Effect of ofloxacin on theophylline pharmacokinetics at clinical dosage in dogs
Abstract
We examined the effects of ofloxacin (OFX) and norfloxacin (NFX) on theophylline (TP) pharmacokinetics in dogs. OFX, as a noncompetitive and mechanism-based inhibitor, and NFX, as a noncompetitive inhibitor, were orally administered (5 mg/kg) for a single dose or multiple doses (12 hourly for 3 days). TP (5 mg/kg, i.v) was injected at 2 h after the final dose of the fluoroquinolones (FQs). The same dose of TP was injected (i.v) 3 weeks before the start of FQs treatment for control. Multiple doses of OFX significantly reduced the total body clearance (ClB) of TP from 0.117 to 0.085 L/h/kg, although a single dose did not change it. Neither a single dose nor multiple doses of NFX changed the TP pharmacokinetics. Plasma NFX concentrations increased after multiple doses. Those of OFX also increased but were still two orders of magnitude below the Ki for noncompetitive inhibition of CYP1A in dogs. Time-dependent reduction in ClB of TP suggests that mechanism-based inhibition of OFX was the major mode to decrease ClB of TP. The mechanism-based inhibition may result in substantial inhibition of CYP1A activities in clinical conditions.
Introduction
From our previous in vitro study, we have reported that some fluoroquinolones (FQs) inhibit cytochrome P-450 1A (CYP1A) activities by a reversible noncompetitive inhibition and mechanism-based inhibition (Regmi et al., 2005) in dogs. The potency of noncompetitive inhibition is based on concentrations of inhibitors at the enzyme site. On the other hand, that of mechanism-based inhibition depends not only on inhibitor concentrations available around the enzyme but also on exposure duration to the enzyme. As FQs are antibiotics, their long-duration use in treatments is common also in dogs. Identification of their major inhibitory mode, therefore, is important to avoid possible drug–drug interactions in clinical conditions in dogs.
The mechanism-based inhibition seems more dominant than the noncompetitive inhibition of FQs at therapeutic dosages in dogs. Intorre et al. (1995) observed a reduction in total body clearance (ClB) of theophylline (TP) by 43% after the co-administration of enrofloxacin (EFX) at 5 mg/kg, i.v. once a day for 5 days. They also observed progressive increments in plasma TP concentrations after each dose of EFX. These findings suggest that the noncompetitive inhibition of EFX was not the cause for the reduction of TP ClB; because they did not observe an accumulation of EFX in the plasma. Alternatively, the mechanism-based inhibition of ciprofloxacin (CFX), the metabolite of EFX, could be the cause; because EFX has only a reversible noncompetitive inhibition and does not have the mechanism-based inhibition and CFX has both inhibitions (Regmi et al., 2005). Similarly, Hirt et al. (2003), reported that multiple doses of marbofloxacin reduced ClB of TP in dogs. On the other hand, there are no reports describing that a single dose of FQs reduces the ClB of CYP1A substrates like TP. Neither there is information that the FQs only with a reversible inhibition reduce CYP1A activities at clinical dosages in dogs.
We, therefore, designed this study to examine the effects of ofloxacin (OFX) and norfloxacin (NFX) on TP pharmacokinetics to clarify the major inhibitory mode of FQs at a therapeutic situation in dogs. We selected OFX as a noncompetitive and mechanism-based inhibitor. We also selected NFX as a reversible noncompetitive inhibitor because its inhibitory constant (Ki) value is similar to that of OFX (Regmi et al., 2005). For the demonstration, both OFX and NFX were orally administered for a single dose or six (multiple) doses. A single dose of OFX was used to examine its noncompetitive effect. OFX multiple doses were used to examine its mechanism-based inhibition.
Materials and methods
Chemicals
Ofloxacin tablets (Tarivid®, 50 mg) were purchased from Daiichi Pharmaceutical Co. Ltd (Tokyo, Japan). NFX tablets (BACCITAL®, 50 mg) were purchased from Kyorin Pharmaceutical Co. (Tokyo, Japan). OFX standard was gifted from Daiichi Pharmaceutical Co. Ltd (Tokyo, Japan). Standards of NFX, TP and 7β-hydroxypropyltheophylline were purchased from Sigma Chemical Co. (St Louis, MO, USA). All other chemicals used as reagents were of analytical or high-performance liquid chromatographic (HPLC) grade.
Animals
Four beagle dogs (female, 2 years old, 9.5–16 kg) were obtained from CSK Research Park Co. Ltd (Nagano, Japan). The dogs were housed individually in metabolic cages. The dogs were supplied water ad libitum and dry food (One Lac Meal®, Morinyu Sun World, Tokyo, Japan) twice a day (at 09:00 and 21:00 hours). The dogs were handled according to the guidelines for the care and use of laboratory animals, Faculty of Agriculture, Tokyo University of Agriculture and Technology.
