Distribution of ceftiofur into Mannheimia haemolytica-infected tissue chambers and lung after subcutaneous administration of ceftiofur crystalline free acid sterile suspension
Abstract
Washburn, K., Johnson, R., Clarke, C, Anderson, K. Distribution of ceftiofur into Mannheimia haemolytica-infected tissue chambers and lung after subcutaneous administration of ceftiofur crystalline free acid sterile suspension. J. vet. Pharmacol. Therap.33, 141–146.
The objective of this study was to evaluate the penetration of ceftiofur- and desfuroylceftiofur-related metabolites (DCA) into sterile and infected tissue chambers, lung tissue and disposition of DCA in plasma across four different sacrifice days postdosing. Twelve healthy calves were utilized following implantation with tissue chambers in the paralumbar fossa. Tissue chambers in each calf were randomly inoculated with either Mannheimia haemolytica or sterile PBS. All calves were dosed with ceftiofur crystalline free acid sterile suspension (CCFA-SS) subcutaneously in the ear pinna. Calves were randomly assigned to 4 groups of 3 to be sacrificed on days 3, 5, 7 and 9 postdosing. Prior to euthanasia, plasma and tissue chamber fluid were collected, and immediately following euthanasia, lung tissue samples were obtained from four different anatomical sites DCA concentration analysis. Results of our study found that, in general, DCA concentrations followed a rank order of plasma > infected tissue chamber fluid > noninfected tissue chamber fluid > lung tissue. Data also indicated DCA concentrations remained above the therapeutic threshold of 0.2 μg/mL for plasma and chamber fluid and 0.2 μg/g for lung tissue for at least 7 days post-treatment.
Introduction
Ceftiofur is a broad-spectrum third generation antibiotic developed for veterinary use. It is currently approved for use in three formulations: a sodium salt, hydrochloride salt and a crystalline free acid-sterile suspension (CCFA-SS). Ceftiofur has previously been shown to treat successfully bovine respiratory disease (BRD) associated with Mannheimia haemolytica, Pasteurella multocida, and Histophilus somni in beef and dairy cattle, and acute bovine foot rot associated with Fusobacterium necrophorum and Bacteroides melaninogenicus. The length of time that ceftiofur and its active desfuroylceftiofur metabolite concentrations remains above 0.2 μg/mL in targeted tissues is routinely used as a therapeutic threshold and for pharmacokinetic comparisons. This threshold exceeds the minimum inhibitory concentration for ceftiofur against major respiratory disease pathogens in cattle by at least 300% and is, therefore, a conservative measure of clinical efficacy (Yancy et al., 1987). The CCFA-SS form demonstrates extended release properties and has been developed as a single administration product.
As a member of the β-lactam group of antimicrobials, ceftiofur distributes well into extracellular compartments such as plasma and interstitial fluid compared with intracellular compartments. Except in the case of septicemia or infections of hematopoietic or lymphatic origin, bacterial pathogens of bovine respiratory disease generally reside within the interstitial fluid compartment (Clarke et al., 1989b; Clarke, 1993). Therefore, the use of lung tissue homogenate concentrations of β-lactam antimicrobials may underestimate true concentrations of drug available to fight extracellular respiratory pathogens (Carbon, 1992). Considering that pneumonic infections often involve accumulation of exudates and development of diffusion barriers, antimicrobial concentrations in infected tissue chambers are thought to be more representative of drug concentrations in infected lung than are plasma concentrations (Clarke et al., 1989a).
Extravasation of albumin, α1-acid glycoprotein, and fibrinoexudates into tissues during inflammation associated with infection can change the interstitial fluid pH, which may affect protein binding and availability of free drug for antibacterial activity (Clarke et al., 1996). Therefore, bacterial infections of soft tissues such as the lung may affect both the distribution of antibacterials and their in vivo activity in the target tissue (Clarke et al., 1989a). Several models have been developed to evaluate antibacterial activity in infected tissues. One well-characterized model is the subcutaneously (s.c.) implanted tissue chamber model (Clarke, 1989d; Clarke et al., 1989a,b,c, 1996; Halstead et al., 1992; Ensink et al., 2005; Boothe et al., 2009). Based on chemical and cytological findings, it is believed that fluid collected from chambers implanted s.c. in cattle is in equilibrium with the interstitial fluid of the tissues of the implantation site (Clarke et al., 1989c). Inoculation of M. haemolytica into s.c. tissue chambers implanted in cattle results in an inflammatory response that is characterized by an accumulation of fibrin and a rapid influx of neutrophils, which are considered important aspects of the pathology associated with pulmonary lesions seen in bovine respiratory disease (Yates, 1982; Slocombe et al., 1985; Clarke et al., 1989e). In a previous study (Washburn et al., 2005), dose of 6.6 mg ceftiofur equivalents (CE)/kg of CCFA-SS s.c. in the caudal ear pinna produced concentrations of ceftiofur and desfuroylceftiofur metabolite that followed a decreasing rank order of plasma > infected > noninfected tissue chamber fluid, with respect to the maximum concentration (Cmax), area under the curve (AUC0-LOQ), and time above 0.2 μg/mL (t<0.2) values. This study was conducted to investigate the correlation between ceftiofur and desfuroylceftiofur metabolite concentrations in plasma, tissue chambers (both noninfected and M. haemolytica-infected), and lung tissues after administration of the same dose of CCFA-SS. This necessitated the euthanasia of animals at regular intervals over a period of 9 days after drug administration to allow determination of lung tissue concentrations.
