Evaluation of the interaction between ivermectin and albendazole following their combined use in lambs
Abstract
Mixtures of drugs from different chemical families have been proposed as a valid strategy to delay the development of anthelmintic resistance. The current work summarizes the outcome of the evaluation of the plasma disposition kinetics of albendazole (ABZ) and ivermectin (IVM) administered either alone or co-administered to lambs infected with gastrointestinal (GI) nematodes resistant to both anthelmintic molecules. Thirty six (36) Corriedale lambs naturally infected with multiple resistant GI nematodes were allocated into six treatment groups: (a) ABZ intravenous (ABZIV); (b) IVMIV; (c) ABZIV + IVMIV; (d) ABZ intraruminal (IR); (e) IVM subcutaneous (SC) and (f) ABZIR + IVMSC. Plasma samples were collected over 15 days post-treatment and analysed by HPLC. The estimated pharmacokinetic (PK) parameters were statistically compared using parametric and non-parametric statistical tests. The presence of IVM did not affect the plasma disposition kinetics of ABZ and its metabolites after the i.v. administration. However, the ABZ sulphoxide (ABZSO) area under the concentration vs. time curve (AUC) was significantly lower (P < 0.01) after the intraruminal (i.r.) administration of ABZ alone compared to that obtained for the combined treatment with IVM [subcutaneous (s.c.) injection]. The IVM plasma AUC obtained after its i.v. co-administration with ABZ was 88% higher (P < 0.05) compared to the treatment with IVM alone. Any marked difference on IVM PK parameters was observed between the treatments ABZ + IVM and IVM alone injected subcutaneously. The data obtained here indicate that the co-administration of ABZ and IVM does not induce an adverse kinetic interaction. This type of pharmacology-based evaluation of drug interactions is becoming highly relevant as drug combinations are now widely used as an alternative to control resistant helminth parasites in livestock.
Introduction
Nematode infection control in livestock has been largely based on the over-use of broad spectrum antiparasitic drugs. Therefore, the anthelmintic resistance of sheep and cattle nematodes is an increasing economic problem in many countries (Kaplan, 2004; Wolstenholme et al., 2004). In this context, mixtures of drugs from different chemical families have been proposed as a valid strategy to delay the development of resistance (Anderson et al., 1988).
Albendazole (ABZ) is a benzimidazole methylcarbamate anthelmintic compound effective against lungworms and gastrointestinal (GI) nematodes, tapeworms and liver flukes (Campbell, 1990; McKellar & Scott, 1990). The intrinsic anthelmintic action of benzimidazole compounds on the parasite relies on a progressive disruption of basic cell functions as a result of their binding to parasite tubulin and depolimerization of microtubules (Lacey, 1990). Ivermectin (IVM), a member of the macrocyclic lactones antiparasitic drugs, exhibits a broad-spectrum of activity against GI and lung nematodes (Benz et al., 1989) as well as against ectoparasites of domestic animals (McKellar & Benchaoui, 1996). IVM acts on ligand-gated channels, including glutamate and GABA-gated chloride channels, which participate in nematode feeding, reproduction and locomotion (Feng et al., 2002; Yates et al., 2003). In an attempt to overcome the potential of the development of resistance to the available anthelmintic chemical groups, several pharmaceutical formulations combining either two or three chemical entities have been developed. Several preparations combining benzimidazole and macrocyclic lactone compounds are available in the veterinary pharmaceutical market (i.e. ABZ + IVM + levamisole or oxfendazole + IVM + levamisole). The rationale behind using combined anthelmintic preparations is based on the fact that individual worms may have a lower degree of resistance to a multiple component formulation (each chemical with different mode of action) compared with that observed when a single anthelmintic compound is used. However, potential pharmacokinetic (PK) and/or pharmacodynamic (PD) interactions between components may occur and need to be addressed.
The most common PK interactions include enzyme induction or inhibition, competition for transporter proteins and protein binding. Evaluation of drug kinetic interactions in ruminant species is scarce. Some information on the kinetic behaviour of ivermectin and closantel co-administered to cattle has been reported (Cromie et al., 2006). However, evaluation of drug-drug kinetic interactions between benzimidazole and macrocyclic lactones has not been assessed. Potential PK interactions between co-administered anthelmintics should be understood before any practical use can be advised. This study reports the outcome of the evaluation of the plasma disposition kinetics of ABZ and IVM administered either alone or co-administered to lambs infected with GI nematodes resistant to both anthelmintic molecules. The work reported here is complementary to a clinical efficacy trial addressed to characterize the clinical efficacy of the ABZ plus IVM combination against GI resistant nematodes in lambs.
