Assessment of endothelium-dependent vasodilation in equine digital resistance vessels
Abstract
Haemodynamic disturbances leading to ischaemia and reperfusion injury of the digit are thought to be involved in the pathophysiology of acute equine laminitis. Identification of physiological regulators of blood flow through the equine digit is important in identifying factors, which may predispose animals to laminitis. A method was developed to assess endothelium-dependent responses of the isolated Krebs-perfused equine digit by co-administration of 5-hydroxytryptamine (5-HT) with vasodilator agents, carbachol (CCh), bradykinin (BK) and substance P (SP). Bolus co-administration of CCh (0.02–2 μmol), BK and SP (0.02–0.2 nmol), caused inhibition of the 5-HT pressor response by 50–60%. The vasodilator responses were abolished by the detergent, CHAPS, indicating endothelium dependency; whereas vasoconstrictor responses to 5-HT were potentiated. CCh-induced relaxation was significantly reduced by the nitric oxide synthase inhibitor l-NAME (79.7 ± 3.4% inhibition), whereas a large proportion of BK and SP-induced relaxation remained (34.1 ± 6.3% and 33.6 ± 5.3% inhibition). l-NAME potentiated vasoconstrictor responses to 5-HT. In conclusion, this study demonstrates that endothelium-derived NO modulates the response to vasoconstrictors such as 5-HT and is likely to be an important regulator of blood flow in the digital resistance vascular bed. Other factor(s) released by the endothelium are also important in regulating blood flow, whose identity remains to be established.
Introduction
Equine laminitis is thought to be a result of multisystemic anomalies in organs often anatomically remote from the foot (Pollitt, 1999). In addition, the complex organization and functions of the digital circulation predispose the horse to regional ischaemic disease of the foot (Allen et al., 1990). These anomalies, together with the fact that the equine digital circulation is more sensitive to vasoconstrictor agents such as 5-HT compared with other vascular beds of the horse (Bailey & Elliott, 1998b), may have implications in the pathogenesis of laminitis. Although ischaemia and reperfusion injury of the distal digit is thought to be one possible pathophysiological mechanism of acute equine laminitis (Hood et al., 1993), the precise mechanisms and the trigger factor(s) have yet to be identified. Identification of physiologically important regulators of nutrient blood flow through the equine digit is important in understanding and identifying factors which predispose animals to laminitis.
Distribution of blood flow to the equine digit is regulated by muscular arterioles and the response of these resistance vessels to vasoactive agents has been reported to be similar to other skin vascular beds (Robinson et al., 1975). The majority of studies on the digital circulation, however, have employed isolated large conductance and capacitance vessels. The large vessels, whilst providing useful information about the receptors present in this important circulation (Elliott, 1997; Bailey & Elliott, 1998a), may not be truly representative of the resistance vessels, which regulate blood flow through the digit. New studies investigating isolated laminar microvascular function are emerging, which will provide useful information regarding this important circulation (Peroni et al., 2005). Furthermore, the importance of the vascular endothelium in regulating blood flow through the equine digit has not been examined in perfused blood vessels where fluid flow interacts with the endothelium and influences local mediator production. Previous studies have employed static systems to study blood vessel function and have concentrated on vasoconstrictor action predominantly (Baxter et al., 1989; Elliott, 1997).
The vascular endothelium is a source of many chemical mediators, including nitric oxide (NO), prostacyclin (PGI2) and endothelium-derived hyperpolarizing factor (EDHF), and has an important role in local control of blood flow (Mombouli & Vanhoutte, 1999). Many vascular diseases result in a dysfunction of the endothelium, manifest by impaired endothelial NO production (Mombouli & Vanhoutte, 1999). Endothelium-dependent vasodilation is impaired in Raynaud's phenomenon (Freedman et al., 2001), which shares a number of clinical and pathological similarities with equine laminitis (Hood et al., 1990). It has been speculated that endothelial cell dysfunction may predispose horses and ponies to laminitis (Bryant & Elliott, 1994; Hinckley et al., 1996). Thus, it would seem important to be able to study endothelial cell function in resistance vessels controlling blood flow to the equine digit.
Limited studies have investigated the role of nitric oxide (NO) in modulating vascular tone of large equine digital vessels, in vitro. Endothelium-dependent vasorelaxant responses to agonists such as carbachol and bradykinin have been demonstrated in equine digital arteries and veins and these responses were shown to be mediated predominantly by NO, as they were virtually abolished by the nitric oxide synthase inhibitor, l-NAME (Elliott et al., 1994; Cogswell et al., 1995). Inhibition of the NO pathway or mechanical removal of the endothelium, however, had no effect on the resting tension or on the responses of isolated conductance and capacitance vessels to vasoconstrictor agents such as 5-HT, thromboxane, endothelin-1 and α-adrenoceptor agonists, suggesting tonic production of NO by these vessels is low (Elliott et al., 1994; Cogswell et al., 1995; Bailey & Elliott, 1998a; Katz et al., 2003a).
