Grapefruit juice, lyophilized grapefruit juice, and powdered whole grapefruit inhibit cytochrome P450-mediated triazolam hydroxylation by beagle dog liver microsomes
Abstract
Hanley, M. J., Cerundolo, R., Radwanski, N., Court, M. H. Grapefruit juice, lyophilized grapefruit juice, and powdered whole grapefruit inhibit cytochrome P450-mediated triazolam hydroxylation by beagle dog liver microsomes. J. vet. Pharmacol. Therap. 33, 189–195.
Coadministration of grapefruit juice (GFJ) has been proposed to enhance the systemic availability and decrease the required dose of drugs such as cyclosporine that are extensively metabolized in the intestine and liver. Although GFJ inhibits human cytochrome P450 (CYP) 3A, effects on dog CYP have not yet been reported. Consequently, we determined whether GFJ inhibits triazolam hydroxylation by Beagle dog liver microsomes (DLM) using human liver microsomes (HLM) as positive control. Results were compared with the effects of lyophilized GFJ and commercially-available powdered grapefruit capsules, which may be more convenient dosage forms. GFJ inhibited α-hydroxytriazolam formation in both DLM and HLM with similar IC50 (inhibitor concentration producing a 50% decrease in reaction velocity) values of 0.56% and 0.52% (v/v), respectively. Lyophilized GFJ and powdered grapefruit also inhibited DLM α-hydroxytriazolam formation with IC50 values of 0.76 and 1.2 mg/mL, respectively. Consistent with mechanism-based enzyme inhibition, preincubation of DLM with any of the grapefruit products for 20 min resulted in significant enhancement of inhibition of triazolam α-hydroxylation by 8–20%. The results indicate that 16 g of lyophilized GFJ or 23 g of powdered grapefruit would be equivalent to dosing 100 mL of GFJ. In vivo pharmacokinetic interaction studies are needed to confirm these in vitro findings.
Introduction
Cyclosporine is an immunosuppressive agent used in both human and veterinary medicine. Although more widely known for its use in organ transplant recipients, there is growing interest in the use of cyclosporine in canine dermatology, including treatment of perianal fistulas and atopic dermatitis (Mathews & Sukhiani, 1997; Mathews et al., 1997; Griffiths et al., 1999; Fontaine & Olivry, 2001; Mouatt, 2002; Olivry et al., 2002a,b; Patricelli et al., 2002; Doust et al., 2003; Steffan et al., 2003; Hardie et al., 2005). Unfortunately, the currently approved canine cyclosporine product (Atopica®; Novartis Animal Health, Greensboro, NC, USA) while effective, is also relatively expensive. When given at a 5 mg/kg daily dose, the approximate monthly cost of medication can range from $60 to $400 depending on the size of the animal (http://1800petmeds.com).
In dogs and humans, cyclosporine has limited systemic availability in part resulting from extensive presystemic metabolism by cytochrome P450 (CYP) in the intestine and liver (Wu et al., 1995; Whalen et al., 1999). Accordingly, coadministration of CYP inhibitors with cyclosporine would be expected to increase plasma concentrations of cyclosporine. Therapeutic drug-drug interactions of this type have been used to decrease the dose of cyclosporine necessary to achieve effective blood concentrations and, in turn, reduce the overall cost of therapy. For example, in a recent study involving treatment of dogs with perianal fistulas, ketoconazole – a potent CYP3A inhibitor – was successfully shown to ‘boost’ cyclosporine concentrations, thereby decreasing the cost of treatment by up to 70% (O’Neill et al., 2004). This finding is in agreement with the results of an earlier study where ketoconazole coadministration reduced the cyclosporine dose by 80–90% and the cost of therapy by as much as 83% (Mouatt, 2002). Patricelli et al. (2002) also found this combination to be a cost-saving and effective method for the treatment of perianal fistulas in dogs. In addition to ketoconazole, the calcium channel-blocker and CYP3A inhibitor, diltiazem, has been used to reduce the required dose of cyclosporine in two dogs with pruritic dermatopathies (A. Bell, personal communication in Robson, 2003). However, coadministration of cyclosporine with either ketoconazole or diltiazem exposes the animal to the inherent risks associated with the boosting agent. In fact, a recent report found a 15% frequency for adverse effects during ketoconazole therapy, with vomiting, anorexia, lethargy and diarrhoea being the most common (Mayer et al., 2008).
