Intracoronary enalaprilat during angioplasty for acute myocardial infarction: alleviation of postischaemic neurohumoral and inflammatory stress?
Abstract.
Aims. Reperfusion after myocardial ischaemia is associated with a distinct ischaemia/reperfusion injury. Since ACE-inhibition, beyond its influence on cardiac angiotensin II formation and kinin metabolism, has been shown to be cardioprotective by decreasing leucocyte adhesion and endothelin-1 (ET-1) release, we investigated the effects of intracoronary (i.c.) enalaprilat during primary angioplasty in acute myocardial infarction.
Methods and Results. Twenty-two patients were randomized to receive i.c. enalaprilat (50 μg) or placebo immediately after reopening of the infarct-related artery (IRA). Plasma concentrations of soluble L-selectin, P-selectin, intercellular adhesion molecule-1 (sICAM-1), vascular cell adhesion molecule-1 (sVCAM-1), ET-1 and nitric oxide metabolite concentrations (NO(x)) were measured in pulmonary arterial blood. Coronary blood flow was assessed using corrected thrombolysis in myocardial infarction (TIMI) frame counts (CTFC). During reperfusion, there was a significant increase in sL-selectin, sP-selectin and ET-1 in the placebo group, which was greatly diminished by enalaprilat. Levels of sVCAM-1 and sICAM-1 were not affected in either group. CTFC in the placebo group remained higher than normal in both the IRA and nonculprit vessels, whereas myocardial blood flow improved with enalaprilat.
Conclusion. Enalaprilat as adjunct to primary angioplasty might be a protective approach to prevent leucocyte adhesion and the release of ET-1, thereby improving coronary blood flow.
Objectives
Coronary artery reperfusion by primary percutaneous coronary intervention (PCI) and thrombolysis are established methods to achieve reopening of an occluded vessel in patients with acute myocardial infarction (AMI). But there is extensive evidence that reperfusion of ischaemic myocardium by itself can result in irreversible tissue injury [1]. The vascular endothelium, strategically located between the circulating blood and the vascular smooth muscle layer, plays a key role in the control of vasomotor tone, local homeostasis and leucocyte adhesion. Postischaemic activation of endothelial cells induces sequential interactions of rapidly expressed adhesion molecules and their cognate ligands on leucocytes and platelets [2]. Raised plasma levels of soluble forms of adhesion molecules have been identified in numerous patients with acute coronary syndromes [3–5]. Upon reperfusion, the inflammatory response, being associated with decreased tissue microvascular perfusion, is largely influenced in an auto-/paracrine fashion by a variety of substances released from the endothelium and myocardium [6]. Notably, the activations of the cardiac renin-angiotensin system (RAS) and the cardiac endothelin (ET-1) system are important identified contributors to a low/no reflow phenomenon and myocardial damage and both systems are known to elicit pro-inflammatory responses [7–10]. ACE-inhibitors (ACE-Is) beyond their protective influence on cardiac angiotensin II formation and kinin metabolism have been shown to reduce the activation of the ET-system, to diminish ET-1-induced coronary constriction, and to reduce ET-1 secretion [11]. In this context, ACE-Is because of their multiple actions provide an ‘appealing approach’ to prevent ischaemia/reperfusion (I/R) injury.
We hypothesized that intracoronary (i.c.) infusion of enalaprilat (50 μg), which has previously been shown to be efficient in potentiating bradykinin (BK) and to be safe in the setting of reperfusion therapy [12], might additionally improve coronary blood flow by preventing leucocyte recruitment and ET-1 release in a BK/NO-dependent pathway. To address this postulate, we measured the levels of nitrate/nitrite (as stable indirect markers of NO), ET-1 and the major soluble adhesion molecules before and during reperfusion, and used corrected TIMI frame counts to assess the coronary flow.
Methods and design
The study population consisted of 22 consecutive patients who were admitted to the coronary care with their first myocardial infarction (MI, ST-segment elevation, refractoriness of angina to nitrates, onset of symptoms persisting no longer than 6 h). Patients were randomly assigned to receive mechanical reperfusion therapy combined with i.c. administration of enalaprilat (50 μg) or placebo as previously reported [12]. Patients were excluded for the following reasons: history of previous MI or cardiomyopathy, severe valvular disease, left bundle branch block or permanent pacemaker, chronic renal failure, previous ACE-inhibitor treatment, cardiogenic shock (Killip class IV) or need of i.v. catecholamines, connective tissue disease, advanced liver disease, malignant disease and infectious disorders. According to standard treatment for AMI, all patients were set on an i.v. heparin (5000 IU bolus followed by continuous infusion of 1000 IU h−1) and a single dose of acetylsalicylic acid (500 mg).