Materials and methods
Animals
A total of 12 Hereford calves (10 steers and two heifers), 4–6 months of age were bought from local sources. Within 24 h of arrival, calves were confirmed healthy by physical examination, weighed, and individually identified with ear tags. Calves were housed in a Biosafety Level 2 facility for the duration of the study and fed free choice prairie grass hay and a commercial grain ration containing 14% protein. Health was monitored daily by assessing rumen fill, attitude, respiratory function, and rectal temperature throughout the study.
This study constituted the second phase of a two-phase project. In the first phase, all calves were utilized in a nonterminal study that compared the total concentrations of ceftiofur and desfuroylceftiofur metabolite in plasma, infected tissue chambers, and noninfected tissue chambers following dose of 6.6 mg CE/kg CCFA-SS s.c. in the caudal ear pinna (Washburn et al., 2005). After a washout period of no less than 14 days from administration of CCFA-SS in the first phase, all calves were enrolled in the second phase of the study and randomly assigned to one of four euthanasia groups (to be euthanized on days 3, 5, 7, or 9 post-CCFA-SS administration), with three calves per group. The study protocol was approved by the Oklahoma State University Institutional Animal Care Use Committee.
Implantation of tissue chambers
Tissue chambers were constructed, sterilized, implanted s.c., and sampled according to previously described methods (Clarke et al., 1989c, 1996). Briefly, the perforated cup-shaped chambers were constructed of thermoplastic (Delrin®, EI du Pont de Nemours & Co, Wilmington, DE, USA) and measured 4.6 cm internal diameter, 5.2 cm outer diameter, and 1.5 cm in depth. Perforations distributed over the base and walls of the chamber cup allowed unrestricted exchange of cells and solutes between interstitial and chamber fluids. The top of the chamber was covered with silicone elastomer sheeting (Technical Products, Decatur, GA, USA) through which samples were collected percutaneously. Two sterile chambers were implanted s.c. approximately 5 cm apart in each paralumbar fossa (total of four chambers per calf). At least 30 days after implantation, sterility of the chambers was monitored by aerobically and anaerobically culturing an aspirate of the chamber fluid. Subsequently, all chambers determined to be infected at this time were surgically removed prior to initiation of the study (two out of 24 total chambers = 8.3%). As long as animals had at least one remaining sterile chamber on each side, they were maintained in the study.
Preparation of inocula
Chambers were inoculated with a field isolate of M. haemolytica serotype 1 (Corstvet et al., 1973). Mannheimia haemolytica isolates were initially streaked for purity and then grown on bovine blood tryptic soy agar for 6 h at 37 °C. Thereafter, bacteria were scraped from the agar and suspended in sterile 0.9% PBS at an approximate concentration of 1 × 106 colony forming units (CFU)/mL, as determined photometrically. Sterile PBS served as the negative control.
Experimental design and collection of samples
All tissue chambers on one side of each animal (right or left side determined randomly) were inoculated with 1 mL of M. haemolytica inoculum and the remaining chambers on the other side received 1 mL sterile PBS. After 24 hours of inoculation, tissue chamber samples were collected to confirm infection or sterility and CCFA-SS (Excede®, Pfizer Animal Health, New York, NY, USA) was administered s.c. in the middle third of the caudal aspect of the ear pinna at a dose of 6.6 mg CE/kg using a 16-gauge one-inch needle attached to an eccentric hub syringe. Tissue chamber fluid samples were collected by percutaneous aspiration and blood samples were collected by jugular venipuncture into heparinized tubes prior to dosing with CCFA-SS, and on days 3, 5, 7, and 9 postdosing prior to euthanasia, for ceftiofur-and desfuroylceftiofur-related metabolite assay.
