The pharmacokinetics of nitazoxanide active metabolite (tizoxanide) in goats and its protein binding ability in vitro
Abstract
Zhao, Z., Xue, F., Zhang, L., Zhang, K., Fei, C., Zheng, W., Wang, X., Wang, M., Zhao, Z., Meng, X. The pharmacokinetics of nitazoxanide active metabolite (tizoxanide) in goats and its protein binding ability in vitro. J. vet. Pharmacol. Therap.33, 147–153.
The pharmacokinetics of tizoxanide (T), the active metabolite of nitazoxanide (NTZ), and its protein binding ability in goat plasma and in the solutions of albumin and α-1-acid-glycoprotein were investigated. The plasma and protein binding samples were analyzed using a high-performance liquid chromatography (HPLC) assay with UV detection at 360 nm. The plasma concentration of T was detectable in goats up to 24 h. Plasma concentrations vs. time data of T after 200 mg/kg oral administration of NTZ in goats were adequately described by one-compartment open model with first order absorption. As to free T, the values of t1/2Ka, t1/2Ke, Tmax, Cmax, AUC, V/F(c), and Cl(s) were 2.51 ± 0.41 h, 3.47 ± 0.32 h, 4.90 ± 0.13 h, 2.56 ± 0.25 μg/mL, 27.40 ± 1.54 (μg/mL) × h, 30.17 ± 2.17 L/kg, and 7.34 ± 1.21 L/(kg × h), respectively. After β-glucuronidase hydrolysis to obtain total T, t1/2ke, Cmax, Tmax, AUC increased, while the V/F(c) and Cl(s) decreased. Study of the protein binding ability showed that T with 4 μg/mL concentration in goat plasma and in the albumin solution achieved a protein binding percentage of more than 95%, while in the solution of α-1-acid-glycoprotein, the percentage was only about 49%. This result suggested that T might have much more potent binding ability with albumin than with α-1-acid-glycoprotein, resulting from its acidic property.
Introduction
Nitazoxanide (NTZ) is a thiazolide compound that has been synthesized based on the structures of niclosamide and metronidazole (Rossignol & Stachulski, 1999). This drug and its deacetyl derivative, tizoxanide (T), exhibit a broad spectrum of activities against intracellular and extracellular protozoa, helminthes, aerobic and anaerobic bacteria, and viruses infecting humans and animals (Hemphill et al., 2006; Rossignol et al., 2006; Korba et al., 2008). Although the mechanism of NTZ activity against helminths has not yet been determined, the antiprotozoal activity of NTZ is believed to be due to interference with the Pyruvate–Ferredoxin Oxidoreductase (PFOR) enzyme dependent electron transfer reaction, which is essential for anaerobic energy metabolism of the parasites (Gilles & Hoffman, 2002). Studies of the anti-viral activity of NTZ suggest that it targets cellular pathways involved in the synthesis of viral proteins (Rossignol & El-Gohary, 2006).
The United States Food and Drug Administration has approved this drug in December 2002 for treatment of diarrhea caused by Cryptosporidium parvum and Giardia lamblia, in children 1–11 years of age (Bobak, 2006). NTZ is used in many areas of the world, especially in Central and South America, as a broad-spectrum parasiticidal agent in adults and children. It is approved for veterinary use (for the treatment of helminthic infections in cats and dogs) in Switzerland and France.
The pharmacokinetics of NTZ have been examined in healthy humans after single- and multiple-dose oral administration of NTZ. Following oral administration, the drug is partially absorbed from the gastrointestinal tract and rapidly hydrolyzed in plasma to form its active circulating metabolite, T, and subsequent conjugation to tizoxanide glucuronide, and tizoxanide sulfate occurs rapidly, so that NTZ is not detectable in plasma (Stockis et al., 1996; Broekhuysen et al., 2000; Prod Info Alinia, 2005). The rate of T formation from the plasma compartment (apparent formation rate constant of 0.57/h) is similar to its elimination rate (apparent elimination rate constant of 0.6/h (Stockis et al., 1996). Plasma protein binding is high, with >99% of T formed following a dose of NTZ estimated to be protein bound (Prod Info Alinia, 2005). The extent of systemic bioavailability of T and tizoxanide glucuronide, the active metabolites of NTZ, was increased when nitazoxanide was administered with food (Stockis et al., 2002a). Tizoxanide glucuronide, excreted in urine and bile, is the main metabolite in any type of sample, and it also seems to be entirely hydrolyzed in the intestine, as only non-conjugated T could be detected in feces (Bobak, 2006). The elimination half-life of T from plasma is approximately 1.5 h (Stockis et al., 2002b).