Coadministration of GFJ represents a potentially safer means of ‘boosting’ cyclosporine concentrations in dogs. Various in vitro and in vivo investigations have implicated the furanocoumarins as the GFJ constituents responsible for CYP3A inhibition in humans (Schmiedlin-Ren et al., 1997; He et al., 1998; Guo et al., 2000; Tassaneeyakul et al., 2000; Paine et al., 2006). While GFJ drug interactions have been extensively studied in humans, far fewer studies have been conducted in dogs. Orally administered liquid or freeze-dried GFJ increased the maximum observed maximum plasma concentration (Cmax) and area under the plasma concentration–time curve (AUC) of praziquantel in beagle dogs by approximately 200% and 150%, respectively (Giorgi et al., 2003). In another study in beagle dogs, bergamottin, an inhibitory furanocoumarin found in GFJ, increased diazepam AUC and Cmax by 89- and 51-fold respectively (Sahi et al., 2002). The results of these two studies suggest that GFJ is capable of inhibiting canine CYP metabolism. Unfortunately, the clinical use of GFJ (up to 100 mL dose is required for an inhibitory effect) is limited by its poor palatability in dogs, while bergamottin is relatively expensive and not currently available in a form for routine clinical use.
A product containing powdered whole grapefruit in capsule form is currently being marketed as a dietary supplement for weight loss (Citrasens™ Grapefruit Solution™ capsules; http://www.vitareason.com/). This product might represent a convenient method for boosting cyclosporine concentrations in dogs, assuming that the capsule contents are capable of inhibiting canine CYP metabolism. Consequently, the main objective of the present study was to use a validated bioassay to determine the potential for inhibition of CYP metabolism in dog liver by powdered grapefruit capsules. Triazolam hydroxylation was used as a surrogate for cyclosporine oxidation as the triazolam hydroxylation assay was readily available, technically less challenging, and both triazolam and cyclosporine are specifically metabolized by the same CYP isoforms (CYP3A subfamily) in humans (Kronbach et al., 1988, 1989; Ducharme et al., 1995; von Moltke et al., 1996; Greenblatt et al., 2000; Perloff et al., 2000). Effects of white GFJ and lyophilized ruby red GFJ on triazolam hydroxylation were also evaluated for comparison. The results of this study provide a starting point for clinical investigations examining cost-effective methods for boosting cyclosporine concentrations in dogs. However, pharmacokinetic data from in vivo studies will be necessary before GFJ-boosted cyclosporine regimens can be adopted.
Materials and methods
Reagents
Triazolam, β-nicotinamide adenine dinucleotide phosphate, isocitric acid, isocitric dehydrogenase, ketoconazole, and 6′,7′-dihydroxybergamottin (dihydroxybergamottin) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Solvents for high-performance liquid chromatography (HPLC) analysis were of HPLC grade and purchased from Fisher Scientific (Pittsburgh, PA, USA). Powdered whole grapefruit capsules were purchased from a commercial source (http://www.vitareason.com/). White GFJ produced by Ocean Spray (Lakeville-Middleboro, MA, USA) was purchased from a local grocery store and stored at 4 °C until use. Ruby red grapefruits were purchased from a local grocery store, the juice extracted by squeezing, and then dried down in a lyophilizer. Human liver microsomes (HLM) and dog liver microsomes (DLM) were from frozen liver banks maintained in the Department of Pharmacology and Experimental Therapeutics, Tufts University, Boston, MA, USA. The preparation of microsomes has been previously described (Court & Greenblatt, 1997). HLM and DLM were suspended in 0.1 m phosphate buffer containing 20% glycerol and were stored at −80 °C until use. Pooled DLM were prepared by combining individual preparations from four male beagle dogs. The collection and use of these livers for this purpose was approved by the Tufts University Institutional Review Board, and Institutional Animal Care and Use Committee, respectively.