Study design
Ofloxacin and NFX tablets were orally administered to dogs resulting in an approximation of 5 mg/kg (5.20–5.56 mg/kg). The dogs up to 10 kg body weight were given one tablet, those from 11 to 15 kg body weight were given one plus a one-half tablets and those above 15 kg body weight were given one plus a two-third tablets. The drugs were administered after 1 h of feeding for single or six (multiple) doses (b.i.d. at 12 h intervals for 3 days). A latin-squared method was used for FQ treatments (Table 1). TP was intravenously injected (5 mg/kg) into the left cephalic vein at 2 h after each final dose of FQs. The injected solution was prepared by dissolving TP in physiological saline. The pH of the solution was adjusted at 8–9 by 2 n NaOH, then the final concentration was adjusted to 20 mg/mL by adding the physiological saline. The washout period was 3 weeks. TP (5 mg/kg) was intravenously injected 3 weeks before the start of FQs treatment for control.
| Dog no. | Treatment with fluoroquinolones | |||
|---|---|---|---|---|
| First | Second | Third | Fourth | |
| 1 | OFX SD | OFX MD | NFX SD | NFX MD |
| 2 | OFX MD | NFX SD | NFX MD | OFX SD |
| 3 | NFX SD | NFX MD | OFX SD | OFX MD |
| 4 | NFX MD | OFX SD | OFX MD | NFX SD |
- OFX, ofloxacin; NFX, norfloxacin; SD single dose; MD, multiple (six) doses. OFX or NFX (5 mg/kg) was orally administered for SD or MD 12 hourly for 3 days.
After TP administration, blood samples (3 mL) were collected in glass test tubes containing Na2 EDTA from the right cephalic vein at 0.5, 1, 2, 3, 4, 6, 8, 10 and 12 h. Plasma was immediately separated by centrifugation (1500 g, 5 min) and stored at −80 °C until assay. One day before the start of each experiment, blood samples were taken from each dog. The samples were examined for interfering peaks in the plasma, if any.
Determination of plasma TP concentrations
Plasma TP concentrations were determined by an HPLC method. After thawing, 0.5 mL of plasma was spiked with 0.1-mL solution of internal standard (7β-hydroxypropyltheophylline, 200 μg/mL) and then 5 mL, a mixture of chloroform and 2-propanol (85:15, v/v). After 1 min vortex, the mixture was centrifuged at 3000 g for 5 min. The organic layer was transferred to a clean pear-shaped flask. Evaporation was performed at 40 °C to dryness under a reduced pressure using a rotary evaporator (Rotavapor® R-114, Shibata Scientific Technology Ltd., Tokyo, Japan). The residue was reconstituted in a 1-mL mobile phase. After filtration with a 0.45-μm filter, 50 μL of the filtrate was injected on to an HPLC column (TSK-gel® ODS-120T, 5 μm particle size, 250 mm × 4.6 mm i.d.; TOSOH Co., Tokyo, Japan). Column effluent was monitored at 254-nm wavelengths using an ultraviolet detector (SPD-6A; Shimadzu Corporation, Kyoto, Japan). The mobile phase was a mixture of acetonitrile and distilled water (10:90, v/v). The pH of the mobile phase was adjusted at 3 with 7 m phosphoric acid. The flow rate was 1 mL/min. The detection limit (LOD) was 60 ng/mL at a signal-to-noise ratio of 3. The intra-day CV value was 1.77% at 10 μg/mL (n = 4). The inter-day CV values were ranged from 0.17% to 6.1% (4 days, four determinations per day) at 10 μg/mL.
Determination of plasma OFX and NFX concentrations
Plasma OFX was determined by the HPLC with a fluorometric detector (RF-535; Shimadzu Corporation) as described by Fabre et al. (1994) with slight modifications. After thawing, 0.5 mL of plasma was spiked with 2 mL of acetonitrile in a glass test tube. The mixture was vortexed for 10 sec. After centrifugation (2000 g, 5 min), the supernatant was collected in a clean pear-shaped flask. Once again, 2 mL of acetonitrile was added into the test tube and the previous procedure was repeated. The supernatant was collected in the same flask. Evaporation was performed at 40 °C to dryness. The residue was reconstituted in 1 mL of 0.02 M sodium acetate buffer (pH 4.5). After filtration with a 0.45-μm filter, 50 μL of the filtrate was injected on to the HPLC column. Column effluent was monitored under the excitation and emission wavelengths being 296 and 504 nm respectively. The mobile phase was a mixture of acetonitrile and 0.1% tetramethyl ammonium chloride solution adjusted pH at 4.5 by 4 n phosphoric acid (30:70, v/v). The flow rate was 1 mL/min. The LOD was 0.91 ng/mL at a signal-to-noise ratio of 3. Recovery was 89.7 ± 2.2% and 93.8 ± 1.3% at 1 and 10 μg/mL concentrations respectively (n = 5). The intra-day CV value was 1.92% at 10 μg/mL (n = 4). The inter-day CV values were ranged from 2.0% to 2.7% (4 days, four determinations per day) at 10 μg/mL.
