Serotonin: a review
Abstract
5-Hydroxytryptamine, or serotonin, is a biogenic amine most noted for its role as a neurotransmitter. Manipulation of serotonin in animal models was used as a tool for studying its role in humans. Through such research serotonin has been shown to modulate gastrointestinal motility, peripheral vascular tone, cerebral vascular tone, and platelet function and has been implicated in the pathophysiology of mood disorders, emesis, migraine, irritable bowel syndrome (IBS), and pulmonary and systemic hypertension. The knowledge gained is being directly applied back to animals in research on drugs that manipulate the serotonergic system in dogs and cats. Increasing use and availability of drugs that manipulate the serotonergic system has created a circumstance through which a novel toxicity was discovered in both humans and animals. Serotonin Syndrome describes the clinical picture seen in humans and animals with serotonin toxicity. This paper provides a review the physiology of serotonin and its involvement in the pathophysiologic mechanisms of various conditions, including the Serotonin Syndrome.
Introduction
Serotonin is a molecule with diverse effects in the central nervous system as well as in the periphery. It acts as a hormone, a neurotransmitter, and a mitogen and is ubiquitous in the animal kingdom. Despite its ubiquitous nature, the importance of serotonin in health and disease has been under recognized in veterinary medicine. This is in sharp contrast to the situation in human medicine where the topic has been heavily studied for over half a century. Most of the original studies investigating serotonin were carried out in animal models, which implies its relevance in veterinary physiology.
Serotonin, otherwise known as 5-hydroxytryptamine (5-HT), was isolated and characterized in 1948 by Maurice Rapport and Irvine Page (Rapport et al., 1948a–c). The isolation of serotonin came after decades of investigation to characterize a vasoconstrictor substance that was suspected to be contained in platelets (Janeway et al., 1918; Reid & Bick, 1942; Zucker, 1944). It was named serotonin after the Latin word serum and the Greek word tonic. In 1937, Italian scientist Vittorio Erspamer extracted a substance from enterochromaffin cells in the gastrointestinal tract that was responsible for causing smooth muscle contraction which he named enteramine (Erspamer & Asero, 1952). In 1952, he demonstrated that enteramine and serotonin were the same (Reid & Rand, 1952). Experiments using the newly discovered indolalkylamine showed that it caused contraction of a variety of smooth muscle strips such as carotid artery, jejunum, uterus, and nictitating membranes (Zucker, 1944; Reid & Rand, 1952). The variety of animal models used included sheep, ox, rabbits, rats, cats, and dogs (Janeway et al., 1918; Reid & Bick, 1942; Zucker, 1944).
An early curiosity was the ability of serum to better induce smooth muscle contraction when compared to plasma (Janeway et al., 1918; Zucker, 1944). This supported the notion of serotonin being stored and released during the clotting process rather than being free in circulation. Janeway et al. in 1918 proposed that the vasoconstrictor substance may be contained in platelets. It was demonstrated that circulating serotonin was carried primarily in platelets in many species of animals (Humphrey & Jaques, 1954) and was suspected to play a role in hemostasis, which would not be elucidated for another decade.
After the original discovery of serotonin, many laboratories were conducting parallel studies identifying the localization of serotonin along with investigating its function. Serotonin was quickly identified as being present in many tissues including brain, lung, kidney, platelets, and the gastrointestinal tract. Around the same time its role in platelet function was being investigated, Brodie & Shore (1957) proposed the role of serotonin as a neurotransmitter. This was based on studies that demonstrated the localization of 5-HT receptors to specific areas of the vertebrate brain (Twarog & Page, 1953; Amin et al., 1954). It was further elucidated that serotonin was principally located in the nerve endings of neurons in isolated portions of the mammalian brain (Michaelson & Whittaker, 1963; Zieher & DeRobertis, 1963). Dahlstrom & Fuxe (1964) were the first to map specific nuclei in the brain that contained serotonin. These clusters of neurons became known as the serotonergic system (Dahlstrom & Fuxe, 1964). Serotonin was only the third neurotransmitter to have been discovered at the time and but then has been linked to a variety of central nervous system functions such as mood, behavior, sleep cycles, and appetite.
It was evident early on that serotonin was an important chemical in the vertebrate system and since its discovery research in the field has ballooned. In this review, we will present what is currently known about the role of serotonin in normal physiology and serotonin involvement in the pathogenesis of certain diseases such as migraine, irritable bowel syndrome (IBS), systemic, and pulmonary hypertension. We hope to demonstrate that much of what is known about serotonin physiology in health and disease has been based on animal models. Furthermore, we will review the emerging problem of serotonin toxicity known as serotonin syndrome in humans and animals.
Origin and metabolism of serotonin
Serotonin is a biogenic monoamine, similar to epinephrine, norepinephrine (NET), dopamine (DAT), and histamine. Serotonin is produced in two steps. The essential amino acid tryptophan is hydroxlyated to 5-hydroxytryptophan (5-HTP) by tryptophan hydroxylase. In a second step 5-HTP is decarboxylated to form 5-HT (Fig. 1; Clark et al., 1954). Early pharmacologic studies demonstrated that hydroxylation and decarboxylation occur almost instantaneously in the presence of tryptophan (Clark et al., 1954).
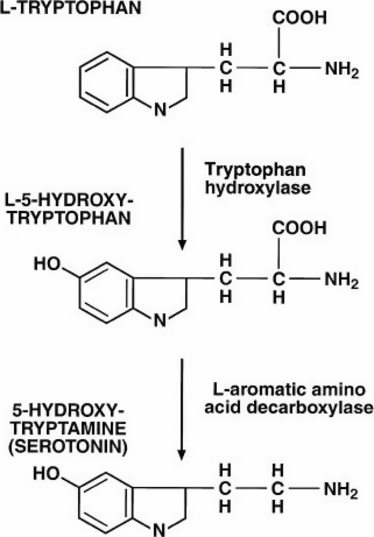
Conversion of tryptophan to 5-hydroxytryptamine (serotonin). With permission from Gwaltney-Brant et al., 2000.