There are limited published reports available on pharmacokinetics of NTZ in animals. In mice, rats, and dogs, NTZ is quickly hydrolyzed by plasma esterases into T, and subsequent conjugation to tizoxanide glucuronide occurs rapidly (Prod Info Alinia, 2005; Stettler et al., 2004). The results from the metabolic study of NTZ in goat after administered with 200 mg/kg body weight showed that there existed similar metabolic profiles as described above (Zhao et al., 2008a). Following oral administration of 1 mg NTZ in mice, serum T increased rapidly and peaked at 1 h postinoculation at a mean concentration of 0.25 μg/mL, and then dropped down to zero at 4–6 h (Stockis et al., 2002b). Studies of radio-labeled NTZ in the rat and the dog have shown that approximately two-thirds of the product is excreted in feces of rats and dogs while one-third is excreted in urine. The primary metabolite in the feces of dogs and goats is T (Stockis et al., 1996; Prod Info Alinia, 2005, Zhao et al., 2008b).
To our knowledge, the pharmacokinetics of NTZ active metabolite, T, in goats have not been reported despite its therapeutic potential in this species. Therefore, we evaluated the in vivo pharmacokinetics of T (free and total) in goats and its protein binding ability in vitro, so as to provide reliable scientific data for designing drug treatment regiments.
Materials and Methods
Chemicals and reagents
Nitazoxanide (>98.5%) and T (>99.9%) were synthesized and characterized by NMR, LC–MS, and high-performance liquid chromatography (HPLC)–UV (Rossignol & Cavier, 1975; Rossignol & Stachulski, 1999), and then NTZ capsule (500 mg/g) preparations were compounded in house. In detail, NTZ and pharmaceutical aids were weighed, respectively, and then mixed together. The resulting powder was filtered by 40-mesh screen, and its concentration was measured and then filled into capules. β-glucuronidase (fluid preparation, type HP–3, 101, 092 units/mL) from Helix pomatia, albumin, and α-1-acid-glycoprotein were bought from Sigma (Sigma–Aldrich, St Louis, MO, USA). HPLC-grade acetonitrile was bought from Fisher. HPLC-grade water was prepared using a Milli-Q water purification system (Millipore, Bedford, MA, USA). All other reagents were of HPLC or analytic grade.
Standard solutions and mobile phase preparation
Stock solution of T (100 μg/mL) was prepared in 100 mL acetonitrile with 1.6 mL N, N-Dimethylformamide (DMF) and was stored at −76 °C.
All of the standard and quality control (QC) samples (10 mL/each) were prepared using the stock solutions from the same source and the same concentration. Separate working solutions (1, 2.5, 5, 12.5, and 50 μg/mL) for standard and QC preparation were prepared by diluting the stock solution of the analyte with acetonitrile. Standards one through five were prepared by adding the correct volume of the working solutions to class A volumetric flasks and diluting to volume with blank plasma. The following concentrations were prepared: 0.2, 0.5, 1, 2.5, and 10 μg/mL. The standards and QCs (0.2, 1, and 10 μg/mL) were aliquoted into polypropylene tubes and stored in freezers maintained at −76 °C.
The mobile phase consisted of buffer and acetonitrile in the ratio (35:65 v/v). The buffer used in the mobile phase contained 0.02 mol/L of KH2PO4 in double-distilled water and adjusted pH to 2.5 with concentrated hydrochloric acid. The mobile phase filtered through a 0.45 μm filter before use.
Plasma pharmacokinetics
Experimental animals and treatment
The study was conducted in six adult male goats, approximately 1 year old weighing between 25 and 30 kg. Five goats received NTZ orally and one goat served as a control. All goats were clinically normal and they were housed in animal sheds with concrete floors (40 m2). They had free access to water and were given antiparasitic free commercial feed (Guangming) indoors. None of the animals was treated with any antiparasitic agents for at least 3 months prior to the start of the experiment. NTZ capsules were administered to the goats at a single oral dose of 200 mg/kg body weight. All treated animals were observed closely for the appearance of any adverse effects of the drug. Care and management of goats were carried out according to ethical guidelines laid down in the Shanghai Veterinary Research Institute (SHVRI), China Academy of Agricultural Sciences (CAAS). Protocols were submitted to and approved by ethics committee of the SHVRI.
