Residue depletion of melamine and cyanuric acid in catfish and rainbow trout following oral administration
Abstract
Reimschuessel, R., Evans, E., Andersen, W. C., Turnipseed, S. B., Karbiwnyk, C. M., Mayer, T. D., Nochetto, C., Rummel, N. G., Gieseker, C. M. Residue depletion of melamine and cyanuric acid in catfish and rainbow trout following oral administration. J. vet. Pharmacol. Therap.33, 172–182.
The intentional addition of triazines such as melamine to animal feeds and the lack of information about residue accumulation in food animals caused global concerns for food safety during 2007 and 2008. We report the results of a good laboratory practices (GLP) study to determine melamine and cyanuric acid residues in catfish and trout filets harvested at 1, 3, 7, 14, 28, and 42 days after a single oral dose of 20 mg/kg body weight of melamine, cyanuric acid, or melamine and cyanuric acid together. Peak melamine concentrations were 12.73 mg/kg (ppm) in catfish (mean = 9.98), 12.26 mg/kg in trout (mean = 7.89) on day 1. Within 7 days (catfish) or 14 days (trout) residues were <2.5 mg/kg, a level in foods accepted by many risk assessors worldwide to be unlikely to pose health risks to consumers. Peak cyanuric acid residues also occurred on day 1, 0.68 mg/kg in catfish (mean = 0.46), 2.59 mg/kg in trout (mean = 0.86). Cyanuric acid muscle residues were <2.5 mg/kg by day 3. The half-lives for melamine and cyanuric acid ranged between 1 and 4 days. Renal crystals formed in fish given both melamine and cyanuric acid, persisting for weeks after the single dose.
Introduction
The practice of adulterating feed ingredients with melamine was brought to light in 2007 (Barboza & Barrioneuvo, 2007). At that time, pet food contaminated with melamine caused hundreds of dogs and cats to develop renal failure (Brown et al., 2007; Reyers, 2007; Cianciolo et al., 2008; Thompson et al., 2008) in the United States, Canada and South Africa. Initially, this was a conundrum because melamine was considered to be relatively nontoxic (Lipschitz & Stokey, 1945; NTP, 1983; Melnick et al., 1984). However, melamine was not only added in pure form but also as scrap melamine, which contains related triazine analogs such as cyanuric acid, ammeline, or ammelide. A second contaminant, cyanuric acid, was later discovered in the pet food linked to animal deaths. Subsequent laboratory studies showed that animals fed both melamine and cyanuric acid form renal melamine-cyanurate crystals (Puschner et al., 2007; Dobson et al., 2008; Reimschuessel et al., 2008). It has been proposed that these crystals may induce renal failure by causing intratubular obstruction in a mechanism similar to uric acid nephropathy (Reimschuessel et al., 2008). Thus, the adulteration of wheat gluten and other feed ingredients by melamine led to the largest recall of animal feeds in history (USFDA, 2007a).
In addition to pet food, fish and livestock feeds were also found to be contaminated with melamine, raising concerns for human food safety (Burns, 2007; USFDA, 2007b,c). The US Food and Drug Administration (USFDA) therefore rapidly developed methods to detect melamine and cyanuric acid in tissues of fish that had been fed these compounds (Andersen et al., 2007, 2008; Karbiwnyk et al., 2009; Smoker & Krynitsky, 2008). Those studies showed that residues of these two triazines, when given in the high concentrations found in the pet food, can accumulate in tissues of fish raised for human consumption. A number of questions, however, remained unanswered. Would fish bioaccumulate triazines if the feed had low levels of those contaminants and if so, how long would it take for the triazines to deplete from edible tissues?.
In September 2008, another incident of melamine adulteration, this time in milk products, was reported in China (WHO, 2008). Over 300 000 infants and children drinking adulterated milk and infant formula developed kidney stones, with some developing serious life-threatening postrenal obstructions because of those stones (Sun et al., 2008; Guan et al., 2009; Kuehn, 2009; Shen et al., 2009). This event led to world-wide recalls of contaminated foods including milk, infant formula and products containing milk powder such as candy and chocolate. This incident again demonstrated the need to understand both the effect of melamine in the body and how rapidly it is eliminated.
Further investigations into the extent of food contamination revealed that some foods, such as whole eggs, also contained melamine, a result of adulterated chicken feed (Barboza, 2008a; Mccabe, 2008). In addition, more reports of contaminated fish feeds surfaced (Anonymous, 2008a; Barboza, 2008b), raising concerns about melamine residues in edible tissues. As a result, many nations have established limits for the amount of melamine that can be present in infant formula and other foods (Anonymous, 2008b; AQSIQ, 2008; Centre for Food Safety, 2008; Commission Decision 2008/757/EC, 2008; New Zealand Food Safety Authority, 2008; USFDA, 2008). Because melamine and related triazines are used in a variety of industrial applications, low levels of these compounds are present in the environment and trace amounts may occur in some foods. Most risk assessors worldwide have concluded that levels below 2.5 mg/kg in foods, other than infant formula, are unlikely to pose health risks to consumers. Most of these risk assessments, however, concluded that there were major data gaps regarding residue information in food animals.