While both enzymes are necessary for the conversion of tryptophan to serotonin, tryptophan hydroxylase is considered the rate-limiting enzyme for several reasons. Tryptophan hydroxylase has a relatively high Km (3 × 10−5 m; Tong & Kaufman, 1975), the enzyme has little affinity for other amino acids (Noguchi et al., 1973), and its distribution is limited to those tissues containing serotonin (Noguchi et al., 1973; Tyce, 1990; Champier et al., 1997).
The specific activity of tryptophan hydroxylase is in contrast to the nonspecific enzymatic activity of tryptophan decarboxylase. Tryptophan decarboxylase has affinity for many l-amino acids. Tryptophan decarboxylase, like tryptophan hydroxylase has a relatively high Km, between 8 × 10−6 m and 8 × 10−4 m, and is present in most tissues (Clark et al., 1954; Tyce, 1990). As it is not the limiting factor in serotonin synthesis, it is difficult to reduce serotonin levels by inhibiting this enzyme.
Interestingly, the conversion of tryptophan into serotonin only accounts for 5% of the total metabolism of tryptophan (Tyce, 1990). This can be explained by a few factors; the localization of tryptophan hydroxylase solely to the brain, enterochromaffin cells and, to a much lesser extent, platelets and enzymatic conversion of dietary tryptophan to kynurenine by tryptophan pyrrolase in the liver which accounts for about 95% of tryptophan metabolism (Tyce, 1990).
Within the central nervous system (CNS), serotonin is synthesized and stored in the presynaptic neurons (serotonergic neurons, pineal gland, and catecholaminergic neurons). Serotonin is located in nine groups of cell bodies isolated to the pons and midbrain (Dahlstrom & Fuxe, 1964). The raphe nuclei represent the major nuclei with both ascending serotonergic fibers projecting to the forebrain and descending fibers that extend to the medulla and spinal cord (Dahlstrom & Fuxe, 1964). A small number of serotonergic nuclei are also present in reticular formation with fibers that remain locally within the medulla (Dahlstrom & Fuxe, 1964).
Serotonin synthesis outside the CNS is limited to enterochromaffin cells and to a lesser extent platelets. Platelets may have very little ability to produce serotonin, however, platelets represent a major storage site for serotonin outside the CNS. It was recognized early on that platelets readily take up serotonin from plasma, leaving very little circulating in plasma (Toh, 1954; Hardisty & Stacey, 1955).
About 90–95% of the body’s serotonin is located in the periphery, mostly stored in platelets and enterochromaffin cells. Ninety-nine percent of total body serotonin is located intracellulary, implying a tight regulation of serotonin. The concentration of serotonin in tissues is dependent upon the rate of synthesis and the rate of metabolism (Tyce, 1990). Metabolism by monoamine oxidase (MAO) is the primary metabolic pathway for serotonin (McIsaac & Page, 1959) as well as many other biogenic amines including NET, DAT, and histamine. MAO is a ubiquitous enzyme that exists in two major forms, MAO-A and MAO-B. Serotonin is primarily inactivated by MAO-A (Sandler et al., 1981). Metabolism by MAO-B represents a small portion of serotonin metabolism and is predominant form of MAO in human platelets (Sandler et al., 1981). McIsaac & Page (1959) demonstrated that the major metabolite of MAO metabolism of serotonin is 5-hydroxyindoleacetic acid (5HIAA) which is excreted primarily in the urine. It was shown that the majority of 5HIAA excretion occurred within 24 h of exogenous serotonin administration, suggesting rapid metabolism by MAO. Major sites of MAO activity include the brain, gastrointestinal tract, lungs, liver, and platelets (Tyce, 1990). Although metabolism occurs very rapidly, storage protects serotonin against metabolism. Glucuronidation and sulfation represent minor metabolic pathways for serotonin and occurs in the liver, lung, kidney, and brain (Tyce, 1990).
In the CNS serotonin is processed in several ways. Upon neuronal depolarization, serotonin is released into the synaptic cleft. It can bind to postsynaptic serotonin receptors (5-HT receptors) or serotonin autoreceptors on the presynaptic membrane (Cerrito & Raiteri, 1979). Binding of serotonin to the autoreceptor acts as a negative feedback against further release of serotonin into the synaptic cleft (Cerrito & Raiteri, 1979). The highly selective serotonin transporter (SERT) located on the presynaptic membrane is responsible for removing serotonin from the synaptic cleft (Fig. 2). Once transported into the presynpatic neuron, serotonin is recycled back into presynaptic vesicles where it is protected from metabolism. Metabolism by MAO occurs within the cytosol of the neuron. An alternative pathway for serotonin in the pineal gland is the conversion to melatonin.
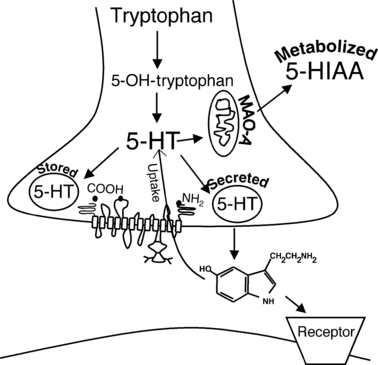
Depiction of serotonergic synapse and handling of 5-HT from synthesis, storage, release, uptake via SERT and metabolism. With permission from Ni & Watts, 2003.
Serotonin that originates from enterochromaffin cells is released into portal circulation and is quickly eliminated from the plasma via uptake into platelets and metabolism by the liver. Serotonin transporters located on the platelet membrane and enterochromaffin cells are responsible for uptake into those cells. Serotonin that escapes uptake and liver metabolism reaches the lung where is it then metabolized (Tyce, 1990).
The 5-HT receptor families
The diverse effects of serotonin are mediated via 5-HT receptors. The 5-HT receptor is a phylogenetically ancient receptor that evolved over 750 million years ago and is present in the lowest of invertebrates as well as the higher mammals (Peroutka & Howell, 1994). The three major subtypes 5-HT1, 5-HT2, and 5-HT3 are only 25% homologous which suggests that divergence of the receptor itself occurred at least 700 million years ago (Peroutka & Howell, 1994). The 5-HT receptors have been identified and cloned in fruit flies, mollusks, round worms, rodents, rabbits, dogs, cats, and humans (Van Den Berg et al., 2003).