Acute catheterization, angiography, angioplasty procedure and blood sampling
In brief, a transfemoral arterial and venous access was gained in all patients and a 7-French ‘multipurpose’ catheter was positioned in the left pulmonary artery. Primary angioplasty was performed in a routine fashion. Prior to positioning the guidewire by a 6-French guiding catheter, blood was drawn from the femoral sheath and pulmonary arterial line. Immediately after reopening the infarct-related artery (IRA) by primary angioplasty (onset of reperfusion, with at least TIMI Flow ≥2), either enalaprilat (50 μg) or placebo was administered through the guiding catheter into the IRA over 2 min. Further blood samples were drawn 2, 10 and 60 min after enalaprilat/placebo administration and repeated balloon inflations or coronary stenting was performed until an acceptable result of the angioplasty (<25% residual stenosis) was achieved. If coronary flow was compromised by visible coronary thrombus, abciximab was additionally administered as previously described [12]. All patients were admitted to the coronary care unit after the procedure. The protocol of the study has been previously approved by the local Ethics Committee and complies with the principles outlined in the Declaration of Helsinky, and all patients gave written informed consent.
Corrected TIMI Frame Count (CTFC)
As a quantitative method of assessing coronary artery flow in coronary angiograms, the number of cineframes required for dye to reach standardized distal coronary landmarks was counted with a frame counter (MEDIS QCA-CMS Version 3.0; ELK Cine-Angio System, Nuenen, the Netherlands), and corrected for filming speed according to Gibson et al. [13, 14]. The CTFC was assessed at the end of the trial and the films of coronary angiograms were given blinded to the investigator. Coronary flow was quantified in all vessels before intervention (occluded vessel) and at the end of the last performed coronary intervention (after i.c. infusion of enalaprilat/placebo and the last performed angioplasty). Additionally, when enalaprilat was infused in the left main coronary artery, CTFCs were determined in the non-IRA (reference vessel, REF) before and after primary angioplasty of the left anterior descending artery (LAD) or left circumflex artery (LCX). That means flow was assessed in the LCX (REF) before and after PCI of the LAD (IRA) and vice versa. Contrast media were injected with hand injections, i.e. 4 mL of dye in the right coronary artery or 7 mL into the left main coronary artery, respectively.
Biochemical measurements
Plasma levels of the soluble forms of L-selectin, P-selectin, vascular cell adhesion molecule-1 and intercellular adhesion molecule-1, as well as the concentrations of ET-1, were measured with commercially available enzyme-linked immunosorbent assays (ELISAs; R&D Systems Europe, Abingdon, UK and Biomedica GmbH-A 1210 Wien, Austria, respectively). Noradrenaline (NA) was quantified by using high performance liquid chromatography (HPLC) with electrochemical detection [15] and NO(x) was determined by using a fluorometric assay according to Sakowitz et al. [16].
Statistical analysis
Data shown in graphs and tables are presented as means ± SEM. Analyses were performed with Fisher's exact test for categorical data and anova with correction for multiple comparisons for continuous data or Student's t-test. Statistical significance was accepted at the 95% confidence level (P-value below 0.05; graphpad Software Inc., San Diego, CA, USA).
Results
There were no differences in clinical characteristics, coronary risk factors, chronic medication, the extent of coronary artery disease and angiographic findings between either groups. Baseline patient characteristics of both groups are shown in Tables 1 and 2. The peak levels of LDH as well as troponin T levels at 60 min of reperfusion documented substantial myocardial damage in both groups.