Material and methods
Animals
Thirty six (36) female Corriedale lambs (7–8 month old, 27.3 ± 4.3 kg), naturally infected with resistant GI nematodes were involved in this trial. The experimental lambs were selected from a farm where the failure of ABZ and IVM to control GI nematodes had been previously demonstrated by the fecal egg counts reduction test (FECRT). The selection of the animals was based on worm egg per gram counts (epg). On day −1, all lambs were checked for epg counts, ear tagged and the individual body weights were recorded. Experimental animals had an average of 2028 ± 1111 epg ranging from 600 to 4860. Animals were allocated in a paddock and fed on a lucerne/white and red clover pasture during the experiment and for 20 days before starting the PK study. All the animals had free access to water. Animal procedures and management protocols were approved by the Ethics Committee according to the Animal Welfare Policy (act 087/02) of the Faculty of Veterinary Medicine, Universidad Nacional del Centro de la Provincia de Buenos Aires (UNCPBA), Tandil, Argentina (http://www.vet.unicen.edu.ar).
Chemicals
Standards of ABZ, ABZ-sulphoxide (ABZSO), ABZ-sulphone (ABZSO2), and oxibendazole (OBZ) used as internal standard, were obtained from Schering Plough (Kenilworth, NJ, USA). IVM pure reference standard was purchased from Sigma Chemical Company (Saint Louis, MO, USA). The commercial formulation of ABZ was provided by Pfizer Animal Health, Argentina (Valbazen®, 10% suspension) and IVM by Merial, Argentina (Ivomec®, 1% injectable solution). ABZ solution for intravenous injection was prepared as a 2% (w/v) solution in propylene glycol/dimethyl sulphoxide (80/20) (Anedra, Buenos Aires, Argentina).
Experimental design, treatments and sampling
All parasitized lambs were randomly allocated into six experimental groups (n = 6). Experimental animals received the following treatments: ABZIV: ABZ (2% solution) intravenously (i.v.) administered at a dose rate of 3.8 mg/kg; IVMIV: IVM (Ivomec®, Merial, 1% solution) administered by the i.v. route at 0.2 mg/kg; ABZIV + IVMIV: ABZ (2% solution, 3.8 mg/kg) plus IVM (Ivomec®, Merial, 1% solution, 0.2 mg/kg), both administered by the i.v. route (two consecutive injections); ABZIR: ABZ (Valbazen®, Pfizer, 10% suspension) intraruminally (i.r.) administered at a dose rate of 3.8 mg/kg; IVMSC: IVM (Ivomec®, Merial, 1% solution) subcutaneously (s.c.) administered at the dose rate of 0.2 mg/kg; IVMSC + ABZIR: ABZ (Valbazen®, Pfizer, 10% suspension, 3.8 mg/kg) plus IVM (Ivomec®, Merial, 1% solution, 0.2 mg/kg), administered by the i.r. and s.c. routes, respectively.
Blood samples were taken from the jugular vein before administration (time 0) and at 5, 15 and 30 min, 1, 3, 6, 9, 12, 24 and 48 h, and 4, 6 and 10 days following the i.v. treatments. After the i.r. or s.c. treatments, jugular blood samples were collected before administration (time 0) and at 1, 3, 6, 9, 12, 18, 24, 36, 48 h and at 4, 6, 10 and 15 days post-treatment. In all the experimental groups, blood samples were collected using 10 mL heparinised Vacutainers® tubes (Becton Dickinson, NJ, USA). Plasma was separated by centrifugation at 2000 g for 15 min, placed into plastic tubes and frozen at −20 °C until analysis by high performance liquid chromatography (HPLC).
Analytical procedures
ABZ/metabolites analysis
Sample clean up: ABZ, ABZSO and ABZSO2 were extracted using disposable C18 columns (RP-18, 100 mg, Strata®, Phenomenex, CA, USA). Ten (10) μL of OBZ (50 μg/mL) was added to 500 μL of plasma in a glass test tube. Spiked samples were placed into a C18 column (preconditioned with 0.5 mL of methanol followed by 0.5 mL water) in a vacuum system (Lichrolut®, Merck, Germany). Samples were washed (2 mL of water) and then eluted with 2 mL of HPLC grade methanol. After elution, all samples were concentrated to dryness in a vacuum concentrator (Speed-Vac®, Savant, MN, USA) and then reconstituted with 300 μL of mobile phase.