Radio-ligand binding studies using receptor-specific agonists and antagonists have enabled the characterization of multiple 5-HT receptors. The nomenclature has evolved into an accepted classification which recognizes seven subtypes of serotonin receptors (5-HT1–7; Hoyer et al., 1994). Most subtypes exhibit heterogeneity and are further divided into 5-HT1A, 5-HT1B, etc. Six of these subtypes involve G-protein-coupled receptors. The 5-HT3 receptor is unique in that it involves a ligand-gated Na+/K+ ion channel similar to gamma-aminobutyric acid (GABA), and N-methyl-d-aspartic acid (Derkach et al., 1989).
5-HT1 and 5-HT5 receptors are negatively coupled with adenylyl cyclase; activation of these receptors downregulates cyclic AMP. 5-HT2 receptor upregulates the inositol triphosphate and diacylglycerol pathways resulting in intracellular Ca2+ release. 5-HT4 and 5-HT7 increase cAMP activity. The Na+/K+ cation channel associated with 5-HT3 results in plasma membrane depolarization (Derkach et al., 1989; Kandel, 2001). Table 1 summarizes the different 5-HT receptor families based on location, function and important receptor agonists and antagonists (Katzung, 2001).
| Receptor subtype | Location/function | Agonist | Antagonist |
|---|---|---|---|
| 5-HT1A | CNS: neuronal inhibition, behavioral effects (sleep, feeding, thermoregulation, and anxiety) | Buspirone* | Yohimbine* |
| 5-HT1B | CNS: presynaptic inhibition, behavioral effectsVascular: pulmonary vasoconstriction | Yohimbine* | |
| 5-HTm | CNS: locomotionVascular: cerebral vasoconstriction | Sumatriptan | Yohimbine* |
| 5-HT2A | CNS: neuronal excitation, behavioral effects, and learning;Smooth muscle: contraction, vasoconstriction/dilatationPlatelets: aggregation | KetanserinCyprohepatdine*Chlorpromazine*LSD | |
| 5-HT2B | Stomach fundus | Chlorpromazine*Yohimbine* | |
| 5-HT2C | CNS: choroid plexus, CSF secretion | ||
| 5-HT3 | Sensory and enteric nerves, emesis | Metoclopramide*Ondasetron*Dolasetron* | |
| 5-HT4 | CNS and myenteric neurons, GI motility | Metoclopramide*Cisapride* | |
| 5-HT5A | CNS: function unknown | ||
| 5-HT6 | CNS: function unknown | ||
| 5-HT7 | CNS, blood vessels, GI tract: function unknown |
- *Drugs that are used off label in veterinary patients.
Serotonin transporter
There are a few important factors that determine the strength and duration of signaling on the postsynaptic serotonin receptor. Abundance of serotonin in the synaptic cleft is the major determinant of its effects. The two mechanisms directly involved in controlling the availability of serotonin in the synaptic cleft are binding of serotonin to its autoreceptor and the activity of the SERT both of which are located on the presynaptic membrane. The negative feedback created by stimulation of the 5-HT autoreceptor decreases further release of serotonin, while the SERT actually removes serotonin from the synaptic cleft. The SERT is a member of a more general class of monoamine transporters. There are specific transporters for DAT and NET. Although these transporters are highly selective for their respective substrates there is some affinity for the other monoamines (Torres et al., 2003; Ni & Watts, 2006).
All monoamine transporters, including SERT, are 12 transmembrane spanning sodium-dependent transporters. The basic mechanism is the transportation of Na+, Cl− and the substrate intracellularly in exchange for K+ (Sneddon, 1973; Torres et al., 2003; Ni & Watts, 2006). SERT has been identified in the CNS, gastrointestinal tract, pulmonary and peripheral vasculature, and platelets. This is the mechanism by which serotonin is taken up by platelets and enterochromaffin cells.
Serotonin in the central nervous system
The functions of serotonin in the CNS are very broad and are related to the action of the serotonergic system on the forebrain, brainstem, and cerebellum. Projections from the rostral nuclei of this system help regulate temperature, appetite, sleep cycles, emesis, and sexual behavior. Projections from caudal nuclei participate in nociception and motor tone. The most clinically relevant aspect has been the role of serotonin in psychological disorders in humans. An evolution of theories regarding the role of biogenic amines in the pathophysiology of psychological disorders led to the consensus that depression, mania, and anxiety disorders are associated with decreased availability of serotonin in the CNS (Kandel, 2001). Although the earliest antidepressants, the MAO inhibitors, and tricyclic antidepressants (TCA), enhanced availability of serotonin as well as other biogenic amines such as DAT and NET, the drugs aimed specifically at altering serotonin levels were considered the most effective.
The most potent and specific antidepressant is the class of drugs known as the selective serotonin reuptake inhibitors (SSRI). These drugs bind specifically to the SERT, increasing availability of serotonin at the synaptic junction for receptor binding. This is in contrast to other SSRIs, such as TCAs, which have varying affinity for DAT and NET transporters as well. SSRIs also have the added effect of inhibiting the presynaptic autoreceptor further enhancing availability of serotonin in the synaptic cleft (Owens, 1996).
The veterinary community can greatly benefit from what has been learned in the human field. Although psychological disorders are less recognized in companion animals, several disorders have been characterized in both dogs and cat. Obsessive compulsive disorder and anxiety disorders have been documented in companion animals. Some examples include storm phobias and separation anxiety in dogs, and inappropriate elimination in cats. Several studies have demonstrated that treatment of these patients with conventional antidepressants such as SSRIs, TCAs can be successful (Pryor et al., 2001; Overall & Dunham, 2002; Crowell-Davis et al., 2003; Hart et al., 2005; Frank et al., 2006).
Recently, several serotonergic agents have been approved by the FDA for treatment of separation anxiety in dogs. Clomipramine (ClomicalmTM; Novartis Animal Health US, Inc., Greensboro, NC, USA), a TCA, and fluoxetine (ReconcileTM; Eli Lilly, Indianapolis, IN, USA), an SSRI, are used in combination with a behavioral program (King et al., 2000; Simpson et al., 2007).