Perfused vascular beds have been used to study endothelial cell function in resistance vessels in other species (Randall & Hiley, 1988). The isolated perfused equine digit preparation was first described by Eyre & Elmes, (1980) where the vasoconstrictor effects of 5-HT and catecholamines were reported. The aim of the present study was to develop this preparation to enable the study of endothelium-dependent relaxant responses within the resistance blood vessels of the equine digit.
Materials and methods
Animals and tissues
Hind limbs collected from mixed breed, healthy adult horses of both sexes killed at an abattoir were used as source of tissue for perfusion experiments. Horses with normal foot conformation and no foot pathology were selected as source of tissue. Only one foot from each horse was used per experiment. The limbs were collected within 10 min of slaughter and 250 mL ice-cold Krebs–Henseleit solution (KHS) was infused into the circulation at the abattoir via a cannula inserted in the digital artery at the level of the fetlock joint, to remove blood. The limbs were then transported to the laboratory with ice-packs and kept in cold room (4 °C) until use (within 24 h).
Preparation of equine digits for perfusion pressure measurement
Equine digits were prepared for in vitro perfusion as follows. Briefly, the digital artery was ligated at the level of the pastern on the lateral side and cannulated on the medial side with a 14-gauge catheter connected to a pressure transducer (Physiological Pressure Transducer model 4-422; Bell & Howell Ltd, Basingstoke, UK). The hoof was placed in water bath (kept at 30 °C; Bailey & Elliott, 1998a), and the digital circulation was perfused via a peristaltic pump (Harvard Apparatus model 1203; South Natick, MA, USA) at a constant flow rate of 100 mL/min with modified Krebs-Henseleit solution which was gassed with 95% O2/5% CO2. The pressure transducer, which was calibrated against a mercury manometer at the start of each experiment, was connected to a pen recorder (Linseis model L6512; Linton Instruments, Norfolk, UK) via an amplifier (6 channel transducer amplifier model PM1000; CWE Inc., New York, NY, USA). The flow rate (100 mL/min) remained constant regardless of the resistance to flow, and changes in vascular resistance within the hoof were recorded as changes in the perfusion pressure. The flow rate of 100 mL/min was chosen as the rate of digital arterial blood flow in vivo was estimated to be the same (Robinson et al., 1975). The baseline perfusion pressure was established after an equilibration period of 30 min.
Effect of bolus co-administration of vasodilators on 5-HT-induced pressor response of the Krebs perfused equine digital vascular bed
Hooves were perfused with drug-free Krebs, and once a steady-baseline perfusion pressure was established, a 30-min equilibration period was allowed before preparations were stimulated with a bolus dose of 5-HT, (6 nmol in 0.2 mL of Krebs) injected through an injection port close to the arterial cannula. This dose was repeated three times at 15 min intervals to ensure stability of the response and then the same bolus dose of 5-HT was administered together with carbachol (CCh, 0.02–2 μmol), bradykinin (BK, 0.02–0.2 nmol), or substance P (SP, 0.02–0.2 nmol) in a randomized order. Each co-administration of vasodilator and 5-HT was followed by a dose of 5-HT alone. The optimal time between each dose was found to be 15 min, allowing sufficient time for baseline pressure to be re-established and ensuring reproducibility of the responses recorded. Trace recordings were scanned and digitized using UNGRAPH computer program (Biosoft Ltd, Cambridge, UK) to obtain peak height and Area under the curve (AUC) for each response. Vasodilatory responses were quantified as the percentage decrease in the peak height and AUC to 5-HT caused by inclusion of the vasodilator.
Effect of time on vasodilatory response of the Krebs perfused equine digit
To establish the reproducibility of the vasodilatory responses measured by the co-administration method, a series of experiments were conducted to ensure that the vasodilatory responses to the agonists were stable following repeated stimulation of the preparation. Three vasodilatory responses to each of the agonists, CCh (0.2 μmol), BK (0.2 nmol) and SP (0.2 nmol), co-administered with the standard dose of 5-HT (6 nmol) in a randomized order, were obtained before and after a standard bolus dose of 5-HT alone in a same preparation.
