Rat eosinophils stimulate the expansion of Cryptococcus neoformans-specific CD4+ and CD8+ T cells with a T-helper 1 profile
Senior author: Diana T. Masih
Summary
Experimental Cryptococcus neoformans infection in rats has been shown to have similarities with human cryptococcosis, revealing a strong granulomatous response and a low susceptibility to dissemination. Moreover, it has been shown that eosinophils are components of the inflammatory response to C. neoformans infections. In this in vitro study, we demonstrated that rat peritoneal eosinophils phagocytose opsonized live yeasts of C. neoformans, and that the phenomenon involves the engagement of FcγRII and CD18. Moreover, our results showed that the phagocytosis of opsonized C. neoformans triggers eosinophil activation, as indicated by (i) the up-regulation of major histocompatibility complex (MHC) class I, MHC class II and costimulatory molecules, and (ii) an increase in interleukin (IL)-12, tumour necrosis factor-α (TNF-α) and interferon-γ (IFN-γ) production. However, nitric oxide (NO) and hydrogen peroxide (H2O2) synthesis by eosinophils was down-regulated after interaction with C. neoformans. Furthermore, this work demonstrated that CD4+ and CD8+ T lymphocytes isolated from spleens of infected rats and cultured with C. neoformans-pulsed eosinophils proliferate in an MHC class II- and class I-dependent manner, respectively, and produce important amounts of T-helper 1 (Th1) type cytokines, such as TNF-α and IFN-γ, in the absence of T-helper 2 (Th2) cytokine synthesis. In summary, the present study demonstrates that eosinophils act as fungal antigen-presenting cells and suggests that C. neoformans-loaded eosinophils might participate in the adaptive immune response.
Introduction
Cryptococcus neoformans is a facultative intracellular pathogen that causes severe meningoencephalitis or disseminated mycosis in immunocompromised patients.1 This encapsulated yeast is also able to persist in healthy hosts, thus causing dormant infections that may later be reactivated under an immunosuppressive disease.2 Cryptococcal infections in rats have been shown to have similarities with human cryptococcosis, revealing a strong granulomatous response and a low susceptibility to disseminated infections.3
T-cell-mediated immunity is a critical component of protective immunity against infection with C. neoformans. Both CD4+ and CD8+ T cells are required for effective immune pulmonary clearance and prevention of extrapulmonary dissemination.4 The cells recruited during the inflammatory response include neutrophils, eosinophils, monocyte/macrophages (Mφ), dendritic cells and lymphocytes [CD4+ T cells, CD8+ T cells, B cells and natural killer (NK) cells]. Of these cells, activated Mφ, neutrophils and lymphocytes are all capable of in vitro killing or growth inhibition of C. neoformans.5 Related to this, previous studies in our laboratory have shown that Mφ from infected rats appear to be able to kill C. neoformans, principally by generating nitric oxide (NO).6 Moreover, the NADPH oxidase system was also found to be very important in the mechanism of C. neoformans killing by rat peritoneal cells, with the superoxide anion, hydrogen peroxide (H2O2) and the hydroxyl radical being involved in this process.7
Eosinophils, in contrast, are implicated as effector cells in helminthic infections, releasing their many cytoplasmic granules, containing toxic molecules, in response to antigenic stimuli.8 Moreover, they notably contribute to allergic inflammation at airway mucosal sites.9 Recent studies have also demonstrated that eosinophils are able to function as antigen-presenting cells (APCs). The eosinophils express major histocompatibility complex (MHC) class I and class II, and the costimulatory molecules CD28, CD40, CD80 and CD86, suggesting that these cells can directly communicate with T cells to regulate immune responses. In addition, eosinophils also secrete a range of cytokines that are not only proinflammatory, but also function as growth factors, stimulants and chemoattractants [e.g. interleukin (IL)-2, IL-4, IL-5, IL-10, IL-12, IL-16, interferon-γ (IFN-γ) and regulated on activation, normal, T-cell expressed, and secreted (RANTES)] for T cells.10 In this sense, eosinophils were demonstrated to present antigens to primed T cells, thus increasing T-helper 2 (Th2) cytokine production.10–14 Furthermore, antigen-loaded eosinophils migrate into local lymph nodes and localize in the T-cell-rich paracortical zones, where they stimulate the expansion of CD4+ T cells.15 It was shown that eosinophil effector responses, the expression levels of MHC class II, CD80 and CD86, and the capability to induce resting T cells to proliferate, can be enhanced by exposing them to specific eosinophil-active cytokines, including the eosinophil growth-factor cytokines, granulocyte–macrophage colony-stimulating factor (GM-CSF), IL-3 and IL-5.16
Although the role of eosinophils in the immune response to fungal infections has not been extensively studied, there are some results suggesting that Coccidioidomycosis, caused by the fungus Coccidioides immitis, may be accompanied by an increase in peripheral blood eosinophils of the order of 3–10%.17 Moreover, during Paracoccidioidomycosis in humans, Wagner et al.18 have shown a clear association among the presence of Paracoccidioides brasiliensis, infiltration of the lesion by eosinophils and deposition of myelin basic protein (MBP) on the fungus. In this regard, Feldmesser et al.19 have demonstrated in vitro that rat eosinophils phagocytose opsonized C. neoformans yeasts, and they also observed a direct interaction between eosinophils and C. neoformans in vivo during an experimental murine intratracheal infection. Even though eosinophils are unlikely to be the predominant effector cells in the immune response to this organism, their occurrence, in intimate association with C. neoformans, suggests a potential function for eosinophils as effector cells.
The aim of this study was to evaluate the ability of rat peritoneal eosinophils to be activated by C. neoformans yeasts in order to present fungal antigens to T cells, thereby promoting the development of an immune response to this pathogen. The results presented here show that eosinophils became activated by C. neoformans, increasing the surface expression of MHC class I and class II and of CD80 and CD86, resulting in the secretion of proinflammatory cytokines, such as IL-12, IFN-γ and tumour necrosis factor-α (TNF-α). Finally, this work demonstrated that these fungal-activated eosinophils induce the development of a C. neoformans-specific T-helper 1 (Th1) immune response.
Materials and methods
Reagents and media
For cell cultures, RPMI-1640 supplemented with 10% fetal bovine serum (FBS), 2 mm glutamine and 50 μg/ml of gentamycin (Sigma-Aldrich Co., St Louis, MO) was used. The mouse monoclonal antibodies (mAbs) anti-rat CD32 (FcγRII), CD18 (WT.3), MHC class II (RT1b), MHC class I (RT1a), CD80 (B7-1), CD86 (B7-2), OX-62, CD11b/c, CD4, CD8a, CD25, IFN-γ (DB-1), IL-4 (OX-81) and IL-10 (A5-4) were obtained from BD Biosciences (San Jose, CA). The glucuronoxylomannan-specific mAb, 3C2 (mouse IgG1), was a generous gift from Thomas R. Kozel (Department of Microbiology and Immunology, University of Nevada, Reno, NV 89557). Recombinant rat GM-CSF was obtained from BioSource (Camarillo, CA), and 2′,7′-dichlorodihydrofluorescein diacetate (DCF) was obtained from Sigma-Aldrich.