Because of the uncertainties regarding bioaccumulation of melamine and cyanuric acid in fish tissues and potential public health effects following the pet food recall of 2007, the Center for Veterinary Medicine (CVM) of the USFDA initiated studies in January 2008 to evaluate the depletion of melamine and cyanuric acid in two fish species given a single dose of 20 mg/kg body weight (BW). This dosage, much lower than the 400 mg/kg BW concentration used to develop tissue residue methods in 2007, is comparable to fish being given feed containing 500 mg/kg (ppm) of the contaminants. We report here the results of a good laboratory practices (GLP) study to determine concentrations of melamine and cyanuric acid found in the edible tissues of catfish and trout at multiple time points after consuming these triazines either individually or in combination.
Materials and methods
Animals
Channel catfish, Ictalurus punctatus, and rainbow trout, Oncorhynchus mykiss, were bought from commercial fish farms and housed in 200 L flow-through glass aquaria (catfish) or 1800 L round fiberglass tanks (trout). Trout were maintained in a freshwater recirculating aquaculture system with partial water changes, catfish were maintained with a continuous freshwater flow. Fish were acclimated for a minimum of 1 month before use. The holding systems and experimental systems were supplied with the same source of freshwater. Catfish were fed a commercially available extruded diet at 35% protein, 8% fat, and 5% fiber (Burris Aquaculture, Franklinton, LA, USA) at 1–2% BW/day. Trout were fed a pelleted commercial diet at 40% protein, 14% fat, and 4% fiber (Rangen Inc. Buhl, ID, USA) at 1–4% BW/day. Prior to use, all feeds were tested for melamine and cyanuric acid by liquid chromatography tandem mass spectrometry (LC-MS/MS) (Heller & Nochetto, 2008). No residues were detected above the limit of quantification (LOQ) of the method, 0.5 μg/gm (ppm). The use of fish in this study was approved by the USFDA/CVM Institutional Animal Care and Use Committee.
Treatment and experimental design
Individual fish were removed from acclimation tanks, weighed, and transferred to 60 L flow-through glass aquaria (1 fish/tank). The fish were allowed to acclimate in the exposure tanks for at least 1 day prior to dosing. A total of 20 fish were used for each withdrawal time: six fish for each of the three chemical treatments and two control fish. Treatments consisted of 20 mg/kg BW of melamine, 20 mg/kg BW of cyanuric acid, or 20 mg/kg BW of melamine plus 20 mg/kg BW cyanuric acid weighed into gelatin capsules (size 4; Torpac, Fairfield, NJ, USA or size 4; Capsuline, Pompano Beach, FL, USA). Gelatin capsules were tested for melamine and cyanuric acid using the same method as for feeds. No residues were detected above the LOQ of the method.
A single capsule containing the chemical was administered to each fish via an intragastric feeding tube. For the combination doses, melamine and cyanuric acid were weighed separately in gelatin capsules then placed in one capsule just prior to dosing. Control fish were given an empty gelatin capsule. Fish were observed for approximately 1 h after dosing. If capsules were regurgitated they were retrieved, and if intact used to dose again. If necessary a new dose was prepared. Actual mean dosages calculated on the basis of BW at necropsy were 21.9 ± 2.3 mg/kg for catfish and 20.6 ± 2.1 mg/kg for trout.
Fish were held for 1, 3, 7, 14, and 28 days after dosing. An additional withdrawal time of 42 days was added for trout. Catfish tank water was maintained at 24.3 ± 1.0 °C, with dissolved oxygen at 8.4 ± 1.3 ppm and pH of 7.2 ± 0.2. Trout water was maintained at 12.5 ± 1.1 °C, with a dissolved oxygen concentration of 9.3 ± 3.4 ppm and pH of 7.0 ± 0.1. Ammonia and nitrite were monitored weekly during treatments. Mean values for ammonia and nitrite were 0.056 ± 0.04 ppm and 0.0021 ± 0.001 ppm, respectively.
Necropsy and sampling
Fish were stunned with a sharp blow to the head and euthanized by severing the cervical spine with a sharp knife followed by double pithing. Weight and length were recorded: catfish (n = 100) were 195.5 ± 40.0 g and 25.3 ± 1.7 cm, and trout (n = 120) were 408.3 ± 168.9 g and 30.2 ± 4.2 cm. Both filets were removed, weighed, wrapped in foil, and stored at −80 °C until analysis. Skin was removed from catfish filets while trout skin was retained but scales were removed.
The caudal kidney was removed and transverse sections were sampled for fresh tissue wet mount and histology from two regions. The first section was taken from the most posterior part of the kidney. In channel catfish, which have a bifurcated caudal kidney, the second wet mount section was sampled near the base of one fork and the second histology section was taken in a similar location from the other fork (Fig. 1). In trout, the second set of samples was taken from a region near the dorsal fin. The remaining kidney tissue from each fish was archived in a tissue cassette and stored at −80 °C.
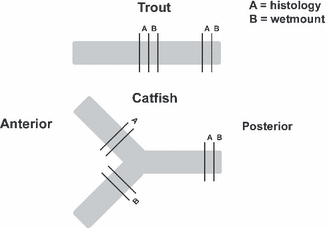
Diagram of trout and catfish kidneys showing where they were sampled for histology and wet mounts.
The wet mount sections were placed on a microscope slide, covered, and flattened with another slide. Wet mounts were viewed with an inverted microscope for crystals previously described in fish following feeding of melamine and cyanuric acid (Reimschuessel et al., 2008). The crystal results were described with a subjective scale from 0 to 5 as follows: 0, none seen; 1, extremely few (1 or 2 in an entire section); 2, few with scattered distribution; 3, moderate numbers seen throughout section; 4, large numbers seen immediately; and 5, extensive numbers obliterating the regular tissue architecture. The histology samples were fixed in 10% neutral buffered formalin. Formalin was replaced with 70% ethanol after 24 h and tissues were processed for routine histologic evaluation, and sections (5–6 μm) were stained with hematoxylin and eosin (Prophet et al., 1992).