Sample collection
Blood samples (3–5 mL) were collected by jugular venipuncture into heparinized glass tubes prior to and at 5, 15, 30 min, and 1, 2, 3, 4, 5, 6, 7, 8, 10, 12, 24, 48, 72, and 96 h after drug administration. Blood samples were centrifuged at 10 000 g for 5 min to harvest plasma, which was stored at −76 °C until assayed.
Sample preparation
For the purposes of this paper, the following definitions are used. Unconjugated T recovered in plasma after extraction only (i.e., no enzymatic digestion) is referred to as ‘free T.’ T recovered from β-glucuronidase treated plasma is referred to as ‘total T’ (free tizoxanides plus tizoxanides released from glucuronide conjugate).
Free T was extracted by acetonitrile (450 μL) with anhydrous sodium sulfate (0.17 g, solid) from 200 μL plasma. A rotating mixer was used for approximately 2 min to facilitate the extraction. After centrifugation at 12 000 g for 10 min, the supernatant was transferred to a new eppendorf centrifuge tube and evaporated to dryness in a vacuum condenser. Residues were reconstituted in 100 μL mobile phase and centrifuged at 12 000 g for 10 min before 10 μL supernatant was injected onto the HPLC system described below.
After optimizing the acidity, temperature, enzymatic content, and the time of hydrolysis, total T was determined by transferring 200 μL of plasma sample into a 1.5 mL eppendorf centrifuge tube with 20 μL acetic acid–sodium acetate buffer (pH 4.3) and treated with 4 μL β-glucuronidase at 37 °C for 16 h to hydrolyze glucuronide conjugate of T. Acetonitrile (426 μL) was added to stop the reactions, and the enzymatic hydrolysis solution was handled following the same procedures.
Protein binding ability of T in vitro
Equilibrium dialysis was applied to determine the protein binding of T in vitro. T at different designed concentrations (1, 2, 4, 8, 12, 16, and 20 μg/mL) was in vitro spiked to goat blank plasma, or to solutions of albumin and α-1-acid-glycoprotein, independently. Each of the resulting samples containing T with a volume of 1.0 mL was inside the bag filter, while outside the bag filter was phosphate buffered solution (PBS) (0.02 mol/L, pH 7.40) mixed with NaCl (0.15 mol/L). A steady concentration of T in both plasma and PBS was attained within 96 h at 4 °C. Thus, a dialysis time of 96 h was used for all subsequent experiments. The protein binding ability of T was determined within a range of 0.2–10 μg/mL concentrations of T. Various protein solutions with different concentrations using HPLC-grade water as dilution were also designed and prepared (albumin = 10, 20, 30, 40 μg/mL, α-1-acid-glycoprotein = 0.125, 0.25, 0.5, 1.0 μg/mL). Finally, a fixed concentration of 4.0 μg/mL of T spiked in the solutions of albumin, or α-1-acid-glycoprotein or the goat plasma were dialyzed under the same conditions.
HPLC–UV analysis
The plasma and plasma protein binding samples were analyzed using the HPLC–UV analysis. HPLC–UV analysis was carried out on a Waters Alliance 2690 HPLC system (Waters, Milford, MA, USA), equipped with a 2487 ultraviolet detector. Separation was achieved by using a Diamonsil C18 reversed-phase column (250 × 4.6 mm, 5 μm; Dikma Technologies, Dalian, China) preceded by a guard column (10 × 5 mm, 5 μm), which was maintained at 30 °C. The mobile phase consisted of acetonitrile–KH2PO4 buffer (0.02 mol/L, pH 2.5) (65:35) at a flow-rate of 1.0 mL/min for 10 min after injection. T was detected at 360 nm wave length. The eluents were helium-degassed. The sample injection volume was 10 μL. The method was validated prior to the analysis of samples. The working standard solutions of T prepared daily were used to spike blank goat plasma. Plasma standards at 0.2, 0.5, 1, 2.5, and 10 μg/mL for T (external standard) were prepared and extracted as described for the experimental samples. T was quantified from its respective peak area and the concentrations in plasma samples were determined by means of calibration curves determined on analysis of blank plasma samples spiked with T. The retention time for T was 6.2 min with limits of detection and quantification in plasma of 0.02 and 0.04 μg/mL, respectively. The respective limits of detection and quantification were determined as 3 and 10 times the signal to noise ratio at the time of elution of the tizoxanide. Mean recovery of T in goat plasma was in the range of 97.2–98.6%. The method was found to be linear and reproducible in the concentration range of 0.2–10 μg/mL with typical r > 0.9999. The intra- and inter-day assay coefficients of variation were <10%. There was no endogenous interference to T in extracted plasma samples treated with or without enzyme.