Animals
Male, 7- to 8-week-old Wistar rats, weighing 250 g, were housed and cared for in the animal resource facilities of the Department of Clinical Biochemistry, Faculty of Chemical Sciences, National University of Cordoba, following institutional guidelines. These animal resource facilities are accredited as an entity that follows international norms of the ‘Guide to the Care and Use of Experimental Animals’ regulation published by the Canadian Council on Animal Care (assurance number A5802-01). All experimental protocols were approved by the Animal Experimentation Ethics Committee, Faculty of Chemical Sciences, National University of Cordoba (resolution number 1135/09).
C. neoformans
Serotype A C. neoformans strain 102/85 (National University of Cordoba stock culture collection) was used. This strain of Cryptococcus is a clinical isolate with a large capsule, typified by a polymerase chain reaction (PCR) multiplex and PCR fingerprinting (Centro de Biotecnologia da Universidade Federal do Rio Grande do Sul, Brasil) as C. neoformans var. grubii, which has been used in previous studies.6,20–23 To perform the experiments, living yeasts of C. neoformans were expanded in liquid Sabouraud media for 24 hr in a gyratory shaker at 30°. Then, the yeasts were washed three times with phosphate-buffered saline (PBS), resuspended at 107 cells/ml and opsonized with 5 μg/ml of mAb 3C2 for 30 min at 37°. After this, the yeasts were washed with PBS and finally resuspended in RPMI-1640 supplemented with 10% FCS, 2 mM glutamine and 50 μg/ml gentamycin for subsequent cultures with eosinophils.
Isolation and culture of eosinophils
Eosinophils were purified from the peritoneal cavity of normal rats by washing it with cold PBS, pH 7·3, containing 0·1% FBS. The cells thus obtained were centrifuged at 400 g for 10 min and resuspended in 2 ml of 1× Hanks’ balanced salt solution (HBSS). Then, the cells were separated on a discontinuous Percoll gradient (2 ml of a solution of Percoll with a density of 1·090 g/ml and 2 ml with density of 1·080 g/ml, carefully overlaid). The tubes were centrifuged at 400 g for 25 min, and the eosinophils were collected from the middle interface between the Percoll layers.24 The percentage of eosinophils was > 90%, as determined by May–Grünwald–Giemsa staining. This population was further purified by negative selection, by incubation for 30 min with anti-CD11b/c- and anti-OX-62-labelled fluorescein isothiocyanate (FITC), and then for a further 15 min with anti-FITC MicroBeads (Miltenyi Biotec, Bergisch Gladbach, Germany). The eosinophil population contained < 1% OX-62+ cells and < 2% CD11b/c+ cells, which was not significantly different from the isotype control (Fig. S1). Finally, the percentage of eosinophil viability was > 95%, as determined by the Trypan Blue dye-exclusion test.
Purified eosinophils were incubated in supplemented RPMI-1640 alone, or with opsonized or non-opsonized live yeasts of C. neoformans at 37° and a 5% CO2 humidified atmosphere, in the presence or absence of GM-CSF (5 ng/ml).
For some comparative experiments, rat peritoneal Mφ were used. These cells were purified from the upper interface of the Percoll layers.
Eosinophil phagocytosis assays
Phagocytosis assays were performed as described in previous studies with some modifications.19,25 In brief, eosinophils were plated at a density of 106 cells/ml in media on a 24-well plate containing 106 opsonized or non-opsonized yeast cells/ml (i.e. at an effector : target cell ratio of 1:1) and with or without 5 ng/ml of GM-CSF. In some experiments, eosinophils were preincubated for 30 min with anti-FcγRII and/or anti-CD18 (5 μg/ml). The plates were incubated for 2 hr at 37° in an atmosphere of 5% CO2. The cells were then collected from the wells, centrifuged in a Cytospin cytocentrifuge (Eppendorf AG, Hamburg, Germany) for 5 min and stained with May–Grünwald–Giemsa, and the number of eosinophils (out of a total of 100) containing ingested cryptococci was determined by counting no fewer than 500 cells.
Flow cytometry assay for eosinophil surface expression of MHC class I and class II, and CD80 and CD86
Three-hundred-thousand cells were plated on a 96-well U-shaped plate with the same number of opsonized or non-opsonized yeast cells, or with medium alone, in the presence or absence of GM-CSF. In some experiments, the eosinophils were preincubated for 30 min with anti-FcγRII and/or anti-CD18 (5 μg/ml). The plates were incubated at 37° and 5% CO2 for 24 hr. The cells were then blocked with anti-(rat FcγRII) (CD32) for 15 min at room temperature and stained with anti-(rat MHC class I), anti-(rat MHC class II), anti-(rat CD80) or anti-(rat CD86) for 30 min under the same conditions. After incubation, the cells were collected by centrifugation, fixed in 1% Paraphormaldehyde, washed three times with wash buffer and then 20 000 events were analyzed by flow cytometry (Cytoron Absolute; ORTHO Diagnostic System, Raritan, NJ). The percentage of positively labelled cells was determined using logarithmic-scale histograms. Autofluorescence was assessed using untreated cells and control isotypes.
Nitrite measurement
Cells were plated at a density of 106/ml in medium with or without GM-CSF (5 ng/ml), on a 24-well plate containing 106 opsonized yeast cells/ml. In some experiments, eosinophils were preincubated for 30 min with anti-FcγRII and/or anti-CD18 (5 μg/ml). Nitrite accumulation, an indicator of NO production, was measured using the Griess reagent.6 Briefly, 100-μl aliquots of 24-hr culture supernatants were mixed with an equal amount of Griess reagent and incubated at room temperature for 15 min. The absorbance at 540 nm was measured using an automated microplate reader (BioRad, Hercules, CA). The concentration of nitrite was calculated from a NaNO2 standard curve.
Flow cytometric detection of intracellular H2O2
To measure the concentration of intracellular H2O2, eosinophils were incubated with DCF, with the non-fluorescent reduced form being converted into a green fluorescent form when oxidized. DCF is oxidized by cellular H2O2, hydroxyl radicals and other free-radical products of H2O2. However, it is relatively insensitive to oxidation by superoxide.26 Eosinophils were treated for 2 hr (because at earlier time-points there was no H2O2 release detected) in the presence or absence of GM-CSF (5 ng/ml), with medium alone or opsonized live yeasts, before being washed with PBS and treated with 10 μm DCF for 20 min at 37°. In some experiments, eosinophils were preincubated for 30 min with anti-FcγRII and/or anti-CD18 (5 μg/ml). A total of 10 000 events were analyzed by flow cytometry (Cytoron Absolute; ORTHO Diagnostic System) for changes in fluorescence.26
Eosinophil cytokine assays
Supernatants from cultures set up as described above were collected after 24 hr in order to measure the concentrations of IL-12p40, TNF-α, IFN-γ, IL-10, IL-4 and IL-13, and were frozen at −70° until analyzed. IL-12p40, TNF-α and IFN-γ were measured using the enzyme-linked immunosorbent assay (ELISA) sandwich CytoSets according to the manufacturer’s protocol (Biosource). Dilutions of recombinant rat IL-12p40, TNF-α and IFN-γ were used as standards. After washing, the plates were reacted with horseradish peroxidase conjugated to streptavidin (Biosource). This was followed by the addition of tetramethylbenzidine (TMB; Biosource) for 5–20 min and stopped with sulphuric acid. The reaction was read using a Microplate Reader (BioRad), and the results were expressed as pg/ml.
