Differences in Ichthyophonus prevalence and infection severity between upper Yukon River and Tanana River chinook salmon, Oncorhynchus tshawytscha (Walbaum), stocks
Abstract
Two genetically distinct populations of chinook salmon, Oncorhynchus tshawytscha (Walbaum), were simultaneously sampled at the confluence of the Yukon and Tanana rivers in 2003. Upper Yukon-Canadian fish had significantly higher infection prevalence as well as more severe infections (higher parasite density in heart tissue) than the lower Yukon-Tanana River fish. Both populations had migrated the same distance from the mouth of the Yukon River at the time of sampling but had significantly different distances remaining to swim before reaching their respective spawning grounds. Multiple working hypotheses are proposed to explain the differences between the two stocks: (1) the two genetically distinct populations have different inherent resistance to infection, (2) genetically influenced differences in feeding behaviour resulted in temporal and/or spatial differences in exposure, (3) physiological differences resulting from different degrees of sexual maturity influenced the course of disease, and (4) the most severely infected Tanana River fish either died en route or fatigued and were unable to complete their migration to the Tanana River, thus leaving a population of apparently healthier fish.
Introduction
Ichthyophonus is a widely distributed protozoan parasite, primarily of marine and cultured fish, that belongs to the class Mesomycetozoea, an ancient phylogenetic group that includes the genera Dermocystidium, Rhinosporidium, Ichthyophonus, Psorospermium and Sphaerothecum (Mendoza, Taylor & Ajello 2002). Ichthyophoniasis, the pathological condition caused by this organism, is an emerging disease of chinook salmon, Oncorhynchus tshawytscha (Walbaum), in several major watersheds throughout Alaska including the Yukon, Kuskokwim and Taku rivers (Kocan, Hershberger & Winton 2003, 2004), as well as sockeye salmon, Oncorhynchus nerka (Walbaum), from British Columbia (Tierney & Farrell 2004).
The epidemiology of Ichthyophonus in Yukon River chinook salmon was described, along with observations of significantly different infection prevalences and parasite density between fish in the upper Yukon River mainstem and those in the Tanana River, a major tributary to the lower Yukon River by Kocan et al. (2003). Fish sampled at river km 1175 (river mile 730) and bound for the upper Yukon River and Canada had infection prevalences ≥39%, while those sampled 64 km (40 miles) downstream at river km 1110 (RM 690) and bound for the Tanana River exhibited <25% infection prevalence and presented with lower parasite density than fish migrating to the upper Yukon River. These differences in prevalence and severity of disease seemed incongruous because all fish were sampled within 64 km of each other (<1 day's migration) and after migrating for a similar period of time.
Fish travelling the north shore of the Yukon River at km 1110 were bound for the upper Yukon River and Canada, while those on the south shore were bound for the Tanana River in Alaska (Bucklis 1981; Bucklis & Barton 1984). A recent study determined that approximately 39% of the metapopulation migrated to Canada while 27% returned to the Tanana River (Eiler 2005), thus accounting for 66% of all chinook salmon entering the Yukon River in any one year. The two populations are considered to be genetically distinct (Gharrett, Shirley & Tromble 1987; Beacham, Murray & Withler 1989; Templin, Wilmot, Guthrie & Seeb 2005) and are morphologically distinguishable at this point in the Yukon River.
The objectives of the present study were to determine whether the observed differences in disease prevalence and severity between upper Yukon-Canada and Tanana River chinook salmon were real and repeatable, and if so, to utilize available data to develop hypotheses to explain the differences. The variables considered were: (1) residence (migration) time in-river, (2) distance travelled from mouth of river, and (3) proximity to spawning streams.
If sampled simultaneously, fish from both shores (north and south) should have migrated the same distance since entering the Yukon River, but the majority of Tanana River fish would have <322 km (200 river miles) remaining in their migration, while upper Yukon River-Canada fish would have 925 km (575 miles) to 1690 km (1050 miles) to travel before reaching their spawning streams (Fig. 1).
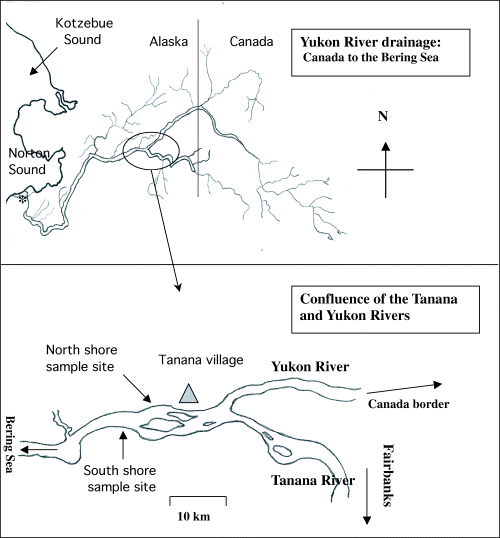
Yukon River (top) and north and south shore sample sites at river km 1110 (bottom) at the Tanana–Yukon River confluence. *Denotes approximate sample site at river mouth (river km 39).