Data analysis and statistical comparison
Plasma concentrations and time data collected from the oral dosing were analyzed with Practical Pharmacokinetic Program-Version 97 (3P97) published by Chinese Pharmacological Association (Beijing, China). One-, two- and three-compartmental analyses with different weighting factors (1, 1/c, 1/c2) were used to determine the best fitting compartmental model based on the random distribution of residuals, the correlation coefficient, and the Aikaike’s information criteria (Yamaoka et al., 1978).
The pharmacokinetic parameters were determined from the individual fitted equations (Giabaldi & Perrier, 1982). The area under the plasma concentration–time curve from zero to 24 h (AUC0–24) was calculated by means of the trapezoidal rule extrapolating to 24 h with terminal elimination rate constant (Ke). Tmax and Cmax were recorded directly from the measured data.
The concentration of T outside and inside bag filter was depicted as Cout and Cin, respectively. The plasma protein biding percentage of T (PT) was calculated according to the following equation: PT = (1 −Cout/Cin) × 100%.
Data were expressed as mean ± SD. A two-way analysis of variance (anova) followed by comparison was used for the statistical comparisons between groups. P < 0.05 was considered as statistically significant.
Results
General observation
Clinical examination of all the goats before and after each administration did not reveal any abnormalities. No local or systemic adverse reactions occurred after oral administration of NTZ.
Pharmacokinetics of T in goats
Semi-logarithmic plots of mean plasma concentration–time curves of free and total T after oral administration of NTZ (200 mg/kg) were shown in Fig. 1, and pharmacokinetic parameters were listed in Table 1. The plasma concentration data of free and total T were described by a one-compartment open model with first order absorption. Peak plasma concentrations of 2.56 ± 0.25 and 5.67 ± 0.17 μg/mL for free and total T, respectively, were reached within 5–6 h. The concentration vs. time curves of T were biphasic, most prominent for total T, with a slow distribution phase and a slower terminal elimination phase lasting up to 24 h after administration, as indicated by its long absorption half-life (t1/2Ka, 2.63 ± 0.12) and longer elimination half-life (t1/2Ke, 4.02 ± 0.54). The AUC [60.32 ± 2.11 (μg/mL) × h] of total T was 2.2-fold greater than that [27.40 ± 1.54 (μg/mL) × h] of free T. The apparent volumes of distribution, V/F(c), of free T in goats (30.17 ± 2.17 L/kg) are larger than that of total T (20.21 ± 0.35 L/kg). The Cl(s) of total T was decreased compared with free T.
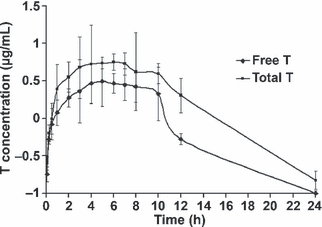
Semi-logarithmic plots of mean plasma concentration–time curves of tizoxanide (T) in goats after oral administration of 200 mg/kg bodyweight nitazoxanide capsules (n = 6).