In vitroantigen presentation by eosinophils to naive orC. neoformans-primed T cells
Naive mononuclear spleen cells (MSCs) were obtained from untreated Wistar rats, and C. neoformans-primed MSCs were collected from rats infected intraperitoneally, 7 days before the experiment with 107 live yeasts of C. neoformans in 1 ml of PBS. Spleens were pressed through wire-mesh screens to separate the cells. Erythrocytes were lysed with a lysis buffer, pH 7·3, and MSCs were obtained after centrifugation on a Hystopaque 1083 (Sigma-Aldrich) gradient and a 6-hr adherence culture to remove adherent cells. For some experiments, purified CD4+ and/or CD8+ T cells were obtained by incubating MSCs for 30 min with FITC-labelled anti-CD4 and/or anti-CD8a, and then for a further 15 min with anti-FITC MicroBeads. By positive selection (MACS; Miltenyi Biotec), > 97% pure T cells were obtained with a viability of 98%.
Eosinophils were cultured in RPMI-1640 supplemented with 5 ng/ml of GM-CSF in the absence (unpulsed eosinophils) or presence of opsonized C. neoformans (C. neoformans-pulsed eosinophils), at a ratio of 1:1, for 24 hr, as described above. Then, these eosinophils were removed from the plates, washed twice with RPMI-1640 supplemented with 2·5 μg/ml of amphotericin B, and fixed in 1% paraformaldehyde to avoid degranulation and to preserve the cells during subsequent co-cultures.11,27 Fixed antigen-pulsed APC have been shown to have unchanged expression levels of MHC class II and to be able to stimulate the proliferation of T cells.28 After 24 hr, the eosinophils were extensively washed with RPMI-1640, and 6 × 104 of these cells were incubated in flat-bottomed 96-well plates containing 3 × 105 naive or C. neoformans-primed MSCs or purified T cells in RPMI-1640 supplemented with 50 μm 2-mercaptoethanol (Merck, Damstadt, Germany). In some experiments, 1 μg of anti-MHC class I or anti-MHC class II was added to 106 cells. The cultures were incubated for 7 days at 37° and 5% CO2. After the addition of 1 μCi of [3H]thymidine (obtained from Comisión Nacional de Energía Atómica, CNEA) to each well, the incorporation of [3H]thymidine by lymphocytes was determined, 16–18 hr later, using a cell harvester and a liquid scintillation counter.
For some comparative studies, similar cultures were performed using unpulsed or C. neoformans-pulsed Mφ as APC.
Furthermore, the production of IFN-γ, TNF-α, IL-4, IL-13 and IL-10 by purified T cells was measured in supernatants of 96-hr cultures using anti-(rat IFN-γ), anti-(rat-TNF-α), anti-(rat-IL-4), anti-(rat-IL-13) and anti-(rat-IL-10) CytoSets (BioSource), as described above.
Immunofluorescent staining for intracellular cytokine detection in CD4+ and CD8+ T cells by flow cytometry
For intracellular cytokine and surface-marker staining, C. neoformans-primed CD4+ and CD8+ T cells (1 × 106 cells) were co-cultured with unpulsed or C. neoformans-pulsed eosinophils (2 × 105 cells), or in medium alone, for 4 days. Then, T cells were stimulated with phorbol 12-myristate 13-acetate (PMA; 50 ng per 2 × 106 cells), ionomycin (500 ng per 1 × 106 cells) and brefeldin A (5 ng/1 × 106 cells) for 3 hr at 37° under a 5% CO2 humidified atmosphere. For cell-surface staining, cells were incubated for 30 min at 4° in the dark with FITC-conjugated mouse mAb specific to rat CD4 (0·5 mg per 106 cells) or CD8 (0·5 mg per 106 cells). The cells were then washed twice (30 min each wash, at room temperature) with PBS containing 3% FCS and then fixed overnight at 4° in PBS containing 1% paraformaldehyde. Before intracellular cytokine staining, the cells were washed with PBS containing 0·5% saponin. Cells in this buffer were incubated with phycoerythrin (PE)-conjugated mouse anti-(rat IFN-γ) (0·125–0·5 μg per 106 cells) and anti-(rat IL-4) (0·1–0·5 μg per 106 cells), or appropriate controls (corresponding to IgG-negative isotypes) in the dark for 30 min at room temperature. The cells were washed twice with PBS containing 0·5% saponin and finally resuspended in 0·2 ml of flow wash.29 The immunofluorescently stained cells were analyzed using a Becton Dickinson FACS Canto II flow cytometer (San José, CA). The percentage of double-positive labelled cells was determined using dot-plot graphs.
Statistical analysis
Data were expressed as means + standard errors of the mean (SEM) and analyzed statistically using the Student’s t-test. Statistical significance was taken to be a P-value of < 0·05. All experiments were repeated and equivalent results were obtained.
Results
Rat eosinophils phagocytose opsonized C. neoformans and increase MHC class II expression through FcγRII and CD18 interactions
First, we evaluated the ability of eosinophils to phagocytose live yeasts of C. neoformans. Purified eosinophils were exposed to yeasts of C. neoformans that were either opsonized with the mAb 3C2 which binds specifically to C. neoformans glucuronoxylomannan, or non-opsonized, at a ratio of 1:1, in the presence or absence of GM-CSF (5 ng/ml) for 24 hr. Eosinophils incubated with opsonized C. neoformans, non-opsonized C. neoformans or medium alone, showed more than 85% viability, as determined using the Trypan Blue dye-exclusion test, and < 10% apoptosis when tested by propidium iodide staining and flow cytometry (data not shown). Figure 1 shows that both non-stimulated and GM-CSF-stimulated eosinophils phagocytosed opsonized yeasts of the fungus, with 20–25% of phagocytosis being carried out by eosinophils in the absence or presence of GM-CSF. However, eosinophils were not able to ingest non-opsonized yeasts (eosinophils plus opsonized C. neoformans versus eosinophils plus non-opsonized C. neoformans, P < 0·05). C. neoformans phagocytosis was blocked by anti-FcγRII and anti-CD18 mAbs (Fig. 1b), suggesting that both receptors are involved in this phenomenon.