Effect of deactivation of the endothelium on agonist-induced vasodilatory responses of the Krebs perfused equine digital vascular bed
Hooves were perfused as described before and the change in the perfusion pressure (vascular resistance of the digital circulation) in response to a bolus dose of 5-HT (6 nmol) alone and when co-administered with the standard doses of CCh (0.2 μmol), BK (0.2 nmol), SP (0.2 nmol) or sodium nitroprusside (SNP, 0.2 μmol) was measured. Preliminary experiments showed these doses of vasodilators gave large, reproducible and equivalent inhibition of the 5-HT response (50–60% inhibition). Preparations that responded to the vasodilators (i.e. CCh, BK or SP) with <40% inhibition of the 5-HT vasoconstrictor responses were rejected from the study at this stage. These equi-effective doses of the three vasodilator agents were used for all subsequent studies described below. The responses were repeated in the same hooves after the preparation was perfused with 0.3% CHAPS (nondenaturing zwitterionic detergent) for 1 min, to functionally deactivate the vascular endothelium (Randall & Hiley, 1988).
Histological examination of effect of CHAPS on Krebs perfused digital vessels
Segments of third-order branches of small digital arteries (diameter ∼1 mm) were dissected from Krebs perfused digits after CHAPS treatment. Following the functional study, hooves were infused with 200 mL of 10% neutral buffered formalin. Small arteries were then dissected free of connective tissue, fixed in 10% neutral buffered formalin and embedded in paraffin. Control vessels were obtained from perfused digits that had not been exposed to CHAPS and in which co-administration of CCh (0.2 μmol) with 5-HT reduced the 5-HT response by >60%. Six micron sections were cut and stained with haematoxylin and eosin (H&E). Staining was visualized with an Olympus model BH2-RFl transmission microscope and images were captured with a JVC KY-F55B colour video camera, recorded with Zeiss KS300 software (version 3.0; Carl Zeiss Vision GmbH, Hallbergmoos, Germany).
Effect of nitric oxide synthase inhibition on agonist-induced vasodilatory response in the perfused hoof
To investigate the contribution of nitric oxide to the agonist-induced endothelium-dependent relaxation of the equine digital resistance vessels, hooves were perfused as described above and the responses to the standard doses of CCh, BK, SP and SNP (co-administered with 5-HT) were obtained. The responses were then repeated in the same preparations after inclusion of l-NAME (nonselective NOS inhibitor at concentration of 100 μm) to the perfusing Krebs solution, 30 min prior to and during stimulation with the above agonists.
Effect of magnitude of 5-HT-induced tone and repeated exposure of agonists on vasodilatory responses in the perfused hoof
In order to investigate whether the magnitude of the 5-HT pressor response influenced the agonist-induced vasodilatory response, the following experiment was carried out. Vasodilatory response to CCh (0.2 μmol) co-administered with 5-HT (6 nmol) was measured before and after inclusion of l-NAME (100 μm, 30 min prior to and during stimulation) in the perfusing Krebs solution. Subsequently CCh (0.2 μmol) was co-administered with 5-HT (3 nmol). This reduced dose of 5-HT was chosen as it gave a similar increase in peak height and area under the curve obtained to 6 nmol of 5-HT in the absence of l-NAME.
Statistical analysis of data
Data are presented as mean ± SEM and n represents the number of animals from which hooves were used for perfusion. All statistical comparisons were performed using GraphPad Prism version 3.00 for Windows. Vasodilatory responses were calculated as the percentage reduction of the AUC of the 5-HT pressor response by the vasodilator compounds. The control 5-HT response (taken as 100%) was the mean of the responses obtained immediately before and immediately after the vasodilator was co-administered with 5-HT. The vasodilatory responses of CCh, BK and SP in the Krebs perfused hoof and the pressor responses to 5-HT following repeated cycles of stimulation were compared using repeated measures anova with Bonfferoni's multiple comparison post hoc test. The effect of l-NAME and CHAPS on agonist-induced vasodilatory responses were compared with control values using two-tailed paired t-test. P-values ≤0.05 were considered to indicate statistical significance.
Drugs and solutions
The modified Krebs–Hensleit solution had the following composition (mm): NaCl 118, KCl 4.57, CaCl2 1.27, KH2PO4 1.19, MgSO4 1.19, NaHCO3 25 and glucose 5.55. 5-hydroxytryptamine creatinine sulphate (5-HT), carbachol (carbamylcholine chloride), bradykinin (acetate salt), substance P (acetate salt), Nω-Nitro-l-Arginine methyl ester hydrochloride (l-NAME), CHAPS {3-[(3-cholamidopropyl)dimethylammonio]-1-propane sulphate}, and sodium nitoprusside (SNP) were all purchased from Sigma-Aldrich company Ltd, Poole, Dorset, UK. All drugs were made as concentrated solutions and subsequently diluted in Krebs solution. All drugs and the Krebs solution were freshly made on the day of the experiment, except bradykinin and substance P, which were made up from aliquots of 1 mm stock solution dissolved in 0.1 m acetic acid and kept at −70 °C. 5-HT was initially dissolved in 0.01 m sulphuric acid, whereas l-NAME, CHAPS, carbachol and sodium nitroprusside (NO donor) were initially dissolved in distilled water.