| Placebo (n = 11) | Enalaprilat (n = 11) | P-value | |
|---|---|---|---|
| Age | 63 ± 4 | 64 ± 5 | 0.896 |
| Male sex n (%) | 7 (64%) | 8 (72%) | 0.995 |
| Body mass index | 27.3 ± 0.7 | 27.8 ± 1.8 | 0.709 |
| Smoker | 6 (54%) | 8 (72%) | 0.948 |
| Hypertension | 6 (54%) | 7 (64%) | 0.995 |
| Diabetes mellitus | 1 (9%) | 1 (9%) | 1.000 |
| Total cholesterol (mmol L−1) | 5.80 ± 0.18 | 5.71 ± 0.4 | 0.786 |
| Family history of CAD | 4 (36%) | 3 (27%) | 0.994 |
| Time between onset of pain and reperfusion | 3.86 ± 0.41 | 4.74 ± 0.45 | 0.155 |
| ST-segment elevation in ECG (mV) | 0.45 ± 0.07 | 0.44 ± 0.07 | 0.933 |
| Troponin T at baseline (ng mL−1) | 0.07 ± 0.022 | 0.09 ± 0.05 | 0.705 |
| Troponin T at 60 min reperfusion (ng mL−1) | 6.04 ± 2.03 | 4.12 ± 2.31 | 0.539 |
| Peak-LDH levels (U L−1) | 1013 ± 584 | 855 ± 557 | 0.539 |
| CRP-levels mg L−1) | 5.96 ± 1.87 | 6.23 ± 1.95 | 0.921 |
| WBCC (n/μL) | 9952 ± 698 | 8987 ± 662 | 0.327 |
| Platelets (n nL−1) | 298 ± 72 | 279 ± 53 | 0.833 |
| Premedication in general: | 7 | 5 | 0.920 |
| Beta blockers | 0 | 1 | 0.980 |
| Alpha blockers | 1 | 1 | 1.000 |
| Calcium channel blockers | 1 | 3 | 0.950 |
| Diuretics | 3 | 1 | 0.950 |
| Acetylsalicylic acid | 1 | 3 | 0.950 |
- The baseline characteristics of the patient population demonstrates no differences in clinical characteristics, coronary risk factors and chronic medication. The peak levels of cardiac enzymes documented substantial myocardial damage in both groups.
| Placebo (n = 11) | Enalaprilat (n = 11) | P-value | |
|---|---|---|---|
| Number of coronary vessel disease 1/2/3 | 6/0/5 | 5/2/4 | 0.996 |
| Infarct related vessel LAD/LCX/RCA | 7/1/3 | 7/0/4 | 0.995 |
| Contrast media used (mL) | 273.2 ± 26.5 | 256.2 ± 25.2 | 0.645 |
| Stenting | 8 (72%) | 7 (64%) | 0.994 |
| Baseline heart rate | 81.2 ± 3.8 | 85.3 ± 6.6 | 0.599 |
| Baseline systolic blood pressure | 131.6 ± 7.6 | 129.8 ± 3.3 | 0.864 |
| Baseline diastolic blood pressure | 81.9 ± 4.6 | 75.7 ± 3.4 | 0.288 |
| Cardiac index (l min m2) | 2.7 ± 0.2 | 2.6 ± 0.1 | 0.934 |
| Pulmonary capillary wedge pressure (mmHg) | 19.2 ± 2 | 17.2 ± 2 | 0.856 |
- Angiographic findings during the baseline assessment indicates no major differences of the extent of coronary artery disease and haemodynamics between both groups. In addition, there were no differences in terms of volume of contrast medium and stent-implantation.
During reperfusion, there was an immediate significant increase in pulmonary arterial ET-1 (P < 0.001), which was greatly diminished by i.c. enalaprilat (only ET-1 at 60 min REP versus baseline, P = 0.032; Fig. 1a). The calculated/normalized net release (equation: pulmonary arterial blood concentration normalized to baseline [%] – central venous blood concentration normalized to baseline [%]) demonstrated that ET-1 was mainly released from the heart. A much lower net release of ET-1 was observed after i.c. enalaprilat (Fig. 1b). The NA levels in the circulation were not influenced by i.c. enalaprilat and remained elevated throughout the observation window (Fig. 1(c,d)) in either group, when compared with patients who had undergone elective angioplasty in the past (263 ± 33 pg mL−1 [15]).
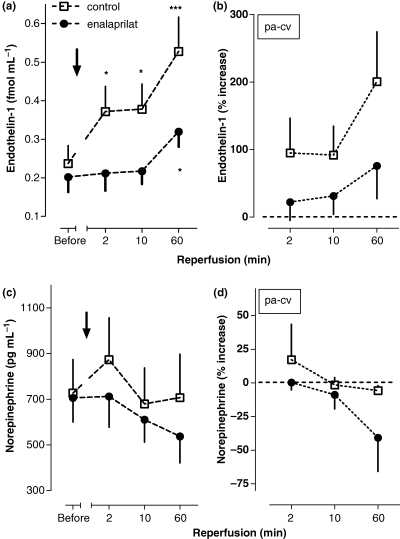
(1a) Plasma levels of endothelin-1 (ET-1) in pulmonary-arterial blood before and after reopening (2, 10 and 60 min after drug administration) of the infarct vessel by primary percutaneous coronary intervention (PCI) combined to intracoronary (i.c.) administration of enalaprilat or placebo in patients with acute myocardial infarction (AMI). (n = 11), Mean ± SEM, *denotes P < 0.05, **<0.01, ***<0.001 referring to absolute values at baseline. (1b) Approximated net release of ET-1 (% to baseline) from the heart, calculated as the difference between pulmonary arterial and central venous blood concentrations either after i.c. enalaprilat or placebo administration (2, 10 and 60 min), (n = 11), Mean ± SEM. (1c) Plasma levels of noradrenaline (NA) in pulmonary-arterial blood before and after reopening (2, 10 and 60 min after drug administration) of the infarct vessel by primary PCI combined to i.c. administration of enalaprilat or placebo in patients with AMI; (n = 11), Mean ± SEM. (1d) Approximated net release of NA (% to baseline) from the heart, calculated as the difference between pulmonary arterial and central venous blood concentrations either after i.c. enalaprilat or placebo administration (2, 10 and 60 min), (n = 11), Mean ± SEM.
