The Endocannabinoid System and Liver Diseases
Abstract
Endogenous cannabinoids (EC) are ubiquitous lipid signalling molecules provided by a number of central and peripheral effects, which are mainly mediated by the specific cannabinoid receptors CB1 and CB2. Although the expression of these receptors is very low or even absent in the healthy liver, a considerable series of experimental studies and some clinical observations have recognised the EC system as an important player in the pathophysiology of liver diseases. The EC system is highly up-regulated during chronic liver diseases and, to date, it has been implicated in the pathogenesis of non-alcoholic fatty liver disease, progression of fibrosis to cirrhosis and the development of the cardiovascular abnormalities of cirrhosis, such as the hyperdynamic circulatory syndrome and cirrhotic cardiomiopathy. Furthermore, the EC system influences the mechanisms responsible for cell damage and the inflammatory response during acute liver injury, such as that resulting from ischaemia-reperfusion. Thus, molecules targeting the CB1 and CB2 receptors may represent potential therapeutic agents for the treatment of liver diseases. At present, the CB1 antagonists represent the most attractive pharmaceutical tool to resolve fat accumulation in patients with non-alcoholic fatty liver disease and to treat patients with cirrhosis, as they may slow the progression of fibrosis and attenuate the cardiovascular alterations associated with the advanced stage of the disease.
Endogenous cannabinoids (EC) are lipid signalling molecules mimicking the activity of Δ9-tetrahydrocannabinol, the main psychotropic constituent of marijuana. They are provided by a series of central and peripheral effects because they influence analgesia and motor function, energy balance and food intake, cardiovascular function, immune and inflammatory responses, and cell proliferation, which are mediated by the specific cannabinoid receptors CB1 and CB2. The CB1 receptor is mainly expressed in brain, but also in several peripheral tissues including heart and vessels, whereas CB2 is mostly found in immune system cells. However, some EC effects result from the interaction with other receptors, such as the vanilloid receptor (1).
Although both hepatocytes and nonparenchymal cells are capable of producing EC, the physiological expression of CB1 and CB2 receptors in the adult liver is at a very low level or even absent (2–4). However, in recent years, several experimental studies and some clinical observations suggest that the EC system plays an important role in the pathophysiology of chronic and acute liver diseases.
A number of clinical conditions, with viral hepatitis, alcohol abuse and non-alcoholic fatty liver being the most predominant, can induce chronic hepatocyte injury and inflammation, thus activating fibrogenesis as wound-healing mechanism. However, the persistence of the damaging insult and the progression of fibrosis disrupt the normal liver architecture, leading to cirrhosis and subsequently to the onset of the life-threatening complications related to liver failure and portal hypertension.
Mounting evidence indicates that the EC system is highly up-regulated in chronic liver diseases, and, to date, it has been implicated in the pathogenesis of non-alcoholic fatty liver disease (NAFLD), progression of fibrosis to cirrhosis and the development of cardiovascular abnormalities, such as the hyperdynamic circulatory syndrome and cirrhotic cardiomiopathy (5).
Finally, the EC system influences the mechanisms responsible for cell injury and the inflammatory response during acute liver damage which occurs during the ischaemia-reperfusion (IR) that almost invariably takes place in hepatic surgery.
The EC system and NAFLD
Non-alcoholic fatty liver disease (NAFLD) is the hepatic feature of the metabolic syndrome, and represents the most frequent cause of hypertransaminasaemia in Western countries. The clinical manifestations of NAFLD range from simple steatosis, which is usually considered as a benign condition, to non-alcoholic steatohepatitis (NASH), which consists of steatosis, liver inflammation and fibrosis, and carries a 20% risk of cirrhosis within 20 years (6, 7).
The maintenance of energy homeostasis and body weight involves both appetite behavior and peripheral energy metabolism (8). Besides the well-known activation of CB1-receptors by EC in the central control of appetite, Osei-Hyiaman et al. (9) demonstrated that endogenous N-arachidonoyl-ethanolamine (anandamide, AEA) favours the development of diet-induced obesity and fatty liver in mice by increasing the de novo fatty acid synthesis through the induction of lipogenic transcription factor and its target enzymes. All these events are blunted when the mice are pretreated with the CB1 receptor antagonist rimonabant or when knockout mice for the CB1 receptor are fed with a high-fat diet (9).
In line with this, prolonged treatment with rimonabant to obese Zucker (fa/fa) rats improves dyslipidaemia, reverses fatty liver and attenuates the serum markers of liver injury compared to the pairfed control animals (10). These findings were also associated with a reduction of the hepatic content of tumour necrosis factor (TNF)-α and an increase in plasma adiponectin (10). At present, the phase III clinical trial ‘An Efficacy and Safety Study of Rimonabant for Treatment of Non-alcoholic Steatohepatitis (NASH) in patients without diabetes’ (NCT00576667) is ongoing.