Analytical methods
Reagents and standards
For both standards and dosing chemicals, melamine was purchased from Sigma-Aldrich (St Louis, MO, USA) (99+% purity) and cyanuric acid (98% purity) was purchased from TCI America (Portland, OR, USA). All other chemicals used were of reagent grade or better. Deionized water was purified to 18.2 MΩ.cm (Millipore, Bedford, MA, USA). Standard stock solutions (100.0 μg/mL (ppm) of melamine and cyanuric acid were prepared in 2% ammonium hydroxide. Solutions were sonicated 20–30 min to aid dissolution. Working standard solutions (10.0 and 1.0 μg/mL) were prepared by diluting with water. Calibration standards of melamine in concentrations ranging from 0.005 to 1.0 μg/mL were prepared by diluting appropriate aliquots of the working standards with 95/5 (v/v) acetonitrile/ammonium formate (20 mm in water). Cyanuric acid matrix standards were prepared by extracting negative control tissue as described below and adding an appropriate amount of cyanuric acid working solution before reconstituting the dried extract. Formic acid solution (0.1%) was then added to produce a final volume of 1 mL. Cyanuric acid matrix standards were prepared in concentrations from 0.010 to 1.0 μg/mL.
Muscle samples
Thawed fish filets were cut into small pieces, which were all blended with dry ice in a blender/homogenizer with pulsed action until contents were uniform and had the consistency of a fine powder. The homogenate was allowed to degas in the freezer overnight, and then was tightly sealed and stored (−80 °C) until analysis. Fortified (25 and 50 ng/mL) and un-spiked (negative control) samples of tissue homogenate from store-bought filets of farm-raised catfish and rainbow trout were extracted and analyzed with each set of depletion study samples to ensure method performance criteria for melamine and cyanuric acid residue determination.
Muscle extraction and clean-up
The determinations of melamine and cyanuric acid residues in catfish and trout muscles were based on previously published methods (Andersen et al., 2008; Karbiwnyk et al., 2009). These methods are outlined briefly below.
Melamine. Twenty-four milliliter of a 50/50 (v/v) solution of acetonitrile/water and 1 mL of 1.0 N hydrochloric acid was added to a 50 mL polypropylene centrifuge tube containing 5.0 g of homogenized muscle. The sample was capped, shaken vigorously for 30 sec and then vortex mixed for 1 min. The sample was centrifuged at 2500 g for 5 min at 5 °C. A 5 mL aliquot of supernatant was removed to a 15 mL polypropylene centrifuge tube containing 10 mL of dichloromethane, and the sample was shaken for 2 min, then centrifuged as above. A portion (2.5 mL) of the upper aqueous layer was carefully removed to a glass culture tube. Water (2.5 mL) was added to the dichloromethane layer and the sample was re-extracted by shaking for 1 min, then centrifuged. The entire upper aqueous layer was removed and combined with the first aqueous extract in the glass culture tube, then vortex mixed briefly. The sample extract was cleaned-up by gravity elution through a conditioned (methanol, water) Oasis MCX SPE cartridge (150 mg, 6 mL; Waters Corp., Milford, MA, USA). The cartridge was washed with 0.1 N hydrochloric acid (5 mL) and methanol (2 mL), then dried under vacuum for 1 min. Melamine was eluted from the cartridge into a glass culture tube using 5 mL of 5% ammonium hydroxide in methanol. The eluate was evaporated to dryness in a water bath at 55 °C under blowing nitrogen at 15 psi for 20 min (Turbo-Vap LV, Zymark, Hopkinton, MA, USA). The dried extract was reconstituted in 1.0 mL of 95/5 acetonitrile/ammonium formate (20 mm), vortex mixed for 15 sec, and filtered through a 0.2 μm nylon syringe filter (Acrodisc 13 mm; Pall Life Sciences, East Hills, NY, USA) into a glass LC vial. Average recoveries for melamine fortified samples in this study were found to be 92.2% (±13.5% RSD, n = 14) for catfish and 88.0% (±19.6% RSD, n = 11) for trout.
Cyanuric acid. Twenty-five milliliter of 0.04% acetic acid solution (v/v) was added to a 50 mL polypropylene centrifuge tube containing 5 g of homogenized muscle. The sample was capped, vigorously shaken for 30 sec and put into a water bath held at 84 °C for 5 min. The heated sample was vortex mixed for 2 min and then centrifuged at 2500 g for 5 min at 5 °C. A 15 mL aliquot of supernatant was transferred to a clean 50 mL centrifuge tube. Hexane (10 mL) was added and the sample was mixed gently for 2 min then centrifuged as above. The hexane layer was then removed by aspiration. The defatted sample was loaded onto a conditioned (dichloromethane, methanol, 0.04% acetic acid) Envi-Carb SPE cartridge (6 mL, 500 mg; Supelco, Bellefonte, PA, USA) followed by a wash with 5 mL of water. The cartridge was dried under vacuum for 5 min, then eluted by gravity with 10 mL of methanol into glass culture tubes. The sample was evaporated to dryness at 55 °C with nitrogen flow for 25–30 min. The dried extract was reconstituted with 1 mL of 0.1% formic acid in water and vortex mixed for 15 sec, then filtered through a 0.2 μm nylon syringe filter (Acrodisc 13 mm) into an LC vial. A threefold analyte concentration was achieved by this extraction procedure. Average recoveries for cyanuric acid fortified samples in this study were found to be 74.0% (±8.8% RSD, n = 11) for catfish and 83.1% (±14.9% RSD, n = 9) for trout.
