Penetration of enrofloxacin into the nasal secretions and relationship between nasal secretions and plasma enrofloxacin concentrations after intramuscular administration in healthy pigs
Abstract
Bimazubute, M., Cambier, C., Baert, K., Vanbelle, S., Chiap, P., Albert, A., Delporte, J. P., Gustin, P. Penetration of enrofloxacin into the nasal secretions and relationship between nasal secretions and plasma enrofloxacin concentrations after intramuscular administration in healthy pigs. J. vet. Pharmacol. Therap.33, 183–188.
The pharmacokinetic behaviour of enrofloxacin (ENRO) in plasma and nasal secretions of healthy pigs was investigated, after a single-dose intramuscular administration of 2.5 mg/kg body weight of the drug. Blood samples and nasal secretions were collected at predetermined times after drug administration. Concentrations of ENRO and its active metabolite ciprofloxacin (CIPRO) were determined in plasma and nasal secretions by high-performance liquid chromatography (HPLC). CIPRO was not detected probably because we investigated young weaned pigs. The data collected in 12 pigs for ENRO were subjected to noncompartmental analysis. In plasma, the maximum concentration of drug (Cmax), the time at which this maximum concentration of drug (Tmax) was reached, the elimination half-life (t½) and the area under the concentration vs. time curve (AUC) were, respectively, 694.7 ng/mL, 1.0 h, 9.3 h and 8903.2 ng·h/mL. In nasal secretions, Cmax, Tmax, t½ and AUC were, respectively, 871.4 ng/mL, 2.0 h, 12.5 h and 11 198.5 ng·h/mL. In a second experiment conducted in 10 piglets, the relationship between concentrations of ENRO measured in the plasma and the nasal secretions has been determined following single-dose intramuscular administration of 2.5, 10 or 20 mg/kg body weight of the drug. It has been demonstrated that, among several variables, i.e., (1) the dose administered, (2) the time between intramuscular injection and blood sampling, (3) the age, (4) the sex, (5) the animal body weight and (6) the plasma concentration of the drug, only the latter influenced significantly the ENRO concentration in nasal secretions. Practically, using a generalized linear mixed model, ENRO concentrations in the nasal secretions (μg/mL) can be predicted taking into account the ENRO concentrations in plasma (μg/mL), according to the following equation: 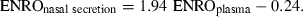
Introduction
Enrofloxacin (ENRO) is a fluoroquinolone antimicrobial agent developed exclusively for use in veterinary medicine. In vitro, ENRO has a high antimicrobial activity against a wide range of Gram-negative and Gram-positive bacteria and Mycoplasma spp (Papich & Rivière, 2001; Walker & Dowling, 2006).
In swine production, bacterial infections associated with respiratory diseases are mainly because of: Pasteurella multocida, Bordetella bronchiseptica, Mycoplasma hyopneumoniae and Actinobacillus pleuropneumoniae (Aarestrup et al., 2008). These pathogenic agents are generally susceptible to fluoroquinolones. For example, in Spain, the level of resistance of Pasteurella strains recently isolated in pigs to ENRO was 0% (Lizarazo et al., 2006).
However, successful treatment of infections of the respiratory tract depends on a number of factors, including concentration of antimicrobials at the site of infection. In the respiratory tract, infection sites comprise several locations including nasal compartment comprising mucosa and secretions, where Bordetella bronchiseptica coupled with toxigenic strains of Pasteurella cause, for example, atrophic rhinitis.
To provide effective therapy and to avoid development of resistance in commensal and pathological strains, it is necessary to obtain knowledge of the penetration of the drug in nasal secretions. Moreover, even when administered in animals for an infectious process located in sites other than nasal cavities, ENRO could diffuse in nasal secretions, where selection of resistant strains of commensals could occur.
The first study described in this work was undertaken to determine the pharmacokinetics of ENRO in the plasma and the nasal secretions of pigs.
As, in pigs and other species (Küng et al., 1993; Anadón et al., 1995, 1999), ENRO is de-ethylated to ciprofloxacin (CIPRO), a potent antimicrobial agent, with mechanism of action and antimicrobial activity similar to those of ENRO, CIPRO concentrations have also been investigated.
Due to the practical difficulties in collecting nasal secretions in pigs, the second study investigated the possibility to predict the concentrations of ENRO in nasal secretions, knowing the concentration of ENRO in plasma.