Plasma NFX was determined by the HPLC with the fluorescence detection as described by Mascher and Kikuta (1998) with slight modifications. After thawing, 0.2 mL of plasma was spiked with an equal volume of acetonitrile. The mixture was vortexed for 30 sec. After centrifugation (2000 g, 2 min), 0.1 mL of supernatant was mixed with 0.2 mL, a mixture of 0.1 m perchloric acid and 0.02 m triethylamime (1:1, v/v). After vortex for a few seconds and then filtration with a 0.45-μm filter, 50 μL of the mixture was injected on to an HPLC column (RP-18 GP®, 5 μm particle size, 250 mm × 3 mm i.d.; Kanto Chemical Co. Ltd, Tokyo, Japan). Column effluent was monitored under the excitation and emission wavelengths being 300 and 450 nm, respectively. The mobile phase was a mixture of 0.1 m perchloric acid, 0.02 m triethylamine and methanol (30:30:40, v/v/v). The flow rate was 1 mL/min. The LOD was 3.16 ng/mL at a signal-to-noise ratio of 3. The recovery was 90.1 ± 1.7% and 108.4 ± 1.4% at 0.5 and 0.05 μg/mL concentrations, respectively (n = 5). The intra-day CV value was 2.85% at 0.1 μg/mL (n = 4). The inter-day CV values were ranged from 2.1% to 4.8% (4 days, four determinations per day) at 0.1 μg/mL.
In vitro plasma protein binding of fluoroquinolones
 (1)
(1)Pharmacokinetic analysis
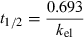 (2)
(2)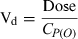 (3)
(3)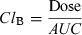 (4)
(4)Statistics
We used a log-normal transformation method for pharmacokinetic parameters because the transformation gives normal distribution to the pharmacokinetic parameters. Geometric mean, therefore, was used. After the transformation, paired, two-tailed Student's t-test was used to test statistical significance. A value of P < 0.05 was considered significantly different.
Results
Effect on TP pharmacokinetics
The intravenous pharmacokinetics of TP was affected by the multiple doses of OFX; however, it was not affected by the single dose (Fig. 1). The elimination t1/2 was increased from 4.08 to 5.50 h, and the ClB was decreased from 0.117 to 0.085 L/h/kg by the multiple doses of OFX (Table 2). These changes were statistically significant (P < 0.05). On the other hand, TP pharmacokinetics was not affected by the single dose or the multiple doses of NFX.
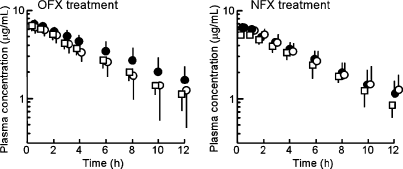
Plasma concentration–time curves of theophylline (5 mg/kg) following i.v. injection in the absence or presence of fluoroquinolones in dogs. Ofloxacin (OFX) or norfloxacin (NFX) was orally administered (5 mg/kg) for single or multiple doses, 12 hourly for 3 days. Theophylline was administered at 2 h after the final dose of fluoroquinolones. Open circles (control), open squares (single dose) and closed circles (multiple doses) represent theophylline plasma concentrations without fluoroquinolone or during fluoroquinolone treatment respectively. Each point and vertical line represent a mean value and SD, respectively (n = 4).