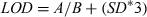
IVM analysis
Sample clean up and derivatization: The extraction of IVM (22,23 dehydro-avermectin B1a), from spiked and experimental plasma samples was carried out following the well established technique (Alvinerie et al., 1993; slightly modified by Lifschitz et al., 1999). Basically, 1 mL-aliquot of plasma sample was combined with 10 ng of the internal standard compound (abamectin) and then mixed with 1 mL of acetonitrile-water (4:1). After mixing for 20 min, the solvent-sample mixture was centrifuged at 2000 g during 15 min. The supernatant was manually transferred into a tube that was then placed on the appropriate rack of an Aspec XL sample processor (Gilson, Villiers Le Bel, France). The supernatant was injected to a Supelclean LC18 cartridge (Supelco, Bellfonte, PA, USA), previously conditioned by passing 2 mL methanol and 2 mL deionized water. The cartridge was flushed with 1 mL of water and 1 mL of water/methanol (4:1). The compounds were eluted with 1.5 mL of methanol and concentrated to dryness under a stream of nitrogen. The re-suspension was carried out with 100 μL of a solution of N-methylimidazole (Sigma Chemical, St. Louis, MO, USA) in acetonitrile (1:1) (De Montigny et al., 1990). Derivatization was initiated by adding 150 μL of trifluoroacetic anhydride (Sigma Chemical, St Louis, MO, USA) solution in acetonitrile (1:2). After completion of the reaction (<30 sec), an aliquot (100 μL) of this solution was injected directly into the chromatograph.
HPLC analysis: IVM concentrations were determined by HPLC using a Shimadzu 10 A HPLC system with autosampler (Shimadzu Corporation, Kyoto, Japan). HPLC analysis was undertaken using a reverse phase C18 column (Phenomenex, 5 μm, 4.6 mm × 250 mm) and an acetic acid 0.2% in water/methanol/acetonitrile (3.8/40/56.2) mobile phase at a flow rate of 1.5 mL/min at 30 °C. IVM was detected using a fluorescence detector (Shimadzu, RF-10 Spectrofluorometric detector, Kyoto, Japan), readings at 365 nm (excitation wavelength) and 475 nm (emission wavelength). IVM concentrations were determined by the internal standard method using the Class LC 10 Software version 1.2 (Shimadzu Corporation, Kyoto, Japan) on an IBM compatible AT computer. The peak area ratios were considered to calculate the IVM concentrations in spiked (validation) and experimental plasma samples. There was no interference of endogenous compounds in the chromatographic determinations. The solvents (Baker, Phillipsburg, NJ, USA) used during the extraction and drug analysis were HPLC grade. A complete validation of the analytical procedures used for extraction and quantification of IVM was performed before starting analysis of the experimental samples obtained during the PK trial. Calibration curves in the range between 0.1–5 ng/mL and 5–100 ng/mL were prepared for each compound. Calibration curves were established using least squares linear regression analysis and correlation coefficients (r) and CV calculated. Linearity was established to determine the IVM concentrations/detector responses relationship. Percentages of IVM recovery from plasma were obtained in the range between 0.1 and 50 ng/mL. The inter-assay precision of the extraction and chromatography procedures was estimated by processing replicate aliquots (n = 6) of pooled sheep plasma samples containing known IVM concentrations (0.2, 10 and 50 ng/mL) on different working days. The LOD and LOQ were calculated similarly as described for ABZ/metabolites. Concentration values below the LOQ were not considered for the kinetic analysis of experimental data. The linear regression lines for IVM analyzed showed correlation coefficients ≥ to 0.998. The mean recovery of IVM from plasma was in a range between 75 and 80%. The inter assay precision of the analytical procedures obtained after HPLC analysis of IVM on different working days showed CV between 3.26 and 7.71%. The LOQ was established at 0.1 ng/mL.
Pharmacokinetic analysis of the data
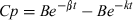
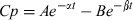
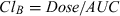
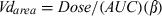
Statistical analysis of the data
The PK parameters and concentration data are reported as arithmetic mean ± SD; however, the half-lives (formation and elimination) and MRT, are presented as harmonic means ± SD. Parametric (Student′s t-test) and/or nonparametric (Mann-Whitney and Wilcoxon) tests were used for the statistical comparison of the PK data obtained from the different experimental groups. A value of P < 0.05 was considered statistically significant. The statistical analysis was performed using the Instat 3.0 Software (Graph Pad Software, CA, USA).