As previously shown, i.c. enalaprilat significantly potentiated pulmonary arterial BK concentrations (P < 0.05, Fig. 2(a,b);[12]), which were accompanied by a ∼20% increase in NO(x) (P = 0.03; Fig. 2(c,d)). In addition, the increase in NO(x) over baseline demonstrated a positive correlation with increasing levels of BK (Fig. 3).
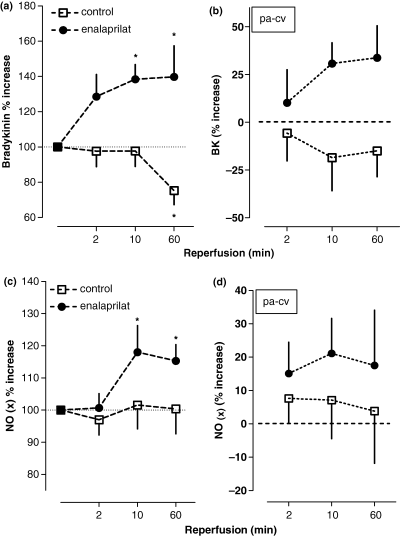
(2a) Relative changes of bradykinin (BK) in pulmonary-arterial blood compared with baseline at 2, 10 and 60 min after reopening of the infarct vessel by primary percutaneous coronary intervention (PCI) and intracoronary (i.c.) administration of enalaprilat or placebo in patients with acute myocardial infarction (AMI); (n = 11), Mean ± SEM, *denotes P < 0.05 referring to absolute values. (2b) Approximated net release of BK (% to baseline) from the heart, calculated as the difference between pulmonary arterial and central venous blood concentrations either after i.c. enalaprilat or placebo administration (2, 10 and 60 min), (n = 11), Mean ± SEM. (2c) Relative changes of NO(x) in pulmonary-arterial blood compared to baseline at 2, 10 and 60 min after reopening of the infarct vessel by primary PCI and i.c. administration of enalaprilat or placebo in patients with AMI; (n = 11), Mean ± SEM, *denotes P < 0.05 referring to absolute values. (2d) Approximated net release of NO(x) (% to baseline) from the heart, calculated as the difference between pulmonary arterial and central venous blood concentrations either after i.c. enalaprilat or placebo administration (2, 10 and 60 min), (n = 11), Mean ± SEM.
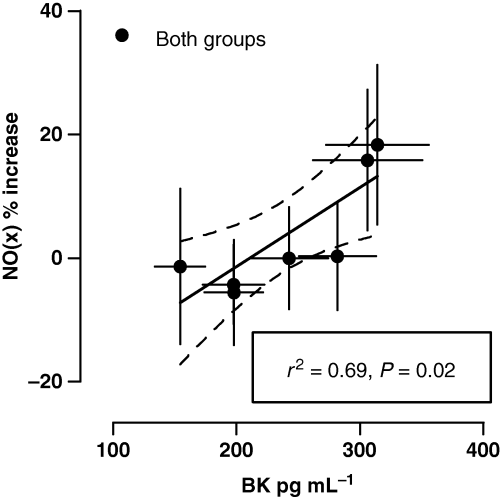
Positive correlation between relative changes of NO(x) in pulmonary-arterial blood and rising levels of bradykinin.
Additionally, i.c. enalaprilat – in contrast to placebo – abolished the observed increase in sL-selectin (placebo, P = 0.027) and sP-selectin (placebo, P = 0.01) during reperfusion (Fig. 4(a,b)), whereas the levels of sICAM-1 and sVCAM-1 did not significantly change in either group during the observation window of 60 min [placebo (ng mL−1): sVCAM-1 before REP: 334.2 ± 21.8; 60 min REP: 335.3 ± 20.0 and sICAM-1 before REP: 213.8 ± 18.9; 60 min REP: 217.5 ± 17.5 vs. enalaprilat (ng mL−1): sVCAM-1 before REP: 328.2 ± 21.8; 60 min REP: 346.7 ± 22.8 and sICAM before REP: 206.8 ± 12.0; 60 min REP: 211.2 ± 9.2].
