Pharmacokinetics of ceftazidime after intravenous, intramuscular and subcutaneous administration to dogs
Ceftazidime, a third generation cephalosporin, is active against Gram negative bacteria, as Enterobacteriaceae (Escherichia coli, Proteus spp., Klebsiella spp.) and Pseudomonas aeruginosa, and some Gram positive cocci as staphylococci and streptococci. Modifications in the pharmacokinetics of antimicrobial agents raise a major concern as successful antimicrobial treatment depends on appropriate plasma and tissue concentrations. Previous studies have determined that, for time-dependent killing antimicrobials, such as beta-lactams, the major determinant for bactericidal activity is the time for which serum drug concentrations remain above the minimum inhibitory concentration (T > MIC) for the offending pathogen (Craig, 1998; Drusano, 2004; McKellar et al., 2004). Occasionally, parenteral administrations are considered interchangeable by the veterinary practitioner. However, it is known that several factors, including route of administration, may alter the rate and extent of drug absorption and disposition. We have recently proved the lack of interchangeability of the intramuscular (i.m.) and subcutaneous (s.c.) routes of administration for cephalexin in cows (Waxman et al., 2008).
Ceftazidime is commercially available for intravenous (i.v.), i.m. and s.c. administration. Ceftazidime pharmacokinetics has been well characterized in cats (Albarellos et al., 2008), calves (Soback & Ziv, 1989), cows (Rule et al., 1996), sheep (Rule et al., 1991) and dogs (Matsui et al., 1984; Kita et al., 1992; Moore et al., 2000). However, to our knowledge, no studies have been conducted to assess T > MIC calculated after the different parenteral administrations in dogs. This study compares the pharmacokinetic behaviour and the efficacy predictor T > MIC against P. aeruginosa of a single dose ceftazidime following i.v., i.m. and s.c. administration to dogs. The aim was to determine whether these administrations would provide T > MIC values that would suggest similar clinical outcomes.
Six female beagle adult dogs (body weight 15.61 ± 5.47 kg) were included in this study. All dogs were in good health, as determined by history, physical examination, haematological and biochemical tests and urinalysis. None of the dogs had been treated with antibiotics in the previous 2 months or had a history of allergy to beta-lactams. The protocol was approved by the Institutional Animal Care and Use Committee of the Veterinary Science School, University of Buenos Aires.
Single doses of an aqueous solution of ceftazidime pentahydrate (Ceftazidima Richet, Laboratorios Richet S.A., Buenos Aires, Argentina) were administered by the cephalic vein (20 mg/kg), and by the i.m. (25 mg/kg) and s.c. (25 mg/kg) routes. Each dog received the three treatments following a crossover design with a 2-week washout period. Blood samples (2 mL) were collected from the jugular vein into heparinized tubes at 0, 0.08, 0.16, 0.33, 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 10, 12 and 24 h after ceftazidime administration. Samples were immediately centrifuged and the plasma was stored at −20 °C until assayed.
Ceftazidime plasma concentrations were determined in triplicate by a microbiological assay (Bennet et al., 1966) using Escherichia coli ATCC 25922 as test organism. Standard curves of ceftazidime were prepared in pooled canine plasma and run simultaneously with test samples. The quantification limit was 3.125 μg/mL. The correlation coefficient for the regression line of the standard solution was 0.98. The within and between-day coefficients of variation and the accuracy (bias) of the assay were <2.5%, 10% and 5%, respectively, in the range of observed concentrations (from 3.125 to 200 μg/mL).
A computer program (pcnonlin 4.0 software; SCI Software, Lexington, KY, USA) was used to calculate noncompartmental pharmacokinetic parameters from the ceftazidime plasma concentration–time curves according to classical equations (Gibaldi & Perrier, 1982). Dose-corrected bioavailability (f) was calculated for the i.m. administration as (AUCi.m/AUCi.v.) (dosei.v./dosei.m.), using similar dose- and half-life correction for the s.c. administration. T > MIC was determined graphically for the previously reported MIC90 (4 μg/mL) and MIC100 (8 μg/mL) values for P. aeruginosa of canine origin (Moore et al., 2000) and was expressed as % of the recommended dose intervals (8 and 12 h) (McKellar et al., 2004). All values are reported as mean ± standard deviation (SD). Analysis of variance (anova) and Tukey’s multiple comparison test was used for the comparison of log-transformed dose-normalized pharmacokinetic parameters (GraphPad Prism 3.0; GraphPad Software Inc., La Jolla, CA, USA). P values ≤0.05 were considered significant.