Results
Effect of bolus co-administration of vasodilator agonists on pressor response to 5-HT in the perfused equine digit
The baseline perfusion pressure of the isolated digit was 104.3 ± 4.7 mmHg (n = 8) and remained stable throughout the experiment. Bolus administration of 6 nmol 5-HT caused an increase of 105.4 ± 6.9 mmHg (n = 8) in the peak height. Bolus co-administration of CCh with 5-HT reduced the pressor response to 5-HT, with the maximum recorded effect of CCh seen at a dose of 0.2 μmol, reducing the AUC of the 5-HT pressor response by 60.1 ± 3.3% (see Fig. 1). Co-administration of both BK and substance P with 5-HT also caused a reduction in the pressor response to 5-HT, as shown in Fig. 2. Preliminary experiments showed that 0.2 nmol BK and 0.2 nmol SP gave similar responses to 0.2 μmol CCh (56.7 ± 3.8 and 53.1 ± 6.1% inhibition of 5-HT induced tone, respectively). These equi-effective doses of the three vasodilator agents were used for all subsequent studies described below. The vasodilatory response to CCh, BK and SP did not change significantly over the time of a typical experiment (i.e. approximately 3.5 h) and repeated exposure to the agonists did not cause a change in the vasodilatory response recorded (data not shown).
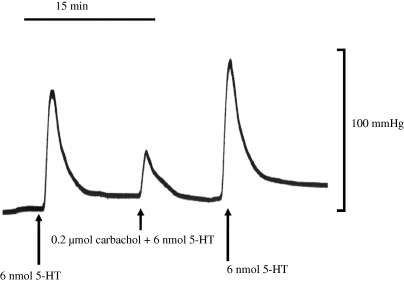
Typical trace record showing a change in the total perfusion pressure in response to a bolus dose of 5-HT administered alone and co-administered with carbachol. Hooves were perfused with drug-free Krebs solution and the arrows indicate administration of drugs. Similar trace records were obtained with co-administration of bradykinin, substance P and sodium nitroprusside.
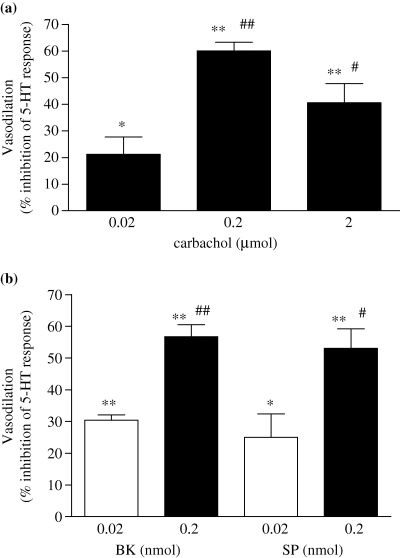
The effect of bolus co-administration of carbachol (CCh), bradykinin (BK) and substance P (SP) on pressor response to 5-HT in the perfused equine digit. The AUC was calculated by scanning the traces, and quantified by computer software. The relaxation responses to carbachol (2a) and bradykinin or substance P (2b), has been calculated as the decrease in the pressor response (AUC) to a standard dose of 5-HT (6 nmol) when co-administered with the agonists, expressed as a percentage of the pressor response obtained to 5-HT alone. Each bar represents the mean ± SEM responses obtained in preparations from five horses. *P < 0.05, **P < 0.001 vs. 5-HT alone. #P < 0.05, ##P < 0.001 vs. lowest dose of vasodilator used. Comparisons were made by repeated measures anova with Bonfferoni's multiple comparison post hoc test.
Effect of deactivation of the endothelium on agonist-induced vasodilatory responses of the Krebs perfused equine digital vascular bed
Infusion of Krebs solution containing the detergent CHAPS (0.3%) for 60 sec, caused a significant increase (P = 0.0028, paired t-test, n = 4) of 33.6 ± 3.7 mmHg, in the baseline perfusion pressure. The pressor response to 5-HT was also significantly potentiated by 176.8 ± 57.9% (peak height, P < 0.05, n = 4) and 156.3 ± 56.1% for the area under the curve (P < 0.01, n = 4). Treatment with CHAPS almost completely abolished the vasodilatory responses to CCh, BK and SP, as shown in Fig. 3. The response to co-administration of 5-HT with the NO donor, sodium nitroprusside (SNP), however, was unchanged.