Results
The mean (± SD) plasma concentrations of ABZ (a) and ABZSO (b) after the i.v. administration of ABZ either alone or co-administered with IVM are shown in Fig. 1. Table 1 summarizes the plasma PK parameters for ABZ and ABZSO obtained after the i.v. administration of ABZ either alone (ABZIV) or co-administered with IVM (ABZIV + IVMIV) to parasitized lambs. After its i.v. administration, ABZ was detected in plasma up to 3 h post-treatment. Its main metabolic product found in plasma (ABZSO) was detected between 5 min and 24 h post-treatment. The sulphone metabolite reached a Cmax of 0.35 ± 0.08 (ABZIV) and 0.32 ± 0.10 (ABZIV + IVMIV) μg/mL at 9.5 and 10 h post-treatment, respectively. The presence of IVM did not affect the plasma disposition kinetics of ABZ/metabolites (Table 1) after the i.v. administration.
Comparative mean (±SD) plasma concentration profiles (n = 6) for (a) albendazole (ABZ), and (b) ABZ-sulphoxide (ABZSO), after the administration of ABZ either alone (3.8 mg/kg) or co-administered with ivermectin (0.2 mg/kg), both given by the intravenous route to parasitized lambs.
| Pharmacokinetic parameters | (ABZIV) | (ABZIV + IVMIV) | ||
|---|---|---|---|---|
| ABZ | ABZSO | ABZ | ABZSO | |
| C0 (μg/mL) | 5.08 ± 2.40 | – | 6.60 ± 4.55 | – |
| Cmax (μg/mL) | – | 3.22 ± 0.71 | – | 2.96 ± 0.64 |
| T½for (h)* | – | 0.10 ± 0.08 | – | 0.14 ± 0.04 |
| AUC0-t (μg·h/mL) | 1.70 ± 0.68 | 30.2 ± 5.31 | 2.03 ± 1.10 | 33.9 ± 6.65 |
| T½el (h)* | 0.29 ± 0.83 | 7.34 ± 0.95 | 0.32 ± 0.38 | 9.31 ± 1.64† |
| MRT (h)* | 0.39 ± 0.60 | 10.4 ± 1.45 | 0.41 ± 0.19 | 13.2 ± 2.45 |
| Vdarea (L/kg) | 1.44 ± 1.16 | – | 1.20 ± 0.51 | – |
| ClB (L/h/kg) | 2.28 ± 0.89 | – | 2.20 ± 1.02 | – |
- *Time-based parameters calculated as harmonic means. †Different from the ABZIV treatment at P < 0.05.
- C0, concentration at time 0; Cmax, peak plasma concentration; T½for, metabolite formation half life; AUC0-t, area under the plasma concentration vs. time curve from 0 to the detection time; T½el, elimination half-life; MRT, mean residence time (obtained by non-compartmental analysis of the data); Vdarea, apparent volume of distribution (area method); ClB, total body clearance.
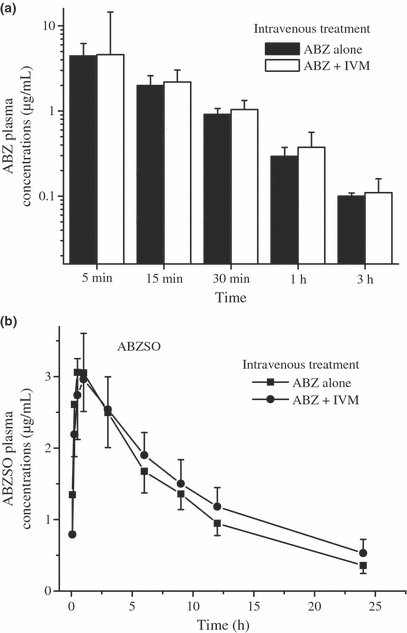
The plasma concentration profiles of IVM observed after its i.v. administration alone or co-administered with ABZ in lambs are shown in Fig. 2. The PK parameters obtained for IVM in lambs, after its administration by the i.v. or s.c. route, either alone or co-administered with ABZ, are shown in Table 2. After the i.v. administration, IVM plasma levels were measured up to 10 days post-treatment. Conversely to that observed for ABZ-related metabolites, the plasma disposition kinetics of IVM was modified by the presence of ABZ/metabolites after the i.v. treatment. A significantly (P < 0.05) higher AUC value was obtained for the ABZIV + IVMIV treatment (AUC = 210.3 ± 80.6 ng·day/mL), compared to that observed in the IVMIV group (AUC = 112.3 ± 37.4 ng·day/mL). No statistical differences between both treatments were observed among other PK parameters, including C0, T½ el, MRT, ClB and Vdarea (Table 2).