No side effects were observed after ceftazidime administration. Moderate pain was observed after the i.m. administration. Pharmacokinetic parameters calculated after the i.v., i.m. and s.c. administration are given in Table 1. Mean ceftazidime plasma concentrations vs. time curves are shown in Fig. 1. Elimination half-life and mean residence time after the s.c. administration, and area under the curve after the i.m. administration were significantly higher when compared with the i.v. administration. Bioavailability was significantly higher for the i.m. administration. Ceftazidime concentrations were measured up to 3 and 6 h (5/6 dogs) after the i.v. and i.m. administrations respectively. The s.c. route showed more inter-subject variability, as drug concentrations were present up to 4 (2/6), 8 (1/6) and 10 (3/6) h. T > MIC against P. aeruginosa calculated for the three routes of administration and expressed as % of the recommended dosing intervals are shown in Table 2.
| Parameters | i.v. | i.m. | s.c. |
|---|---|---|---|
| t max (h) | – | 1 ± 0.27ns | 1.12 ± 0.54ns |
| C max (μg/mL) | – | 80.2 ± 20.7 ns | 52.5 ± 17.5ns |
| λz | 0.710 ± 0.15 | 0.651 ± 0.18 | 0.52 ± 0.29* |
| t 1/2λ (h) | 1.02 ± 0.27 | 1.13 ± 0.29 | 1.68 ± 0.79 * |
| AUC 0–last (μg·h/mL) | 119 ± 8.19 | 202 ± 25.50* | 156 ± 47.75 |
| AUC 0–∞ (μg·h/mL) (% extrapolated) | 126 ± 9.04 (8.62 ± 1.21) | 210 ± 24.6* (3.66 ± 0.94) | 177 ± 61.1 (10.05 ± 6.39) |
| Vd(ss) (l/kg) | 0.206 ± 0.045 | – | – |
| Vd(z) (l/kg) | 0.233 ± 0.045 | – | – |
| Cl t (ml·kg/min) | 2.66 ± 0.173 | – | – |
| MRT (h) | 1.31 ± 0.31 | 2.20 ± 0.43 | 2.95 ± 1.18* |
| f (%) | – | 134 ± 18.1** | 70.3 ± 10.6 |
- t max, time to reach peak plasma concentration; Cmax, peak plasma concentration, λz, terminal rate constant; t1/2 λ, terminal half-life; AUC0–last, area under the plasma concentration time-curve from time zero to last sample point; AUC0–∞, area under the plasma concentration time-curve from time zero to infinity; Vd(z), apparent volume of distribution; Vd(ss), volume of distribution at the steady state; Clt, total body clearance; MRT, mean residence time from time zero to infinity; f, bioavailability; ns, nonsignificant. Data are presented as mean ± SD. *Significant differences (P ≤ 0.05) from i.v. administration. **Significant differences (P ≤ 0.05) between i.m. and s.c. administration.
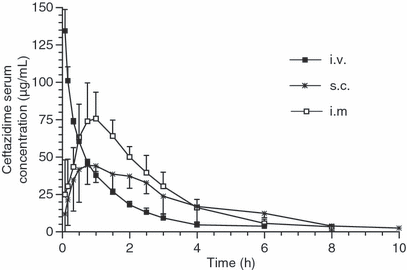
Mean (SD) ceftazidime serum concentration vs. time after intravenous (i.v.), intramuscular (i.m.) and subcutaneous (s.c.) administration of 20 (i.v.) or 25 (i.m. and s.c.) mg/kg of ceftazidime to six dogs.
| Dosing interval (h) | MIC = 4 μg/mL | MIC = 8 μg/mL | ||||
|---|---|---|---|---|---|---|
| i.v. (20 mg/kg) | i.m. (25 mg/kg) | s.c. (25 mg/kg) | i.v. (20 mg/kg) | i.m. (25 mg/kg) | s.c. (25 mg/kg) | |
| 8 | 58.3 ± 5.1 | 71.8 ± 5.1 | 83.1 ± 27.6 | 39.6 ± 5.1 | 65.6 ± 8.6 | 72.9 ± 25.9 |
| 12 | 38.7 ± 3.5 | 48.0 ± 5.1 | 55.5 ± 18.6 | 26.4 ± 3.4 | 43.7 ± 5.7 | 48.6 ± 17.2 |
- Data are expressed as percentage of the recommended dosing intervals (8 or 12 h) for MIC90 and MIC100 for Pseudomonas aeruginosa and presented as mean ± SD.