On days 3, 5, 7, and 9 postdosing with CCFA-SS, all three calves in one of the groups were stunned with a captive bolt pistol and exsanguinated at approximately the same time of day following collection of the aforementioned plasma and tissue chamber samples. At least 50 g of lung tissue was collected from each of four anatomical sites from all calves immediately following euthanasia. Lung samples were collected from the ventral aspects of each of the right and left cranial lobes and the dorsal aspects of each of the right and left caudal lung lobes.
Plasma, tissue chamber fluid, and lung tissue samples were stored at −70 °C prior to shipping to Pfizer Animal Health and then analyzed for ceftiofur and desfuroylceftiofur metabolite concentrations.
Sample analyses
Tissue chamber fluid, plasma, and homogenized lung tissue samples were analyzed for ceftiofur and desfuroylceftiofur metabolites by derivatization of both the parent drug and the active metabolites to the stable analyte, desfuroylceftiofur acetamide (DCA), which was quantitated by high-performance liquid chromatography with UV detection (HPLC-DCA), according to a previously described method (Jaglan et al., 1990). Standard concentrations prepared in plasma ranged from 0.1 to 10.0 μg CE/mL. Recovery ranged from 79 to 101% and the inter-assay precision ranged from 14.2% at the low end of the standard curve (0.1 μg CE/mL) to 3.62% at the upper end of the curve (10.0 μg CE/mL). Accuracy at 0.1 μg CE/mL was 78.6%, whereas accuracy at all other standard concentrations ranged from 94.6 to 99.0%. Limits of detection (LOD) (0.042 μg CE/mL) and quantitation (LOQ) (0.139 μg CE/mL) were estimated at the signal-to-noise ratios of 3:1 and 10:1, respectively (Keith et al., 1983; Taylor, 1987; Miller & Miller, 1993; Stanko et al., 1993). However, considering that the lowest concentration standard had acceptable accuracy and precision, this concentration (0.1 μg CE/mL) was used as the LOQ. The same assay methodology was used for measurement of chamber fluid and homogenized lung tissue concentrations with the exception that standards were prepared in these respective fluids. The LOQ of the HPLC-DCA assay for lung tissue was determined by the lowest fortified sample used in the validation assay to be 0.10 μg CE/g. The LOD was chosen to be three times the square root of the mean square error of the lowest fortified sample (0.107 mg/g); therefore, the LOD was 0.015 μg CE/g. Estimates of assay validation parameters in these samples were identical to those generated in plasma.
Statistical analyses
Recognizing calves as experimental units, DCA concentrations in plasma, tissue chamber fluids, and lung tissue samples were analyzed according to the general linear model, using the MIXED procedure of SAS (SAS Institute Inc, 1999). When applicable, the model included: fixed effects of tissue (plasma, tissue chamber fluid, lung tissue), tissue chamber or lung sample location, infection, day of sampling; random effects of animal and residual; and relevant interactions between these effects. Based on previous experience involving statistical analysis of tissue chamber concentrations (Washburn et al., 2005), data were log transformed prior to analysis to address the expectation that variance and concentration are positively correlated. In the case of tissue chamber concentrations, zero values required addition of a constant (the value of one) to allow log transformation. Additionally, concentrations of DCA in lung were subjected to linear regression analysis to evaluate the effect of time (days) after drug administration. To compare DCA concentrations of plasma, infected chamber fluids, sterile chamber fluids and lung tissue, concentrations within each calf were averaged over the chambers of similar treatment or lung sample location. Effects were considered significant at P ≤ 0.05.
Results
All chambers inoculated with M. haemolytica were confirmed to be culture positive, whereas all chambers that received negative PBS control were still sterile immediately prior to administration of CCFA-SS. During the study period, rumen fill, attitude, respiratory function and rectal temperature remained within normal limits and no significant findings or adverse events following administration of CCFA-SS were noted.