LC-MS/MS parameters
For both melamine and cyanuric acid methods (Andersen et al., 2008; Karbiwnyk et al., 2009), the LC-MS/MS consisted of a Thermo (San Jose, CA, USA) TSQ Quantum triple quadrupole mass spectrometer coupled to a Thermo Surveyor LC-MS pump and autosampler. A metal needle sample kit was installed on the electrospray source; the orientation of the spray to the orifice was set at the second notch (approx 62 deg offset). XCaliber V2.0 software (Thermo, San Jose, CA, USA) was used to acquire and analyze the data.
Melamine. The LC-MS/MS was operated in positive ion mode with selected reaction monitoring (SRM) performed on the protonated molecule for melamine using the following general parameters: source spray voltage = 5 kV; capillary temperature = 270 °C; sheath gas (nitrogen) = 14 (arbitrary units); auxiliary gas (nitrogen) = 0 (arbitrary units); Q1 peak width m/z = 0.7; Q3 peak width m/z = 0.7; collision gas = 1.5 mTorr Argon; scan width m/z = 1, and scan time = 0.5 sec. To optimize the signal for the m/z 127 precursor ion, the electrospray skimmer potential was set to 20 V. Two SRM transitions of m/z 127 → 85 (collision energy = 7 V) and m/z 127 → 68 (collision energy = 23 V) were monitored. The LC column was an Atlantis HILIC Silica column, 3 μm, 3.0 × 50 mm (Waters Corp.) and the mobile phase program consisted of a binary gradient of acetonitrile and 20 mm aqueous ammonium formate. The composition started out at 95% acetonitrile and decreased linearly to 50% acetonitrile over 5 min. The mobile phase was then returned to 95% acetonitrile between 5 and 7 min, and the column was re-equilibrated for 5 min. The flow rate was 350 μL/min. The column was kept in an insulated compartment, but the temperature was not controlled. The injection volume was 10 μL.
Cyanuric acid. The electrospray interface was operated in negative ion mode with SRM performed on the m/z 128 [M-H]− ion using the following general parameters: source spray voltage = 2.6 kV; capillary temperature = 270 °C; sheath gas (nitrogen) = 80 (arbitrary units); auxiliary gas (nitrogen) = 3 (arbitrary units); Q1 peak width m/z = 0.7; Q3 peak width m/z = 0.7; collision gas = 1.5 mTorr Argon; scan width m/z = 1, and scan time = 0.5 sec. To optimize the signal for the m/z 128 precursor ion, the electrospray skimmer potential was set to 5 V. Collision energies were optimized on SRM transitions m/z 128 → 42 (collision energy = 20 V) and 128 → 85 (collision energy = 11 V). Sample and standard solutions were separated on a 5 μm, 100 × 2.1 mm Hypercarb column (Thermo) at 30 °C using a solvent gradient and flow rate of 200 μL/min. Mobile phase (A) consisted of 0.1% formic acid in water (v/v). Mobile phase (B) was acetonitrile. At the start of each analysis, the acetonitrile concentration was 10% of the mobile phase flow. The acetonitrile was increased to 100% of the mobile phase flow between 0 and 10 min and held at 100% for an additional 2.5 min. The instrument was then returned to the starting mobile phase conditions and the column was re-equilibrated for 5 min prior to subsequent analyses. The injection volume was 10 μL.
Detection and Confirmation of melamine and cyanuric acid in muscle by LC-MS/MS
For melamine and cyanuric acid residues to be positively confirmed in a sample, samples had to meet specific confirmation criteria. The retention time of melamine or cyanuric acid found in a sample had to match within 5% of that for standards analyzed on the same day, and the relative abundance of the two transitions had to match within 10% of that for the standards (USFDA, 2003).
Melamine. Quantitative data were determined by comparison of the area counts of the chromatographic peak observed for the m/z 127 → 85 SRM transition to the 7 point calibration curve generated for that transition from melamine standards with concentrations ranging from 0.005 to 1.0 μg/mL (ppm). For depletion study samples with low concentrations of melamine (levels below 0.060 mg/kg, (ppm), the concentration was calculated using a 5 point calibration curve with calibration standards ranging from 0.005 to 0.100 μg/mL. Samples with more than 1.0 mg/kg of melamine were appropriately diluted in 95/5 acetonitrile/ammonium formate and re-analyzed. For confirmation, peak area counts from the m/z 127 → 85 and m/z 127 → 68 SRM transitions were generated and the resulting chromatographic peaks were integrated. Relative abundances were calculated from these peak areas and compared with contemporary standards.
Cyanuric acid. Concentrations in samples were calculated from the peak area of the m/z 128 → 42 transitions using a calibration curve generated from 5 or 6 cyanuric acid matrix standards with concentrations ranging from 0.010 to 1.0 μg/mL. Samples with more than 1.0 mg/kg of cyanuric acid were appropriately diluted in 0.1% formic acid and re-analyzed. For confirmation, peak area counts from the m/z 128→ 42 and m/z 128→ 85 SRM transitions were generated and the resulting chromatographic peaks were integrated. Relative abundances and analyte retention times were determined from these peak areas and compared with standards analyzed that day.