Material and methods
Experimental animals
A total of 12 healthy, 52- to 55-day-old, male (castrated) and female Pietrain piglets were used in the first study. The average weight of the pigs was 10.0 kg (range: 8.5–12.2 kg).
Ten other healthy, 47- to 49-day-old, male (castrated) and female Pietrain piglets were used in the second study. At first administration time, the average weight of the pigs was 8.2 kg (range: 6.7–9.8 kg).
All piglets were obtained from a single farm, i.e., the breeding farm of the University of Liège, Faculty of Veterinary Medicine. During both studies, the piglets were housed together on duckboards. The pigs had ad libitum access to water and pelleted, antibiotic-free food. On the days of drug administration and intensive sampling, food was withheld overnight. The pigs were weighed and clinically inspected to ensure healthy condition, prior to each day of drug administration.
Protocol
In the first study, all pigs (n = 12) received ENRO, as Baytril®(Bayer AG, Leverkussen, Germany) 50 mg/mL enrofloxacin injectable solution, at a dose of 2.5 mg/kg by intramuscular route. Intramuscular injections were given in the dorsal aspect of the side of the neck.
Nasal secretions and blood were sampled at the same time, from 10 min to 24 h after administration. To sample nasal secretions, it was necessary to anaesthetize pigs. While the sampling procedure was rather short by itself, the total duration of anaesthesia was around 90 min. Anaesthesia was achieved in most pigs twice a day, with at least 8 h between two consecutive anaesthesia. Thirteen minutes prior to samples collection, a sedative mixture of xylazine (Xyl-M®, VMD, Brussels, Belgium) at 2 mg/kg body weight and ketamine (Ketamine®, CEVA, Brussels, Belgium) at 10 mg/kg body weight were administered to the pigs, which were anaesthetized after 8 min by an intravenous injection of thiopental (Pentothal®, ABBOT Laboratories, North Chicago, IL, USA). The anaesthesized animals were allocated in three groups (A, B and C) of four pigs. In group A, blood was sampled 10, 20, 30, 45, 60, 90 min and 8 h after ENRO administration. In group B, blood was sampled 2, 2.5, 3, 3.5 and 12 h after ENRO administration. In group C, blood was sampled 4, 4.5, 5, 5.5 and 24 h after ENRO administration. Nasal secretions were absorbed on a swab fixed at the end of a flexible rod inserted deeply into the nasal cavity for 10 min, 5 min before and 5 min after blood sampling.
During the second study, all animals received ENRO, as Baytril® 5%, by intramuscular route, on three occasions. At first administration time, the animals received a dose of 2.5 mg/kg of ENRO. After 6 days of washout, the same animals received a dose of 10 mg/kg of ENRO (second administration time). Finally, after 7 days of washout, the same animals received a dose of 20 mg/kg of ENRO (third administration time). As described in the first study, it was necessary to anaesthetize the pigs to sample nasal secretions. At each administration time, blood was sampled at only one occasion in each pig, i.e., at 0.5, 1, 2, 6 or 12 h after administration. Two pigs were thus sampled at each time after administration. Nasal secretions were collected as described previously, at the same time as blood sampling.
Blood was always collected into heparinized tubes, by venipuncture at the level of the jugular vein. Plasma was separated after centrifugation and stored frozen at −80 °C until analysis.
After centrifugation of nasal secretions from the swab, the supernatant was frozen at −80 °C until analysis.
Enrofloxacin assays
Plasma and nasal secretion concentrations of ENRO were measured using a validated analytical procedure coupling the on-line sample clean-up on restricted access material (RAM) to high-performance liquid chromatography (HPLC) (Bimazubute et al., 2008).
The chromatographic system was composed of a model 230 ternary pump, a model 210 isocratic pump, a model 410 auto-sampler including a programmable column oven and a model 363 fluorescence detector, all from Prostar modules of Varian (Walnut creek, CA, USA). The switching valve of the chromatographic auto-sampler was equipped with a 100-μL injection loop. The separation was performed on a PURSUIT analytical column (150 × 4.6 mm, i.d.) preceded by a guard column (12.5 × 4.6 mm, i.d.), both packed with C18 (5 μm) stationary phase from Varian. The sample was pretreated on a precolumn consisting of LiChroCart column (25 × 4 mm, i.d.) packed with LiChrospher RP-18 ADS (25 μm) from Merck and was fitted to a VICI model E60-220 six-port switching valve (VICI Valco Instruments Co., Inc. Houston, TX, USA). The control system was performed using a resident software Star from Varian loaded on a DELL computer (Dell Computer Corporation, Round Rock, Tx, USA). The only off-line sample preparation was of a 50-fold dilution of nasal secretions and plasma samples in the washing liquid composed of 25 mm phosphate buffer of pH 7.4. A volume of 10 μL of diluted sample was injected directly onto the precolumn for sample pretreatment. By rotation of the switching valve, the analyte of interest was eluted in the back-flush mode with the LC mobile phase, which consisted of a mixture of 25 mm phosphate buffer of pH 3.0 and acetonitrile, according to a segmented gradient elution. The flow rate was 0.8 mL/min for the washing liquid and 1.5 mL/min for the LC mobile phase. ENRO was detected at excitation and emission wavelengths of 278 and 445 nm, respectively.