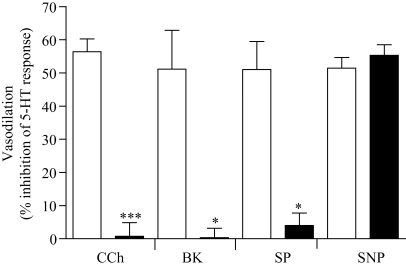
The effect of CHAPS treatment on agonist-induced vasodilatory responses. Hooves were perfused with drug-free Krebs solution and once the baseline perfusion pressure was established, a bolus dose of 5-HT (6 nmol) was administered into the digital circulation alone and together with either carbachol (CCh, 0.2 μmol), bradykinin (BK, 0.2 nmol) substance P (SP, 0.2 nmol) or sodium nitroprusside (SNP, 0.2 μmol) in a randomized order (open columns). The responses to the above agonists were also obtained after the same hooves were perfused with Krebs solution containing 0.3% CHAPS for, 1 min prior to stimulation with the above agonists (CHAPS, closed columns). Vasodilatory responses are expressed as the percentage decrease in the area under the curve of the pressor response to 5-HT. Each column represents the mean ± SEM value obtained with perfused digits from four horses. *P < 0.05, ***P < 0.0001 vs. control compared by two-tailed paired t-test.
Histological examination of the effect of CHAPS on Krebs perfused digital vessels
Histological sections of vessels from the control and CHAPS treated Krebs perfused digits are shown in Fig. 4. Treatment with CHAPS (0.3%, for 1 min) caused loss of integrity of the endothelium (loss of endothelial cells) (see Fig. 4b). Furthermore, CHAPS caused partial separation of the internal elastic lamina from the underlying smooth muscle layer. Cellular morphology of the smooth muscle, however, was unaffected. As Fig. 4a shows, perfusion of digits with drug-free Krebs alone did not cause loss of integrity of the endothelium.
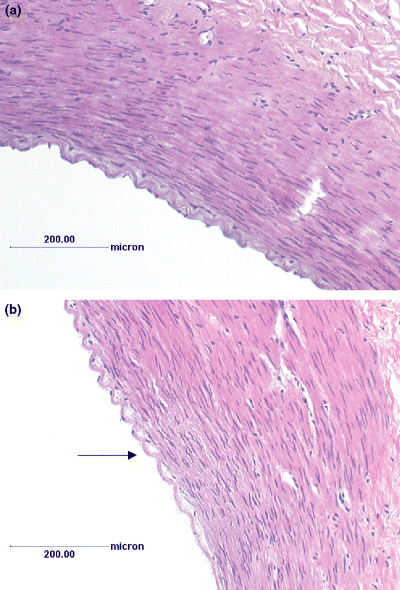
H&E staining of control Krebs perfused and CHAPS treated equine digital vascular bed (×40 magnification). Hooves were perfused with control Krebs and vasodilatory response to standard dose of CCh established in the absence (a) and presence (b) of CHAPS treatment (0.3%, for 1 min). The digits were then infused with 10% neutral buffered formalin (200 mL) and third-order branches of digital artery (diameter ∼1 mm) dissected free of connective tissue and fixed with 10% neutral buffered formalin. Vessels were then embedded in paraffin and 6 μm sections were cut and stained with haematoxylin and eosin (H&E). Staining was visualized with an Olympus model BH2-RFl transmission microscope and images were captured with a JVC KY-F55B colour video camera connected to Zeiss KS300 version 3.0 programme. A (control), B (CHAPS treated). Arrow indicates loss of endothelial layer and partial separation of the internal elastic lamina from the underlying smooth muscle layer in CHAPS treated digit. Cellular morphology of smooth muscle appears unaffected.
Effect of l-NAME on endothelium-dependent agonist-induced vasodilator responses in the perfused equine digit
Infusion of Krebs solution containing 100 μml-NAME did not cause any change in the baseline perfusion pressure. However, the area under the pressor response of 5-HT (6 nmol) was significantly potentiated by 1.8 ± 0.11-fold (P = 0.0029, paired t-test, n = 4). As Fig. 5 shows, in the presence of l-NAME (100 μm), CCh-induced relaxation was significantly reduced (79.7 ± 3.4% inhibition), whereas a large proportion of BK and SP-induced relaxation was relatively resistant to NOS inhibition (34.1 ± 6.3% and 33.6 ± 5.3%, inhibition in the area under the curve of the vasodilatory responses, P = 0.122 and 0.112 respectively).