Statistics, elimination half-life and rate constant determination
The mean residue levels of melamine and cyanuric acid from each treatment group on each sample day were used to calculate the tissue half-life (t½) and elimination rate constant (Kel). Calculations were carried out by regression of the semi-logarithmic concentration vs. time data using PK functions for Microsoft Excel developed by Usansky et al. (Department of Pharmacokinetics and Drug Metabolism, Allergan, Irvine, CA, USA).
The residue levels (ppm) of melamine or cyanuric acid were analyzed separately for trout and catfish with two-way analysis of variance tests using sigmastat 3.5 (San Jose, CA, USA). The two-way interaction of Treatment and Day were analyzed. For each Day, the melamine or cyanuric acid levels following the individual compound dose and combination dose were compared with each other and to the control dose using pairwise multiple comparison procedures (Tukey test).
Results
Graphs of the overall residues are shown in 2, 3 and individual residues found in catfish and trout tissues are shown in Tables 1–4. In the group given only melamine, the highest concentration of melamine was found on day 1 following administration, 12.73 mg/kg in catfish, 12.26 mg/kg in trout. These values rapidly declined in both species, with the mean concentrations on day 1, 9.98 mg/kg (catfish) and 7.89 mg/kg (trout), declining to 0.32 mg/kg (catfish) and 1.63 mg/kg (trout) on day 7. Melamine residues in each individual fish were below 2.5 mg/kg on day 7 following administration.
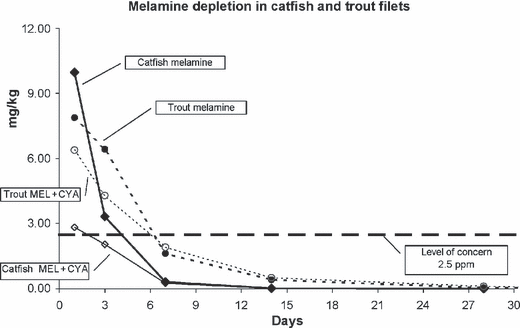
Depletion of melamine in fish given either melamine alone (20 mg/kg BW) or melamine in combination with cyanuric acid (20 mg/kg BW of each chemical).
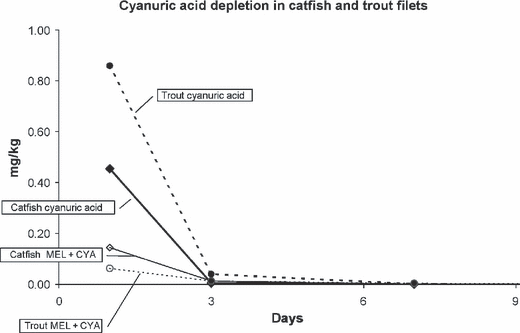
Depletion of cyanuric acid in fish given either cyanuric acid alone (20 mg/kg BW) or cyanuric acid in combination with melamine (20 mg/kg BW of each chemical).
| Days | 1 | 3 | 7 | 14 | 28 |
|---|---|---|---|---|---|
| Melamine only | 12.73 | 5.44 | 0.70 | 0.03 | 0.00 |
| 10.42 | 5.16 | 0.69 | 0.03 | 0.00 | |
| 9.89 | 4.62 | 0.24 | 0.03 | 0.00 | |
| 9.31 | 3.59 | 0.12 | 0.02 | 0.00 | |
| 8.96 | 1.15 | 0.10 | 0.02 | 0.00 | |
| 8.55 | 0.02 | 0.05 | 0.02 | 0.00 | |
| Mean | 9.98 | 3.33 | 0.32 | 0.03 | 0.00 |
| SD | 1.51 | 2.25 | 0.30 | 0.00 | 0.00 |
| Melamine + cyanuric acid | 5.01 | 3.71 | 0.62 | 0.05 | 0.00 |
| 4.06 | 2.74 | 0.42 | 0.01 | 0.00 | |
| 3.78 | 2.60 | 0.27 | 0.01 | 0.00 | |
| 2.99 | 1.62 | 0.16 | 0.01 | 0.00 | |
| 0.64 | 1.12 | 0.05 | 0.00 | 0.00 | |
| 0.54 | 0.51 | 0.03 | 0.00 | 0.00 | |
| Mean | 2.84 | 2.05 | 0.26 | 0.02 | 0.00 |
| SD | 1.86 | 1.18 | 0.23 | 0.02 | 0.00 |
| Days | 1 | 3 | 7 | 14 | 28 | 42 |
|---|---|---|---|---|---|---|
| Melamine only | 12.26 | 8.29 | 2.39 | 0.66 | 0.17 | 0.00 |
| 12.11 | 7.65 | 2.18 | 0.54 | 0.06 | 0.00 | |
| 7.92 | 5.97 | 1.39 | 0.50 | 0.02 | 0.00 | |
| 5.42 | 5.79 | 1.39 | 0.30 | 0.02 | 0.00 | |
| 5.28 | 5.70 | 1.30 | 0.27 | 0.02 | 0.00 | |
| 4.32 | 5.22 | 1.10 | 0.21 | 0.01 | 0.00 | |
| Mean | 7.89 | 6.44 | 1.63 | 0.41 | 0.05 | 0.00 |
| SD | 3.54 | 1.23 | 0.53 | 0.18 | 0.06 | 0.00 |
| Melamine + cyanuric acid | 9.64 | 7.23 | 3.43 | 1.37 | 0.56 | 0.01 |
| 7.32 | 6.00 | 2.99 | 0.99 | 0.06 | 0.01 | |
| 6.85 | 5.01 | 1.94 | 0.24 | 0.05 | 0.00 | |
| 6.81 | 3.02 | 1.37 | 0.20 | 0.02 | 0.00 | |
| 5.56 | 2.97 | 1.34 | 0.15 | 0.01 | 0.00 | |
| 2.20 | 1.57 | 0.44 | 0.12 | 0.01 | 0.00 | |
| Mean | 6.40 | 4.30 | 1.92 | 0.51 | 0.12 | 0.00 |
| SD | 2.45 | 2.14 | 1.12 | 0.53 | 0.22 | 0.00 |
| Days | 1 | 3 | 7 | 14 | 28 |
|---|---|---|---|---|---|
| Cyanuric acid only | 0.68 | 0.03 | 0.00 | 0.00 | 0.00 |
| 0.48 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 0.46 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 0.44 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 0.37 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 0.30 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Mean | 0.46 | 0.01 | 0.00 | 0.00 | 0.00 |
| SD | 0.13 | 0.01 | 0.00 | 0.00 | 0.00 |
| Cyanuric acid + melamine | 0.31 | 0.05 | 0.00 | 0.00 | 0.00 |