| Parameters (unit)* | Tizoxanide concentration (μg/mL) | |
|---|---|---|
| Free | Total | |
| A (μg/mL) | 26.45 ± 1.60 | 50.60 ± 2.44† |
| K e (1/h) | 0.18 ± 0.02 | 0.22 ± 0.02 |
| K a (1/h) | 0.027 ± 0.03 | 0.28 ± 0.04 |
| Lag time (h) | 0.053 ± 0.04 | 0.06 ± 0.05 |
| t 1/2Ka (h) | 2.51 ± 0.41 | 2.63 ± 0.12 |
| t 1/2Ke (h) | 3.47 ± 0.32 | 4.02 ± 0.54† |
| T max (h) | 4.90 ± 0.13 | 5.91 ± 0.23† |
| C max (μg/mL) | 2.56 ± 0.25 | 5.67 ± 0.17† |
| AUC [(μg/mL) × h] | 27.40 ± 1.54 | 60.32 ± 2.11† |
| V/F(c) (L/kg) | 30.17 ± 2.17 | 20.21 ± 0.35† |
| Cl (s) [L/(kg × h)] | 7.34 ± 1.21 | 5.50 ± 0.61† |
- *A, zero time drug concentration intercepts of biphasic disposition curve; Ke, terminal elimination rate constant; Ka, absorption rate constant; Lag time, absorption time lag; t1/2ke, the elimination half-life; Tmax, the time to reach maximum plasma concentration; Cmax, the maximum plasma concentration; AUC, the area under the plasma concentration–time curve; V/F(c), the apparent volume of distribution calculated using AUC; Cl(s), the total body clearance. †P < 0.05 compared with free tizoxanide.
Protein binding rate of T
The results of the protein binding percentage of T (4 μg/mL) in vitro in goat plasma and in solutions of albumin and α-1-acid-glycoprotein were shown in Table 2. To determine the optimal concentrations of T for protein binding in goat plasma, seven concentrations (0.5, 1, 2, 4, 6, 8, and 10 μg/mL) of T were tested. The results showed that within the range of T concentrations from 0.5 to 8 μg/mL, protein binding percentages show parabola kinetics, and are all more than 60%, and the maximal protein binding percentage is 98.01% (mean value) when 4 μg/mL, while with 10 μg/mL concentration of T, the protein binding percentage was markedly decreased down to about 60%. This indicated that the concentrations of T from 0.5 to 8 μg/mL could be treated as the optimal concentrations for the protein binding experiments. Hence, 4 μg/mL concentration of T was chosen for further experiments with solutions of albumin and α-1-acid-glycoprotein. In the albumin test, 10, 20, 30, and 40 μg/mL concentrations of albumin were examined with 4 μg/mL T, and the results demonstrated that the protein binding percentage of T was more than 95% within the range of 20 to 40 μg/mL concentrations of albumin in the solution. However, with the concentrations of 0.125, 0.25, 0.5, and 1.0 μg/mL of α-1-acid-glycoprotein in the solution plus 4 μg/mL T, the protein binding percentage was between 39.5% and 51.2%. These results indicate that T has greater affinity for protein binding in goat plasma and to albumin than to alpha-1-acid-glycoprotein.
| Goat plasma | Albumin (20 μg/mL) | α-1-acid-glycoprotein (0.5 μg/mL) | |
|---|---|---|---|
| P T (%) | 98.01 ± 1.12 | 96.39 ± 1.97 | 49.20 ± 2.35 |
Discussion
The pharmacokinetics of NTZ’s active metabolite, T, in goat after oral administration of a single dose of 200 mg/kg NTZ were described by a one-compartment open model with first order absorption. To our knowledge, this is the first report describing the pharmacokinetics of NTZ in goats and they were characterized by a long terminal elimination half-life (3.47 ± 0.32 h), moderately slow clearance [7.34 ± 1.21 L/(kg × h)] and a large volume of distribution (30.17 ± 2.17 L/kg) for free T. After oral administration of NTZ at a dose of 200 mg/kg, the pharmacokinetic profiles of NTZ determined with β-glucuronidase hydrolysis were shown in Fig. 1 and Table 1. The mean plasma level of T determined without β-glucuronidase hydrolysis was much lower than that determined after β-glucuronidase hydrolysis. The maximum plasma concentration of T without β-glucuronidase hydrolysis was 2.56 ± 0.25 μg/mL, and this was only 50.4% of that measured with β-glucuronidase hydrolysis. The AUC of T determined after β-glucuronidase treatment was 2.2-fold greater than that found without β-glucuronidase hydrolysis. After oral administration, NTZ is rapidly converted to T, and T is further metabolized to tizoxanide glucuronide, which is the major metabolite in goat plasma (Zhao et al., 2008a). Tmax was observed at 5.91 ± 0.23 h for β-glucuronidase hydrolysis group.
However, V/F(c) and Cl(s) for β-glucuronidase hydrolysis group decreased significantly (P < 0.05). This phenomenon could be due to the formation of phase II metabolism, mainly tizoxanide glucuronide, and then drug excretion increased.