Comparative mean (±SD) plasma concentration profiles (n = 6) for ivermectin (IVM) obtained after its administration (0.2 mg/kg) either alone or co-administered with albendazole (ABZ, 3.8 mg/kg), both given by the intravenous route to parasitized lambs.
| Pharmacokinetic parameters | IVM treatments | |||
|---|---|---|---|---|
| (IVMIV) | (IVMIV + ABZIV) | (IVMSC) | (IVMSC + ABZIR) | |
| C0 (ng/mL) | 281.2 ± 32.6 | 314.2 ± 94.8 | – | – |
| Cmax (ng/mL) | – | – | 21.3 ± 13.3 | 18.2 ± 5.71 |
| Tmax (days) | – | – | 2.80 ± 1.5 | 1.80 ± 1.20 |
| AUC0-t (ng·day/mL) | 112.3 ± 37.4 | 210.3 ± 80.6† | 131.1 ± 70.5 | 139.7 ± 28.6 |
| T½el (days)* | 1.35 ± 0.19 | 1.45 ± 0.42 | 2.67 ± 1.21 | 4.15 ± 1.11 |
| MRT (days)* | 1.25 ± 0.33 | 1.51 ± 0.36 | 5.38 ± 1.19 | 6.64 ± 1.26 |
| Vdarea (L/kg) | 3.85 ± 1.30 | 2.44 ± 1.20 | – | – |
| ClB (L/day/kg) | 1.97 ± 0.72 | 1.15 ± 0.69 | – | – |
- *Time-based parameters calculated as harmonic means. †Different from the IVMIV treatment at P < 0.05.
- C0, concentration at time 0; Cmax, peak plasma concentration; T½for, metabolite formation half life; AUC0-t, area under the plasma concentration vs. time curve from 0 to the detection time; T½el, elimination half-life; MRT, mean residence time (obtained by non-compartmental analysis of the data); Vdarea, apparent volume of distribution (area method); ClB, total body clearance.
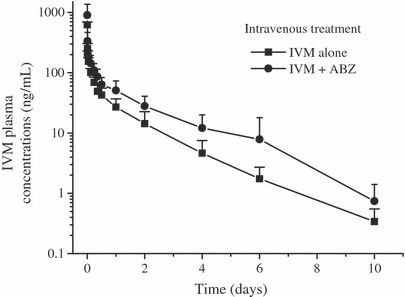
The comparative mean ABZSO and IVM plasma concentrations profiles measured after the administration of ABZ (i.r.) alone, IVM (s.c.) alone or after their co-administration to lambs are shown in 3, 4. The plasma PK parameters obtained for ABZSO and ABZSO2 after the i.r. administration of ABZ to lambs alone or co-administered with IVM are shown in Table 3. ABZ administered by the i.r. route did not affect the plasma PK behavior of IVM administered by the s.c. route. However, the presence of IVM induced changes in the plasma levels of ABZSO. The ABZSO AUC value increased 42% after administration of the combination ABZIR + IVMSC (19.8 ± 2.55 μg·h/mL), compared to the treatment with ABZIR alone (28.2 ± 3.72 μg·h/mL). Furthermore, the MRT and T½for values obtained for ABZSO2 resulted longer after the ABZIR + IVMSC treatment compared to ABZIR administration (Table 3).
Comparative mean (±SD) plasma concentration profiles (n = 6) for albendazole sulphoxide (ABZSO) obtained after the administration of albendazole (ABZ) by the intraruminal route (3.8 mg/kg) either alone or co-administered with ivermectin (IVM) given by the subcutaneous route (0.2 mg/kg) to parasitized lambs.
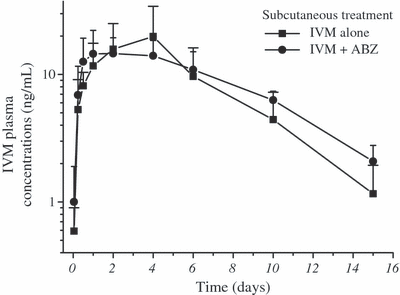
Comparative mean (±SD) plasma concentration profiles (n = 6) for ivermectin (IVM) obtained after its subcutaneous administration (0.2 mg/kg) either alone or co-administered with albendazole (ABZ) given by the intraruminal route (3.8 mg/kg) to parasitized lambs.