| 0.28 | 0.02 | 0.00 | 0.00 | 0.00 | |
| 0.16 | 0.01 | 0.00 | 0.00 | 0.00 | |
| 0.07 | 0.01 | 0.00 | 0.00 | 0.00 | |
| 0.03 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 0.03 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Mean | 0.14 | 0.01 | 0.00 | 0.00 | 0.00 |
| SD | 0.13 | 0.02 | 0.00 | 0.00 | 0.00 |
| Days | 1 | 3 | 7 | 14 | 28 | 42 |
|---|---|---|---|---|---|---|
| Cyanuric acid only | 2.59 | 0.14 | 0.01 | 0.00 | 0.00 | 0.00 |
| 0.75 | 0.04 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 0.68 | 0.03 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 0.47 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 0.41 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 0.26 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Mean | 0.86 | 0.03 | 0.00 | 0.00 | 0.00 | 0.00 |
| SD | 0.87 | 0.05 | 0.01 | 0.00 | 0.00 | 0.00 |
| Cyanuric acid + melamine | 0.15 | 0.05 | 0.03 | 0.00 | 0.00 | 0.00 |
| 0.11 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 0.05 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 0.04 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 0.02 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Mean | 0.06 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 |
| SD | 0.06 | 0.02 | 0.01 | 0.00 | 0.00 | 0.00 |
The highest concentration of melamine in fish given both melamine and cyanuric acid also occurred on day 1. The mean day 1 melamine tissue concentration in the fish given melamine alone was higher than those of fish dosed with both melamine and cyanuric acid, but the difference was only significant in catfish (9.98 vs. 2.83 mg/kg catfish, P < 0.001; 7.89 vs. 6.40 mg/kg trout, P = 0.228). Muscle concentrations of melamine in individual fish given both compounds together were below 2.5 mg/kg within 7 days following dosing for catfish and 14 days following dosing for trout.
The highest concentration of cyanuric acid, like melamine, occurred on day 1 following administration, 0.68 mg/kg in catfish, 2.59 mg/kg in trout with mean cyanuric acid concentrations declining rapidly during the first week. Mean cyanuric acid tissue concentrations of fish given both melamine and cyanuric acid were consistently less than those of fish given only cyanuric acid. Other than one trout on day 1, no fish had cyanuric acid muscle residues greater than 2.5 mg/kg at any time point.
The interaction of withdrawal day and treatment was statistically significant although the effect on different withdrawal days depended on the treatment. Table 5 lists the P-values for the pairwise comparison of the treatments on withdrawal days 1 and 3. No statistical differences were found on days 7, 14, and 28. In trout on days 1 and 3, the melamine levels were not statistically different in fish given melamine alone or the combination dose, although both differed from the controls.
| Species | Comparison | P-value | |
|---|---|---|---|
| Day 1 | Day 3 | ||
| Trout | mel-alone vs. mel-combo | 0.228 | 0.053 |
| mel-alone vs. control | <0.001* | <0.001* | |
| mel-combo vs. control | <0.001* | 0.004* | |
| cya-alone vs. cya-combo | <0.001* | 0.964 | |
| cya-alone vs. control | <0.001* | 0.959 | |
| cya-combo vs. control | 0.904 | 0.995 | |
| Catfish | mel-alone vs. mel-combo | <0.001* | 0.100 |
| mel-alone vs. control | <0.001* | <0.001* | |
| mel-combo vs. control | 0.005* | 0.054 | |
| cya-alone vs. cya-combo | <0.001* | 0.967 | |
| cya-alone vs. control | <0.001* | 0.951 | |
| cya-combo vs. control | 0.006* | 0.991 | |
- *Significant difference, P < 0.05.
- mel-alone, melamine residues following melamine-only dose; mel-combo, melamine residues following combination dose; cya-alone, cyanuric acid residues following cyanuric acid-only dose; cya-combo, cyanuric acid residues following combination dose.
Cyanuric acid residues in trout following the individual dose were only statistically different from the combination dose or the control on day 1. Cyanuric acid residues from trout given the combination dose were very low, and not significantly different from the controls even on day 1. By day 3 all the cyanuric acid levels were similar to those of the controls.