Microsomal incubations
Microsomal incubations were carried out in a final volume of 250 μL as described in detail previously (von Moltke et al., 1996; Perloff et al., 2000). Incubations contained 50 mm phosphate buffer (pH 7.5), 5 mm magnesium, 0.5 mm nicotinamide adenine dinucleotide phosphate and an isocitrate dehydrogenase regenerating system. For studies using white GFJ, a series of incubation tubes containing 0%, 0.05%, 0.1%, 0.25%, 0.5%, 1% and 2.5% (volume/volume) juice were preincubated with HLM or DLM (0.25 mg of microsomal protein per mL of incubation volume) for 20 min at 37 °C. Tubes containing 0.1 μm ketoconazole or 2.5 μm of the inhibitory furanocoumarin, dihydroxybergamottin, were also included as positive control inhibitors. At the conclusion of the preincubation period, the triazolam hydroxylation reaction was initiated by transferring the mixture to tubes containing triazolam (250 μm final concentration). The incubation mixtures were immediately returned to the 37 °C water bath for an additional 20 min. Reactions were then stopped by the addition of 100 μL of ice-cold acetonitrile containing 500 ng of the internal standard, diazepam. After centrifuging, 300 μL of the supernatant was transferred to an HPLC vial for quantitation of hydroxytriazolam content. Incubations were performed in duplicate and results averaged.
For studies using powdered grapefruit capsules, the contents of each of eight randomly selected capsules were weighed; 10 mg from each capsule was combined, and then added to 8 mL of Millipore-filtered water to yield a 10 mg/mL stock solution. As the powder did not completely dissolve after vigorous mixing, the stock solution was centrifuged and the supernatant stored at −20 °C until use. Incubations using the supernatant were then performed as described for the aforementioned GFJ assays. The final concentrations of powdered grapefruit tested were 0.005, 0.01, 0.025, 0.05, 0.1, 0.25, 0.5, 1.25, 2.5 and 5 mg/mL.
For studies using lyophilized ruby red GFJ, a 10 mg/mL stock solution was prepared in Millipore-filtered water, centrifuged after vigorous mixing, and the supernatant was stored at −20 °C until use. The final concentrations of lyophilized ruby red GFJ tested were 0.005, 0.01, 0.025, 0.05, 0.1, 0.25, 0.5, 1.25, 2.5 and 5 mg/mL.
Effect of inhibitor preincubation on the extent of inhibition
The effect of 20 min preincubation at 37 °C of DLM with inhibitor and cofactor mix (but without triazolam) on the extent of inhibition of triazolam hydroxylation was tested by comparing rates of hydroxylation with and without a 20 min preincubation period. Incubations were performed as described above, except that for incubations without preincubation, both triazolam and inhibitor were added at the same time. Reactions (performed in triplicate) were initiated using DLM at a final concentration of 0.25 mg of microsomal protein/mL of incubation volume. Inhibitor concentrations tested were 0.1 μm for ketoconazole, 2.5 μm for dihydroxybergamottin, 0.5% (v/v) for GFJ, 0.7 mg/mL for powdered grapefruit and 0.375 mg/mL for lyophilized ruby red GFJ.
Capsule-to-capsule variability in CYP inhibition by powdered grapefruit
A 10 mg/mL powdered grapefruit solution was prepared from each of eight capsules as described above. The effects of each of these eight solutions at a final concentration of 1.25 mg/mL on triazolam hydroxylation by DLM were determined in triplicate.
Quantitation of α-hydroxytriazolam and 4-hydroxytriazolam formation by HPLC
Incubation mixtures were analysed using a Thermo-Finnigan Surveyor HPLC system operated by the ChromQuest software package, version 4.0 (Thermo Fisher Scientific, Waltham, MA, USA) as previously described with minor modifications (von Moltke et al., 1996). The column was a 4 μm 250 × 4.60 mm Phenomenex Synergi Hydro-RP (Phenomenex, Torrance, CA, USA). The HPLC mobile phase consisted of 50% (v/v) 20 mm phosphate buffer (pH = 2.2), 31% methanol and 19% acetonitrile delivered at a flow rate of 0.850 mL/min for 40 min. The injection volume was 20 μL. Column effluent was monitored by measuring ultraviolet absorbance at a wavelength of 220 nm. Analyte peaks were identified by injecting pure standards. Typical retention times were 17.2 min for α-hydroxytriazolam, 18.1 min for 4-hydroxytriazolam, 26.8 min for triazolam and 35 min for diazepam.
Data analysis
Peak height ratios of α-hydroxytriazolam and 4-hydroxytriazolam relative to the internal standard, diazepam, were calculated for each incubation mixture. Relative reaction velocities were calculated using the average peak height ratio for each inhibitor concentration expressed as a percentage of the analogous ratio resulting from control incubations that contained no inhibitor. The concentration producing a 50% decrease in reaction velocity (IC50) was determined by nonlinear regression analysis using SigmaPlot 11.0 (Systat Software, San Jose, CA, USA) as previously described (von Moltke et al., 2002). In the preincubation study, differences in the α-hydroxytriazolam formation rate with and without preincubation were compared with a two-tailed Student’s t-test. Differences with a P value <0.05 were considered statistically significant.