Platelet aggregation
Several research groups demonstrated that serotonin induced platelet aggregation (Mitchell & Sharp, 1964; O’Brien, 1964; Hilton & Cumings, 1971); however, the exact mechanism was not understood. Other substances being studied at the time included ADP, thrombin, and NET (O’Brien, 1964). 5-HT receptors on platelet membranes were characterized in many species of animal. Cerrito et al. demonstrated that serotonin alone is a weak stimulator of platelet aggregation; however, its effects are greatly potentiated in the presence of ADP and Thromboxane A, two other substances released during platelet activation. They concluded that serotonin plays an important role in the amplification of platelet aggregation, by recruiting additional platelets once platelet aggregation has been initiated. Their study as well as others have shown that this latter effect is mediated via 5-HT2 receptor located on the platelet surface. Furthermore, platelet aggregation can be almost entirely blocked with addition of 5-HT2-specific antagonists (DeClerk, 1990; Noble & Drake-Holland, 1990; Cerrito et al., 1993).
Vascular tone
The original intent of investigating serotonin was for its vasoconstrictor effects. Early studies in dogs established a triphasic response to serotonin when injected i.v.: (i) an initial fall in arterial blood pressure with bradycardia followed immediately by (ii) a pressor response, and finally (iii) another fall in blood pressure (Page & McCubbin, 1953). This can, in part, be explained by the receptors involved as well as the activity of the vascular endothelium at the time of serotonin exposure.
With few exceptions, isolated vascular smooth muscle strips contract when exposed to serotonin. This effect is mediated by 5-HT2 receptors on the vascular smooth muscle surface. In vivo, serotonin released from activated platelets can induce vasoconstriction in most large arteries, large veins, and venules. Serotonin also indirectly contributes to vasoconstriction by amplifying the contractile response of other vasoactive substances such as NET, angiotensin II, and histamine (Vanhoutte, 1987).
Vasodilatory effects on the arterial tree are most prominent at the arteriolar level and in a few isolated larger vessels. Smooth muscle relaxation is due to stimulation of the 5-HT1 receptor and resultant release of nitric oxide from the endothelium. Additionally, activation of inhibitory prejunctional 5-HT1 receptors on smooth muscle adrenergic nerve endings reduces the release of NET (Vanhoutte, 1987). Vasodilation on the venous side is thought to be mediated by 5-HT3 receptors, but the importance of this mechanism is unclear.
Central serotonergic neurons also contribute to peripheral vasomotor tone via sympathetic outflow mainly through 5-HT1 and 5-HT2 receptors and are involved in blood pressure regulation (Skop & Brown, 1996).
As previously mentioned the health and activity of the endothelium is a determining factor in how serotonin affects vascular smooth muscle. The endothelium is the source of two important products, nitric oxide and MAO-A. Vascular disease results in phenotypic changes to the endothelium such as increased cell adhesion, fat deposition, decreased NO release, and decreased MAO activity. NO is not only released as a result of 5-HT1 stimulation, but also acts as a counterregulatory mechanism against serotonin’s natural vasoconstrictor effects (Vanhoutte, 1990). In basic terms the lack of NO may dampen vasodilatory response to serotonin predisposing to vasocontriction. Decreased MAO activity may prolongs serotonin’s effects and even intensify it. From a clinical perspective, vascular disease not only sensitizes patients to serotonin but may enhance its ill effects, namely, systemic hypertension.
Hypertension
Like control of vasomotor tone, the role of serotonin as a regulator of peripheral blood pressure is not straightforward. The simplest explanation is that the pressor effects of serotonin are both central and peripheral in origin. Experimentally, serotonin injected directly into the CNS has been reported to cause both hypertension and hypotension. This was attributed to the distribution of serotongeric neurons to areas of the brain which produce divergent responses (Saxena et al., 1987).
Early experiments with specific 5-HT receptor antagonists indicated that 5-HT1-like antagonism as well as 5-HT2 antagonism corrected hypertension in animal models. Several mechanisms were proposed to explain this including direct vasodilation, inhibition of adrenergic input, and stimulation of central areas contributing to correction of vasomotor tone (Saxena et al., 1987). There are few clinically relevant agonists and antagonists for manipulating vascular tone. Most drugs are for research purposes only. And, many clinically relevant 5-HT2 and 5-HT1 antagonists such as cyproheptadine and propanolol have proven to be ineffective antagonists at peripheral 5-HT receptor sites. However, one notable exception is ketanserin. Ketanserin is a 5-HT2 antagonist also causes α1-adrenoreceptor blockade and reliably results in brief resolution of naturally occurring and experimentally induced hypertension. In fact, the hemodynamic effects of ketanserin are remarkably similar to those seen with administration of prazosin, a well known α1-adrenergic antagonist (Bolt & Saxena, 1985). This likely explains why specific 5-HT antagonists have no clinical effect on hypertension.
The possible involvement of adrenergic receptors in hypertension further clouds the exact role of serotonin in the development of essential hypertension. However, the effect of serotonin on a hypertensive vascular bed has been well documented. A hypertensive vascular bed is markedly more sensitive to the vasoconstrictive effects of serotonin when compared to a normal vascular bed. The threshold for the constrictor effect of serotonin is lower, the dose–response curve is steeper and the maximal response is much greater in hypertensive animals than normotensive ones. Additionally, there is decreased inhibition of adrenergic neurons which contributes to vasoconstriction (Saxena et al., 1987).
Pulmonary hypertension
The discovery of the serotonin receptors led scientists to believe that serotonin can only exert its actions through these receptors. However, a unique mechanism of action was discovered in the cardiopulmonary system. Serotonin has mitogenic activity on aortic and pulmonary arterial smooth muscle cells, inducing hypertrophy and proliferation (Torres et al., 2003; Ni & Watts, 2006). There was speculation that SERT was important in this mechanism. Interest grew after the popular diet drug fenfluramine/phentermine, which increases SERT activity, caused serious (sometimes fatal) cardiovascular disease, including pulmonary hypertension (Belohlavkova et al., 2001). The drug was subsequently taken off of the market. Several studies have since demonstrated the necessity of uptake of serotonin by the transporter in mediating the mitogenic effects on pulmonary arterial smooth muscle (Eddahibi et al., 2000; Marcos et al., 2003). This research refuted the decades old dogma that serotonin only exerted its action via receptors and that the singular role of the transporter was to recycle serotonin.