Although the contribution of CB2 receptors to the pathogenesis of NAFLD is much less established, a recent study reported increased hepatic CB2 receptor expression in patients with NAFLD (11), and preliminary data indicate that CB2 receptor antagonism reduces obesity, insulin resistance and fatty liver in an experimental model of obesity-induced NAFLD (12).
The EC system and liver fibrosis
Liver fibrosis is the common wound-healing response to chronic hepatic injury, regardless of the underlying etiology. The chronic persistence of stimuli to fibrogenesis induces an exaggerated deposition of extracellular matrix, which progressively replaces the hepatocytes and disrupts the normal liver architecture, ultimately leading to cirrhosis.
In the last two decades, many signalling pathways have been shown to activate the fibrogenic cells and contribute to the accumulation of the extracellular matrix in the liver (13). Recent evidence clearly indicates that the EC system participates in this complex network system (14). The CB1 and CB2 receptors are markedly up-regulated in the cirrhotic human liver, predominantly in hepatic myofibroblasts within fibrotic septa (15, 16). Experimental studies in which CB1 and CB2 receptors were blocked using both genetic and pharmacological approaches have provided important information on the EC system-dependent regulation of both pro- and anti-fibrogenic responses in the liver (15, 16). Indeed, in mice models of liver fibrosis induced by carbon tetrachloride (CCl4), thiocetamide or bile duct ligation (BDL), CB1-deficient animals, as well as those treated with the CB1-receptor antagonist rimonabant, show a significant decrease of fibrosis compared to their wild-type littermates (15). By contrast, CB2-deficient mice present a significant increase in fibrosis following exposure to CCl4 (16).
The molecular mechanisms that mediate the activity of the EC system in liver fibrogenesis are poorly defined. EC appears to influence cell death and proliferation in fibrogenic cell types as well as modulate the immune responses involved in the wound-healing process in the liver. Recent experimental studies in isolated cell models showed that AEA induces necrosis and 2-arachidonoyl glycerol (2-AG) causes apoptosis in activated hepatic stellate cells via an increased generation of reactive oxygen species. However, these effects of EC are likely to be independent of the interaction with CB1 and CB2 receptors (17, 18).
Taken together, these data indicate that, at least in experimental liver fibrosis, CB1 and CB2 receptors have opposing effects on fibrogenesis. Interestingly, patients with chronic hepatitis C and daily marijuana consumption presented a more advanced liver fibrosis than nonconsumers, and daily cannabis use was found to be an independent predictor of fibrosis severity (19). These results suggest that the profibrogenic CB1 signals dominate over the antifibrogenic CB2 signals, and warrant the design of clinical trials aiming to evaluate the available CB1 antagonists as antifibrotic molecules. However, the results of further investigations in experimental models are awaited to answer a series of open questions, such as the identification of the EC or other ligands that interact with the CB1 and CB2 receptors, the cellular targets and the molecular mechanisms that modulate the fibrogenic process.
The EC system and cardiovascular abnormalities in cirrhosis
Hyperdynamic circulatory syndrome
Patients with advanced cirrhosis present a hyperdynamic circulatory syndrome, which results from the reduction of peripheral vascular resistance and compensatory increase in cardiac output (20). It represents the pathophysiological background of several complications of cirrhosis, such as renal retention of sodium and water, ascites and hepatorenal syndrome, hepatopulmonary syndrome and increased susceptibility to hypovolaemic and distributive shock. Moreover, the hyperdynamic circulatory syndrome, by favouring an increased splanchnic blood inflow, contributes to the onset and maintenance of portal hypertension as anterograde component.
The pathophysiological background of haemodynamic abnormalities of advanced cirrhosis is represented by vasodilation, which involves resistance arteries and is mainly located in the splanchnic circulatory area (21). As a result, a reduction in effective volaemia ensues, which, in turn, evokes the compensatory activation of neuro-humoral systems able to promote vasoconstriction and renal retention of sodium and water, such as the renin-angiotensin-aldosterone system, the sympathetic nervous system, and the secretion of arginin-vasopressin by posterior hypophysis (21).