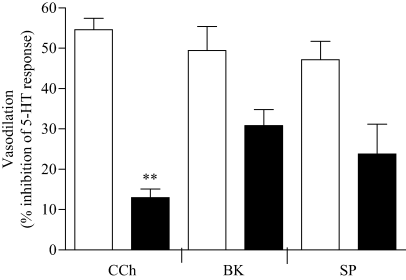
The effect of l-NAME on agonist-induced vasodilatory responses of the isolated Krebs perfused equine digit. Digits were perfused with drug-free Krebs solution and once the baseline perfusion pressure was established, a bolus dose of 5-HT (6 nmol) was administered into the digital circulation alone or together with either CCh, BK or SP in a randomized order (Control, open columns). The vasodilator responses to CCh (0.2 μmol), BK (0.2 nmol), SP (0.2 nmol) and SNP (0.2 μmol) were also obtained in the same digits perfused with Krebs solution containing (l-NAME) 100 μm, 30 min prior to and during stimulation with the above agonists (filled columns). Trace records were scanned and digitized and area under the curve calculated. Vasodilatory responses are expressed as the percentage decrease in the area under the curve of the pressor response to 5-HT. Each column represents the mean value ± SEM obtained using preparations from four horses. CCh (carbachol), BK (bradykinin), SP (substance P). **P < 0.01, vs. control (compared by two-tailed paired t-test).
Effect of magnitude of 5-HT pressor response and repeated exposure of agonists on vasodilatory response of the Krebs perfused equine digit
The magnitude of the 5-HT pressor response had no influence on the agonist-induced vasodilatory response. Lowering the dose of 5-HT to 3 nmol, in the presence of l-NAME gave similar magnitude of increase in peak height and area under the curve obtained to the standard dose of 5-HT (6 nmol) in the absence of l-NAME (101.8 ± 16.4 vs. 98.9 ± 11.2 mmHg, respectively). Similar magnitude of inhibition of CCh-induced vasodilatory response was also obtained to 3 nmol 5-HT in the presence of l-NAME compared with that found when 6 nmol 5-HT was used to provide tone in the presence of l-NAME (31.6 ± 3.5 vs. 29.3 ± 2.1% inhibition of 5-HT-induced tone, respectively).
Discussion
The present study provides information on the normal physiological function of the vascular endothelium in the small resistance vessels of the equine digit, an area which has not previously been studied. Understanding and identifying factors that regulate blood flow in the digital circulation may be crucial to determine what factors predispose horses to laminitis. The main finding of the present study is that endothelium-dependent vasodilatory responses can be demonstrated in the resistance vascular bed of the Krebs perfused isolated equine digit. Agonist-induced endothelium dependent vasodilatory responses were demonstrated by bolus administration of CCh, BK and SP, which opposed the pressor response to a bolus dose of 5-HT, in a dose dependent manner. The responses were sufficiently large in magnitude and stable over time, and following repeated stimulation, to allow this method to be used to investigate the nature of the mediators involved in these responses by the use of selective inhibitors.
The experimental model developed in the present study uses perfusion of the digital circulation with a physiological salt solution to examine the importance of endothelium-derived mediators in regulating blood flow, in the resistance vessels of the equine digit. Several investigators have used whole blood perfused models (which mimic physiological conditions) to examine the importance of endothelium-derived mediators, such as NO, in regulating blood flow in other resistance beds, such as the superior mesenteric artery of the rat (Randall & Hiley, 1988). Both nitric oxide and 5-HT are known to interact with blood components (Houston & Vanhoutte, 1986; Moncada et al., 1991) and their actions in the Krebs perfused hoof may be over estimated. In addition, blood has greater viscosity than Krebs solution, and the shear force at the endothelial surface would be greater in the blood perfused model and the whole animal. However, previous studies by other researchers have shown that perfusing with Krebs solution provides reproducible results and does not lead to significant oedema formation over a 3 h period (Eyer & Elmes, 1980).
Use of Krebs solution to perfuse this preparation requires nonphysiologic aeration of the perfsuing fluid with 95% O2/5% CO2. However, the use of 95% O2/5% CO2 to aerate modified Krebs solution is well established and widely used in various isolated vascular bed functional studies to examine the role of EDRFs, including nitric oxide and EDHF (Elliott et al., 1994; Mombouli et al., 1996) making this an appropriate strategy to adopt in the present study. Another limitation of the present experimental model is that neuronal modulation of vascular responses would be absent in the isolated digit. The use of whole blood, in the present study was not possible, as the perfusion model is not re-circulating. However, the model developed more closely represents the situation in vivo than the isolated vessel rings used in most previous studies (Elliott et al., 1994; Cogswell et al., 1995).