| Pharmacokinetic parameters | ABZSO | ABZSO2 | ||
|---|---|---|---|---|
| (ABZIR) | (ABZIR + IVMSC) | (ABZIR) | (ABZIR + IVMSC) | |
| T½for (h)* | 2.25 ± 1.33 | 4.30 ± 0.54 | 1.88 ± 0.58 | 2.69 ± 0.44† |
| Cmax (μg/mL) | 1.08 ± 0.22 | 1.22 ± 0.37 | 0.39 ± 0.23 | 0.33 ± 0.10 |
| Tmax (h) | 9.50 ± 2.26 | 14.0 ± 5.90 | 24.0 ± 0.00 | 30.0 ± 6.57 |
| AUC0-t (μg·h/mL) | 19.8 ± 2.55 | 28.2 ± 3.72† | 6.25 ± 2.75 | 7.88 ± 1.92 |
| T½el (h)* | 14.1 ± 7.30 | 9.82 ± 3.22 | 1.93 ± 0.65 | 2.73 ± 0.50 |
| MRT (h)* | 27.1 ± 9.13 | 20.9 ± 3.76 | 20.1 ± 0.97 | 25.7 ± 2.15† |
- *Time-based parameters calculated as harmonic means. †Different from the ABZIR treatment at P < 0.05.
- T½for, metabolite formation half life; Cmax, peak plasma concentration; Tmax, time to the Cmax; AUC0-t, area under the plasma concentration vs. time curve from 0 to the detection time; T½el, elimination half-life; MRT, mean residence time (obtained by non-compartmental analysis of the data).
Discussion
The interaction between co-administered drugs may induce changes on the PK behavior of either molecule. The overall disposition kinetics and pattern of tissue residues are often modified when drug-drug interactions occur. Both a PK and/or a PD interaction may be established following the combined use of ABZ and IVM in ruminant species. The kinetic assessment of a potential interaction undertaken in the current work is useful to characterize the pharmacological basis of the combination of both anthelmintic compounds. ABZ was rapidly depleted from the bloodstream following its i.v. administration to lambs, being detected up to only 3 h post-treatment. The metabolites ABZSO (active metabolite) and ABZSO2 (inactive metabolite) appeared rapidly (5 min) in the bloodstream after the i.v. administration of ABZ. This disposition pattern agrees with those previously reported in sheep (Galtier et al., 1991; Alvarez et al., 1999), after the intravascular administration of ABZ. A rapid and efficient microsomal biotransformation, and an extensive ABZ tissue distribution (Vd = 1.44 ± 1.16 L/kg, ABZIV), account for the observed fast depletion from the bloodstream. IVM was detected in the bloodstream up to 10 days after its i.v. administration. This extended IVM plasma detection is related to its high lipophilicity and affinity for fatty tissues, low metabolism and extensive enterohepatic recycling, which prolong the organic elimination of the drug (Lanusse et al., 1997; Lifschitz et al., 2000). IVM is excreted in high concentration in the bile of sheep (Bogan & McKellar, 1988), which constitutes the primary route of excretion for this compound. The estimated Vd for IVM after its i.v. administration was 3.85 ± 1.30 L/kg (IVMIV), which correlates with its well characterized extensive tissue distribution (Lifschitz et al., 2000). Low metabolic rate and extended enterohepatic recycling account for the low total body clearance (1.97 ± 0.72 L/day/kg) obtained for IVM intravenously injected to lambs.
No significant PK changes were observed for ABZ/metabolites after its i.v. co-administration with IVM in lambs. Only the ABZSO T½el resulted significantly (P < 0.05) longer after the co-administration of ABZIV + IVMIV compared with that observed after the i.v. administration of ABZ alone (Table 1). Nevertheless, the observed 26% longer T½ el does not seem to have any clinical relevance (Entrocasso et al., in press). Conversely, an increase on IVM plasma concentrations was observed in the presence of ABZ, after the i.v. co-administration of both compounds. The enhanced IVM plasma concentrations observed after the i.v. co-administration of both anthelmintics resulted in an increase in the AUC value (87%). No other changes on the PK disposition after the i.v. administration were observed.
Consistently with kinetic data previously obtained in sheep (Marriner & Bogan, 1980; Hennessy et al., 1989; Lanusse et al., 1995), ABZSO and ABZSO2 were the main metabolites recovered in plasma after the i.r. administration of ABZ, which has been related to a first-pass oxidation occurring mainly in the liver. ABZSO was the main metabolite recovered in plasma for a period of 36 h (Fig. 3) post-treatment, accounting for either 76 (ABZIR) or 78% (ABZIR. + IVMSC) of the total analytes found in sheep plasma after the i.r. administration of ABZ. After s.c. administration of IVM to lambs, a Cmax value of 21.3 ± 13.3 ng/mL was obtained at 2.8 ± 1.5 days post-treatment (IVMSC) (Table 2). These results agree with that previously reported for IVM administered by the s.c. route to sheep (Marriner et al., 1987; Imperiale et al., 2004). Unlike the observations after the i.v. treatments, the presence of IVM (s.c. injection) modified the plasma PK behavior of ABZSO and ABZSO2. The ABZSO plasma AUC was significantly (P < 0.05) higher (>42%) following the ABZ + IVM parenteral co-administration compared to that observed after ABZ i.r. alone treatment (Table 3). Additionally, longer MRT and T½ for values were observed for ABZSO2 in the presence of IVM (Table 3). Oppositely, ABZ did not modify the plasma disposition of IVM after their co-administration by the i.r. and s.c. routes, respectively.