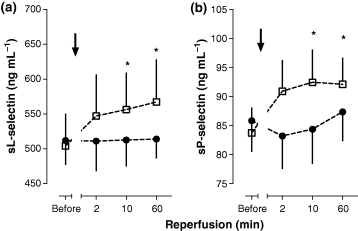
(4a) sP-selectin and (4b) sL-selectin in pulmonary-arterial blood before and after reopening of the infarct vessel by primary percutaneous coronary intervention combined to intracoronary administration of enalaprilat or placebo (before, 2, 10 and 60 min after drug administration) in patients with acute myocardial infarction; (n = 11), Mean ± SEM, *denotes P < 0.05, referring to absolute values at baseline.
In each group, total coronary occlusion (TIMI 0) was observed in nine patients and two patients had partial reperfusion during the baseline assessment (TIMI 1–2; E: CTFC 66 and 47: P CTFC 42 and 43). PCI was performed in 14 anterior wall infarctions (C: n = 7: E: n = 7), 1 postero-lateral infarction (E: n = 1) and 8 inferior infarctions (C: n = 4, E: n = 3). The assessment of CTFC post-PCI revealed that after i.c. enalaprilat infusion coronary blood flow improved to a borderline significance in the IRA (seven patients with <28 frames, four patients with >28 frames) compared with the control group (only three patients obtaining < 28 frames and eight patients having > 28 frames; Fig. 5; P = 0.054). Three patients received abciximab after completion of the experimental protocol because of persistent elevated CTFC in the IRA (C n = 2: CTFC 58 and 52; E n = 1: CTFC 43). Additionally, nonculprit coronary arteries demonstrated a distinct flow reduction throughout the whole coronary vascular bed during the baseline assessments (normal 21 [14–28 frames] [13, 14, 17, 18]; E: 26.9 ± 1.2 and C: 26.8 ± 1.2). Enalaprilat if injected into the left main coronary artery, significantly increased the flow (−5.3 frames) in nonculprit vessels (i.e. the LCX), after PCI of the LAD (IRA) was successfully performed and vice versa, indicating improved blood supply to the infarct border zone. Conversely, flow remained unchanged after placebo administration into the left main coronary artery (nonculprit vessel +1.7 frames; see also Table 3 and Fig. 6; P < 0.01, see Table 3). Fifteen patients received stents (n = 8 placebo vs. n = 7 enalaprilat). In general, CTFC was not related to stent implantation, duration of ischaemia, the degree of vessel stenosis and CKmax. (data not shown). There were no differences concerning CRP-levels, WBCCs, platelet counts between both groups during the subsequent 10 days of observation (data not shown). No patient had a cardiac event until hospital discharge. The IRA was always found open at follow-up angiography (with no differences in CTFC; data not shown).
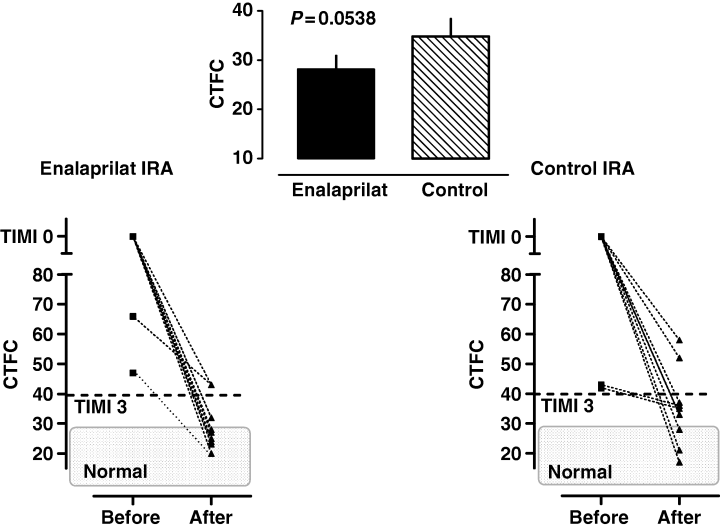
Corrected TIMI frame counts (CTFC) in the infarct-related artery before percutaneous coronary intervention and at the end of intervention (incl. administration of enalaprilat or placebo). Mean ± SEM of achieved CTFC at the end of intervention are shown in the inset between both graphs; (n = 11).
