacious and resulted in maximum plasma concentrations that were typically greater than 40 ng/mL. Plasma concentrations fell below the therapeutic concentrations for a portion of the 48 h dosing interval, contributing to clinical tolerability in dogs. Toceranib concentrations were proportional with dose and there was no accumulation or any other change of PK parameters for toceranib after 13 weeks of dosing in the range of 2.0 to 6.0 mg/kg every other day. There were no significant food effects on any PK parameter. Toceranib had moderate clearance and a large volume of distribution resulting in a t1/2 of 31 h. Dosing toceranib phosphate to dogs with naturally-occurring mast cell tumors resulted in concentrations similar to those found in laboratory beagle dogs. The PK results reported herein support the dosing regimen of 3.25 mg/kg administered every other day to the canine for the treatment of mast cell tumors.
Acknowledgments
The authors wish to thank G. Scott Grover for kinetic analyses; Thomas D. Cox, Philip J. Cutshaw, Donald E. DeLong, Thomas W. DeRyke, Tamalyn F. Flook, Jady J. Nye, Michael J. Prough, David J. Schlukebir, John J. Stone, and Terri L. VandeGiessen for assistance during the in-life phases; Lavern F. Krabill for statistical support; Gearey R. Halverson, Marie A. Jonaitis, Sandra M. Sims, and Chang-Quan Sun for tablet preparation and formulation support; Stacey Follis and Phyllis Malpas for their involvement with the field efficacy study; Matthew Krautman for his involvement with the margin of safety study; Joseph A. Robinson, and Michael Stegemann for critical reviews of this manuscript.