In catfish, melamine residues in fish given melamine alone or in combination with cyanuric acid were significantly different from each other and controls on day 1. By day 3, only the fish given melamine alone had melamine residues which differed statistically from the controls. Cyanuric acid residues of fish given cyanuric acid alone or in combination with melamine were significantly different from each other and controls on day 1. Cyanuric acid residues in all dosage groups on day 3 had decreased to a level similar to the controls.
The t½ for melamine was between 1 and 2 days in catfish and between 3 and 4 days in trout. Cyanuric acid t½ was less than 1 day in catfish and between 1 and 2 days in trout. In general, if both chemicals were given together the half-lives increased (Table 6).
| MEL alone | MEL with cya | CYA alone | CYA with mel | |
|---|---|---|---|---|
| Catfish | ||||
| t½ (day) | 1.51 | 1.67 | 0.32 | 0.59 |
| Kel (per day) | 0.46 | 0.41 | 2.19 | 1.18 |
| Trout | ||||
| t½ (day) | 3.62 | 4.04 | 0.71 | 1.71 |
| Kel (per day) | 0.19 | 0.17 | 0.97 | 0.41 |
Renal crystals were observed in fish given both melamine and cyanuric acid (Fig. 4). Crystals appear by day 1 in some catfish, but the prevalence and intensity were greater by 3 days postdosing (Table 7). Crystals persisted in kidneys of some of the animals that were euthanatized 4 and 6 weeks following administration of the dose.
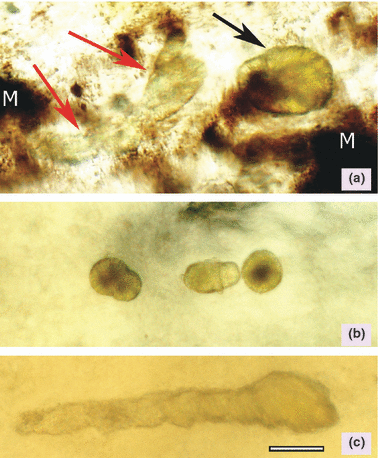
Fresh tissue preparations showing spherulite crystals in trout and catfish kidneys. (a) Trout kidney – one large crystal spherulite (black arrow) adjacent to a tubular shaped conglomeration of crystals (red arrows). Large dark melanin deposits (M) also seen in the photomicrograph are normally present in trout kidneys. (b) Catfish kidney – multiple spherulites. (c) Catfish kidney – tubular shaped conglomerate of multiple crystals. Bar = 20 μm.
| Day | Catfish n = 6 | Intensity | Trout n = 6 | Intensity |
|---|---|---|---|---|
| 1 | 3 | 1, 1, 1 | 0 | |
| 3 | 5 | 2, 2, 2, 4, 4 | 3 | 1, 2, 2 |
| 7 | 3 | 1, 2, 3 | 1 | 2 |
| 14 | 4 | 2, 2, 2, 4 | 4 | 2, 2, 2, 2 |
| 28 | 2 | 1, 3 | 1 | 2 |
| 42 | not dosed | 5 | 2, 2, 2, 2, 4 | |
| Total | 17 of 30 | 14 of 36 |
- No control fish developed renal crystals, 1 of 30 catfish given only melamine (day 3) and 2 of 30 catfish receiving only cyanuric acid developed renal crystals (days 14 and 28). Intensity: 1, extremely few (1 or 2 in an entire section); 2, few with scattered distribution; 3, moderate numbers seen throughout section; 4, large numbers seen immediately; and 5, extensive numbers obliterating the regular tissue architecture.
Discussion
There is rising concern worldwide that melamine or other triazines could enter the human food supply as a result of animal feed contamination (Bhalla et al., 2009; Kuehn, 2009). Little is known about the bioaccumulation and potential transfer of melamine and its analogs through the food chain. During both the pet food recall of 2007 and the tainted milk incident in China of 2008, there were scattered reports of feed contamination with melamine and other triazines (Luengyosluechakul, 2007, Anonymous, 2008a,c; Chen & Lifei, 2008). Without a clear understanding of the depletion patterns of melamine and cyanuric acid in edible tissues, it is difficult for public health officials to assess risks imposed by such contamination incidents.
Fish tend to excrete chemicals more slowly than mammals (Reimschuessel et al., 2005, 2007). This slower metabolism makes fish a very conservative animal model for residue depletion following feed contamination. It was for this reason, and the fact that fish feeds had been contaminated, that fish were chosen as the starting point for depletion studies in food animals.
During the 2007 pet food recall, CVM conducted a study in which fish were given high doses of melamine and cyanuric acid [400 mg/kg BW, comparable to 10 000 mg/kg (ppm) in feed] to simulate concentrations found in the contaminated pet food (Reimschuessel et al., 2008). That dose was administered for three consecutive days. In that study we were endeavoring to overwhelm the normal excretory processes to ensure that residues would accumulate in edible tissues because we needed those tissues to confirm that the chemical methods being developed would detect melamine that had been ‘processed’ by the body.
Our current study was designed to determine the depletion patterns of melamine and cyanuric acid in edible fish tissues following a single oral dose of 20 mg/kg BW (comparable to approximately 500 mg/kg melamine in feed for fish consuming about 4% of their BW). This dose was chosen because preliminary experiments in 2007 had shown that approximately half of the fish given both melamine and cyanuric acid at that 20 mg/kg BW developed renal crystals, a critical biomarker of effect for these compounds. In addition, this dose was near the lower range of reported melamine concentrations found in pet feeds associated with toxic responses (10–3200 mg/kg in feed) (Puschner et al., 2007).