Absorption of T was slow, as indicated by the long absorption half-life and time to attain maximum concentration (t1/2Ka, 2.51 ± 0.41 h and Tmax, 4.90 ± 0.13 h) for free T. This slow absorption of T could be explained by the reason as described below. Absorption of a drug from a solution in a capsule is considered to involve liberation of the drug from the capsule into the aqueous luminal fluid followed by transport across the gastrointestinal epithelium, and the transfer from the capsule to the aqueous phase may become the rate determining process in the bioavailability pathway (Alhamami et al., 2006). As NTZ and its active metabolite, T, are insoluble in water (Prod Info Alinia, 2005), the amount of drug initially available for absorption by controlling its release to the gastrointestinal fluids was reduced, and consequently the rate of appearance of the drug in the small intestine was decreased. This latter site is regarded normally as the optimum site of absorption for most drugs even if the drugs are readily absorbed from the stomach (Levy, 1961), and even if the drug is ionized in the intestine and nonionized in the stomach (Benet, 1973). The rate of absorption of T could also be affected by the fact that goats are small ruminants and have their own physiological features. Single-dose oral administration distributes benzimidazole throughout the fluid and particulate phases of digesta in the rumen, which then effectively functions as a large reservoir of drug (Bogan & Marriner, 1987), and the same is true of nitazoxanide. The persistence of this reservoir depends on several complex interactions, including the degree of association of drug with the particulate phase, the rate of fiber digestion by microbes in the rumen, drug solubility in the fluid phase, and absorption rate across the rumen epithelium (Hennessy, 1997; Steel & Hennessy, 1999). All of these factors affect directly or indirectly on the mean residence time of drug in rumen digesta and the proportion of the dose, which is ultimately transported from the reticulo-rumen to the abomasum and intestines. Once in the rumen, the duration of drug availability as it is absorbed from the rumen and as it flows to more distal sites of absorption by host largely depends on the flow rate of the digesta (Hennessy, 1997). As rumen volume remains essentially constant, an inverse relationship between feed intake and digesta residence time exists (Kay, 1986) and a large food intake, particularly if the feed has a high water content, increases gastric transit and reduces the duration of drug availability. In addition, the solubility of nitazoxanide is low at normal rumen pH (6.5–7.0) (Ngomuo et al., 1984; Prod Info Alinia, 2005) but increases substantially at the lower pH (2–3) encountered in the abomasum and upper small intestine, which presumably facilitates absorption at these sites.
The elimination half-life of free T (t1/2ke) in goat (3.47 ± 0.32 h), determined in the current study is longer than that in humans (Stockis et al., 1996, 2002a; Prod Info Alinia, 2005). The difference suggested a slow elimination rate of the drug in goats. The delay in the rate of elimination of T in goats could be explained by the following facts. The first order elimination rate constant (ke) is concentration dependent. Therefore, the higher the peak plasma concentration following oral administration of NTZ is, the higher ke would be. In addition, the apparent elimination half-life determined in goats was longer than that of humans suggesting a higher rate of drug elimination and/or metabolism in humans than in goats. This could also be attributed to breed differences in the absorption, distribution, and elimination of the drug. These differences in elimination kinetics may, in part, reflect differences in metabolic pathway, analytical method, and environmental conditions employed in these studies.