| Pharmacokinetics | Control | During OFX treatment | During NFX treatment | ||
|---|---|---|---|---|---|
| Single dose | Multiple doses | Single dose | Multiple doses | ||
| Vd (L/kg) | 0.690 (0.64–0.74) | 0.731 (0.69–0.78) | 0.687 (0.65–0.73) | 0.838 (0.80–0.88) | 0.691 (0.64–0.75) |
| K el (h−1) | 0.170 (0.13–0.22) | 0.154 (0.13–0.19) | 0.126* (0.09–0.17) | 0.155 (0.13–0.18) | 0.160 (0.12–0.22) |
| AUC (0-inf) (μg·h/mL) | 42.66 (31.0–58.6) | 44.58 (36.0–55.2) | 58.79* (41.4–83.5) | 37.94 (30.4–47.4) | 45.61 (35.9–58.0) |
| t 1/2 (h) | 4.08 (3.1–5.3) | 4.51 (3.8–5.4) | 5.50* (4.1–7.4) | 4.48 (3.8–5.3) | 4.33 (3.2–5.8) |
| Cl B (L/h/kg) | 0.117 (0.09–0.16) | 0.112 (0.09–0.14) | 0.085* (0.06–0.12) | 0.132 (0.11–0.17) | 0.110 (0.09–0.14) |
- Values are represented in geometric mean. Values inside parentheses represent mean − SD and mean + SD (n = 4). Significantly different compared with control at *P < 0.05. Vd, apparent volume of distribution; Kel, elimination rate constant; AUC(0–inf), area under the curve from time 0 h to infinity; t1/2, elimination half-life; ClB, total body clearance. TP (5 mg/kg) was administered at 2 h after the final dose of fluoroquinolones. OFX or NFX (5 mg/kg) was orally administered for single or multiple doses.
Plasma concentration–time curves of fluoroquinolones
The AUCs of OFX or NFX after multiple doses were compared with those after a single dose (Fig. 2). The concentrations of OFX at time points after multiple doses were increased compared with a single dose; however, the increases were not significant. On the other hand, high standard deviations for Cmax and AUC after a single dose of OFX treatment were observed (Table 3) which may be because of a variation in the absorption of OFX after oral administration. Mean plasma concentrations of OFX (AUC2.5–14h divided by 11.5 h, Table 3) during TP pharmacokinetic study was increased by 1.8-fold after multiple doses compared with a single dose, but the increase was not significant. Plasma NFX concentrations at time points also increased after multiple doses compared with the single dose; however, the increases were not significant except at 3 h.
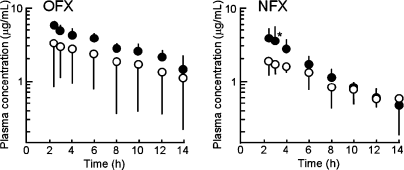
Ofloxacin (OFX) and norfloxacin (NFX) plasma concentration–time curves in dogs. Each fluoroquinolone (5 mg/kg) was orally administered for single or multiple doses 12 hourly for 3 days. Open (single dose) and closed circles (multiple doses) represent plasma concentrations of OFX or NFX after single or multiple doses respectively. Each point and vertical line represent a mean value and SD respectively (n = 4). Significant difference at *P < 0.05 compared with a single dose.
| Parameters | After OFX treatment | After NFX treatment | ||
|---|---|---|---|---|
| Single dose | Multiple doses | Single dose | Multiple doses | |
| C max tot (μg/mL) | 2.74 (1.4–5.2) | 5.60 (5.0–6.3) | 0.177 (0.12–0.26) | 0.340 (0.23–0.51) |
| AUC (2.5–14h) tot (μg·h/mL) | 19.2 (10–36) | 35.3 (28–45) | 1.10 (0.83–1.5) | 1.57 (1.1–2.2) |
| C m tot (μg/mL) | 1.67 (0.89–3.1) | 3.07 (2.4–3.9) | 0.096 (0.07–0.13) | 0.136 (0.10–0.19) |
| C max fu (μg/mL) | 2.05 (1.1–3.9) | 4.20 (3.7–4.7) | 0.106 (0.07–0.16) | 0.204 (0.14–0.31) |
| AUC (2.5–14h) fu (μg·h/mL) | 14.4 (7.7–27) | 26.5 (23–35) | 0.659 (0.50–0.87) | 0.941 (0.67–1.3) |
| C m fu (μg/mL) | 1.25 (0.66–2.3) | 2.30 (1.8–2.9) | 0.057 (0.04–0.07) | 0.082 (0.06–0.11) |
| t 1/2 (h) | 7.24 (6.5–8.0) | 6.25 (4.9–7.9) | 3.85 (2.9–5.0) | 3.48 (2.4–4.9) |
- Values are represented in geometric mean. Values inside parentheses represent mean − SD and mean + SD (n = 4); Cmax tot, maximum total plasma concentrations (observed); AUC(2.5–14h)tot, area under curve from 2.5 to 14 h based on total plasma concentrations; Cm tot, mean plasma concentrations from 2.5 to 14 h based on total concentrations; Cmax fu, maximum unbound plasma concentration (calculated), AUC(2.5–14 h) fu, area under curve from 2.5 to 14 h based on unbound plasma concentrations; Cm fu, mean plasma concentrations from 2.5 to 14 h based on unbound plasma concentrations; t1/2, elimination half-life. Both fluoroquinolones were orally administered for single or multiple doses.