Briefly, preliminary experiments were performed on plasma and nasal secretion samples collected after i.m. administration of 2.5, 10 and 20 mg/kg bwt of ENRO at 1 h and 24 h, from three pigs per each dose. The active metabolite CIPRO was not found in any sample. This method was consequently validated for the determination of only ENRO simultaneously in plasma and nasal secretions. During the same preliminary experiments, the estimated lower LOQ was in the range of approximately 50–70 ng/mL in plasma and 100–115 ng/mL in nasal secretions. For the upper LOQ, ENRO concentrations were in the range of 3000–4500 ng/mL in plasma and 5000–7500 ng/mL in nasal secretions. The calibration range fixed arbitrary at 30–15 000 ng/mL in plasma was large enough to allow the standardized quantification of ENRO in all future unknown plasma and nasal secretion samples from pigs treated by i.m. 2.5, 10 and 20 mg/kg bwt of the drug. As nasal secretions were not used for calibration and considering the estimated lower LOQ in nasal secretions described above, the validated LLOQ in this biological fluid was fixed at 90 ng/mL.
PK data analysis
During the first study, several pharmacokinetic parameters were computed by means of the program WinNonlin® version 5.01 (Pharsight, Mountain View, CA, USA) for both matrices (plasma and nasal secretions) using the noncompartmental model after extravascular administration with sparse data sampling. These parameters included terminal half-life (t½), maximum plasma concentration (Cmax), time to reach peak levels (Tmax) and area under the concentration-time curve (AUC). The standard error was calculated for the Cmax and the AUC. Volume of distribution (V/F) was also calculated in plasma.
Study of the relationship between enrofloxacin plasmatic concentrations and enrofloxacin nasal secretion concentrations
The relationships between the concentration of ENRO in the nasal secretions and potentially predictive covariates, including dose administered, time between administration and sampling, age, sex, body weight of the animal and plasma concentration, were first examined graphically. Then, the relationship was analysed by means of the general linear mixed model (GLMM) approach, which accounts for repeated measurements on each pig. Calculations were always carried out on the maximum number of data available. Missing data were not replaced. Results were considered to be significant at the 5% critical level (P < 0.05). Data analysis was carried out using SAS (version 9.1 for Windows, Cary, NC, USA) statistical package.
Results
Enrofloxacin disposition in plasma and nasal secretions
Mean plasma and nasal secretion concentrations of ENRO obtained after intramuscular administration of 2.5 mg/kg were determined (Fig. 1).
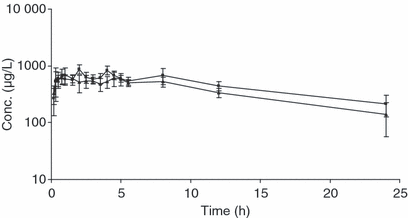
Mean enrofloxacin plasmatic (triangles) and nasal secretion (squares) concentrations after intramuscular administration of enrofloxacin at a dose of 2.5 mg/kg. Concentrations are expressed in μg/L, as mean ± SD for 12 pigs. Time is expressed in hours.
Values for kinetic variables that described absorption and disposition kinetics of ENRO were determined for plasma and nasal secretions (Table 1).
| Variable | Plasma | Nasal secretions |
|---|---|---|
| t 1/2β (h) | 9.3 | 12.5 |
| Cmax (μg/L) | 695 ± 117 | 871 ± 82 |
| Tmax (h) | 1.0 | 2.0 |
| AUC (h·μg/L) | 8903 ± 511 | 11 198 ± 710 |
| Vd/F (L/kg) | 3.22 | |
| Cl/F (L/h·kg) | 0.240 |
- PK calculations were performed on average data, the sparse data sampling option providing SE for Cmax and AUC.