Materials and methods
Sampling
Chinook salmon were sampled from three sites on the Yukon River in 2003 with a minimum sample size goal of 100 fish per site. The first sample was taken at river km 39 (river mile 24) by gill net during the third week of June, and the second and third samples were taken simultaneously at km 1110 by fish wheel during the first 2 weeks of July. Fish at km 39 are a mix of all Yukon River stocks, but as they migrate upriver a portion of them divert into lower Yukon tributaries. As fish migrate upriver and approach the Tanana River, the upper Yukon-Canadian-bound fish follow the north shore while Tanana River fish follow the south shore (Bucklis 1981; Bucklis & Barton 1984). The upper Yukon and Canada-bound fish (hereafter referred to as ‘north shore’) and Tanana River fish (hereafter referred to as ‘south shore’) were compared to determine whether a real difference in infection or disease occurred between the two populations. Fish from the south shore were collected by fish wheel [U.S. Fish and Wildlife Service test wheel located just below the confluence of the Tanana River (65°09.159′N, 152°16.685′W)], while north shore fish were collected by fish wheel operated by a Tanana Village subsistence fisher (65°10.288′N, 152°05.984′W).
Data collection
Fish were necropsied within 6 h of capture and sex, weight, length and clinical disease (presence of white lesions on internal organs) were recorded. Samples of heart tissue were fixed in 10% formalin for histology and cultured in MEM-5 medium (tris-buffered Eagle's minimum essential medium supplemented with 5% foetal bovine serum, 100 IU mL−1 penicillin, 100 μg mL−1 streptomycin and 100 μg mL−1 gentamycin) to confirm Ichthyophonus infection and to determine the prevalence of subclinical infections. The cultured tissue was used to confirm visual diagnoses and to determine the total infection prevalence (clinical + sub-clinical).
Fish were classified as: (1) ‘infected’ when the organism was isolated by in vitro explant culture or histologically identified from tissue, (2) ‘clinically infected’ or ‘diseased’ when visible white lesions were observed on at least one organ and confirmed to be Ichthyophonus by culture or histology, (3) ‘sub-clinically infected’ when visible lesions were not apparent but Ichthyophonus was detected in culture or histologically, or (4) ‘negative’ when Ichthyophonus could not be identified by any of the above techniques. Because of the possibility of missing some low-level infections, the data presented represent minimum infection prevalence.
Histological evaluation
Based on positive explant cultures, Ichthyophonus-positive tissues from km 39 (Emmonak) (n = 27) and km 1110 (north shore and south shore) (n = 33) were selected for histological determination of infection severity. Sections of formalin-fixed heart tissue were stained with haematoxylin and eosin and periodic acid-Schiff, then evaluated using brightfield microscopy. A severity index for cardiac muscle infection was designed based on previously published studies (Marty, Freiberg, Meyers, Wilcock, Farver & Hinton 1998). To accurately quantify the relative infection severity in each infected heart, five sections from each heart, separated by 50 μm of tissue, was evaluated. The infection severity index was then defined as the mean of all five sections and given the numerical values of: ‘0’ (no organisms observed), ‘1’ (1–2 organisms per 10X field × 5 sections), ‘2’ (3–4 organisms per 10X field × 5 sections) or ‘3’ (5 + organisms per 10X field × 5 sections).
Statistical analyses
To evaluate infection prevalence, returning adult chinook salmon were treated as a binomial population consisting of infected and uninfected individuals. To compare groups a null hypothesis (Ho) was established, which stated that the two groups were not different. To test this hypothesis we used a 2 × 2 chi-square statistic with 1 degree of freedom (Leaverton 1978; Gordis 2000). The first comparison was conducted to determine whether fish diverting to spawning tributaries of the lower Yukon River (below the Tanana River confluence) skewed the prevalence and severity of Ichthyophonus infections in the chinook salmon population at km 1110. Fish from the mouth of the Yukon River at km 39 are a composite of all upriver populations; therefore fish sampled over a 7-day period from km 39 (n = 97) were used as a baseline to which all sampled fish from the north and south shore at km 1110 were compared (n = 438).
Once fish reached km 1110, north shore and south shore fish were compared using the same chi-square statistic to determine whether the prevalence of Ichthyophonus infections were significantly different between upper Yukon-Canadian fish and Tanana River fish.