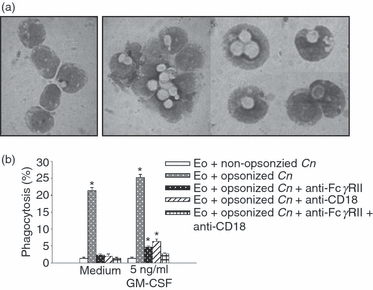
Exposure of eosinophils to opsonized live yeasts of Cryptococcus neoformans leads to fungal phagocytosis. Eosinophils (1 × 106 cells/ml) were incubated for 2 hr with 1 × 106 yeasts/ml, with or without opsonizing monoclonal antibody (mAb) (5 μg/ml) and granulocyte–macrophage colony-stimulating factor (GM-CSF) (5 ng/ml). In some experiments, anti-FcγRII and/or anti-CD18 were added to the culture. (a) Cytospin preparations of eosinophils in medium alone (left photographs) or cultured with opsonized C. neoformans (right photographs), May–Grünwald–Giemsa staining (magnification 1000 ×). (b) Percentage of eosinophils with ingested cryptococci, calculated by counting no fewer than 500 cells. Bars represents the means ± standard error of the mean (SEM) of triplicates from three independent experiments. *P < 0·05 (eosinophils + opsonized C. neoformans versus eosinophils + non-opsonized C. neoformans). Cn, Cryptococcus neoformans; EO, eosinophils.
Flow cytometric analysis of MHC class II surface expression demonstrated that the ingestion of opsonized yeasts stimulated the increase of both the percentage and the mean fluorescence intensity (MFI) of MHC class II on eosinophils (Fig. 2a) (eosinophil plus opsonized C. neoformans versus eosinophil plus non-opsonized C. neoformans; P < 0·02). According to the observations for C. neoformans phagocytosis, MHC class II expression by eosinophils incubated with opsonized yeasts was completely inhibited by FcγRII and CD18 (Fig. 2b). Furthermore, the increased expression of MHC class II on eosinophils treated with opsonized C. neoformans was significantly higher in cultures with GM-CSF than in its absence (60% versus 20%; P < 0·02) (Fig. 2b).
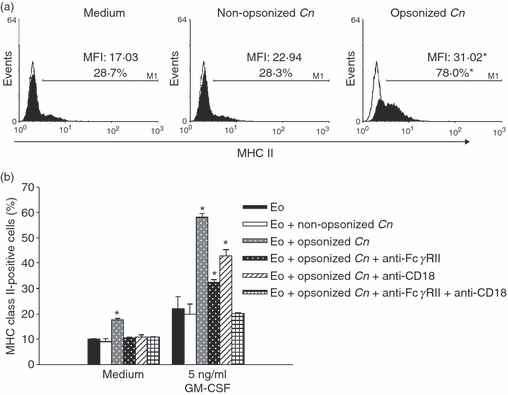
Phagocytosis of Cryptococcus neoformans increases the expression of major histocompatibility complex (MHC) class II in eosinophils. (a) Eosinophils (3 × 105 cells) were incubated for 24 hr with opsonized or non-opsonized fungal yeasts (at a ratio of 1:1), or with medium alone, in the presence of granulocyte–macrophage colony-stimulating factor (GM-CSF) (5 ng/ml). The eosinophils were then stained with anti-(MHC class II), and 20 000 events were analyzed by flow cytometry. The percentage of positively labelled cells and the mean fluorescence intensity (MFI) were determined using logarithmic-scale histograms (open histograms indicate the isotype control). (b) Anti-FcγRII or/and anti-CD18 blocking monoclonal antibodies (mAbs) were added to the culture 2 hr before the addition of the yeasts. Data are representative of five independent experiments. *P < 0·02 (eosinophils + opsonized C. neoformans versus eosinophils + non-opsonized C. neoformans). Cn, Cryptococcus neoformans; EO, eosinophils.
C. neoformans promotes eosinophil activation, inducing increased expression of MHC class I, CD80 and CD86 and increased production of IL-12 p40, TNF-α and IFN-γ
We further analyzed the expression of MHC class I, CD80 and CD86 on the surface of eosinophils incubated with opsonized or non-opsonized C. neoformans, in the presence or absence of GM-CSF. Figure 3a demonstrates that in the presence of GM-CSF, opsonized C. neoformans drastically increased the percentage and MFI of MHC class I expression on eosinophils (eosinophil plus opsonized C. neoformans versus eosinophil plus non-opsonized C. neoformans; P < 0·01). Moreover, opsonized C. neoformans significantly up-regulated the surface expression of CD80 and CD86 on these cells (eosinophil plus opsonized C. neoformans versus eosinophil plus non-opsonized C. neoformans; P < 0·05). Similar results were observed in cultures performed in the absence of GM-CSF (Fig. 3b). Therefore, in contrast to that observed for MHC class II, opsonized C. neoformans up-regulated the expression of MHC class I and costimulatory molecules, regardless of the presence of GM-CSF in the medium.
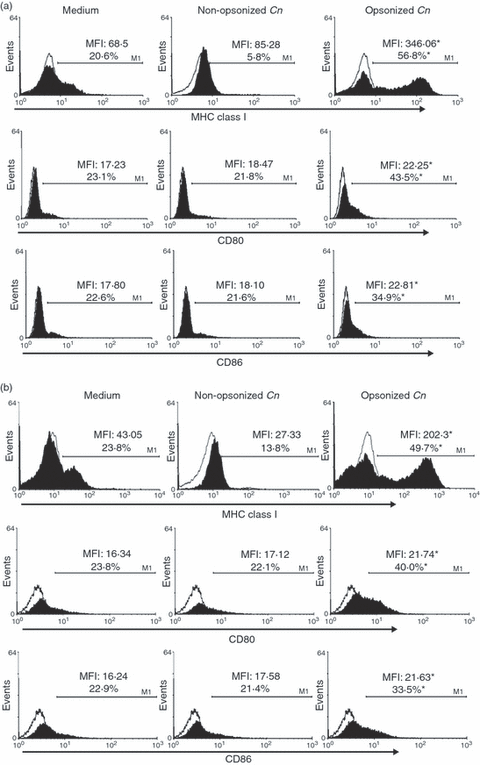
Cryptococcus neoformans promotes eosinophil activation, resulting in an increased expression of major histocompatibility complex (MHC) class I, CD80 and CD86. Eosinophils (3 × 105 cells) were incubated for 24 hr with opsonized or non-opsonized fungal yeasts (at a ratio of 1:1), or with medium alone, in the presence (a) or in the absence (b) of granulocyte–macrophage colony-stimulating factor (GM-CSF) (5 ng/ml). Eosinophils were then stained with anti-(MHC I), anti-CD80 or anti-CD86, and 20 000 events were analyzed by flow cytometry. The percentage of positively labelled cells and the mean fluorescence intensity (MFI) were determined using logarithmic-scale histograms (open histograms indicates isotype control). Data are representative of five independent experiments. *P < 0·02 (eosinophils + opsonized C. neoformans versus eosinophils + non-opsonized C. neoformans). Cn, Cryptococcus neoformans.