- t 1/2β, half-life at elimination phase; Cmax, maximal concentration after intramuscular administration; Tmax, time needed to reach Cmax; AUC, area under the concentration-time curve; Vd, volume of distribution.
Mean drug concentrations in plasma 20 and 30 min after i.m. administration were, respectively, 579 and 546 ng/mL. Plasma concentration of ENRO peaked (695 ng/mL) at 1 h after administration, this time corresponding to the Tmax. Plasma drug concentrations exceeded 300 ng/mL (mean: 338 ng/mL) and 100 ng/mL (mean: 140 ng/mL) at 12 and 24 h, respectively.
In plasma, the mean value of t½ of ENRO after intramuscular administration was 9.3 h.
Mean drug concentration in nasal secretions 20, 30 and 60 min after i.m. administration was 533, 599 and 589 ng/mL, respectively. Concentrations of ENRO in nasal secretions peaked (871 ng/mL) at 2 h after administration, this time corresponding to the Tmax. Nasal secretion drug concentrations exceeded 400 ng/mL (mean: 444 ng/mL) and 200 ng/mL (mean: 217 ng/mL) at 12 and 24 h, respectively.
In nasal secretions, the mean value of t½ of ENRO after intramuscular administration was 12.5 h.
The nasal secretion to plasma ratio of ENRO concentrations (based on AUC values) was 1.26.
CIPRO was not found in plasma and nasal secretions.
Relationship between enrofloxacin concentrations in plasma and nasal secretions
When applying GLMM to the data, it appeared that only the plasmatic concentration of ENRO contributed significantly to the prediction of the concentration of ENRO in nasal secretions (P = 0.0004). A close look at 2, 3, however, reveals that unexpected profiles may adversely influence the statistical results. This is probably because of the low number of piglets used in this study (n = 10). In both figures, the smoothed line, obtained by a nonparametric smoothing technique robust to outlying profiles, and the dotted predicted line, obtained by a generalized linear mixed model, differ quite markedly. A closer examination of the data revealed that piglet numbered 3 has a very different evolution compared with that of the other piglets. Therefore, this piglet was excluded from study and the statistical analysis was repeated. However, both models could be used and confirmed that plasma concentrations of ENRO were the sole predictor of concentration of ENRO in nasal secretions and the following equation was obtained: 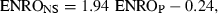 , where ENROP stands for the ENRO concentration in plasma (μg/mL) and ENRONS for the ENRO concentration in nasal secretions (μg/mL).
, where ENROP stands for the ENRO concentration in plasma (μg/mL) and ENRONS for the ENRO concentration in nasal secretions (μg/mL).
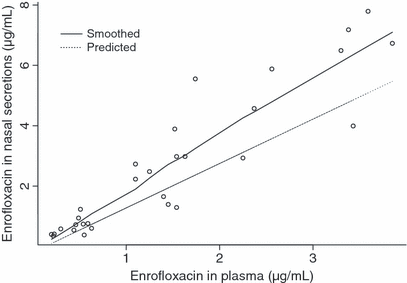
Relationship between concentrations of enrofloxacin in nasal secretions and plasma using a generalized linear mixed model and considering all the piglets (n = 10).
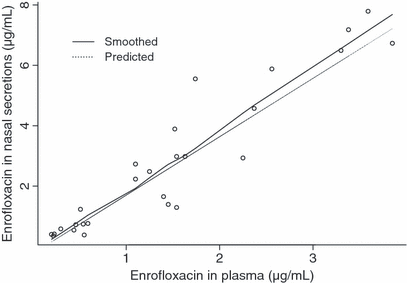
Relationship between concentrations of enrofloxacin in nasal secretions and plasma using a generalized linear mixed model and excluding the piglet numbered 3 (n = 9).
Discussion
Fluoroquinolones are considered to be among the most effective drugs for the treatment of bacterial infections. Among them, ENRO has been developed exclusively for use in veterinary medicine.
To obtain maximal efficacy and to avoid development of resistance in commensal and pathogenic organisms, rational use of antimicrobial drugs should be based on the knowledge of the structures and biochemical characteristics of microorganisms and on the pharmacodynamic and pharmacokinetic properties of antimicrobial drugs.
Most of the time, the pharmacokinetic studies investigate only the concentration of the active substance in plasma. However, it is very important to define the concentrations of antimicrobials at their potential action sites.