There has already been much progress in the treatment of pulmonary arterial hypertension through the manipulation of nitric oxide pathways with phosphodiesterase inhibitors. The manipulation of SERT is proving to be a novel approach in the management of pulmonary arterial hypertension. Knockout mice lacking the transporter have been shown to be relatively resistant to hypoxia-induced pulmonary hypertension while those that overexpress SERT develop pulmonary arterial smooth muscle hypertrophy (Marcos et al., 2003; Ni & Watts, 2006). Identification of abnormal serotonin receptor expression may also help identify those at risk for developing the disease. In mice and people overexpression of 5-HT2B in the pulmonary arterial tree is associated with the development of pulmonary hypertension (O’Callaghan & Gaine, 2007). It is possible that new information or drugs emerging from such research could potentially help veterinarians in the management of pulmonary hypertension in dogs.
Intestinal motility and emesis
Gastrointestinal motor and sensory functions have been shown to be regulated by neurotransmitters such as catecholamines, DAT, and serotonin. The enteric nervous system (ENS) utilizes sympathetic and parasympathetic afferent and efferent pathways for bidirectional communication between the gut and the brain.
The release of serotonin from enterochromaffin cells is stimulated by mechanical and neural input in the intestinal tract. Release of serotonin initiates peristaltic and secretory reflexes via 5-HT receptors associated with primary afferent neurons. These primary afferent neurons synapse in the myenteric plexus. The terminal result is a coordinated stimulation of ascending cholinergic neurons which cause circular muscle contraction and descending cholinergic neurons which cause muscle relaxation. Grider et al. (1998) demonstrated the role of 5-HT4 receptor ascending contraction and descending relaxation in the jejunum of humans, guinea pigs, and rat jejunum. 5-HT3 has also been shown to be involved in motility and secretions. Just as in the CNS nerve terminals, the SERT removes serotonin from gastrointestinal interstitium, thereby terminating its actions (Crowell, 2004).
There are several important agonists and antagonists that have been utilized in human and veterinary medicine for their effects on motility. Cisapride is a 5-HT4 agonist that is not readily used in humans because of its arrhythmogenic effects, however, it is routinely used off label in rabbits and guinea pigs. A 2001 study compared the potential arrhythmogenic activity of cisapride and mosapride, another 5-HT4 agonist. It was demonstrated that cisapride, albeit at supratherapeutic doses, resulted in a prolonged QT interval in rats, guinea pigs, and cats, whereas mosapride was shown to have very little arrhythmogenic potential (Kii et al.,2001).
Metoclopramide is also a 5-HT4 agonist. However, it is classified as a selective agonist because appears to have more effect on upper GI motility (esophageal sphincter, stomach, and duodenum) than colonic motility (De Winter et al., 1999). A recent study has speculated that its prokinetic effects may not be as potent as previously thought. Wilson et al. (2006) demonstrated that metoclopramide can be effective at reducing gastroesophageal reflux, but at much higher doses than routinely used. Their study was performed on anesthetized dogs, so perhaps a consideration needs to be made on how pharmacokinetics and receptor–ligand interaction change in that patient population. Cisapride is thought to be a stronger prokinetic than metoclopramide and has more effect on colonic movement than metoclopramide.
An important class of drugs that would have antagonist effects on 5-HT4 receptors would be anticholinergics. Their concurrent use with 5-HT4 agonists is generally contraindicated.
Abnormalities in previously described gut–brain–gut bidirectional communication have been the topic of much interest in the last decade. Irritable bowel syndrome is one such intestinal disorder whose etiopathogenesis lies in abnormal 5-HT signaling. One hypothesis is that the SERT is deficient on enterocytes of people with IBS. An additional hypothesis points to decreased numbers of enterochromaffin cells in the GI tract of people with IBS (Crowell, 2004).
Emesis is a complex process which involves peripheral and central input coordinated with somatic and visceral motor output. The main components of the emesis reflex include a portion of the area postrema known as the chemoreceptor trigger zone (CRTZ), the poorly defined vomit center located in the lateral reticular formation and visceral and somatic motor nuclei (Andrews et al., 1988).
It used to be thought that the main mediators of emesis were histamine, catecholamines, and DAT (Hesketh & Gandara, 1991). However, blockade of these receptors only partially abated emesis. In an effort to find a powerful antiemetic to combat chemotherapy-induced emesis, it was discovered that 5-HT receptors played a central role in the triggering of emesis. Studies demonstrated 5-HT3 receptors are abundant in the area postrema and receptor antagonism inhibited the vomit reflex (Higgins et al., 1989). 5-HT3 mediates many, but not all, triggers for emesis. Several 5-HT3 antagonists are widely used today in both human and veterinary medicine. Examples of strong 5-HT3 antagonists are ondansetron and dolasetron.
Metoclopramide was originally marketed as a DAT receptor (D2) antagonist. At low doses it was used with success for many triggers for emesis. Only at higher doses it was shown to be an effective antiemetic against chemotherapy-induced emesis. This is due to weak 5-HT3 antagonism at higher doses (Hesketh & Gandara, 1991; Andrews et al., 1998).
Serotonin toxicity
Therapeutic manipulation of transport, binding, or metabolism of serotonin will result in clinically significant increases in serotonin levels. Usually this results in a desired effect such as in the treatment of depression with drugs such as SSRIs, MAO-inhibitors, or TCAs. However, excess serotonin can lead to a potentially life-threatening condition described as the serotonin syndrome, or serotonin toxicity.
Serotonin toxicity as a clinical syndrome was first noted in the 1960s during studies involving a single or combination therapy of antidepressants. Side effects such as euphoria, hyperreflexia, and myoclonus were observed in human subjects. The incidence of toxicity and severity of signs increased when MAO inhibitors were given concurrently with either a TCA or l-tryptophan, the most severe cases resulting in death. Parallel studies carried out in animal models supported the development of specific clinical signs including tremors, rigidity, myoclonus, generalized seizure, hyperthermia, salivation, and sometimes death (Sternbach, 1991; Brown et al., 1996).
The past decades have seen the development of newer, more potent antidepressants. The overall incidence of serotonin toxicity paralleled the increased use and availability of these drugs (Boyer & Shannon, 2005). The increase in both the number of human cases and the evidence of the danger of serotonin toxicity necessitated the development of diagnostic criteria to enable physicians to recognize the syndrome.