The mechanisms responsible for arterial vasodilation are not fully clarified, but it is widely accepted that increased production of nitric oxide (NO) plays a crucial role (22). However, this is a multifactorial phenomenon in which other mediators are implicated, such as carbon monoxide and prostacyclin (23, 24). In 2001 and 2002, two experimental studies provided evidence indicating that the EC system contributes to the vasodilation and arterial hypotension of advanced liver cirrhosis (24, 25). First, the selective antagonism of CB1 receptor with rimonabant increases arterial pressure and vascular resistance in cirrhotic rats, which also present a concomitant decrease of the mesenteric arterial flow and portal pressure (25, 26). This pressor activity was peripherally mediated because injection of rimonabant in the cisterna magna of the cerebral fourth ventricle failed to influence blood pressure (26). Furthermore, infusion of monocytes isolated from cirrhotic but not from control rats induces a marked hypotensive effect in normal recipient animals (25). Finally, monocytes isolated from patients or rats with cirrhosis contain significant amount of AEA in contrast to those isolated from healthy subjects or normal animals (25, 26). As lipopolysaccharide (LPS) represents a major stimulus for EC generation in monocytes and platelets (27), it can be hypothesised that the elevated circulating levels of endotoxin, which are frequently observed in patients with advanced cirrhosis, stimulate these cells to produce large amounts of EC. These, in turn, mediate vasodilation and arterial hypotension by acting on vascular CB1 receptors, which are located in endothelial and smooth muscle cells and in perivascular nerves (28, 29).
Additional insights on the cellular mechanisms implicated in the cardiovascular effect of EC in cirrhosis have been provided by Domenicali et al. (30) who used mesenteric resistance arteries isolated from rats with CCl4-induced cirrhosis and ascites. AEA causes a greater relaxation in vessels isolated from cirrhotic rats than in vessels isolated from control animals. This effect was not influenced by the pretreatment with the NO inhibitor nitro-l-arginine methyl ester or endothelial denudation, suggesting that the endothelium-derived NO does not play a major role in this response (Fig. 1). Interestingly, pretreatment with capsaicin, which blocks the response of primary sensory nerves, fully abolished the AEA-induced relaxation. This demonstrated that the EC act on vessel adventitia, where the sensory nerves are located, rather than in the endothelial layer (Fig. 1).
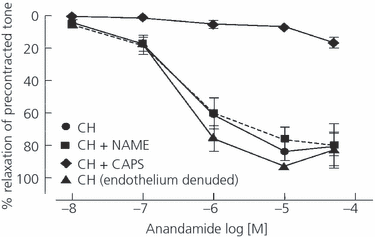
Log concentration–response curves for relaxation to anandamide in phenylephrine (10 μm) precontracted intact mesenteric arteries [cirrhotic rats (CH), n = 7], mesenteric arteries incubated with nitro-l-arginine methyl ester (l-NAME; 100 μm, n = 10), endothelium denuded mesenteric arteries (n = 5), and mesenteric arteries incubated with capsaicin (CAPS; 10 μm, n = 8) of cirrhotic rats. P < 0.001: CH + CAPS versus all other conditions. Reprinted with permission from ref. 30.
However, the CB1 receptor does not appear to be the only ligand implicated in the EC-mediated vasorelaxation. AEA can also interact with the transient receptor potential vanilloid type 1 protein (TRPV1), which is also known as VR1 receptor and is expressed in perivascular nerves (31). Both the CB1 receptor antagonist rimonabant and capsazepine, which blocks the vanilloid receptor, produce a rightward shift of the dose–response curve to AEA, and their concomitant use almost fully prevents the AEA-induced vasorelaxation (Fig. 2). Thus, both CB1 receptor and TRPV1 likely mediate this AEA effect in mesenteric arteries of cirrhotic rats (30).

Log concentration–response curves for relaxation to anandamide in phenylephrine (10 μm) precontracted intact mesenteric arteries [cirrhotic rats (CH), n = 6], mesenteric arteries incubated with SR141716A (SR; 3 μm, n = 6), mesenteric arteries incubated with capsazepine (CZ; 5 μm, n = 4), and mesenteric arteries incubated with SR141716A plus capsazepine (CZ + SR; n = 5) of cirhotic rats. P < 0.01: CH versus CH + SR and CH versus CH + CZ + SR; P < 0.05, CH versus CH + CZ. Reprinted with permission from ref. 30.
The haemodynamic alterations that give rise to the hyperdynamic circulatory syndrome do not involve all the vascular regions to the same extent. There is unanimous agreement on the fact that the renal circulation is characterised by vasoconstriction, whereas the splanchnic circulation is vasodilated (21) Interestingly, the AEA-mediated vasodilation observed in mesenteric resistance arteries cannot be reproduced in femoral arteries. Moreover, although the CB1 mRNA and protein expression is enhanced in mesenteric vessels of cirrhotic rats, no expression can be detected in femoral arteries, irrespective of whether they are isolated from cirrhotic or control animals (30).