In vitro plasma protein bindings of fluoroquinolones
The in vitro experiment showed that the plasma protein bindings of OFX or NFX were independent of their plasma concentrations. Plasma protein binding of OFX (25%) was less than that of NFX (40%) (Table 4). Using these binding percentages, the calculated unbound Cmax and AUC2.5–14h of OFX or NFX have been shown in Table 3.
| Plasma concentration (μg/mL) | Binding percentage | |
|---|---|---|
| OFX | NFX | |
| 1 | 25.1 ± 1.44 | 38.9 ± 2.49 |
| 3 | 26.3 ± 0.981 | 37.4 ± 0.833 |
| 10 | 26.8 ± 1.79 | 40.6 ± 1.31 |
- Values represent mean ± SD (n = 5). Pooled plasma from four dogs was used.
Discussion
In the previous study, we have demonstrated by in vitro experiments that OFX inhibits CYP1A activities by both reversible (noncompetitive) and irreversible manners (mechanism-based inhibition) in dogs. The present in vivo study in dogs suggests that the mechanism-based inhibitory effects of OFX may be substantial in clinical conditions.
After multiple doses of OFX for 3 days at a clinical dose (5 mg/kg), ClB of TP significantly decreased in dogs. However, a single dose of OFX did not affect ClB of TP. This suggests that the decrease in ClB was caused by the mechanism-based inhibition of OFX, because mechanism-based inhibition shows effects based on exposure time. Although the average plasma concentration of OFX after multiple doses was increased by 1.8-fold compared with that of a single dose (Table 3), neither this value nor the observed Cmax of the drug was so high to result in inhibitory effects on CYP1A activities by a reversible manner. It may be, therefore, suggested that the reduction in ClB of TP was caused by the mechanism-based inhibition of OFX.
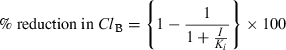 (5)
(5)In contrast to OFX, NFX did not affect the ClB of TP even after multiple dosing (Table 2). The drug has only noncompetitive inhibition on CYP1A with a Ki value of 4.75 mm and lacks the irreversible inhibitory effect in dogs (Regmi et al., 2005). Considering that the Ki-value is much larger than the plasma concentrations (Table 3), the lack of inhibitory effect of NFX suggests that the FQs only with a reversible inhibition are unable to inhibit CYP1A activities in in vivo conditions even in a long-duration treatment. Alternatively, this result may suggest that the FQs only with the mechanism-based inhibition are able to inhibit CYP1A activities in clinical dosage in dogs.
In animal species, other than dogs, there are also several reports describing inhibitory effects of FQs at clinical doses on CYP1A substrate clearance. Wijnands et al. (1986) reported that orally co-medicated enoxacin and CFX after multiple doses resulted in 63.6% and 30.4% decrease in ClB of TP, respectively, in human patients with chronic obstructive pulmonary disease. Schwartz et al. (1988) observed a progressive increment in plasma trough concentrations of TP at steady state after multiple oral doses of CFX at 750 mg in healthy humans. Although they did not report plasma concentrations of CFX, they observed 31% reduction in ClB of TP in this study. These results may also suggest an exposure duration-dependent inhibitory effect of CFX on TP metabolism in humans.
Although, limited information is available regarding the inhibitory effects of FQs on the other isozymes of CYPs, CFX and NFX have competitively inhibited the CYP3A-mediated metabolism in human and rat microsomes, respectively (McLellan et al., 1996). In addition, Vaccaro et al. (2003) have recently reported EFX as a mechanism-based inhibitor primarily of CYP3A in the hepatic microsomes of sea bass fish. These reports of FQs on CYP3A activities in those animal species suggest the need of a study about the inhibitory effects of the drugs on CYP3A activities also in dogs.
In conclusion, oral multiple dosing of OFX at clinical dosage decreased ClB of TP in dogs. Its mechanism-based inhibition was likely the main contributor. As mechanism-based inhibition is time dependent and cumulative, a long treatment with OFX may result in more decrease in CYP1A activities, and thereby more decrease in TP clearance. Clinicians, therefore, should be much careful to avoid drug–drug interactions during OFX therapy with CYP1A substrates like TP. Also CFX, the metabolite of EFX, and orbifloxacin have the mechanism-based inhibition (Regmi et al., 2005). These FQs, therefore, must be considered similar to OFX. Similarly, attention must be paid to marbofloxacin because it also reduces the ClB of TP.