From the results obtained in the current trial, it is evident that some type of PK interaction between ABZ and IVM occurs after their coadministration to lambs. This interaction determines that a) higher IVM plasma concentrations were measured after its co-administration with ABZ (both administered by the i.v. route) and, b) higher ABZSO plasma availability was obtained after the i.r. administration of ABZ co-administered with IVM given subcutaneously. Although it is unclear at which level ABZ/metabolites and IVM may interact, two possible explanations would help to explain the observed PK changes: 1) IVM-induced inhibition of ABZ metabolism, or 2) drug to drug interaction via drug efflux transporter-mediated mechanisms.
Albendazole is extensively metabolized in the sheep liver microsomal fraction; the flavin-mono-oxygenase (FMO) (Galtier et al., 1986) and cytochrome P-450 (CYP) enzymatic systems (Souhaili-El Amri et al., 1987) are primarily involved in ABZ sulphoxidation to ABZSO. Recently, it has been demonstrated that FMO-mediated sulphoxidation accounted for up to 60% of ABZSO production from ABZ, whilst the CYP contributed with 40% in sheep liver microsomes (Virkel et al., 2004). A relatively high CYP3A activity (measured as 6ß-tetosterone hydrolase activity) was described in ovine liver microsomes (Szotákováet al., 2004). Since CYP3A is the most important drug-metabolising CYP, its involvement on ABZ metabolism could be expected. In fact, it has been suggested that CYP 3A4 may be the key contributor to ABZSO formation (Rawden et al., 2000). On the other hand, ABZSO undergoes a second irreversible oxidative step (sulphonation) which forms the inactive ABZSO2 metabolite (Galtier et al., 1991), in which CYP1A is mainly involved (Souhaili-El Amri et al., 1988; Delatour et al., 1991). A low rate of IVM metabolism under in vitro conditions has been reported using rat liver microsomes (Zeng et al., 1996). It is estimated that only a 8.5% of the total parent drug detected in cattle plasma is related to IVM metabolites (Lanusse et al., 1997). The main enzymatic system involved on IVM metabolism includes CYP3A and 1A in rats (Zeng et al., 1996) and CYP3A4 in humans (Zeng et al., 1998).
The co-administration of fenbendazole with piperonyl butoxide (PB), a potent inhibitor of the CYP mediated oxidation, in sheep and goats, markedly affected the PK disposition of fenbendazole/metabolites, potentiating their nematodicidal activity against benzimidazole-resistant strains of Teladorsagia circumcincta (Benchaoui & McKellar, 1996). Similarly, methimazole a metabolic inhibitor of FMO, potentiates the efficacy of netobimin (pro-drug of ABZ) against mixed GI nematodes in cattle (Lanusse & Prichard, 1992). It could be hypothesized that in the current trial IVM may have interfered with both, ABZ sulphoxidation and ABZSO sulphonation by mean of a CYP 3A and 1A competition/inhibition. Such a possible metabolic interaction between both anthelmintic drugs does not seem to achieve great practical relevance.