Ceftazidime was selected for this study to provide an alternative to aminoglycoside or fluoroquinolone treatments, as ototoxicity and nephrotoxicity (Riviere & Spoo, 2001), gastrointestinal and central nervous toxicity and arthropathy in young animals (Brown, 1996; Papich & Riviere, 2001), respectively, have been described for therapeutic doses of these antibiotics. Ceftazidime is active against several pathogens, however, we chose P. aeruginosa for this study because we consider ceftazidime antipseudomonal activity its major indication in veterinary medicine.
Our results are in accordance with those previously published for ceftazidime administration by the i.v. and s.c. routes to dogs (Matsui et al., 1984; Kita et al., 1992; Moore et al., 2000), however, following the i.v. administration, the elimination half-life value here reported was slightly higher than those calculated by other investigators, i.e., 0.86 h (Kita et al., 1992), 0.54 h (Moore et al., 2000) and 0.81 h (Matsui et al., 1984). Ceftazidime is eliminated predominately by the kidneys, with about 87% of the dose being excreted in the urine within 24 h of i.v. administration in dogs (Matsui et al., 1984; Kita et al., 1992) thus, differences in the renal function may account for these results. The clearance calculated in this study for ceftazidime after i.v. administration is very similar to the glomerular filtration rate reported for dogs (Moe & Heiene, 1995), suggesting that this drug is excreted mainly by glomerular filtration. In addition, previous studies demonstrated that ceftazidime does not interact with renal organic transporters (Ganapathy et al., 2000).
Volume of distribution reflected the expected distribution of β-lactam antibiotics and was in good agreement with those previously reported (Matsui et al., 1984; Kita et al., 1992; Moore et al., 2000). Ceftazidime Cmax and tmax calculated in this study for the s.c. administration are in good agreement with values previously reported after this drug was administered at 30 mg/kg (Moore et al., 2000).
The s.c. route provided higher inter-subject variability, lower mean plasma concentrations and significantly lower bioavailability than the i.v. and i.m. injections. A flip-flop phenomenon due to muscular tissue irritation may account for the high bioavailability calculated after the i.m. administration. Elimination was significantly prolonged after s.c. administration when compared with the i.v. administration.
For achieving the maximal bactericidal effect cephalosporin concentrations should be maintained for 60–70% of the dosing interval, whereas for a bacteriostatic effect cephalosporins must exceed the MIC for 35–40% of the dosing interval (Craig, 1998; Drusano, 2004; McKellar et al., 2004). Maximal bacterial killing have further been demonstrated when serum concentrations are maintained at least equal to four to six times the MIC (Manduru et al., 1997). Our results indicate that with the recommended 25 mg/kg dose, the appropriate dosing interval would be of 8 h for both i.m. and s.c. administrations, as they both showed a T > MIC value higher than 60% for most of the P. aeruginosa strains (MIC = 4 μg/mL), however, no bactericidal effect for low susceptibility strains (MIC = 8 μg/mL) would be expected.
Two limitations of this study deserve consideration. First, values of T > MIC indicating successful outcomes have been determined in rabbit, rat and mice experimental models, thus, the therapeutic outcomes obtained with these experiences may not be achieved in clinical treatments in dogs. Second, our study was conducted in healthy dogs. Altered pharmacokinetics of ceftazidime, mostly increases in volume of distribution and half-life and altered clearance, have been reported in human critically ill patients compared with healthy volunteers (Gómez et al., 1999). Despite these limitations, our study provides insight as to the interchangeability of the s.c. and i.m. ceftazidime administration when treating P. aeruginosa infections in dogs, and suggests that i.v., i.m. or s.c. ceftazidime administered at a dosage of 20 (i.v.) or 25 (i.m. and s.c.) mg/kg every 8 h should prove a useful alternative to aminoglycosides and fluoroquinolones for the empirical treatment of the majority of the P. aeruginosa infections in dogs. For successful treatment of low susceptibility P. aeruginosa infections, although, higher doses may be needed. Clinical trials must be conducted to verify these recommendations.
Acknowledgments
This study is part of the UBACYT grant of A. Monfrinotti, Proyect 010, 2005–2007. The authors wish to thank Richet S.A., Buenos Aires, Argentina, for kindly providing the ceftazidime used in this experience, and Claudio Pacheco, Georgina Brandi, Mariela Eisenacht, and Daniela Schenck for technical assistance with dogs.