Although peak height and the area under the curve (AUC) were measured, AUC of the responses was used to quantify 5-HT pressor response and vasodilatory responses, as this reflects the total vasoconstrictory response. In addition, the peak height might be influenced by the speed of injection in the co-administration method. Furthermore, any lag of activation of vasodilator mediators might be masked by measurement solely of peak height. AUC and peak height gave comparable results in this model (data not shown) and the results presented are data based on AUC measurement only.
5-HT has been identified as a potent vasoconstrictor of both isolated equine digital arteries and veins (Baxter et al., 1989) and the pump perfused equine digit (Robinson et al., 1975). It has also been shown to have selectivity for the digital vascular bed, when compared with facial, tail and coronary arteries (Bailey & Elliott, 1998b) and its involvement in the pathogenesis of laminitis has been hypothesized. Preliminary work in our laboratory has also demonstrated that both 5-HT1 and 5-HT2 receptors play a role in the responses of the Krebs perfused equine digit to 5-HT (Bailey, 1998).
Co-administration of bolus doses of CCh, BK and SP reduced the pressor response to 5-HT. Although a complete dose response was not possible to perform due to the duration of time in which the preparations were stable, there was a trend for a dose-dependent inhibition of 5-HT pressor response with the bolus doses of CCh, BK and SP used in this study. The responses to these agonists were completely abolished by chemical destruction of the endothelium, by the zwitterionic detergent CHAPS, suggesting endothelium-dependency of the responses. CHAPS has been used in several perfused vascular preparations to chemically destroy the integrity of the endothelium (Hjelmeland, 1980); and this was demonstrated functionally and histologically (Randall & Hiley, 1988). In the present study, preliminary experiments demonstrated that perfusion with 0.3% CHAPS for 60 sec was sufficient to functionally deactivate the endothelium as assessed by the vasodilator response to CCh. Furthermore, the integrity of the endothelium was destroyed when examined histologically in the present study. The integrity of the underlying smooth muscle in the perfused equine digit was confirmed by the response to the endothelium-independent NO donor, sodium nitroprusside (SNP), which was unaffected by CHAPS treatment. SNP yields nitric oxide by spontaneous decomposition and therefore does not require endothelium for its vasodilator action (Bates et al., 1991).
Another important finding of the present study is that chemical destruction of the endothelium, by the zwitterionic detergent CHAPS, resulted in an increase in the contractile response of the perfused vascular bed to 5-HT. Furthermore, the baseline perfusion pressure of the preparation was markedly increased following perfusion with CHAPS. The magnitude of the pressor response to 5-HT was also potentiated in the presence of l-NAME, suggesting NO production is important in modulating response to 5-HT. The possible explanation for this finding is that the vascular endothelium of the equine digit is important in releasing relaxing factors either tonically, and/or in response to 5-HT receptor stimulation, that regulate vascular tone. Thus endothelial dysfunction would lead to increased vascular resistance, as demonstrated in other experimental models in vivo and in vitro (Mombouli & Vanhoutte, 1999).
In studies using large conductance/capacitance digital vessels, however, inhibition of NOS had no influence on the magnitude of vasoconstriction to agonists such as 5-HT, phenylephrine, the thromboxane mimetic U44069 and norepinephrine (Elliott et al., 1994; Cogswell et al., 1995; Bailey, 1998). Two explanations would account for this difference. First, tonic NO production would be minimal or absent in the isolated vessel segments as they are under static conditions, whereas the shear forces generated by flow of fluid through the vessels would stimulate NO production as demonstrated in other resistance vascular beds (Griffith et al., 1987). Secondly, 5-HT has also been shown to stimulate NO production by endothelial cells through activation of 5-HT1-like, 5-HT2 and 5-HT7 receptor subtypes on endothelial cells of blood vessel of differing origin from various species (Verbeuren et al., 1991). Equine coronary arteries have also been shown to relax in response to 5-HT, partially mediated by endothelial 5-HT2 receptors (Obi et al., 1994; Bailey, 1998). Preconstricted isolated large equine digital arteries, on the other hand, failed to relax in response to 5-HT (Bailey, 1998).