Other possible explanation for the observed ABZ and IVM PK interaction could be based in a drug-drug interaction via a transporter/s mediated drug efflux mechanism. As part of the ABC superfamily, P-glycoprotein (Pgp) is a transmembrane protein located in the apical side of cells that participate in the ATP-dependant efflux of a broad range of structurally and functionally unrelated compounds out of the cell (Gerlach et al., 1986). Pgp is physiologically expressed in different organs/tissues including adrenals, kidneys, liver (biliary canalicular surface of hepatocytes), small intestine (epithelial cells) and blood brain barrier (Balayssac et al., 2005). Pgp plays a key role in the protection of the organism against ingested toxins and contribute to the biliar, urinary and intestinal elimination of different unrelated compounds. IVM is largely excreted in bile and feces as the parent drug, with less than 2% excreted in the urine (Chiu et al., 1990). IVM has been described as a specific Pgp substrate (Didier & Loor, 1996; Pouliot et al., 1997) which is actively secreted from the rat intestine (Laffont et al., 2002). Besides, the co-administration of IVM with loperamide (a Pgp modulating-agent) resulted in changes on the pattern of bile-fecal excretion, which accounted for an enhanced IVM availability in rats (Lifschitz et al., 2004). Recently, IVM was proposed as a multidrug resistance protein 1 and 2 (MRP 1 and 2) substrate (Lespine et al., 2006). Additionally, it is known that ABZSO is actively secreted into the intestinal lumen (Redondo et al., 1999), probably because of a combination of passive diffusion and active transport. Pgp, MRP2 and the breast cancer resistance protein (BCRP) have been proposed as the main candidate proteins involved on ABZSO intestinal efflux transport (Merino et al., 2003). A highly efficient transport of ABZSO by murine BCRP1 has been described (Merino et al., 2005). In contrast, ABZ parent drug does not seem to interact with Pgp (Merino et al., 2002, 2005; Dupuy et al., 2006), MRP2 or BCRP1 (Merino et al., 2005).
ABZ-sulphoxide is mainly eliminated by urine, although biliar excretion (free and conjugated) in sheep has been described (Hennessy et al., 1989; Alvarez et al., 1999). In fact, high intestinal concentrations of ABZSO were detected in sheep (Alvarez et al., 1999) and cattle (Lanusse et al., 1993) after the i.r. administration of ABZ or netobimin, respectively. The relative involvement of the biliary and intestinal excretion mechanisms for ABZ metabolites and IVM in sheep needs to be elucidated. However, since both compounds (at least ABZSO and IVM) have been indicated as Pgp substrates, an interaction at this level should be considered. IVM competition on ABZSO efflux (at intestinal or biliar level) may help to explain the significantly higher ABZSO AUC value observed in lambs treated with ABZIR + IVMSC. There was no change in the ABZSO plasma concentrations after the intravenous co-administration of IVM and ABZ. In agreement with our findings, similar results were reported by Merino et al. (2003), using verapamil (a Pgp inhibitor), which did not have a clear effect on the ABZSO intestinal elimination after their i.v. co-administration in rats; however, after the oral administration of ABZ plus verapamil, a significantly increase on ABZSO availability was observed both in rats and sheep.
The existence of a synergistic effect of metabolic enzymes and efflux transporters at the intestinal level has been proposed (Suzuki & Sugiyama, 2000). The CYP system and Pgp may act synergistically in reducing the bioavailability of their substrates after oral administration. In the case of ABZ, after being taken up by the enterocytes, some of the parent drug suffers a CYP-mediated (mainly CYP3A or CYP1A) first intestinal oxidative step. The ABZSO formed may be eliminated from the cell into the intestinal lumen via Pgp. Drug molecules (ABZ or ABZSO) which escape intestinal metabolic conversion or efflux, would be absorbed via the portal vein system. The effect of IVM on either ABZ metabolism or ABZSO efflux in the intestine after the i.r. administration of ABZ, may enhance the ABZ/ABZSO concentrations reaching the liver. A similar interaction between ABZ and IVM (both administered orally) has been suggested (Merino et al., 2003), where enhanced ABZSO concentration was attributed to a combined effect on metabolism and drug efflux transporter interactions. Since no significant differences in the plasma disposition of ABZ metabolites were observed after the i.v. combined treatment, an interaction at the intestinal level between ABZ and IVM would be more important after i.r. administration of ABZ, when intestinal absorption is critical for the resultant ABZSO systemic availability. Finally, it is likely that other transporter protein (s) different to Pgp may be involved on ABZ efflux at the intestinal wall. If this should be the case, any IVM-mediated efflux competition could result in enhanced ABZ systemic absorption. Further in vivo/in vitro investigation is necessary to confirm interactions between antiparasitic compounds and cellular transporter proteins in ruminant species. Some follow up work on the assessment of the potential PD interaction of IVM and ABZ (clinical efficacy implications) are currently undergoing in our lab. This type of pharmacology-based evaluation of drug interactions are becoming highly relevant since drug combinations are now widely used as an alternative to control resistant helminth parasites in livestock.
Acknowledgments
This work was partially supported by Fundación Antorchas, (Project 14248-127), CONICET (PIP 6489), and Agencia Nacional de Promoción Científica y Técnica (ANPCyT) (PICT 08-13763), all from Argentina. The authors appreciate the collaboration of Ing. Solanet (INTA Balcarce) on the animal phase of the reported trial.