We found the highest melamine or cyanuric acid concentrations occurred at the first sampling time, 1 day postdosing. In all individual fish, melamine residues were below 2.5 by day 7 in catfish and by day 14 in trout. This difference is probably because of the different metabolic rates of these two species, one being a warm water fish and the other a cold water fish. Muscle cyanuric acid levels exceeded 2.5 mg/kg in only one trout (2.59 mg/kg) 1 day postdose and dropped below 0.2 mg/kg 3 days after dosing.
The residue concentrations in edible filets of fish given melamine alone were slightly higher (means: catfish 9.98 mg/kg, trout 7.89) than those given both melamine and cyanuric acid (means: catfish 2.84 mg/kg, trout 6.40) on day 1, although this was only statistically significant for catfish. This was despite the fact that fish given both melamine and cyanuric acid were given 20 mg/kg of each compound, thus 40 mg/kg BW total triazine. Absorption may have been decreased if the triazines combined and precipitated in the intestinal tract. Moreover, as melamine-cyanurate crystals formed in the kidneys there would be less chemical available to accumulate in the muscle. The muscle t½ of the triazines, when given together, were slightly longer than if they were administered separately. This may have been due to reabsorption of some of the chemicals from crystals slowly dissolving in the kidneys.
The t½ of melamine in fish filets ranged between 1.5 days and 4 days. In fish, residue t½ in edible tissues is frequently longer than those in plasma (Reimschuessel et al., 2005, 2007;. The t½ of melamine in fish filets is certainly much longer than the t½ of melamine in mammalian plasma. Melamine is rapidly absorbed in mammals and has a t½ of approximately 3 h in rats and 4 h in pigs (Mast et al., 1983; Sugita et al., 1991; Baynes et al., 2008).
The cyanuric acid t½ in fish filets was shorter than that of melamine, ranging between 0.3 days and 1.7 days. In mammals the t½ of cyanuric acid in plasma is also shorter than that of melamine. The t½ in rats is 1–2.5 h, dogs 1.5–2 h and humans approximately 3 h (Allen et al., 1982; Barbee et al., 1983, 1984). It is difficult to make comparisons between tissue levels and plasma levels of chemical residues, but in general, the t½ of cyanuric acid was shorter than that of melamine, in fish tissues or in mammalian plasma.
Fish are cold-blooded animals that absorb and excrete chemicals more slowly than mammals (Reimschuessel et al., 2005, 2007). Therefore, the estimated t½ for melamine and cyanuric acid in fish is expected to be longer than those of terrestrial livestock animals. Further studies are recommended which assess the t½ of triazines in edible tissues of other food animals. However, as a worse-case-scenario compared with mammals, the data reported here can help risk assessors faced with potential feed contamination events.
Effects on the kidney of combined administration of melamine and cyanuric acid confirmed results from previous studies performed in our laboratory (Reimschuessel et al., 2008). Typical melamine-cyanurate crystals formed in the kidneys of many of the fish to which both triazines had been administered. More fish had crystals on day 3 than on day 1 postdosing. Renal concentrations of the chemicals may increase during the first 3 days as the compounds are cleared through the urinary tract. Once the crystals form, however, they are retained for prolonged periods, even after a one-time exposure. This is similar to what was seen in cats 8 weeks after ingesting contaminated pet feeds (Cianciolo et al., 2008).
Crystal formation within the kidney may be dependent on the concentrations of melamine and cyanuric acid within the renal tubules. Only limited information is available about melamine and other triazine levels in kidneys (Puschner et al., 2007; Reimschuessel et al., 2008) and their effect on crystal formation. Studies to determine a threshold dose for crystal formation in fish and pigs are currently being conducted in our laboratory.
Melamine-uric acid stones form in animals exposed to melamine in feed (Heck & Tyl, 1985; Ogasawara et al., 1995) and in children exposed to high levels of melamine in formula (Sun et al., 2008; Guan et al., 2009; Shen et al., 2009). There is a pressing need to develop more information regarding renal melamine concentrations during normal excretion. In addition, a more complete understanding of how melamine-uric acid stones form and the potential for intermediate crystal formation is needed. Previous animal studies used formalin to preserve renal tissues. As uric acid crystals and melamine-cyanurate crystals are soluble in formalin (Reimschuessel et al., 2008), historical information regarding renal effects and crystal formation may not be reliable. Ogasawara et al. (1995) reported that microcrystals were observed in urine sediments, but their histopathology did not report crystals in the kidneys. Future studies examining the effects of melamine and other triazines should incorporate methods such as fresh tissue wet mount examination into their methods.
In summary, we report here the concentrations of melamine and cyanuric acid in filets of catfish and trout given 20 mg/kg BW melamine, or cyanuric acid or both 20 mg/kg BW melamine and 20 mg/kg BW cyanuric acid. Filet concentrations of melamine declined to less than 2.5 mg/kg by day 7 in catfish and day 14 in trout. Renal crystals formed in fish given both melamine and cyanuric acid and some of these persisted for weeks following the initial dose. It must be noted that these results are for a single dose, while in a real world scenario contaminated feeds would result in multiple doses, presumably for a period of weeks or months. It is therefore necessary to also evaluate the effects of multiple doses over longer periods.
Acknowledgments
The authors would like to acknowledge the technical assistance on this project provide by S. Matthews, V. Mills, S. Rill, M. McDonald, N. Hasbrouck, C. Stine, and K. Elsaid.