Infection severity in fish from km 39 and 1110 (north and south shore) was based on histological evaluation of heart tissue from known infected individuals, and was evaluated using a one-tailed t-test to compare the mean severity index for each group.
Results
A total of 535 Yukon River chinook salmon (367 males and 168 females) were evaluated for the presence of Ichthyophonus during 2003. Fish were sampled from km 39 at Emmonak (n = 97), from the north shore at km 1110 at Tanana Village (n = 267), and from the south shore at km 1110 across the river from Tanana Village (n = 171). Fish from all sites exhibited both clinical and subclinical infections, with clinical infection prevalence approaching the total infection prevalence (i.e. clinical + subclinical). Gross (visual) examination of heart tissue revealed ≥95% clinical infections at all three sample sites in 2003, which was the highest disease prevalence observed since surveys began in 1999 (Kocan et al. 2004). As previously reported there was no difference in infection prevalence based on age or size.
Ichthyophonus prevalence in combined north and south shore samples was 34%, which was not different from a 33% prevalence observed at km 39 (χ2 = 0.063, P = 0.80, n = 535) (Fig. 2). However, infection prevalence in north shore fish was significantly higher (36%) than in south shore fish (25%) (χ2 = 5.60, P = 0.018, n = 438). Clinical infections accounted for all of the difference observed between north and south shore fish (Fig. 3). Clinical disease and total infection prevalence was higher in both males and females from the north shore compared with the south shore.
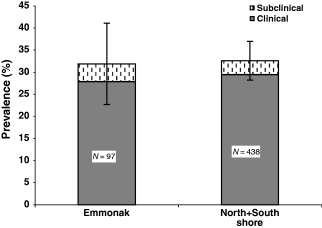
Comparison of Ichthyophonus infection and disease from Emmonak (river km 39) and combined north and south shore fish from the Yukon River near Tanana Village (river km 1110). Bars = 95% confidence interval.
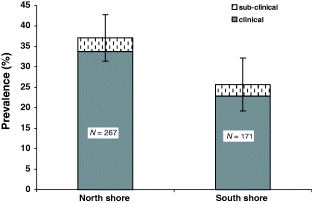
Comparison of Ichthyophonus infection and disease in north shore (upper Yukon-Canadian) and south shore (Tanana River) chinook salmon at Tanana Village (river km 1110). Bars = 95% confidence interval.
Severity of infection in cardiac muscle (based on a severity index scale of 0–3) progressed from 0.74 (±0.06) at the river mouth to 1.10 (±0.05) at km 1110 (north and south shore fish combined). This increase would be expected because the infection had 20 to 25 days to progress during the upriver migration. However, infection severity was significantly greater in north shore fish (1.29 ± 0.06) than in south shore fish (0.80 ± 0.04) (one tail t-test, P < 0.003, n = 438) (Table 1).
| Site | River km | n | Severity indexa (mean ± SE) |
|---|---|---|---|
| Emmonak | 39 | 135 | 0.74 ± 0.06 |
| North shore | 1110 | 105 | 1.29 ± 0.06b |
| South shore | 1110 | 60 | 0.80 ± 0.04 |
- a Severity index (parasite density in heart tissue): ‘0’ = none, ‘1’ = mild, ‘2’ = moderate, ‘3’ = severe.
- b Significantly different from Emmonak and south shore samples.
Discussion
This study verified our original observation that a difference in disease prevalence and severity occurred between upper Yukon and Tanana River chinook salmon, but offered no insight into the mechanism responsible for the difference between the two stocks. The lower prevalence and less severe infections observed in Tanana River fish could have been the result of this population having been exposed to fewer parasites, or conversely, that the more severely infected fish died or failed to reach the Tanana River due to early fatigue. The explanation for this disparity probably lies within biological difference(s) between the two populations, differences in local environment or possibly the parasite itself. Until controlled studies are conducted to elucidate which factor(s) are responsible for the differences between upper Yukon and Tanana River stocks, we can only offer the following hypotheses as a guide to further research into the problem. A number of variables were considered in formulating hypotheses to explain the observed differences between the two stocks: (1) genetic relatedness of the two stocks, (2) exposure time and location, (3) strain of parasite, (4) time and distance of migration at the time of sampling, and (5) physiological differences.