The findings arising from experiments in isolated vessels have been substantially confirmed in an investigation conducted in vivo using rats with cirrhosis induced by bile duct ligation (31), supporting the assumption that EC, mainly AEA, contribute to hyperdynamic circulation in cirrhosis by inducing vasodilation in the splanchnic circulation via activation of the CB1 receptor and TRPV1.
Beside liver fibrosis, arterial vasodilation represents the pathophysiological background of fluid retention and ascites formation in cirrhosis, as they promote portal hypertension and impair effective volemia, eventually leading to renal retention of sodium and water (21). Preliminary data from our group indicate that the prolonged administration of the CB1 receptor antagonist Rimonabant increases urinary sodium excretion and reduces the incidence and accumulation of ascites in a rat model of pre-ascitic CCl4-induced cirrhosis, and such a result is mainly achieved by blunting the hyperdynamic circulatory syndrome (32).
Taken together, these data clearly indicate that the EC triggers deleterious effects in advanced cirrhosis, and suggest that the pharmacological CB1 receptor antagonism can represent a future approach for the treatment of the hyperdynamic circulatory syndrome and related complications.
It should be noted, however, that almost all the knowledge on the EC system in cirrhosis derives from experimental studies, and only scarce and somewhat disappointing information is available in human cirrhosis. The concentration of AEA has been reported to be elevated in monocytes (25) and plasma (33) of cirrhotic patients, but no correlations have been found between its plasma levels and the extent of arterial vasodilation, the severity of portal hypertension or the degree of hepatic and renal dysfunction (33). We also found very high plasma concentrations of two other EC, N-oleoyl-ethanolamine (OEA) and N-palmitoyl-ethanolamine, in cirrhotic patients compared to those measured in age- and sex-matched healthy individuals (P. Caraceni, unpublished data), but the clinical significance of this finding remains elusive.
Cirrhotic cardiomiopathy
The study of cardiac function in patients with cirrhosis is currently receiving growing interest. Abnormalities in systolic and mainly diastolic function have long been recognised, irrespective of the aetiology of the disease; these findings have led to the adoption of the term cirrhotic cardiomyopathy, which, however, still awaits a precise and universally accepted definition. The contractility abnormalities are usually subclinical, but can be unveiled in certain conditions, such as physical exercise, transjugular intrahepatic porto-systemic shunt, and liver transplantation, or contribute to severe complications of cirrhosis, such as hepatorenal syndrome (34). In addition to altered contractility, electrophysiological abnormalities have also been described, such as electromechanical uncoupling, chronotropic incompetence and, more recently, the electrocardiographic QT interval prolongation, which represents the potential substrate for life threatening ventricular arrhythmias (35).
The first indication that the EC system is implicated in the pathogenesis of the cirrhotic cardiomiopathy was provided by Gaskary et al. (36) who showed that cardiac papillary muscles isolated from rats with BDL cirrhosis present a blunted contractile response to isoprotenerol, and that this is restored by the administration of the CB1 receptor antagonist AM 251. Although dose–response curves to AEA, as well as CB1 and CB2 receptor mRNA and protein expressions, do not differ between control and cirrhotic rats, indirect experimental evidence suggests that, in the latter case, heart EC synthesis in response to stress, such as tachycardia and haemodynamic overload, is increased (36). A recent study in rats with CCl4-induced cirrhosis indicates that the inhibition of β-adrenergic responsiveness is also present in vivo and is mediated via the activation of the CB1 receptors as a result of an increased production of AEA in the heart (37). As in isolated papillary muscles, treatment with AM251 improves contractile function (37), suggesting a therapeutic potential of CB1 receptor antagonists.
The EC system and liver IR injury
Normothermic IR injury is an important determinant in the pathogenesis of liver damage occurring during surgical procedures, such as hepatic resection and liver transplantation, and during clinical conditions, including ischaemic hepatitis and multiple organ failure syndrome. Interruption of blood flow to the liver is necessary to control bleeding during partial liver resection. Healthy livers can safely tolerate up to 60 min of normothermic ischaemia but longer periods may cause postoperative liver failure (38). Warm ischaemia is also an important component of the liver injury associated with the transplant procedure. Indeed, a prolonged rewarming time during implantation of the organ in the recipient is considered an important factor for predicting early graft dysfunction (39).