Diagnosis
Harvey Sternbach, a neuropsychiatrist from UCLA, was the first to publish diagnostic criteria for serotonin toxicity and propose an etiopathogenesis for the condition. The original diagnostic criteria included a patient being on one or more serotonergic drugs, the presence of three of 10 specific clinical signs, and ruling out possibility of metabolic disease, drug withdrawal, or the addition of a neuroleptic agent (Table 2; Sternbach, 1991). This algorithm has evolved over the last two decades. And, today there are several different diagnostic criteria that are in use (Dunkley et al., 2003; Boyer & Shannon, 2005). They differ primarily in the order of importance of specific clinical signs such as myoclonus, hyperthermia, and GI signs. In human patients, unlike veterinary patients, subtle differences in regional muscle activity (e.g. sustained vs. inducible myoclonus) can be more easily assessed. These subtleties can distinguish between serotonin toxicity and other differentials such as anticholinergic poisoning, malignant hyperthermia, and neuroleptic malignant syndrome.
| A. Coincident with the addition of or increase in a known serotonergic agent to an established medication regimen, at least three of the following clinical features are present |
| a. Mental status change |
| b. Agitation |
| c. Myoclonus |
| d. Hyperreflexia |
| e. Diaphoresis |
| f. Shivering |
| g. Tremor |
| h. Diarrhea |
| i. Incoordination |
| j. Fever |
| B. Other etiologies have been ruled out |
| C. A neuroleptic agent has not been started or increased in dosage prior to the onset of the signs and clinical signs listed above |
Serotonin toxicity is now thought of as a triad of clinical signs consisting of autonomic hyperactivity (diarrhea, mydriasis, and tachycardia), neuromuscular signs (hyperreflexia, myoclonus, tremors, and rigidity) and altered mental status (Dunkley et al., 2003; Boyer & Shannon, 2005). Human literature stresses that there is a spectrum of clinical signs ranging from very mild (just diarrhea) to severe including hypertension, hyperthermia, and death (Dunkley et al., 2003; Boyer & Shannon, 2005). This is the reasoning for advocating the term toxicity rather than syndrome.
Sternbach’s original criteria recognized circumstances in which certain individuals would be predisposed to developing serotonin toxicity. These predisposing factors could include inherited or acquired MAO dysfunction, liver disease, and cardiovascular disease (Sternbach, 1991).
Pathogenesis
Serotonin toxicity is mostly an iatrogenic condition. Naturally occurring serotonin toxicity can be seen with some types of carcinoid tumors, as these tumors can produce large quantities of serotonin. However, these tumors are uncommon in humans and more rare, but still reported, in dogs (Morrell et al., 2002). The pathogenesis of serotonin toxicity is more commonly the overdose of a single serotonergic drug or the synergism of multiple drugs that act to influence serotonin levels.
Serotonergic drugs act to increase serotonin levels by a variety of mechanism. The main categories include drugs that increase serotonin production, increase serotonin release from storage, decrease serotonin metabolism, and decrease serotonin reuptake. Table 3 shows drugs that are used in humans and veterinary patients and their serotonergic mechanism. Some of these have been implicated in serotonin toxicity while others, like selegiline, have the potential to cause toxicity, although there are no reported cases.
| Increased serotonin production |
| l-Tryptophan |
| Increased serotonin release |
| Amphetamine |
| Bromocriptine |
| l-dopa |
| Selective serotonin reuptake inhibitor |
| Fluoxetine*† (Reconcile®) |
| Paroxetine |
| Sertraline |
| Citalopram |
| Fluvoxamine |
| Impaired reuptake of serotonin |
| Dextromethorphan£ |
| Meperidine |
| Tricyclic antidepressants |
| Amitriptyline† (Elavil®; Astra-Zeneca PLC, Wilmington, DE, USA) |
| Clomipramine*† (Clomicalm®) |
| Tramadol£ |
| Chlorpheniramine†£ |
| Inhibition of serotonin metabolism |
| MAO-A inhibitor |
| MAO-B inhibitor |
| Selegiline* (Anipryl®; Pfizer Animal Health, Exton, PA, USA) |
- MAO, monoamine oxidase. *Drugs that are EDA approved for use in dogs; †Drugs that are used off label in cats; £ Drugs that are used off label in dogs.
Current theory of serotonin toxicity involves stimulation of central 5-HT1A and 5-HT2A receptors as the main mediators of clinical signs (Sternbach, 1991; Brown et al., 1996; Dunkley et al., 2003; Boyer & Shannon, 2005). The peripheral 5-HT receptors most likely do contribute to the clinical signs seen in toxicity, however, their importance is poorly understood. The distribution of 5-HT receptors in the GI tract and blood vessels likely accounts for peripheral signs such as gut hypermotility and vasoconstriction. Although SSRIs do decrease the ability of platelets to uptake serotonin (resulting in decreased platelet concentrations of serotonin), thrombocytopathia has not been documented. There are a few case reports of spontaneous hemorrhage in patients using an SSRI, however, the relevance of coagulopathy in serotonin toxicity is not known (Ottervanger et al., 1994; Hergovich et al., 2000).
The severity of clinical signs is dose related but may also be affected by individual factors such as intrinsic MAO activity and vascular disease. A damaged vascular endothelium may not have the normal NO activity which would otherwise acts as a counterregulatory mechanism against the vasoconstrictive effect serotonin. Decreased MAO activity associated with vascular disease may also decrease the ability to metabolize serotonin, prolonging its effects. From a clinical perspective, vascular disease may sensitize a patient to developing serotonin toxicity. For example, there are several case reports of people with known vascular disease being treated with an SSRI that developed serotonin toxicity after taking an over-the-counter cold medicine containing dextromethorphan (Skop et al., 1994). It is possible that a person with a healthy endothelium would not develop toxicity given the same combination of medications.
Serotonin toxicity in veterinary medicine
Serotonin toxicity has been documented in laboratory animals as well as companion animals (Gwaltney-Brant et al., 2000). The source for ingestion is usually the owner’s medication or medication(s) prescribed by a veterinarian. As physicians rely on history and clinical signs for an accurate diagnosis of serotonin toxicity, the same can be said for diagnosis of toxicity in companion animals.