The upper Yukon and Tanana River chinook stocks have been shown to be genetically distinct (Gharrett, Shirley & Tromble 1987; Beacham et al. 1989; Templin et al. 2005) and therefore potentially different in their response to infection. The difference in resistance to infection and disease in genetically distinct chinook salmon populations is supported by reports that widely separated stocks exhibit differences in susceptibility to Ichthyophonus. Natural infections of Ichthyophonus have never been reported in Puget Sound chinook salmon even though several local forage species are infected (Hershberger, Stick, Bui, Carroll, Fall, Mork, Perry, Sweeney, Wittouck, Winton & Kocan 2002), and repeated attempts to infect Puget Sound chinook salmon with the local Ichthyophonus as well as Yukon River isolates have been unsuccessful (Kocan et al. 2004). Similar results have been reported for a British Columbia stock (Big Qualicum River) of chinook salmon (Jones & Dawe 2002). This suggests that genetically distinct populations, such as the upper Yukon River and Tanana River (lower Yukon) salmon, could be differentially resistant to infection by Ichthyophonus. Differences in stock resistance can best be resolved by controlled exposure studies using the same strain or isolate of parasites and different strains of salmon.
Different stocks may also have genetically influenced behavioural differences that result in the stocks being infected at different times prior to entering the Yukon River. A difference in timing of exposure would result in the parasite having a different incubation period in each stock, which could account for the apparent difference in disease severity, but not for difference in infection prevalence. There are presently no data to support differential exposure timing, but radio-tagging data shows the two stocks migrate at slightly different rates. The upper Yukon fish (north shore) travel 55 km day−1 (34.1 miles day−1) while Tanana River fish travel at 46.0 km day−1 (28.6 miles day−1) (Eiler 2005). This difference in migration speed would result in upper Yukon fish reaching km 1110 approximately 3.7 days earlier than Tanana River fish, even though they travelled the same distance. It is doubtful that this small difference in incubation time could account for such dramatic differences in disease severity, and would have no effect on infection prevalence.
All Ichthyophonus isolates from the north-east Pacific, with the exception of isolates from several coastal rockfish species, have been shown to be genetically similar at the 18s rDNA region (Criscione, Watral, Whipps, Blouin, Jones & Kent 2002; Halos, Hart, Hershberger & Kocan 2005), but until controlled virulence studies are conducted with each isolate it will not be possible to determine if different strains of Ichthyophonus are infecting the two Yukon River stocks.
An obvious difference between north and south shore fish was proximity to their respective spawning streams and thus their physiological condition. We have consistently observed that fish entering the Tanana River are darkly pigmented, have hooked jaws, large teeth, protruding ovipositors and pale flesh (i.e. low lipid reserves), indicating that they are nearing their spawning streams, whereas fish from the north shore appear either silver or ‘blush’ and have red flesh (high lipid reserves), indicating that they are still some distance from their spawning streams. As the Tanana River fish are closer to the end of their migration, their energy reserve is lower than in upper Yukon fish that still have over 1610 km (1000 miles) to swim. The combination of low lipid reserves (Ellis, Roberts & Tytler 1989) and reduced stamina from cardiac damage due to Ichthyophonus infection could result in the more severely infected fish failing to complete their migration, thus leaving fewer and less severely infected fish to enter the Tanana River. Conversely, upper Yukon fish would not begin to fatigue until they neared the end of their migration when the combination of reduced lipid reserves and cardiac damage resulted in fatigue or mortality. This is supported by the previously observed low prevalence of Ichthyophonus-infected fish at the Whitehorse Rapids hatchery at river km 2817 (river mile 1750) (Kocan et al. 2003) and aerial surveys showing fewer fish on spawning grounds than was predicted from upper Yukon River mainstem estimates (Templin et al. 2005). If there were physiological differences between the two stocks, this would support the idea that the more severely affected fish in the Tanana River population were unable to complete their migration and therefore never arrived at the mouth of the Tanana River – hence were underrepresented in the south shore samples.
Alternatively, physiological and hormonal changes associated with proximity to spawning streams may influence parasite growth and pathogenicity. Corticosteroids have been demonstrated to suppress the immune response of fish to infectious agents, including Ichthyophonus (Perry, Kocan, Winton & Hershberger 2004). If hormonal changes associated with proximity to spawning grounds do affect the fish immune response, then fish sampled from the north and south shore of the Yukon River at its confluence with the Tanana River should reveal differences in infection prevalence and pathogenicity which was found to be true.
This study emphasizes the complexity of host–parasite interactions as well as the importance of having multi-year biological data from multiple sites and multiple populations. If Ichthyophonus data existed for only the upper Yukon River or Puget Sound chinook salmon, or from a single sample site, a misleading picture of the true nature of this host–parasite relationship would exist. It also points out the limitations of field observations to explain host–parasite interactions, and emphasizes the need for controlled studies to test the hypotheses generated from field observations.
Acknowledgements
The authors thank the following for providing assistance in field collections: T. Lingnau (Alaska Department of Fish & Game), W. Fliris and P. Moore (Tanana, AK), and J. Kocan (Stillwater, OK).