Results
In agreement with their known ability to inhibit human CYP3A metabolism (Guo et al., 2000; Tassaneeyakul et al., 2000; Venkatakrishnan et al., 2000), the positive control inhibitors, ketoconazole (0.1 μm) and dihydroxybergamottin (2.5 μm), reduced the rate of α-hydroxytriazolam formation by HLM to 63 ± 2% and 41 ± 2% of control (respectively). 4-hydroxytriazolam formation was reduced to 65 ± 0.5% (ketoconazole) and 47 ± 1% of control (dihydroxybergamottin). In DLM, ketoconazole reduced α-hydroxytriazolam and 4-hydroxytriazolam formation to 78 ± 3% and 76 ± 3% of control, respectively. The analogous values for dihydroxybergamottin were 53 ± 2% and 75 ± 4% of control.
As shown in Fig. 1, white GFJ inhibited α-hydroxytriazolam formation in both HLM and DLM in a concentration-dependent manner with inhibition potency (IC50) values (Table 1) that were essentially identical (about 0.5% v/v) for both species. On the other hand, the potency of inhibition by white GFJ of 4-hydroxytriazolam formation in DLM was almost half that observed for inhibition of 4-hydroxytriazolam formation in HLM and also half that for inhibition of α-hydroxytriazolam formation in DLM (Table 1). Lyophilized ruby red GFJ and powdered grapefruit also inhibited both α-hydroxytriazolam and 4-hydroxytriazolam formation by DLM in a concentration-dependent manner (Fig. 2). However, the potency of inhibition was about 40% less for powdered grapefruit as compared with lyophilized ruby red GFJ (Table 1). Again, 4-hydroxytriazolam formation was less potently inhibited than was α-hydroxytriazolam formation in DLM for both grapefruit products.
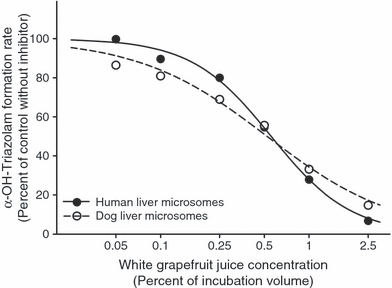
Inhibition by white grapefruit juice of α-hydroxytriazolam formation in human liver microsomes and pooled dog liver microsomes. Each data point represents the mean of two preincubation experiments in which each inhibitor concentration was assayed in duplicate. Nonlinear regression analysis was used to derive the curve.
| Preparation | Triazolam α-hydroxylation (IC50 ± SE) | Triazolam 4-hydroxylation (IC50 ± SE) |
|---|---|---|
| Human liver microsomes | ||
| White grapefruit juice | 0.56 ± 0.05% (v/v) | 0.58 ± 0.08% (v/v) |
| Dog liver microsomes | ||
| White grapefruit juice | 0.52 ± 0.18% (v/v) | 1.1 ± 1.1% (v/v) |
| Lyophilized ruby red grapefruit juice | 0.76 ± 0.14 mg/mL | 1.1 ± 0.31 mg/mL |
| Powdered whole grapefruit | 1.2 ± 0.38 mg/mL | 1.7 ± 1.2 mg/mL |
- IC50 values were determined by nonlinear regression and represent the inhibitor concentration producing a 50% reduction in triazolam hydroxylation rate as compared with the rate measured without added inhibitor.
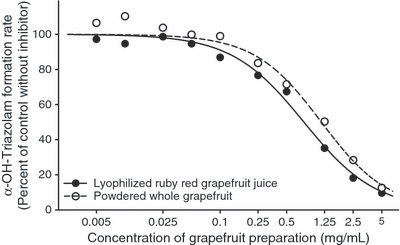
Inhibition by lyophilized ruby red grapefruit juice and powdered whole grapefruit of α-hydroxytriazolam formation in pooled dog liver microsomes. Each data point represents the mean of duplicate incubations. Nonlinear regression analysis was used to derive the curve.
Dog liver microsomes assays conducted to examine the impact of preincubation on the inhibition of α-hydroxytriazolam formation revealed a greater degree of inhibition when dihydroxybergamottin (by 27%), white GFJ (by 10%), lyophilized ruby red GFJ (by 8%), and powdered grapefruit (by 20%) were preincubated with microsomal protein prior to the addition of triazolam (Fig. 3). No difference in the inhibitory effect of ketoconazole was noted when incubations were conducted with or without preincubation. Similar results were observed for 4-hydroxytriazolam formation (data not shown).