The levels of IFN-γ, TNF-α and IL-12p40 were also quantified in the supernatants of eosinophils obtained 24 hr after culture with opsonized or non-opsonized C. neoformans in the presence or absence of GM-CSF. Figure 4 shows the production of cytokines in cultures containing GM-CSF, revealing that in the presence of opsonized C. neoformans, eosinophils secreted significant amounts of IFN-γ, TNF-α and IL-12p40, compared to cells incubated in medium alone or with non-opsonized yeasts (P < 0·03). In contrast, Th2 cytokines (such as IL-4, IL-10 and IL-13) were not detected in these culture supernatants. Almost the same results were obtained in the absence of GM-CSF (data not shown).
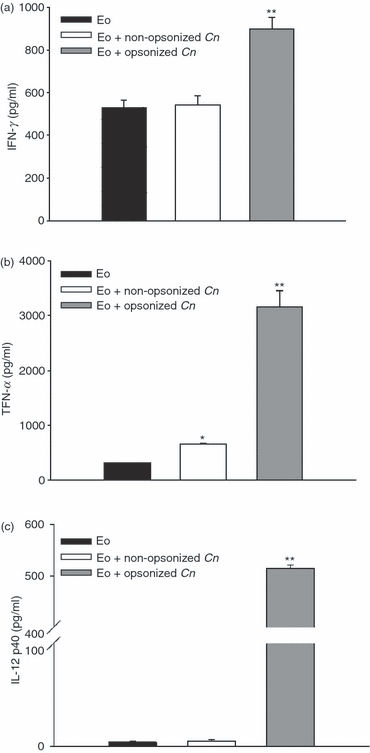
Cryptococcus neoformans promotes eosinophil activation, resulting in the production of proinflammatory cytokines. Eosinophils (3 × 105 cells) were incubated for 24 hr with opsonized or non-opsonized fungal yeasts (at a ratio of 1:1), or with medium alone, in the presence of granulocyte–macrophage colony-stimulating factor (GM-CSF) (5 ng/ml). (a) Interferon-γ (IFN-γ), (b) tumour necrosis factor-α (TNF-α) and (c) Interleukin (IL)-12p40 production by eosinophils were measured in the supernatants using enzyme-linked immunosorbent assay (ELISA) sandwich CytoSets. Data are representative of at least four independent experiments. *P < 0·002 (eosinophils + non-opsonized C. neoformans versus eosinophils) and **P < 0·04 (eosinophils + opsonized C. neoformans versus eosinophils + non-opsonized C. neoformans). Cn, Cryptococcus neoformans; Eo, eosinophils.
C. neoformans inhibits the GM-CSF-stimulated production of H2O2 and NO by eosinophils through FcγRII
In order to evaluate the production of fungicidal molecules by GM-CSF-stimulated eosinophils incubated with opsonized C. neoformans, we measured the production of H2O2 using DCF oxidation and flow cytometry, and the synthesis of NO using the Griess reaction. Figure 5a shows that opsonized C. neoformans drastically inhibited the production of H2O2 by GM-CSF-stimulated eosinophils (P < 0·03; eosinophils plus opsonized C. neoformans versus eosinophils in medium alone). This phenomenon was exclusively dependent on FcγRII, because, in the presence of a blocking antibody, opsonized C. neoformans were unable to suppress H2O2 production. To a lesser extent, opsonized C. neoformans also inhibited NO production by GM-CSF-stimulated eosinophils (Fig. 5b; P < 0·05; eosinophils plus opsonized C. neoformans versus eosinophils in medium alone) through FcγRII interactions. Similarly, in the absence of GM-CSF, opsonized C. neoformans also inhibited the basal production of H2O2 or NO by eosinophils (data not shown).
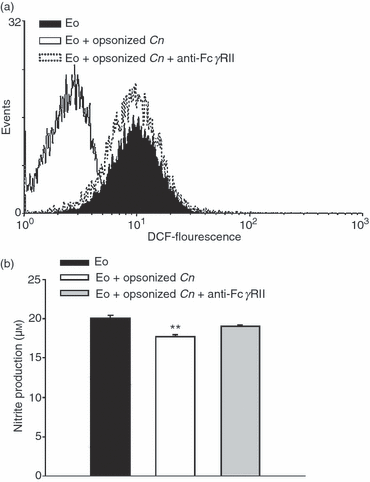
Cryptococcus neoformans inhibits the release of radical oxygen species and nitric oxide by eosinophils through FcγRII interactions. Eosinophils were incubated with granulocyte–macrophage colony-stimulating factor (GM-CSF) (5 ng/ml), in medium alone or with opsonized C. neoformans (at a ratio of 1:1). Anti-FcγRII was added to the culture. (a) After 2 hr of culture, eosinophils were washed with phosphate-buffered saline (PBS), treated with 10 μm 2′,7′-dichlorodihydrofluorescein diacetate (DCF) for 20 min at 37° and then 10 000 events were analyzed by flow cytometry for changes in fluorescence. (b) After 24 hr of culture, the supernatants were analyzed for nitrite accumulation using the Griess reagent. Data are representative of at least three independent experiments. **P < 0·04 (eosinophils + opsonized C. neoformans versus eosinophils and eosinophils + opsonized C. neoformans + anti-FcγRII). Cn, Cryptococcus neoformans; Eo, eosinophils.