The Animal Poison Control Center (APCC) of the American Society for the Prevention of Cruelty to Animals (ASPCA) has been collecting data on intoxications involving serotonergic drugs over the last decade. The first comprehensive case series from 2000 describes 21 dogs that ingested 5-HTP, a precursor of 5-HT, in the form of an herbal supplement (Gwaltney-Brant et al., 2000). The 19 dogs that developed clinical toxicoses exhibited the triad of clinical signs as described in the human literature. Mental alteration was usually accompanied by autonomic signs such as hyperthermia, diarrhea, mydriasis, tachycardia, and hypersalivation. Neuromuscular signs included tremors, ataxia, hyperreflexia, and seizures. Onset from ingestion to clinical signs was 10 min to 4 h. Three dogs died and the two necropsies performed did not yield any significant findings that could be directly attributable to serotonin toxicity. Much like in human cases, treatment resulted in a quick recovery for the surviving dogs; the longest time to clinical sign resolution was 36 h (Gwaltney-Brant et al., 2000).
The APCC has compiled data regarding other single agent toxicoses involving serotonergic drugs in dogs (Tables 4 & 5). The most common signs seen with SSRIs were lethargy, vomiting, mydriasis, and agitation. Of the 189 cases of SSRI intoxications, two deaths and two euthanasias were reported. Knowledge of the toxic dose is important as severity of clinical signs exhibits a dose-dependent relationship. According to APCC data minor signs such as salivation and lethargy were noted at doses between 1 and 3 mg/kg while more severe signs such as tremors and hyperthermia were seen at doses higher than 8 mg/kg. The most serious intoxications involved doses over 25 mg/kg and resulted in seizure activity.
| SSRIs (189 cases) |
| Lethargy 31.7% |
| Vomiting 12.2% |
| Mydriasis 11.6% |
| Agitation 11.1% |
| Hyperactive 9.5% |
| Ataxia 9.5% |
| Depression 8.7% |
| Tachycardia 8.5% |
| Tremors 7.9% |
| Vocalization 6.8% |
| Somnolence 6.3% |
| Hypersalivation 6.3% |
| Anorexia 5.2% |
| Hyperthermia 3.7% |
| Tachypnea 3.7% |
| Restlessness 3.7% |
| Seizure 3.2% |
| Hyperesthesia 2.6% |
| Disorientation 2.0% |
| MAO-I (4 cases) |
| Restlessness (75%) |
| Ataxia (50%) |
| Disorientation (25%) |
| Seizure (25%) |
| Tachypnea (25%) |
| Tremors (25%) |
| St John’s Wort (4 cases) |
| Depression (75%) |
| Vomiting (25%) |
| Tachycardia (25%) |
| Polydipsia (25%) |
| Vomiting (25%) |
| Sertraline (Zoloft®) |
| 3 mg/kg; lethargy, mydriasis, tachycardia |
| 6 mg/kg hyperactivity, vocalization (while others will still be sedate) |
| 8 mg/kg tremors |
| Seizures at doses >25 mg/kg |
| Fluoxetine (Prozac®) |
| <8 mg/kg primarily drooling, lethargy, vomiting |
| >10 mg/kg tremors, tachycardia, agitation, hyperthermia |
| Seizures at doses >25 mg/kg |
| Paroxetine (Paxil®) |
| 1–3 mg/kg vomiting, drooling, lethargy |
| >3 mg/kg agitation and seizures |
| <4 mg/kg sustained release product, sedation, drooling, vomiting |
| >4.5 mg/kg agitation |
| No seizures reported from the sustained release |
There is no clinical data regarding multidrug therapy inducing serotonin toxicity in veterinary patients but this is an increasing concern. As serotonergic agents, such as TCAs and SSRIs, become more commonly used to treat pet behavior problems, the possibility of serotonin toxicity becomes more likely. Although the package insert for Reconcile warns against concurrent use of fluoxetine with SSRIs, TCAs, and MAOIs (including selegiline and amitraz), and suggests conservative wash-out periods, practitioners, or clients may unwittingly co-administer drugs in these classes (Reconcile® package insert, Eli Lilly, Indianapolis, IN, USA). This may result in serotonin toxicity.
Potentially dangerous interactions could occur if more than one drug in the SSRI or TCA family is used concurrently or failing to wait long enough after discontinuing one medication before starting another. It is clear the most dangerous situation involves the use of two SSRIs or an SSRI and a TCA with an MAO inhibitor. But there are other less well recognized interactions that could also be potentially harmful. Selegiline is an MAO-B inhibitor that is approved for use in dogs (Simpson & Simpson, 1996). It is marketed under the trade name Anipryl and is used mostly for canine cognitive dysfunction but has gained popularity for use in some dogs with hyperadrenocorticism. Although there is only a little activity of MAO-B for serotonin, selegiline has been implicated in serotonin toxicity in humans on concurrent SSRI therapy (Garay et al., 2002).
Buspirone is mostly used in cats with inappropriate elimination issues and although there are no reports of serotonin toxicity in cats, clinicians should be aware this drug is a partial 5-HT1A agonist (Eison et al., 1986; Trulson & Arasteh, 1986).
The emerging area of concern is opioids and the possibility of inducing serotonin toxicity. Experimental data with opioid drugs indicates that the phenylpiperidine class of opioids (meperidine, fentanyl, and congeners), tramadol, and dextromethorphan are all weak SSRIs (Gillman, 2005). And, some evidence suggests that Tramadol may also act as a serotonin releaser (Bamigbade et al.,1997). All of these drugs have been implicated in human cases of serotonin toxicity when used concurrently with an SSRI or TCA (Table 3; Skop et al., 1994; Kitson & Carr, 2005). Morphine and its analogs, however, have not been shown to cause serotonin reuptake inhibition and most likely cannot participate in toxicity (Codd et al.,1995; Gillman, 2005).
An appropriate clinical question might address the use of fentanyl or tramadol in a patient that is on a TCA or SSRI. The possibility exists that the patient could develop signs of serotonin toxicity, because one of the two drugs is a potent serotonergic drug. However, the use of tramadol and fentanyl together without other drugs may be a low risk combination given the minor serotonergic effect of each drug. Clinicians should be aware, however, that the appearance of diarrhea after starting multidrug therapy could be an indication of minor serotonin excess.