Desfuroylceftiofur acetamide concentrations in plasma and tissue chamber fluid prior to administration of CCFA-SS were below the LOD or LOQ of the assay, confirming that the washout period following the first phase of the research project was of sufficient duration to allow elimination of previously administered CCFA-SS. dose of 6.6 mg CE/kg CCFA-SS in the present phase of the project resulted in plasma concentrations that remained above 0.2 μg/mL for 9 days (Table 1), attaining the highest concentration on day 3 and then declining thereafter. Similarly, overall tissue chamber fluid DCA concentrations were highest on day 3 and remained above the 0.2 μg/mL threshold for the duration of the 9-day sampling period. Mean concentrations in infected and noninfected tissue chambers on day 3 were 1.45 μg/mL (SD = ±0.37) and 0.94 μg/mL (SD = ±0.42), respectively, but overall there was no statistically significant effect of infection on distribution of ceftiofur and desfuroylceftiofur metabolites into tissue chambers.
| Time after CCFA-SS administration | Plasma | Noninfected tissue chamber | Infected tissue chamber |
|---|---|---|---|
| Day 3 | 2.59 ± 1.10a | 0.94 ± 0.42d | 1.45 ± 0.37d |
| Day 5 | 1.04 ± 0.11b | 0.54 ± 0.16e | 0.50 ± 0.17e |
| Day 7 | 0.68 ± 0.24b,c | 0.37 ± 0.13e,f | 0.39 ± 0.14e,f |
| Day 9 | 0.44 ± 0.11c | 0.23 ± 0.03f | 0.15 ± 0.21f |
- a,b,c,d,e,fValues without a common superscript within a table row or column were significantly different (P < 0.05). HPLC-DCA, high-performance liquid chromatography-desfuroylceftiofur acetamide; CCFA-SS, crystalline free acid-sterile suspension.
Overall, lung tissue DCA concentrations decreased significantly with time after drug administration (Table 2). In general, the concentration in the right cranial lung lobe was significantly lower than the concentrations in other lung sampling locations. Observed lung tissue DCA concentrations across anatomical sites were higher than 0.2 μg/g for 7 days following CCFA-SS administration, with the exception of the right cranial lung lobe where the concentration exceeded the threshold for only 5 days (Table 2). It should be noted, however, that the therapeutic level of drug in μg/g of tissue, may not have the same efficacy in vivo, as does drug in plasma or interstitial fluid reported in μg/mL.
| Time after CCFA-SS administration | Right cranial lobe | Left cranial lobe | Right caudal lobe | Left caudal lobe |
|---|---|---|---|---|
| Day 3 | 0.53 ± 0.23a | 0.68 ± 0.34b | 0.84 ± 0.13b | 0.75 ± 0.33b |
| Day 5 | 0.27 ± 0.03a | 0.30 ± 0.04b | 0.32 ± 0.02b | 0.34 ± 0.03b |
| Day 7 | 0.16 ± 0.05a | 0.22 ± 0.08b | 0.26 ± 0.08b | 0.24 ± 0.08b |
| Day 9 | 0.13 ± 0.00a | 0.14 ± 0.02b | 0.17 ± 0.04b | 0.16 ± 0.02b |
- a,bWithin each euthanasia day, values without a common superscript were significantly different (P < 0.05). HPLC-DCA, high-performance liquid chromatography-desfuroylceftiofur acetamide; CCFA-SS, crystalline free acid-sterile suspension.
Comparison of DCA concentrations in plasma, tissue chamber fluids, and lung tissues revealed similar dispositional profiles with the highest concentrations occurring on day 3 and concentrations declining thereafter (Fig. 1). DCA concentrations in plasma were significantly higher than those in infected and noninfected tissue chamber fluid and lung tissues at all sampling intervals. There were no significant differences between tissue chamber fluid and lung tissue DCA concentrations across euthanasia days, however, in general DCA concentrations appeared to follow a rank order of: plasma, infected chamber fluid > noninfected chamber fluid > lung tissues. Ceftiofur concentrations of plasma, tissue chamber fluid, and lung tissue were above the therapeutic threshold of 0.2 μg/mL or 0.2 μg/g for 7 days following CCFA-SS administration.
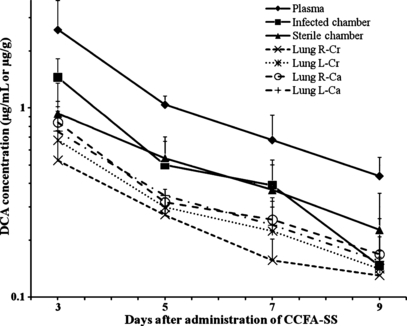
Concentrations (Mean ± SD) of DCA (ceftiofur plus desfuroylceftiofur metabolite) in plasma (Plasma), infected tissue chamber fluid (Infected tissue chamber), noninfected tissue chamber fluid (Sterile chamber) and samples collected from the right cranial (R-Cr), left cranial (L-Cr), right caudal (R-Ca), and left caudal (L-Ca) regions of the lungs. *Values significantly different from corresponding values in samples derived from tissue chambers or lung.