C. neoformans-pulsed eosinophils promote the proliferation of C. neoformans-primed CD4+ and CD8+ T cells in an MHC class II- and MHC class I-dependent manner, respectively
Experiments were then performed in order to evaluate the ability of eosinophils to present fungal antigens. Taking into account that the expression of MHC class II was significantly higher on eosinophils cultured with C. neoformans in the presence of GM-CSF than in its absence (Fig. 2b), eosinophils were pulsed with opsonized C. neoformans in the presence of GM-CSF for 24 hr before being fixed with paraformaldehyde. Then, they were cultured with MSCs or purified T lymphocytes (CD4+ and CD8+) obtained from untreated rats (naive lymphocytes) or from rats infected with 107 yeasts 7 days previously (C. neoformans-primed lymphocytes). Seven days after culture, the lymphoproliferation was measured by thymidine incorporation. The results showed that C. neoformans-primed lymphocytes (MSCs or purified CD4+ plus CD8+ T cells), but not naive lymphocytes, proliferated significantly in the presence of C. neoformans-pulsed eosinophils, compared with MSCs or T cells cultured in medium alone, or with fixed C. neoformans yeasts or unpulsed eosinophils (Fig. 6a,b). Moreover, in the absence of eosinophils, neither MSCs nor T cells proliferated, even when incubated with C. neoformans alone, discounting any possible effect of APC contamination within the eosinophil preparation or among the purified T cells. In addition, Fig. 6b shows that C. neoformans-pulsed peritoneal Mφ did not stimulate T-cell proliferation. In this regard, it has been previously demonstrated that monocytes pretreated with encapsulated cryptococci have little or no ability to stimulate T-cell proliferation.30
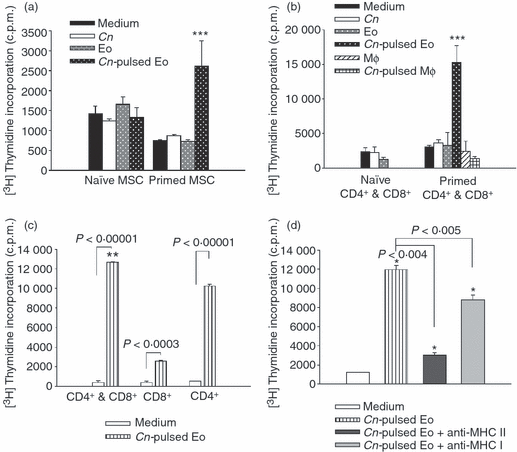
Cryptococcus neoformans-pulsed eosinophils promote C. neoformans-specific CD4+ and CD8+ T cells to proliferate in a major histocompatibility complex (MHC) class II- and MHC class I-dependent manner. (a) Mononuclear spleen cells (MSCs) from the spleens of normal rats or from rats infected with 107 live yeasts of the fungus were co-cultured with fixed C. neoformans-pulsed eosinophils, unpulsed eosinophils and yeasts of C. neoformans. (b) Purified CD4+ and CD8+ T cells from normal rats or from rats infected with 107 live yeast of the fungus were co-cultured with fixed C. neoformans-pulsed eosinophils, unpulsed eosinophils, C. neoformans-pulsed macrophages, unpulsed macrophages or yeasts of C. neoformans. (c) Purified CD4+ or/and CD8+ T cells were co-cultured with C. neoformans-pulsed eosinophils or medium alone. (d) In a separate experiment, C. neoformans-pulsed eosinophils were fixed and incubated for 30 min at 37° with monoclonal antibodies (mAbs) specific to MHC class II and MHC class I before culture with primed CD4+ and CD8+ T cells. Then, 1 μCi of [3H]thymidine was added to each well, and the incorporation of [3H]thymidine by lymphocytes was determined 16–18 hr later, using a cell harvester and a liquid scintillation counter. Data are representative of four independent experiments. *P < 0·04 (C. neoformans pulsed-eosinophils versus medium), **P < 0·001 (CD4+ and CD8+ versus CD4+ and CD8+) and ***P < 0·04 (C. neoformans pulsed-eosinophils versus medium, C. neoformans and eosinophils). Cn, Cryptococcus neoformans; c.p.m., counts per minute; Eo, eosinophils; Mφ, macrophages.
To evaluate if C. neoformans-primed CD4+ or CD8+ T cells were responsible for the lymphoproliferation observed in Fig. 6b, the CD4+ and CD8+ T-cell proliferations were measured separately in the presence of C. neoformans-pulsed eosinophils. Figure 6c shows that both CD4+ and CD8+ T cells proliferated in the presence of C. neoformans-pulsed eosinophils compared with CD4+ and CD8+ T cells cultured in medium alone. However, CD4+ T cells were the main population sensitive to the stimulation of C. neoformans-pulsed eosinophils, showing a higher incorporation of thymidine incorporation than that observed by CD8+ T cells (10 000 c.p.m. versus 3000 c.p.m.; P < 0·03).
From these data, along with those shown in 2, 3, we speculate that eosinophils not only present antigens to CD4+ T cells in an MHC class II pathway, but also present antigens to CD8+ T cells by using their MHC class I molecules. To test this hypothesis, experiments were performed to determine whether the induction of C. neoformans-primed T-cell proliferation was caused by the presentation of antigens by eosinophils in conjunction with MHC class I and MHC class II molecules. C. neoformans-pulsed eosinophils were treated with anti-MHC class I or anti-MHC class II mAbs before incubation with C. neoformans-primed CD4+ and CD8+ T cells. The blocking of MHC molecules on the eosinophil surface was found to suppress the ability of C. neoformans-pulsed eosinophils to stimulate C. neoformans-primed T-cell proliferation (Fig. 6d). Moreover, the suppression seen in the lymphocyte proliferation was more pronounced with anti-MHC class II, which coincided with the higher proliferation of CD4+ T cells shown in Fig. 6c.
In conclusion, C. neoformans-pulsed eosinophils stimulated C. neoformans-primed MSCs and T cells (CD4+ as well as CD8+) in an MHC class II- or class I-dependent manner. This stimulation of proliferation, however, was not observed for naive T cells or when C. neoformans-pulsed Mφ were used as APCs.
C. neoformans-pulsed eosinophils mainly stimulate the increase of an IFN-γ-producing T-cell population
To characterize and differentiate the T-cell profile seen after co-culture with C. neoformans-pulsed eosinophils, C. neoformans-primed purified T cells (CD4+ and CD8+) were analyzed by flow cytometry to determine the intracellular expression levels of IFN-γ and IL-4 after 4 days of culture with C. neoformans-pulsed eosinophils or medium alone. Figure 7 shows a significant increase in the percentage of IFN-γ-producing cells when T cells were incubated with C. neoformans-pulsed eosinophils compared with T cells cultured in medium alone (6·56% versus 1·61%; P < 0·02). With regard to the IL-4-producing T-cell population, the percentage with C. neoformans-pulsed eosinophils (2·42%) was similar to that for medium alone (2·35%). These results allowed us to conclude that C. neoformans-pulsed eosinophils were able to induce the expansion of IFN-γ-producing Th1 cells, but not of IL-4-producing Th2 cells.
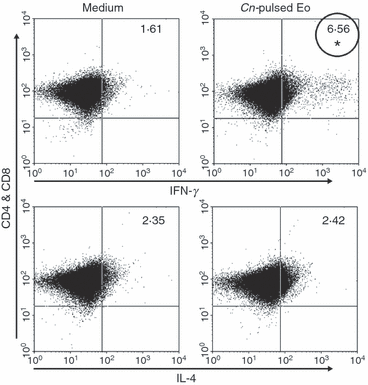
Cryptococcus neoformans-pulsed eosinophils stimulate the increase of C. neoformans-specific interferon-γ (IFN-γ) production by CD4+ and CD8+ T cells. Splenic CD4+ and CD8+ T cells purified from infected rats (C. neoformans-primed) were co-cultured with C. neoformans-pulsed eosinophils or cultured in medium alone. After 4 days, intracellular IFN-γ and interleukin (IL)-4 were detected by flow cytometry. Data are representative of four independent experiments. *P < 0·05 (C. neoformans-pulsed eosinophils versus medium). Cn, Cryptococcus neoformans; Eo, eosinophils.
C. neoformans-pulsed eosinophils promote the production of IFN-γ and TNF-α by C. neoformans-primed CD4+ and CD8+ T cells
To analyze the production of cytokines by CD4+ and CD8+ T cells in supernatants, the concentrations of IFN-γ, TNF-α, IL-4, IL-10 and IL-13 were measured after 4 days of culture. The results presented in Fig. 8(a,b) show that there was a significant increase in the production of IFN-γ and TNF-α generated by C. neoformans-primed T cells cultured with C. neoformans-pulsed eosinophils compared to the cytokine production by T cells cultured in medium alone, with fixed yeasts of C. neoformans or with unpulsed eosinophils. In contrast, no differences in the levels of IL-4, IL-13 or IL-10 were detected in supernatants of C. neoformans-primed T cells cultured with C. neoformans-pulsed eosinophils or in medium alone (Fig. S2). In addition, IFN-γ production by naive T cells incubated with C. neoformans-pulsed eosinophils was similar to controls (Fig. 8a). However, the production of TNF-α by these cells showed a significant increase in the presence of C. neoformans-pulsed or unpulsed eosinophils (Fig. 8b).