| IRA (stenosis/CTFC) | REF (stenosis) | CTFC REF before/after PCI | |
|---|---|---|---|
| Placebo | |||
| #1 | LAD (100%– TIMI 0) | LCX (<25%) | 32/26 |
| #2 | LAD (100%– TIMI 0) | LCX (25%) | 25/25 |
| #3 | LAD (99%– 43) | LCX (50%) | 28/37 |
| #4 | LAD (100%– TIMI 0) | LCX (25%) | 26/43 |
| #5 | LAD (99%– 42) | LCX (<25%) | 25/23 |
| #6 | LAD (100%– TIMI 0) | LCX (<25%) | 31/31 |
| #7 | LAD (100%– TIMI 0) | LCX (<25%) | 21/22 |
| #8 | LCX (100%– TIMI 0) | LAD (25–50%) | 40/35 |
| CTFC mean | 28.5/30.25 | ||
| Enalaprilat | |||
| #1 | LAD (100%– TIMI 0) | LCX (25–50%) | 25/21 |
| #2 | LAD (99%– 66) | LCX (<25%) | 26/21 |
| #3 | LAD (100%– TIMI 0) | LCX (25–50%) | 30/20 |
| #4 | LAD (100%– TIMI 0) | LCX (25–50%) | 24/20 |
| #5 | LAD (99%– 47) | LCX (<25%) | 20/22 |
| #6 | LAD (100%– TIMI 0) | LCX (25%) | 35/26 |
| #7 | LAD (100%– TIMI 0) | LCX (<25%) | 25/18 |
| CTFC mean | 26.4/21.1 | ||
- Subset of patients that were treated for acute coronary occlusion of the left coronary artery system. Coronary flow as assessed by CTFC and the grade of coronary stenosis are shown before and after PCI in the infarct-related artery as well as in the reference vessel. Enalaprilat, if injected into the left main coronary artery, significantly increased the flow (−5.3 frames) in nonculprit vessels. Conversely, flow remained unchanged after placebo administration into the left main coronary artery (nonculprit vessel +1.7 frames; P < 0.01; see also Fig. 4).
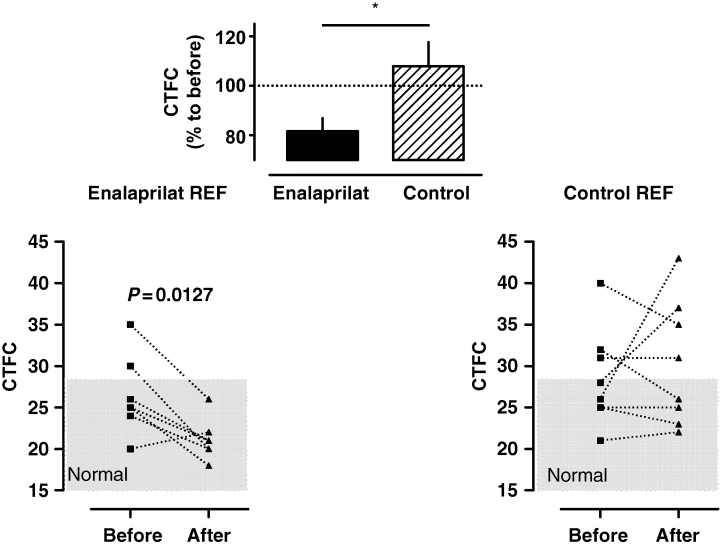
Corrected TIMI frame counts (CTFC) in nonculprit vessels of left coronary artery branches before and after intervention of the IRA (incl. administration of enalaprilat or placebo into the left main coronary artery). Only the reference vessels of posterolateral (i.e. LAD) and anterior wall infarctions (i.e. LCX) are analysed. Per cent changes to before percutaneous coronary intervention of CTFC after completion of the protocol are shown in the inset between both graphs; (n = 7–8).
Discussion
There is a growing body of evidence that infiltrating leucocytes, released ET-1, and the activation of the cardiac RAS are key factors in early I/R-injury [19–22]. In this context, ACE-inhibiton might be particularly protective during I/R [23]. In the past, ACE inhibitors have been described to reduce myocardial ischaemia significantly through modulation of neurohormonal and sympathetic activation and, subsequently, by preventing systemic vasoconstriction [24, 25].
We previously reported that i.c. administration of low-dose enalaprilat during primary angioplasty reduces ventricular arrhythmias, stabilizes haemodynamics, and improves left ventricular ejection fraction in association with potentiation of BK [12]. In addition, favourable effects of i.c. enalaprilat infusion (increase in lumen diameter and coronary blood flow [26] as well as alleviation of diastolic dysfunction [27]) have previously been reported by others. As the ET-system and RAS are known to be tightly interrelated[11, 22, 28], we hypothesized that the infusion of enalaprilat might be associated with a decrease of leucocyte recruitment and ET-1 release in a BK/NO-dependent manner, thereby possibly improving coronary blood flow. Indeed, the principal findings of this study are that the adjunctive infusion of enalaprilat to primary PCI reduces postischaemic leucocyte adhesion (indicated by reduced shedding of adhesion molecules), diminishes early neurohumoral activation, and improves myocardial perfusion.