Hepatic cytochrome (CYP) P450 pathways may play a role in the development of serotonin toxicity. Most drugs are metabolized by one of the many isoforms of cytochrome P450. Although well identified in humans, CYP 450 isoforms have not been well characterized in dogs and cats. However, drugs such as ketoconazole and cimetidine, that inhibit CYP 450 pathways, may inhibit metabolism of serotonergic agents, such as fluoxetine. With concurrent use, the result may be serotonin toxicity. In addition, it has been shown in humans that fluoxetine itself inhibits a CYP 450 isoform that metabolizes other antidepressants, such as the TCA amitriptyline. If TCAs and SSRIs are administered together, metabolism of the TCA may be inhibited leading to serotonin toxicity (Simpson & Papich, 2003).
Vascular disease in particular seems to be a predisposing factor to developing serotonin toxicity in humans. There are no studies regarding vascular disease and serotonin toxicity in companion animals but this may raise a legitimate concern. Spontaneous atherosclerosis has been documented in dogs with hypothyroidism and diabetes mellitus (Liu et al., 1986; Sottiaux, 1999; Hess et al., 2003). Thus, clinicians should be aware of the relationship between vascular disease and increased sensitivity to serotonin levels that has been documented in humans.
Treatment
The most important part of therapy for serotonin toxicity is discontinuation of the offending medication(s). It can take as little as an hour for clinical signs to develop in the instance of an acute overdose, up to days if the case is one of drug synergism/interaction. The mainstay of therapy is supportive care. Decontamination of clinically normal animals with activated charcoal may help prevent or reduce clinical signs. Treatment of specific clinical signs such as hyperthermia, hypertension, tachycardia, and tremors is sometimes necessary. Empirical therapy includes fluids for hyperthermia and benzodiazepines for muscle activity and seizures.
Specific receptor antagonists are also available and surprisingly are already in use in veterinary medicine for their other purposes. Cyproheptadine is a readily available veterinary drug and is usually used as an appetite stimulant. It also is the most commonly used drug in treatment of hyperserotonergic syndromes specifically because of its 5-HT antagonist properties.
Cyproheptadine is a first-generation histamine-1 receptor antagonist with nonspecific antagonist properties at 5-HT1A and 5-HT2A receptors (Nisijima et al., 2001). Although the use of cyproheptadine is more than anecdotal, there is limited data available regarding the dose/efficacy relationship. A 2001 study showed a single injection of high-dose cyproheptadine (10 mg/kg) but not a lower dose (5 mg/kg) prevented death in a lethal model of serotonin toxicity in rats (Nisijima et al., 2001). Although this is useful information regarding the safety and efficacy of cyproheptadine, much lower doses have been used with great success in human cases. The recommendation for cyproheptadine in the human literature is a loading dose of 8–12 mg then 2 mg every 2 h for severe cases or 8 mg ever 6 h for milder cases (Dunkley et al., 2003; Boyer & Shannon, 2005). There are several case reports of this treatment alone resulting in complete resolution of signs within 24 h (Lappin & Auchincloss, 1994; Kapur et al., 1997; Graudins et al., 1998). The dose for dogs and cats recommended by the APCC is 1 mg/kg p.o. or PR q4–8 h until clinical signs resolve.
Chlorpromazine is another 5-HT antagonist that has also been used with success in the treatment of serotonin toxicity in people (Gillman, 1996; Graham, 1997). Chlorpromazine is the prototypical phenothiazine proved to have a wide variety of effects on the CNS, ANS, and endocrine systems. It causes blockade of a large number of receptors including dopaminergic, alpha-adrenergic, histaminergic, and serotonergic (5-HT2). Dosage information for use as an antiemetic in veterinary patients is well published (Plumb, 2002). If nausea and vomiting is part of the clinical picture for a patient, chlorpromazine may be a good drug to use at the published doses. It may also help with management of muscle activity and hypertension.
Other drugs which may prove useful are the more centrally acting antiemetics, ondansetron, and dolasetron, both of which also have 5-HT2 receptor antagonism in the CRTZ. Neither of these has been shown to reverse signs of serotonin toxicity. Propranolol is a nonselective β-adrenergic blocker which also has some 5-HT1A antagonism properties. Although it may be used to treat pathologic tachycardia and supraventricular arrhythmia secondary to any number of conditions including a hyperserotonergic state, there is little data to support its utility for treatment of serotonin toxicity.
The relationship between nitric oxide and serotonin may prove useful for future research regarding the use of exogenous nitric oxide to treat serotonin toxicity in patients with vascular disease. There is one case report of a man with known vascular disease who developed serotonin toxicity after addition of a narcotic to his SSRI therapy (Brown & Skop, 1996). He was treated with nitroglycerin after he developed signs of cardiac ischemia. Although nitroglycerin was not specifically used to treat the serotonin toxicity, there was a temporal relationship between the administration of the nitroglycerin and resolution all of his clinical signs. In retrospect, it was speculated that giving exogenous nitric oxide in the form of nitroglycerin may have counteracted the peripheral effects of serotonin in this patient (Brown & Skop, 1996).
Conclusion
Until recently the serotonergic system was overlooked as clinically relevant in companion animal medicine. Recognizing that much of the research on serotonin physiology and pathophysiology is done in animal models validates the pertinence of this subject in veterinary medicine. Veterinarians are using an increasing number of drugs that alter serotonin levels. It is important to acknowledge that there are currently no comprehensive reviews of serotonin physiology in companion animals to help clinicians with a basic understanding of how these drugs alter serotonin levels.
There are no reports of combination therapy of drug resulting in serotonin toxicity, however, clinicians should be well aware of possible drug interactions. Advances in veterinary health care allow many of our patients to live longer with chronic conditions. Diseases such as hypothyroidism, diabetes mellitus, renal failure, and hyperlipidemia are associated with vascular damage. Although these patients may have subclinical disease, diagnosing an endocrinopathy may help identify individuals who might be at increased risk for developing serotonin toxicity because of combination therapy.
Besides its well-known effects on affect, sleep, and appetite, serotonin helps mediate GI motility, peripheral and cerebral vascular tone, and platelet aggregation. This article only highlights what is known about serotonin’s major functions, however, serotonin has also been shown to have effects on cardiac contractility, and renal function as well.