The apparent volume of distribution at steady-state of a drug (Vdss) is an indication of its diffusion into body tissues (Gilman et al., 1980). The mean apparent volume of distribution, V/F(c), of free T in goats was 30.17 ± 2.17 L/kg. The high value of V/F(c) indicated that T was well distributed in body tissues and fluids of goats. The average t1/2Ke value of 3.47 ± 0.32 h after oral dosing was markedly longer than those reported in horses, dogs, rats, and humans and reflected higher V/F(c) and lower CL in the goat. However, the detailed reason of these relatively high volumes of distribution of T in goats is unknown. A possible mechanism is ion trapping in saliva. The secretion of large volumes of alkaline saliva is characteristic of ruminants, and weakly acidic drugs such as the active metabolite of NTZ, T, should achieve high concentrations in this fluid. Subsequent secondary absorption from the gastrointestinal tract would be anticipated. In addition, this phenomenon may also be related with glucuronidation in vivo. For example, the major metabolic route for furosemide inhumanis glucuronidation (Cutler & Blair, 1979; Vree et al., 1994). However, camels have a decreased capacity for drug metabolism including conversions involving phase II glucuronyl transferases to form furosemide glucuronide, and it is an important factor which may contribute to lower CL (EI Sheikh et al., 1988). The presence of furosemide glucuronide was reported in human plasma (Cmax 0.21 mg/L, Tmax 0.78 h) after administration of an 80 mg oral dose (Vree et al., 1994), but this metabolite was not detected in camel plasma. The average t1/2 values of 118 min and 109 min after iv. and i.m. dosing, respectively, were markedly longer than those reported in horses, dogs, rats, and humans and also reflect higher Vss and lower CL in the camel (Ali et al., 1998). The camel physiology is likely similar to other pseudoruminants and ruminants, and therefore the principles discussed above may apply to goats. The large volume of distribution for T in goats might be due to enterohepatic circulation. Broekhuysen et al. (2000) showed that T in the human was excreted in bile, conjugated with glucuronic and sulfuric acids. Similarly, T in the goat was excreted in urine, conjugated with glucuronic and sulfuric acids (Zhao et al., 2008a). All such conjugates are thereby potential candidates of enterohepatic circulation. However, glucuronidation by itself is not sufficient to ensure that enterohepatic circulation occurs. As indicated by Priymenko et al., 1998, the stereoselectivity of the hepatic uptake, glucuronide conjugation, and intestinal or rumen hydrolysis of the glucuronide by microflora enzymes (Jamali & Mehvar, 1989; Sweeny & Nellans, 1992; Lapicque et al., 1993; McKillop et al., 1993; Zhou et al., 2009) may all contribute to this process. Therefore, enterohepatic circulation of metabolites does not appear to be a major feature of T metabolism. Recirculation is normally reflected in the blood concentrations of free and total T, although the early peak concentrations occurring in the plasma around 5 h after dosing may be indicative of a limited amount of enterohepatic circulation; there is no evidence to suggest that recirculation of metabolites is sustained for any length of time. Whether enterohepatic circulation of tizoxanide and its conjugated metabolites contribute to the overall pharmacokinetic characteristic remains to be determined.
Binding of drugs to plasma proteins, mostly to serum albumin and α-acid-glycoprotein, is one of many factors that influences drug absorption, distribution, metabolism, and excretion (Wilkinson & Shand, 1975; Pike et al., 1984; Kratochwil et al., 2002). Therefore, plasma protein-binding experiments advance our understanding of ADME properties to aid in candidate selection and development by determining the unbound drug blood concentrations as well as (potentially) drug concentration at the site of action. Variation in the levels of these two proteins in plasma perhaps associated with different sex, ages and /or disease conditions could significantly affect free drug concentrations (Woodford-Williams et al., 1964; Verbeeck et al., 1984; Kremer et al., 1988). In general, acidic drugs are more likely to bind with albumin, while the basic drugs favor binding to α-1-acid-glycoprotein (Verbeeck et al., 1984). The active metabolite of NTZ, T, is characteristically acidic. Its plasma protein binding in goats has not been characterized; human plasma protein binding was reported to be about 99% (Prod Info Alinia, 2005). Thus species difference may produce different pharmacokinetic characteristics between humans and animals. These results indicate that T has greater affinity for protein binding in goat plasma and to albumin than to alpha-1-acid-glycoprotein. With 4 μg/mL T concentration in the goat plasma and in the solution of albumin, the protein binding percentage was generally greater than 95%, while in the solution of α-1-acid-glycoprotein, such percentage was only about 49%. Although physiologically relevant binding may not be to albumin, albumin binding is similar in vitro with goat plasma (Kragh-Hansen, 1981). In this study, the equilibrium dialysis was performed at 4 °C, not at body temperature (37 °C/38 °C). However, we assumed that the binding would be the same despite the temperature difference but would take longer to reach equilibrium. Therefore, the present data could mimic the protein binding property in vivo. These results have provided us more information for understanding the properties of T on pharmacokinetics and could at least partially explain the somewhat high AUC, low Cl, and the large volume of distribution observed in goat. The plasma protein binding rate of T in goat is lower than that in human. This small variation may be due to species differences, the assay method used, and the extent of the period between blood sampling.
Acknowledgments
The authors specially appreciated the personal of the experimental farm of Shanghai Veterinary Research Institute, CAAS, Shanghai, China for assistance in animal handling and collecting samples. The authors also like to thank Yang Hong, Anping Li, and Ligang An for their technical assistance.