The potentiation of 5-HT pressor response in the presence of l-NAME, in the present study, therefore could be due to inhibition of NO production linked to stimulation of 5-HT receptors on endothelial cells of the digital circulation. Inhibition of the 5-HT-induced NO production, which would normally antagonise with the 5-HT-induced tone, would then lead to enhanced vasoconstriction. The magnitude of the potentiation was, however, greater after CHAPS pretreatment suggesting more than just NO is important. This could be explained by the fact that the endothelium is metabolically active taking up 5-HT preventing its access to smooth muscle layers (Bailey et al., 2003). In addition, destruction of the endothelium by CHAPS treatment would cause functional loss of 5-HT receptors on the endothelial cells, in turn leaving the 5-HT-induced vasoconstriction unopposed.
The co-administration experimental protocol for assessing vasodilatory responses in the equine isolated perfused digit was reproducible. The vasodilatory responses to CCh, BK and SP were stable during the course of an experiment such that repeated stimulation of the preparation on three occasions to a standard dose of the vasodilator compounds did not change the magnitude of their response. This confirms that the vasodilatory responses were not affected by receptor desensitization or tachyphylaxis and is an important finding when interpreting the effects of l-NAME on these responses.
The finding of l-NAME-resistant vasodilatory responses, particularly to BK and SP in the equine digit, is in contrast to the findings in large conductance digital vessels of the horse (Elliott et al., 1994; Katz et al., 2003b). l-NAME inhibited BK and CCh-induced relaxations by about 90% in digital arteries (Elliott et al., 1994) and SP-induced relaxation was also inhibited by about 90% in digital arteries (Katz et al., 2003b). The finding of l-NAME resistance in the present study suggests that mediators other than NO are involved but it could also be explained by incomplete inhibition of endothelial nitric oxide synthase (eNOS). Increasing the l-NAME concentration from 100 to 300 μm did not appear to cause further inhibition of the CCh, BK and SP-induced relaxations (data not shown). l-NAME (300 μm), which is thought to be the maximally effective concentration for NOS inhibition (Vanheel & Van de Voorde, 2000), caused partial but significant inhibition of the CCh-induced relaxation (similar to 100 μml-NAME) and a large proportion of the BK and SP-induced response was resistant.
In the present study, the existence of residual NOS activity in the presence of l-NAME could not be ruled out, as direct measurement of NO release was not performed in the Krebs perfused digital vascular bed. However, previous work in our laboratory has shown that agonist-induced cGMP release (an index of NO production) by cultured endothelial cells derived from equine digital circulation (EDVECs) was completely inhibited by 100 μml-NAME (Berhane et al., 2003b), suggesting that the concentration of l-NAME used (100 μm) was sufficient to block NO production and the l-NAME-resistant relaxation in the equine digital vascular bed is likely to be mediated by a factor(s) other than residual NO.
The possible candidates for the l-NAME-resistant relaxation in the equine digit may be PGI2 and EDHF. Our previous studies indicated that the role of PGI2 in the agonist-induced endothelium-dependent relaxation of the Krebs perfused digit was equivocal as the reduction in the BK-induced relaxation was augmented by the combined inhibition of NOS and cyclo-oxygenase (COX) with l-NAME (100 μm) and ibuprofen (10 μm), respectively, compared with l-NAME alone (Berhane et al., 2003a). This is despite the fact that BK clearly stimulates PGI2 production by culture equine digital endothelial cells and ibuprofen inhibits this production (Berhane et al., 2003b). Furthermore, there was no further potentiation of the 5-HT pressor response, with combined NOS and COX inhibition compared with l-NAME alone (data not shown). Indeed, in most blood vessels the contribution of PGI2 to the endothelium-dependent relaxation is minor (Lüscher & Vanhoutte, 1990). In most medium to resistance-sized arteries, l-NAME-resistant component of endothelium-dependent relaxation is often mediated by EDHF (Taylor & Weston, 1988), whose identity still remains illusive. Data from previous study in our laboratory also indicate that the l-NAME-resistant endothelium-dependent component of relaxation in the Krebs perfused equine digit is mediated by an EDHF, although its chemical nature still remains to be established (Berhane et al., 2003a).
In conclusion, a model has been developed to examine endothelium-dependent vasodilatory responses in the resistance vascular bed of the equine digit. NO modulates vascular response to vasoconstrictor agents such as 5-HT and is likely to be an important regulator of blood flow in the resistance vascular bed of the equine digit. Other factor(s) released by the vascular endothelium are also important in regulating blood flow in the equine digit. The identity of the factor(s) is not yet known, and remains to be established.
With this model, it should be possible to further characterise the l-NAME-resistant proportion of agonist-induced vasodilatory responses, which may help to increase our knowledge in targeting potential interventions that can be utilized to modulate blood flow through the equine digit.