In our first study, we sampled plasma and nasal secretions in pigs to define and to compare the pharmacokinetics parameters in both these matrices, after intramuscular injection of 2.5 mg/kg of ENRO, i.e., the dose currently used in therapy. Nasal secretions and plasma have been sampled at the same times in a considered animal. As sampling of nasal secretions can only be performed in anaesthetized pigs and as anaesthesia cannot be repeated too often in a same subject, our protocol was designed as described by Agersø and Friis (1998). Practically, all the piglets were treated at the same time but they were divided in several groups based on the times of sampling of nasal secretions and plasma. This methodology was an interesting approach allowing pharmacokinetic analysis of data, although avoiding repetition of anaesthesia in individuals.
The values of plasmatic elimination half-life, Cmax and Tmax obtained in this study were compared with those obtained by Zeng and Fung (1997), Richez et al. (1997), Pijpers et al. (1997), Anadón et al. (1999) and Wiuff et al. (2002), who also administered ENRO at a dose of 2.5 mg/kg, by intramuscular route, in pigs. In our study, the Cmax and Tmax were, respectively, 694.7 ng/mL and 1.0 h. In the studies published by other authors, the highest concentrations were seen from 0.92 to 1.81 h after administration and were from 630 to 1170 ng/mL. These values seem to be in agreement with our study. Values of elimination half-life, however, seem to show more variability between studies, being 9.3 h in our study and from 4.1 to 13.1 h in the studies published by other authors. It has been described that large intra-species differences in the half-life of ENRO occur. Changes in age and correlated maturity of kidneys and liver seem to be an important cause of this variability (Cox et al., 2004). When reviewing the age of the animals included in the studies mentioned above, this parameter could, however, not be confirmed as the cause of the observed variability in the elimination half-life. Other factors, such as breed or physiological state, could also be responsible of the variability.
Ciprofloxacin is the main metabolite of ENRO, resulting from ethyl de-alkylation at the para-nitrogen on the piperazinyl ring (Post et al., 2002). In our study, CIPRO was not detected in plasma, in contrast to the findings of Anadón et al. (1999) in which CIPRO concentrations in plasma made up to 52% of the parent drug. The difference between this study and that of Anadón et al. (1999) could be the result of different ages of the pigs, possibly affecting the drug metabolism. In the study of Anadón et al. (1999), body weight of the pigs (Landrace x Large White) was between 76 and 86 kg. In this study, the average weight of the pigs (Pietrain) was 10 kg. The fact that, in the study of Zeng and Fung (1997) and Wiuff et al. (2002), the metabolite CIPRO was detected only in traces (<0.1 μg/mL) or at very low concentration, corresponding to a maximum of 3% of the ENRO concentration in pigs weighing 15–30 kg, seems to confirm the hypothesis of a different metabolism of ENRO in pigs, depending on age. Post et al. (2002), using swine liver microsomes and liver S10 preparations, demonstrated that CIPRO was not observed.
It is well-known that in general, fluoroquinolones are extensively distributed with high volumes of distribution. The volume of distribution value (V/F) in this study agrees with this general statement and indicates good penetration of biological membranes and tissue distribution.
To the best of our knowledge, concentrations of ENRO in nasal secretions have not been studied by other authors. Reference values are thus not available in the literature for pharmacokinetic parameters calculated for nasal secretions.
The penetration of the drugs into various tissues is best described by the use of the AUC, because this estimate responds to variation in concentration with time (Agersø & Friis, 1998). The ratio of nasal secretions to plasma (based on AUC values) was 1.26.
Perhaps more than with other class of antimicrobial agents, dosage of fluoroquinolones should be based on the susceptibility of the bacterial target. As ‘dose-dependent antimicrobial’, clinical efficacy of the fluoroquinolones is dependent on dose and bacterial pathogen. To maximize clinical efficacy and reduce selection of resistant bacteria, Cmax/MIC ratio ≥ 10:1 or AUC0–24:MIC 90 ratios ≥ 125:1 may be required (Walker & Dowling, 2006). In the present case, Cmax/MIC and AUC/MIC ratios for ENRO in nasal secretions would meet suggested ratios for a targeted MIC of 0.09 μg/mL.