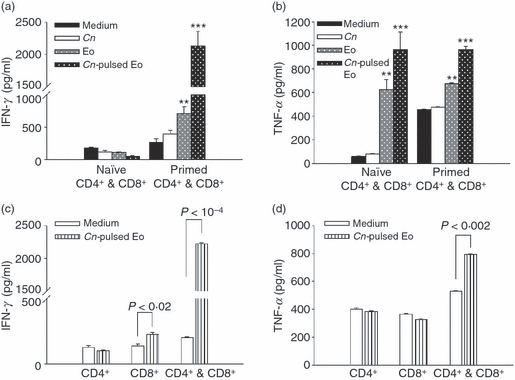
Cryptococcus neoformans-pulsed eosinophils promote the production of interferon-γ (IFN-γ) and tumour necrosis factor-α (TNF-α) by C. neoformans-specific CD4+ and CD8+ T cells. Purified CD4+ and/or CD8+ T cells from normal or infected rats were cultured in medium alone, or with C. neoformans-pulsed eosinophils, unpulsed eosinophils, or yeasts of C. neoformans. After 96 hr of culture, (a and c) IFN-γ and (b and d) TNF-α production was measured in supernatants using enzyme-linked immunosorbent assay (ELISA) capture kits. Data are representative of four independent experiments. **P < 0·02 (eosinophils versus C. neoformans) and ***P < 0·01 (C. neoformans-pulsed eosinophils versus eosinophils). Cn, Cryptococcus neoformans; Eo, eosinophils.
Finally, we decided to investigate which T-cell population (CD4+ or CD8+) was involved in the production of IFN-γ and TNF-α. Surprisingly, only C. neoformans-primed CD8+ T cells cultured with C. neoformans-pulsed eosinophils produced IFN-γ. However, when both primed CD4+ T cells and CD8+ T cells were incubated with C. neoformans-pulsed eosinophils, large amounts of IFN-γ and TNF-α were produced (Fig. 8c,d). These results suggest that cooperation between C. neoformans-primed CD4+ and CD8+ T cells is very important in the case of IFN-γ and necessary for TNF-α production in the presence of C. neoformans-pulsed eosinophils. C. neoformans-pulsed eosinophils not only stimulated the proliferation of C. neoformans-primed CD4+ and CD8+ T cells, but also produced a Th1 microenvironment where cooperation between these two T-cell populations could take place.
Discussion
This study provides the first evidence that rat eosinophils are capable of phagocytosing and presenting C. neoformans antigens to primed T cells, which then trigger a fungal-specific Th1 immune response. Eosinophils have been shown to be components of the inflammatory response to C. neoformans infection in the rat lung,3 and we have previously observed the presence of a large number of eosinophils in the granulomas surrounding C. neoformans-encapsulated yeasts during disseminated cryptococosis in rats (unpublished data). Moreover, although rat peritoneal eosinophils are unable to significantly phagocytose C. neoformans in vitro in the absence of opsonizing antibody, initial phagocytosis is rapidly completed in the presence of a specific mAb as an opsonin.19
Eosinophils constitutively express a variety of Fc receptors, including FcγRII, FcεRII and FcαR, with this expression varying according to the cytokine stimulation. Cross-linking of Fc receptors results in a variety of effects, including the induction of cytotoxicity, phagocytosis, immune complex binding and respiratory burst.19 Herein, we have demonstrated that eosinophils phagocytose opsonized live yeasts of C. neoformans and that this phenomenon involves the engagement of FcγRII and CD18, because the blocking of these receptors together caused the almost complete inhibition of fungal phagocytosis. These results are in agreement with previous reports which showed that Mφ and dendritic cells take up C. neoformans yeasts and the capsular polysaccharide via FcγRII and CD18.23,25,31,32 Furthermore, our results demonstrate that the phagocytosis of opsonized C. neoformans triggers eosinophil activation, as indicated by (i) the up-regulation of MHC class I, MHC class II and co-stimulatory molecules, and (ii) the increased production of IL-12, TNF-α and IFN-γ, without IL-4, IL-10 and IL-13 synthesis being modified. This, however, is in contrast with previous studies, which reported that eosinophils mainly secrete Th2-type cytokines in response to parasite antigens and allergens.33,34
The GM-CSF is a cytokine expressed by a variety of cells, including activated T cells, Mφ, fibroblasts and epithelial cells. GM-CSF is required for the recognition of pathogens, the timely development and proper compartmentalization of the immune response and the control of pulmonary growth of C. neoformans.35 Furthermore, GM-CSF stimulates the functional activity of eosinophils and maintains the maximum viability of cells,13 and GM-CSF-activated eosinophils have been reported to be capable of acting as specific APCs to a T-cell clone derived from mice infected with Mesocestoides corti.27 The results of the present study showed that GM-CSF only modified the MHC class II expression levels on eosinophil surfaces cultured with C. neoformans. Moreover, C. neoformans-pulsed eosinophils in the presence of GM-CSF expressed threefold more MHC class II than C. neoformans-pulsed eosinophils in the absence of this stimulating factor (Fig. 2b). In contrast, GM-CSF did not modify phagocytosis of the fungus, the expression of MHC class I, CD80 or CD86, cytokine production or the fungicidal molecules released by eosinophils incubated with the fungus. Related to this, Feldmesser et al.19 have demonstrated that short-term incubation with IL-5, GM-CSF and lipopolysaccharide (LPS) did not appear to enhance eosinophil phagocytosis.