IR is a complex phenomenon resulting from the interaction of a series of pathogenetic events occurring during the transient ischaemia and the subsequent resupply of blood. In the early phase of reperfusion, liver injury is related to Kupffer cell activation, the release of pro-inflammatory mediators and the generation of reactive oxygen/nitrogen species. These events subsequently induce infiltration of the liver by activated polymorphonuclear leukocytes, which are responsible for the tissue injury occurring after 6–12 h from reperfusion (40).
Recent evidence from animal experimental models indicates that EC are involved in the pathogenesis of IR injury. Reperfusion, but not ischaemia alone, triggers a marked elevation of the plasma levels of AEA and 2-AG (41), as well as of the hepatic concentrations of AEA, 2-AG, and OEA (2), to which both hepatocytes and nonparenchymal cells (Kupffer and endothelial cells) contribute. Well-known mediators of IR injury, such as oxidative/nitrosative molecules (H2O2 and peroxynitrite) and inflammatory stimuli (LPS and TNF-α), are capable of inducing EC production in primary cultured cells. Consistently, the hepatic content of AEA and 2-AG was found to correlate with the plasma concentrations of transaminases and pro-inflammatory cytokines (2).
Batkai et al. (2) proposed that the activation of the hepatic EC system following IR can limit the degree of tissue injury by stimulating the CB2 receptors. Indeed, pretreatment of mice with the CB2 receptor agonist JWH133, which has been reported to be 200-fold more selective to CB2 than CB1 receptor (42), decreases the degree of liver tissue injury and inflammatory cell infiltration, as well as tissue and serum levels of cytokines/chemokines/adhesion molecules and the hepatic extent of lipid peroxidation. Furthermore, genetical ablation of the CB2 receptor is associated with increased injury and inflammatory phenotype in mice subjected to IR (2).
As a result of these findings, targeting the CB2 receptors and stimulating their immunomodulatory activity in the inflammatory response represents a promising therapeutical approach against IR in the liver.
Reperfusion injury during liver surgery and transplantation, ischaemic hepatitis and haemorragic shock is frequently complicated by endotoxaemia as a result of bacterial and toxin translocation from the intestinal lumen into the portal blood following the loss of the gut barrier function (43, 44). Increased LPS levels have been also demonstrated in both organ donors and liver transplant recipients. Indeed, donor patients often present a shock-like state that favours intestinal bacterial translocation (45, 46), whereas most liver transplant candidates are patients suffering end-stage liver disease with high circulating endotoxin levels (47). Endotoxaemia amplifies the inflammatory response triggered by IR itself and the consequent leukocyte recruitment, which represents a rate-limiting step of this process (48).
Our group has shown that pretreatment with the Rimonabant reduces the extent of both tissue necrosis and neutrophil infiltration following rat liver partial IR with superimposed endotoxaemia. This suggests that Rimonabant may interfere with the inflammatory response implicated in reperfusion injury. Whether these effects are solely related to the interaction with the CB1 receptor or also involve other non-CB1/non-CB2 receptors remains to be determined (49).
Thus, beside activation of CB2 receptors, the antagonism of CB1 receptors may be effective against hepatic IR injury if complicated by endotoxaemia. Interestingly, this similar dual role of the EC system parallels what has been described in experimental models of chronic liver disease with inflammation and progressive fibrosis (14–16).
Conclusions
In recent years, accumulating evidence has indicated that EC and their major receptors CB1 and CB2 play a major role in the pathophysiology of liver diseases. Activation of the CB1 receptors promotes fat accumulation and triggers inflammation in NAFLD, contributes to the progression of chronic hepatitis to cirrhosis by stimulating fibrogenesis, and favours the development of severe complications of cirrhosis, such as portal hypertension, ascites formation and cirrhotic cardiomiopathy. The stimulation of CB2 receptors induces anti-fibrogenic and anti-inflammatory effects in acute and chronic conditions, including experimental liver fibrosis, NAFLD and IR.
As a result, molecules targeting the CB1 and CB2 receptors may represent potential therapeutic agents for the treatment of liver disease. At present, the CB1 antagonists possess the greatest potential and could be used to resolve fat accumulation in NAFLD and treat patients with cirrhosis as they may slow the progression of fibrosis and attenuate the cardiovascular alterations associated with the advanced stage of the disease.
Conflicts of interest
Financial support was provided in part by the Ministero dell’Istruzione, dell’Università e della Ricerca (MIUR) – Progetto di ricerca di interesse nazionale 2001 (ex-40%), by the Fondazione Cassa di Risparmio in Bologna, Italy, and by a research grant from Sanofi-Aventis. PC has received research grants and acted as consultant for Sanofi-Aventis, France, Italy. MD and MB have declared no conflicts of interest.