However, antimicrobial action is dependent on unbound rather than total drug concentration, free drug at the site of action being the most important predictor of efficacy (Davis et al., 2007). Villa et al. (1997) showed that, in pigs, the free fraction of ENRO in plasma was 73%. No data are available in the literature concerning protein binding of ENRO in nasal secretions. Data published in calves on danofloxacin, another fluoroquinolone, however, showed that protein binding was lower in nasal secretions than that in plasma (Friis, 1993). It could thus be expected that in nasal secretions, there will not be much difference between the total concentration and the unbound (active) concentration.
The collection of nasal secretion samples in pigs requires anaesthesia. Moreover, anatomically, nasal sockets of pigs are hardly approachable because they are very narrow and snaky, implicating a lot of precautions to avoid contamination of the nasal secretion sample with blood during swabbing. Moreover, small quantities of nasal secretions are collected, particularly in healthy subjects. In this context, the second study investigated the possibility to predict the concentrations of ENRO in nasal secretions, knowing the concentration of ENRO in plasma. By modelling repeated measurements within piglets and discarding abnormal evolution profiles, we found that the concentration of ENRO in the nasal secretions could be quite satisfactorily predicted from ENRO levels in plasma. The quality of the adjustment, assessed by the coefficient of determination (r2), was 94%. The model for prediction of concentrations of ENRO in nasal secretions that takes into account the plasmatic concentration can thus be used to define the ENRO at the action site.
Practically, ENRO concentrations in nasal secretions can be predicted by the plasma concentrations using the following equation: 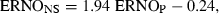 , where ENROP stands for the ENRO concentration in plasma (μg/mL) and ENRONS for the ENRO concentration in nasal secretions (μg/mL). This equation does not allow to conclude that the nasal secretion concentrations of ENRO are about twice that of plasma. The slope of 1.94 is obviously because of the lag time between the nasal secretion profile and the plasma concentration profile, and not because the nasal concentrations are twice higher than the plasma concentrations. Based on the fact that the AUC ratio of nasal secretions to plasma is 1.26, it can be considered that nasal secretion concentrations are nearly equal to the plasma concentrations.
, where ENROP stands for the ENRO concentration in plasma (μg/mL) and ENRONS for the ENRO concentration in nasal secretions (μg/mL). This equation does not allow to conclude that the nasal secretion concentrations of ENRO are about twice that of plasma. The slope of 1.94 is obviously because of the lag time between the nasal secretion profile and the plasma concentration profile, and not because the nasal concentrations are twice higher than the plasma concentrations. Based on the fact that the AUC ratio of nasal secretions to plasma is 1.26, it can be considered that nasal secretion concentrations are nearly equal to the plasma concentrations.
In conclusion, after intramuscular injection of 2.5 mg/kg of ENRO in 7 weeks weaned piglets, the values in plasma of t½, Cmax and Tmax obtained in this study were 9.3 h, 695 ng/mL and 1.0 h, respectively. In nasal secretions, the values of t½, Cmax and Tmax were, respectively, 12.5 h, 871 ng/mL and 2.0 h. The ratio of nasal secretions to plasma (based on AUC values) was 1.26. CIPRO was not detected in plasma and nasal secretions. This could be related to the age of the pigs, possibly affecting the drug metabolism.
It could be concluded that Cmax/MIC and AUC/MIC ratios for drug concentration in nasal secretions would met suggested ratios for a targeted MIC of 0.09 μg/mL. This value is lower than the MIC 90 values determined for Pasteurella strains isolated in pigs, i.e., 0.03 μg/mL, but higher than the MIC 90 values determined for Bordetella bronchiseptica strains isolated in pigs, i.e. 0.5 μg/mL (Kadlec et al., 2004; Wallmann et al., 2004).
Concentrations of ENRO in the nasal secretions can be satisfactorily predicted from ENRO levels in plasma, using the following equation: 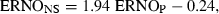 , where ENROP stands for the ENRO concentration in plasma (μg/mL) and ENRONS for the ENRO concentration in nasal secretions (μg/mL). The quality of the adjustment, assessed by the coefficient of determination (r2), amounted to 94%.
, where ENROP stands for the ENRO concentration in plasma (μg/mL) and ENRONS for the ENRO concentration in nasal secretions (μg/mL). The quality of the adjustment, assessed by the coefficient of determination (r2), amounted to 94%.
Acknowledgments
This work was supported by the Ministère fédéral belge des Classes moyennes et de l’Agriculture (Grants S-5989, 6116) and the Région wallonne. The authors thank Dr. Laurence Janssens, Michèle Belleflamme, Isabelle Dizier and Marie-Pierre Liardet for their assistance.