Neutrophil adhesion is largely believed to be responsible for the production of microvascular dysfunction and no-reflow during I/R. Accordingly, neutrophil depletion or prevention of leucocyte adhesion with monoclonal antibodies, directed against specific membrane-associated adhesive glycoproteins, is known to exert a marked cardioprotection in animal experiments [19–21]. Unfortunately, adjunctive treatment with an anti-CD18 antibody in conjunction with fibrinolysis has failed to exert beneficial cardiac effects in humans in two randomized multicentre studies, making dubious at least the isolated concept of inhibiting neutrophil adhesion in humans (HALT, LIMIT AMI) [29, 30].
In general, soluble forms of adhesion molecules are considered to be indicators of activated endothelial cells, platelets and leucocytes [20]. In patients with AMI, unstable angina, and coronary spasm, increased levels of selectins[4, 31, 32], ICAM-1 [33–35] and VCAM-1 [36–39] have been reported. Likewise after recanalization, we found a rapid increase in plasma concentrations of sL-selectin and sP-selectin in the control group. L-selectin and P-selectin are known to be up-regulated (P selectin is additionally released from Weibel–Palade bodies of endothelial cells and alpha-granules of platelets) within minutes during I/R and they are significantly involved in increased leucocyte adherence within 20 min of reperfusion [40, 41]. In contrast, because of their transcriptional regulation, VCAM-1 and ICAM-1 are mainly expressed hours later, with a slow increasing kinetics. Accordingly, it is not surprising that the levels of sICAM-1 and sVCAM-1 remained unchanged within 60 min of reperfusion in both groups. In the present study, i.c. enalaprilat was able to suppress the increases in sL-selectin and sP-selectin. This anti-inflammatory action of enalaprilat is probably the result of kinin-potentiation in local endothelial compartments [12]. A possible simultaneous local inhibition of angiotensin II-formation cannot be ruled out, but pulmonary arterial angiotensin II-levels remained unchanged in both groups as previously shown [31]. Both, BK-potentiation and inhibition of angiotensin II-formation are known to reduce inflammatory responses [42]. It is important to note that the protective effects of BK are divergent and strongly concentration dependent, with lower concentrations of BK leading to protection (decrease in infarct size, attenuation of postischaemic leucocyte adhesion and microvascular barrier disruption), whereas higher concentrations of BK are pro-inflammatory (increased protein extravasation [43], accelerated P-selectin expression and enhanced chemotactic activity for neutrophils [44]) and pro-arrhythmogenic because of accelerated NA spill-over [45]. To sum up, only a modest increase in BK is needed to exert a protective profile. The finding that i.c. enalaprilat does not influence the pulmonary arterial NA concentration strengthens the hypothesis that only a modest increase in BK, most likely contained in the intravascular compartment, was induced. Nevertheless, NA levels were significantly elevated before and during reperfusion, illustrating the well known fact of increased sympathetic stress during I/R [15]. In this study, reperfusion was associated with only minor effects on plasma NO levels as indicated by small changes in the plasma nitrite/nitrate concentration in the control group, whereas i.c. enalaprilat significantly increased NO(x)-levels. This observation is in accordance with the dynamic changes that are influenced in a protective fashion with i.c. enalaprilat. Postischaemic leucocyte recruitment, microvascular barrier dysfunction and organ dysfunction are usually associated with a reduction in NO production [46, 47]. Hence, diminished shedding of selectins might involve a mechanism whereby BK-induced NO production inhibits postischaemic leucocyte sequestration after i.c. enalaprilat. Indeed, expression of endothelial P-selectin is reduced by the administration of NO donors [48]. Thus, BK might act to limit leucocyte rolling, which is a prerequisite of firm adhesion and emigration via NO-mediated inhibition of P-selectin expression. Beside the pro-coagulant activity of P-selectin other cell–cell interactions such as bridging of different cell types (leucocyte–leucocyte, platelet–platelet, platelet–monocyte) might additionally be influenced by i.c. enalaprilat, thereby taking impact on microvascular blood flow. The assumption that BK is very likely to be potentiated after i.c. injection of enalaprilat (50 μg) in humans is supported by the finding of Kuga et al., demonstrating that even in the absence of myocardial ischaemia, i.c. injection of enalaprilat (50 μg) potentiated a BK-induced dilatation of coronary arteries (increase in coronary diameters, blood flow and vascular conductance). Pretreatment with NG-monomethyl-l-arginine (l-NNMA) significantly reduced BK-induced vasodilatation in their study, indicating that BK mediates its effects mainly by endothelium-derived NO in vivo [27].