Discussion
Generally, peak concentrations of drugs in plasma are higher than those attained in tissue chambers, but thereafter plasma concentration often decreases below tissue chamber concentration, depending on the lipid solubility of the drug and its protein binding (Clarke, 1989d). This pattern of drug disposition results from the slow rates of drug diffusion between the vascular and tissue chamber compartments, which generally are lower than the corresponding rates of drug absorption (from the site of drug administration) and elimination (via metabolism and/or excretion) (Clarke et al., 1993). In the present study, total plasma concentration of ceftiofur and active metabolites was persistently higher than corresponding tissue concentrations, both tissue chamber and lung. The failure of plasma drug concentration to decrease below tissue concentrations can be explained by the ‘flip-flop’ phenomenon of drug disposition, whereby a very slow rate of drug absorption (often associated with slow-release formulations such as CCFA-SS) becomes the rate-limiting factor that determines the rate at which plasma drug concentration decreases with time. Under these pharmacokinetic conditions, diffusion between vascular and tissue compartments occurs more rapidly than drug absorption, allowing the drug to equilibrate between these compartments, depending on factors such as protein binding and intracellular distribution. Considering that desfuroylceftiofur metabolite is extensively bound to plasma proteins (Brown et al., 1991), concentration of DCA can be expected to be lower in tissue chamber and lung tissue samples than in plasma, which has a higher protein concentration. Indeed, considering the limited intracellular distribution of ceftiofur and the relatively low albumin concentration in pulmonary interstitium, lung concentrations can be expected to be even lower than those in infected tissue chambers; an expectation that is consistent with the results of the present experiment.
Ceftiofur is rapidly metabolized in vivo by cleavage of a thioester bond to desfuroylceftiofur (Jacobson et al., 2005), which has antibiotic activity similar to that of the parent compound (Salmon et al., 1996). The method of analysis of ceftiofur involves conversion to desfuroylceftiofur, followed by derivitization to the stable DCA. Concentrations of DCA in samples, therefore, represent total antibacterial activity related to ceftiofur and its desfuroylceftiofur metabolite (often expressed as ceftiofur-free acid equivalents, CE). A previous study involving M. haemolytica-infected tissue chambers indicated that both concentrations of DCA and antibiotic activity of CE were higher in infected chambers than in noninfected chambers. Considering that desfuroylceftiofur binds to albumin, it appears that while this binding may affect its distribution between various body and tissue compartments, it is sufficiently reversible not to adversely affect antibiotic activity (Jaglan et al., 1992), (Clarke et al., 1996).
Desfuroylceftiofur acetamide concentrations remained above 0.2 μg/mL for 9 days in plasma and tissue chamber fluid samples and above 0.2 μg/g for 7 days in lung tissue samples with the exception of the right cranial lung lobe. Plasma and tissue chamber results were consistent with our previous study (Washburn et al., 2005) which reported these concentrations to be above 0.2 μg/mL for at least seven and almost 8 days. Presence of infection in tissue chambers dramatically alters the composition of chamber fluid as a result of endothelial cell damage and accumulation of fibrinopurulent exudates, cellular infiltrates, and protein (Clarke et al., 1989a). These changes to the environment of the infected tissue chambers explain the increased influx of ceftiofur and defuroylceftiofur metabolites observed in previous studies (Clarke et al., 1996; Washburn et al., 2005); as endothelial damage promotes diffusion of drug across capillary walls and binding to protein creates a drug reservoir at the site of infection. These observations may also explain why DCA concentrations in healthy, normal lung tissue samples were not above 0.2 μg/g for longer than 7 days and suggest that concentrations may be higher in clinical settings involving naturally occurring BRD due to the binding of drug to increased proteins at the infection site.
In summary, the results of this study indicated that ceftiofur-and desfuroylceftiofur-related metabolites remained above 0.2 μg/mL in plasma, infected, and noninfected tissue chamber fluid and above 0.2 μg/g in lung tissue for at least 7 days following s.c. administration of CCFA-SS in the ear pinna in healthy feedlot calves.
Acknowledgments
This work was supported by Pfizer Animal Health. The authors gratefully acknowledge the following individuals for their contributions to this work: W. Lawrence Bryson, PhD; Edward J. Robb, DVM, MS; Joseph A. Robinson, PhD; Kenneth. Callahan, BS; Larry. Hubbard, BS; and Merlyn J. Lucas, DVM, MS.