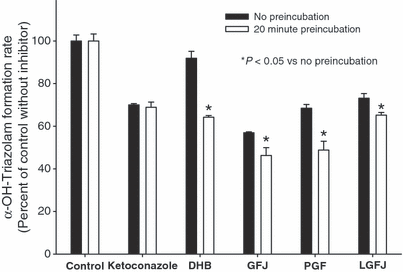
Effect of 20 min preincubation on inhibition of α-hydroxytriazolam formation measured in pooled dog liver microsomes. For preincubation assays, the inhibitor was preincubated with microsomal protein for 20 min prior to the addition of triazolam. The inhibitor concentrations tested were 0.1 μm for ketoconazole, 2.5 μm for 6′,7′-dihydroxybergamottin, 0.5% of incubation volume for white grapefruit juice (GFJ), 0.7 mg/mL for powdered grapefruit (PGF) and 0.375 mg/mL for lyophilized ruby red GFJ. Data are presented as mean ± SEM for incubations performed in triplicate. *Significant difference (P < 0.05) between the two conditions for a given inhibitor tested by Student’s unpaired t-test.
As natural products and dietary supplements are not as stringently regulated as pharmaceuticals, an important concern regarding their potential use in clinical patients is variability between preparations. Consequently, we weighed the contents of eight randomly selected grapefruit powder capsules as well as determined the ability of the contents of each of these eight capsules to inhibit triazolam hydroxylation by DLM. As shown in Table 2, capsule content weights ranged from 533 to 591 mg, with a coefficient of variation of only 3%. Inclusion of 1.25 mg/mL (final concentration) powdered grapefruit resulted in decreased α-hydroxytriazolam formation ranging from 55% to 63% of control rates, while 4-hydroxytriazolam formation decreased to 51% to 62% of control rates with coefficients of variation of 4% and 6%, respectively.
| Capsule | Content weight (mg) | Triazolam α-hydroxylation (% of control without inhibitor) | Triazolam 4-hydroxylation (% of control without inhibitor) |
|---|---|---|---|
| #1 | 557 | 63 | 62 |
| #2 | 533 | 57 | 54 |
| #3 | 558 | 55 | 51 |
| #4 | 557 | 57 | 56 |
| #5 | 585 | 58 | 55 |
| #6 | 578 | 59 | 58 |
| #7 | 591 | 58 | 58 |
| #8 | 558 | 61 | 59 |
| Mean | 565 | 59 | 57 |
| Standard deviation | 19 | 3 | 3 |
| Coefficient of variation (%) | 3 | 4 | 6 |
- A 10 mg of capsule contents per millilitre of water mixture was prepared for each capsule. The resulting supernatant, at a final concentration of 1.25 mg/mL, was then used in preincubation assays with pooled dog liver microsomes. Values are expressed as the percentage ratio of the reaction velocity for incubations containing inhibitor to the control velocity obtained when no inhibitor was present and are the mean of triplicate incubations per capsule.
Discussion
The results of the present study indicate that white GFJ, lyophilized ruby red GFJ, and powdered whole grapefruit are all capable of inhibiting triazolam hydroxylation by CYP in beagle DLM, albeit with differing inhibition potencies. Substantiating our assay system, IC50 values obtained for inhibition by GFJ of triazolam hydroxylation in HLM are in good agreement with previously published values (Farkas et al., 2007). GFJ also inhibited triazolam hydroxylation in DLM with generally similar IC50 values to HLM, suggesting that triazolam is metabolized by enzymes with similar catalytic properties in both species. The only difference was somewhat less potent inhibition of 4-hydroxytriazolam formation in DLM compared with HLM, which suggests that this metabolic pathway could be different in dogs compared with humans. Future studies could resolve this by evaluation of recombinant canine CYP isoforms.