In addition, enalaprilat strongly reduces ET-1 release during reperfusion, demonstrating that ACE-inhibition is a valuable approach to interrupt the interacting pathways of the cardiac RAS and ET-system. ET-1 is a very potent prolonged acting constrictor of coronary arteries both in vitro and in vivo [49] and elevated ET-1 tissue expression/immunoreactivity and plasma levels of ET-1 have been observed in numerous studies [50, 51]. In general, ET-1 is one of the recognized key mediators during myocardial ischaemia in association with impaired reperfusion [15, 22, 52]. In addition to its direct vasoconstrictor effects, ET-1 enhances NA-induced vascular contractions [53], aggravates adhesiveness of leucocytes to endothelial cells [54], and stimulates surface expression of CD11b/CD18 on human neutrophils [10]. Previous animal studies have demonstrated that ACE-Is prevent the activation of the ET-system [55], diminish ET-1-induced coronary constriction [28], and reduce ET-1 secretion through both removal of endogenous angiotensin II and accumulation of endogenous BK/NO [11, 56]. Therefore, the finding of an enalaprilat-related reduction of ET-1 release in human reperfused MI is an exciting observation and we suggest that the suppressed ET-1 release after i.c. enalaprilat is linked to increases in BK/NO with subsequent improved coronary blood flow. In fact, we found a smaller CTFC (28.2 ± 2.0) after i.c. enalaprilat compared with placebo (34.8 ± 2.4). Multiple studies have shown that CTFC is quite reproducible with only one to two frames difference between various observers. Increases in CTFC post-PCI (culprit flow) are strongly related to increases in mortality [13, 57]. Furthermore, it has been shown that flow remains elevated (>28 frames) in culprit vessels during uncomplicated reperfusion therapy. Our measurements demonstrate similar frame counts compared with previous reports. We observed that PCI in conjunction with i.c. enalaprilat improved flow in the IRA, thus achieving a similar CTFC range as all nonculprit arteries before PCI (26.8 ± 1.1 frames). Importantly, recent studies have demonstrated that the flow in nonculprit arteries in the setting of acute coronary syndromes is reduced by 40% compared with normal (30.5 frames) [14, 17, 18]. It is notable that a slower flow throughout all three arteries in MI is associated with adverse outcomes, poorer wall motion scores [17], and poorer tissue perfusion (DSA) [18]. Here, we demonstrate that enalaprilat significantly increases the flow (−5.3 frames) in nonculprit vessels thereby possibly increasing oxygen supply to the infarct border zone. In contrast, the flow remained persistently slowed (+1.7 frames) in the nonculprit arteries after placebo administration. A steal phenomenon after enalaprilat is unlikely, as the flow in the culprit artery as well as in the reference vessel is improved compared with placebo. It could be speculated that the poorer flow in the control group (IRA and nonculprit arteries) might be the result of more a pronounced vasoconstriction mediated through both an increased ET-1 release and enhanced leucocyte trapping in the shared microvasculature. We previously reported that CTFC after successful PCI correlated with ET-1-levels (P < 0.01; r = 0.61) and NA (P = 0.01; r = 0.58) during reperfusion [15]. In addition, Gregorini et al. [58] demonstrated that the CTFC and fractional wall shortening was improved in both the culprit and nonculprit arteries after administration of α-blockers. Most likely, an intense neurohumoral activation with multiple cross-talking systems, augmenting postischaemic leucocyte adhesion, is at least partly, if not largely, responsible for a decreased epicardial and microvascular blood flow after PCI.
Limitations
The short observation window and the small number of investigated patients might be considered as a limitation. Furthermore, we did not cannulate, as requested by the local ethical committee, the coronary sinus to take coronary venous samples. However, it is tempting to speculate that the differences we observed would have been even more pronounced.
In conclusion, intracardiac infusion of enalaprilat as adjunct to PCI might be a promising approach to prevent leucocyte adhesion and the release of ET-1 in addition to its favourable effects on haemodynamics, and heart rhythm, thereby improving coronary blood flow and LV-function after AMI. Elucidation of the clinical relevance awaits larger studies with later end-points.
Conflict of interest statement
No conflict of interest was declared.
Acknowledgements
We are very grateful to Cindy Krause for her technical assistance. We disclose any conflicts of interest, the study was in part supported by a grant (KU 774/3–1) from the Deutsche Forschungsgemeinschaft.