Phagocyte–microbe contact is accompanied by intracellular signals that trigger cellular processes as diverse as cytoskeletal rearrangement, alterations in membrane trafficking, activation of microbial killing mechanisms, production of pro- and anti-inflammatory cytokines and chemokines, activation of apoptosis and the production of molecules required for efficient antigen presentation to the adaptive immune system.36,37 In this regard, it has been shown that eosinophils are able to produce H2O2 in response to phagocytosis of heat-killed Staphylococcus aureus38 and excretory–secretory products (ESP) from interacting with Fasciola hepatica.8 In addition, Phipps et al.39 suggests that eosinophil-derived NO contributes to innate protection against the respiratory syncytial virus. In fact, in cryptococosis, the generation of NO is required for resistance to primary fungal infections. Moreover, mice deficient in inducible nitric oxide synthase (iNOS) did not survive a primary infection.40 Snelgrove et al.41 have shown that NADPH oxidase-deficient mice elicited a heightened Mφ-driven Th1 response with the containment of cryptococci within pulmonary granulomatous lesions. They also observed improved clearance of pathogen in lung and airways, with reduced dissemination to the brain. In the present study, opsonized C. neoformans down-regulated NO and H2O2 synthesis by eosinophils in an FcγRII-dependent manner. In particular, FcγRII was essential in order to inhibit H2O2 production (Fig. 5). Down-regulation of NO and H2O2 by eosinophils could be a mechanism for protecting neighbouring eosinophils from the high toxicity and lack of specificity of this species, as H2O2 is involved in the spontaneous apoptosis of eosinophils.8 Moreover, when performing as an APC there might be a benefit for individual eosinophils to down-regulate toxic molecules in order to prolong survival and therefore function. We observed 85% viability of eosinophils after culture for 24–48 hr with opsonized C. neoformans, similar to that observed for eosinophils in medium alone. In contrast, it has been demonstrated that live yeasts of C. neoformans inhibit NO production by Mφin vitro through efficient free-radical scavengers.42 Moreover, we have previously reported that FcγRII blockade up-regulates the production of NO by rat Mφ incubated with glucuronoxylomannan, the major component of Cryptococcus capsular polysaccharide.23
The present work demonstrates that MSCs and purified T cells isolated from spleens of infected rats and cultured with C. neoformans-pulsed eosinophils proliferate in an MHC class I- and MHC class II-dependent manner, producing a large quantity of Th1-type cytokines, such as TNF-α and IFN-γ, in the absence of Th2 cytokine synthesis. However, although naive T cells did not proliferate or increase IFN-γ production, they did produce TNF-α in response to C. neoformans-pulsed and unpulsed eosinophils. Therefore, fungally activated eosinophils induced the growth and activation of C. neoformans-specific CD4+ and CD8+ Th1 cells. In contrast, it has been previously demonstrated that antigen-loaded eosinophils present antigens to primed T cells and increase the production of Th2 cytokines.10,11 In this regard, eosinophils pulsed with Strongyloides stercoralis antigen stimulated antigen-specific primed T cells and CD4+ T cells to increase the production of IL-5.13,14 However, in a pulmonary cryptococcosis developed in BALB/c mice, Huffnagle et al.43 observed that infiltrating T cells secreted significant amounts of Th2-type cytokines (IL-4, IL-5 and IL-10) in addition to Th1-type cytokines (IFN-γ and IL-2). These results suggest that the phenotype of CD4+ T cells recruited into the lungs included a combination of Th1, Th2 and/or T-helper 0 (Th0) cells. Nevertheless, recent studies have associated eosinophils with protective immunity to respiratory virus infections. In this regard, Handzel et al.44 has demonstrated that human eosinophils bind rhinoviruses (RV), present viral antigens to RV16-specific T cells, and induce T-cell proliferation and IFN-γ secretion. Moreover, Davoine et al.45 has shown that the concentration of both, IFN-γ and GM-CSF appeared to increase when human eosinophils were added to the co-culture of T cells, parainfluenza virus type 1 and dendritic cells. In addition, Phipps et al.39 demonstrated that the expression of both IFN-γ and IFN-β was significantly increased in the lung of hypereosinophilic mice 1–3 days after infection with respiratory syncytial virus. Therefore, further studies are being carried out in our laboratory to investigate the ability of C. neoformans-activated eosinophils to develop a protective Th1 immune response in vivo.
The current work demonstrates that C. neoformans is taken up by an exogenous pathway (phagocytosis), with a considerable, subsequent, increase of MHC class II and MHC class I molecules, which promote the expansion of CD4+ and CD8+ T-cell populations in an MHC class II- and MHC class I-dependent pathways. These results suggest the possibility that cross-presentation of C. neoformans antigens to CD8+ T cells could occur in the C. neoformans-loaded eosinophils. In this regard, there is a consensus that activating types of FcγRs on APCs are internalized upon binding to IgG immune complexes (as in the case of opsonized yeasts), thereby inducing dendritic cell maturation and leading to a significant enhancement of the MHC class II-restricted presentation of antigen to CD4+ T cells as well as to a class I-restricted cross-presentation to CD8+ T cells.46 Furthermore, it is well known that C. neoformans is a facultative intracellular pathogen that survives in various intracellular compartments,47 with Lindell et al.48 having reported CD4+ T-cell-independent CD8+ T-cell activation, suggesting that both endogenous and exogenous antigen-presentation pathways are probably active during C. neoformans infection.
In the present study, we observed that co-operation between CD4+ and CD8+ T cells is necessary for IFN-γ and TNF-α production in the presence of C. neoformans-treated eosinophils. In agreement with this finding, it has been demonstrated that both CD4+ and CD8+ T cells are required for inflammatory cell recruitment, phagocyte activation, pulmonary clearance and protection against extrapulmonary dissemination of C. neoformans.4,5,48,49 The absence of either or both T-cell subsets resulted in the reduction or ablation of inflammation, suggesting that CD4+ and CD8+ combine to mediate a protective inflammatory response to C. neoformans in the lungs.43 Therefore, the present study indicates that C. neoformans-loaded eosinophils could participate in the protective adaptive immune response to these fungi. In this regard, we have previously mentioned that the cells recruited during the initiation of the inflammatory response to C. neoformans infection include neutrophils, eosinophils, monocyte/Mφ, dendritic cells and lymphocytes.5 This immune response peaks 2 weeks after infection and coincides with the beginning of gradual clearance of the pathogen.43 Moreover, it has been shown that dendritic cells internalize, process and ultimately initiate a T-cell response to C. neoformans in a more efficient way than alveolar and monocyte-derived macrophages.31 The present work suggests that eosinophils, present since the initiation of the inflammatory response to these fungi,5 would be capable of phagocytosing and presenting C. neoformans antigens to primed T cells after the immune response had peaked and the immunoglobulin switch from the initial IgM had also occurred, thereby helping the development of a more effective protective Th1 immune response.
In summary, the present study demonstrates that opsonized live yeasts of C. neoformans activate eosinophils, inducing the expression of MHC class I, MHC class II and costimulatory molecules. Furthermore, although the secretion of proinflammatory cytokines is also increased, the production of oxygen and nitrogen radicals is down-regulated. These activated eosinophils can also stimulate CD4+ and CD8+ T cells to produce an antigen-specific immune response, thus creating a Th1 microenvironment. These results suggest that, in addition to their role as effector cells, eosinophils may also serve as specific APCs during fungal infection. Moreover, the fact that eosinophils are able to communicate with T cells suggests that they could be involved in the adaptive immune response to C. neoformans.
Acknowledgements
The present work was supported by grants from Agencia Nacional de Promoción Científica y Tecnológica (PICT 33326); Consejo Nacional de Investigaciones Científicas y Técnicas de Argentina (PIP 6327); Secretaría de Ciencia y Tecnología (SeCyT), Universidad Nacional de Córdoba (Grant 69/08); and Ministerio de Ciencia y Tecnología de la Provincia de Córdoba (Grant 2008). A. P. Garro and J. L. Baronetti are PhD fellows of Consejo Nacional de Investigaciones Científicas y Técnicas, and L. S. Chiapello and D. T. Masih are members of the Research Career of Consejo Nacional de Investigaciones Científicas y Técnicas. We would like to thank native speaker, Paul Hobson for revision of the manuscript.
Disclosures
The authors have no conflicts of interest to disclose.