An important objective of this study was to approximate the dose of powdered grapefruit capsules that would be needed to achieve the same level of drug metabolism inhibition as a dose of GFJ. In the study by Giorgi et al. (2003), a GFJ dose of 100 mL given to adult beagle dogs was sufficient to cause a 3-fold increase in the AUC of praziquantel. Using the IC50 values we obtained for inhibition of α-hydroxytriazolam formation in DLM, we estimate the concentration of powdered grapefruit that is equivalent to GFJ to be 231 mg/mL – [IC50 powdered grapefruit (1.2 mg/mL)]/[IC50 GFJ (0.52 mL/100 mL)]. Consequently, the amount of powdered grapefruit equivalent to 100 mL of GFJ would be 23.1 g or 41 capsules, assuming an average of 565 mg contents per capsule as we had measured. A similar calculation for lyophilized ruby red GFJ yields a dose equivalent mass of 15.7 g (about 31 capsules at approximately 500 mg each). Both of these estimates appear to be similar to, although somewhat higher (i.e. less potent) than, the dose of 10 g of lyophilized GFJ reported by Giorgi et al. (2003) to result in an increase in praziquantel AUC (similar increase to that of 100 mL of juice). Reasons for these differences may be related to the type of grapefruit used, and the grapefruit parts used to prepare the juice and/or powdered extract.
The powdered grapefruit product uses the entire fruit including the albedo (white rind) which is generally low in inhibitory furanocoumarins (Decastro et al., 2006). This may explain the lower inhibition potency of powdered grapefruit. The furanocoumarin content of grapefruit can also vary depending on variety and also from lot to lot (Ho et al., 2000; Decastro et al., 2006). Ruby red grapefruit has been reported to contain higher amounts of furanocoumarins than pink or white grapefruit (Decastro et al., 2006). Although our study was not designed to make this comparison directly, assuming the white GFJ we used contained 10 g of dry matter per 100 mL of liquid and there is no potency loss from lyophilization (as reported by Giorgi et al., 2003), then the dry weight IC50 value for inhibition of triazolam α-hydroxylation by DLM would be 0.52 mg/mL. This is slightly lower than the value we obtained for lyophilized ruby red GFJ (0.76 mg/mL) and suggests that there may be no advantage in using ruby red over white grapefruit for inhibition. We also evaluated the variability in capsule content weight and inhibition capacity, and showed remarkably low variability between the capsules obtained for the present study. However, these capsules were from a single lot and it is possible that more variability could be encountered between lots differing by year of production. Consequently, if such products are to be used as pharmaceutical boosting agents, it may be necessary to standardize the dose by either measurement of furanocoumarin content or inhibition capacity by bioassay (as in this study).
Another important result is that we observed time-dependent enhancement of canine CYP inhibition by all grapefruit products tested, as well as by dihydroxybergamottin, but not by ketoconazole. Such findings are consistent with mechanism-based inhibition (metabolic activation), which is typical of inhibition by furanocoumarins of CYP3A in humans and other species (Letteron et al., 1986; Schmiedlin-Ren et al., 1997; He et al., 1998; Guo et al., 2000; Tassaneeyakul et al., 2000; Greenblatt et al., 2003; Kakar et al., 2004). Ketoconazole, while a relatively potent CYP inhibitor, is not a mechanism-based inhibitor and preincubation results in unchanged (as in this case) or even decreased inhibition associated with conversion of ketoconazole to inactive metabolites. Time-dependent furanocoumarin inhibition tends to be irreversible, requiring de novo synthesis of CYP3A enzyme for recovery of CYP3A function in affected tissues. In humans, the half-life of recovery of CYP3A activity following GFJ inhibition is approximately 23 h (Greenblatt et al., 2003).
In conclusion, the results of this study demonstrate that a variety of grapefruit products are capable of mechanism-based inhibition of CYP-mediated triazolam hydroxylation in dog liver. As triazolam and cyclosporine are substrates for the same enzyme (CYP3A) in humans and other species, concomitant administration of these grapefruit products with cyclosporine to dogs might be expected to enhance cyclosporine systemic availability through decreased enteric metabolism. In turn, this would decrease the required dose of cyclosporine and treatment cost. Powdered grapefruit capsules represent a more convenient dosing form, as compared with GFJ or lyophilization. Although the number of grapefruit capsules that we predict are needed to have an effect equivalent to 100 mL of GFJ is relatively high (41 capsules), it is possible that lower doses might also be effective. A clinical study investigating the impact of coadministration of these grapefruit capsules on the pharmacokinetics of cyclosporine in dogs is currently in progress.
Acknowledgments
This work was funded in part by an American College of Veterinary Dermatology Resident Research grant to Dr Radwanski. Dr Court was supported by grant GM-061834 from the National Institute of General Medical Sciences (NIGMS), National Institutes of Health (Bethesda, MD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.




