A comparison between isolated blood dendritic cells and monocyte-derived dendritic cells in pigs
Summary
Various dendritic cell (DC) populations exist that differ in phenotype and ability to present antigen to T cells. For example, plasmacytoid DCs (pDCs) are less potent T cell activators compared with conventional DCs (cDCs). Here, we compared porcine blood DCs (BDCs), containing pDCs and cDCs, and monocyte-derived DCs (MoDC), consisting of cDCs, in their phenotype, ability to uptake antigen, activation and maturation and their ability to present antigen to autologous T cells. Pigs represent an important animal model, whose immune system in many respects closely resembles that of humans. For example, the distribution of Toll-like receptors is similar to that of humans, in contrast to that of mice. Here we demonstrate that both populations endocytose foreign material. Following lipopolysaccharide stimulation, CD80/86 and chemokine receptor (CCR)7 expression was increased in both populations as was the expression of the chemokine ligands (CCL)-2, CCL-4, CCL-20 and CXCL-2. Although basal and post-stimulation protein concentrations of interleukins 6 and 8 and tumour necrosis factor-α were higher in MoDCs, protein concentrations showed a higher fold increase in BDCs. Antigen-specific proliferation of autologous T cells was induced by MoDCs and BDCs. Interestingly, while MoDCs induced stronger proliferation in naive T cells, no difference in proliferation was observed when primed T cells were studied. These results demonstrate that isolated porcine BDCs are highly responsive to stimulation with lipopolysaccharide and are functionally able to drive primed T-cell proliferation to the same extent as MoDCs.
Introduction
Dendritic cells (DCs) are important cells of the immune system involved in the uptake and presentation of foreign antigens, stimulation of both innate and acquired immunity, as well as modulation of the immune response towards a T helper type 1 (Th1), Th2, Th17 or T regulatory type of response.1,2 At steady state, DC subtypes include type-1 interferon-producing plasmacytoid DCs (pDCs) and conventional DCs (cDCs), both of which are present in lymphoid and non-lymphoid tissues as well as in blood.3 In contrast, monocyte-derived DCs (MoDCs) are generated during inflammation.4,5 Dendritic cells have been extensively characterized in a variety of species and protocols for obtaining DC subtypes range from in vitro culture methods to direct isolation of DCs from blood and tissues. Isolation, however, is complicated in humans and large animal species resulting in limited availability of functional studies. In pigs, blood DCs (BDCs) have only been investigated in a few studies and very little is known about the function of these DCs in antigen presentation and T-cell activation. The objectives of the present study were to compare directly isolated porcine BDCs with traditionally generated porcine MoDCs in terms of phenotype and functionality.
Various porcine DCs have been described including bone marrow-derived (BM) DCs,6 Langerhans-type cells7 and MoDCs.6–11 The MoDCs are the most widely used subtype and can be phenotyped as CD1+, CD14+/−, CD16+, CD80/86+, CD172+, major histocompatibility complex (MHC) I+, MHC II+, CD4−, CD3−, and CD8−.6,7 Initially MoDCs were generated by isolation of peripheral blood mononuclear cells (PBMCs) followed by overnight plastic adherence. Non-adherent cells were then removed and the remaining monocytes were cultured in the presence of interleukin-4 (IL-4) and granulocyte–macrophage colony-stimulating factor (GM-CSF).6 More recent protocols, however, involve the isolation of monocytes using antibodies against CD1412,13 or CD172a,14 a porcine marker known as SWC3 that is present on myeloid cells15 including cDCs and pDCs.16 Porcine BDCs, on the other hand, comprising pDCs and cDCs, were originally described by Summerfield et al.,16 by flow cytometric analysis of PBMCs as being CD172a+, MHC II+, CD80/86+, CD1+/− and CD14− with pDCs being CD4+ and cDCs being CD4−. Subsequently, this approach was further developed by isolating BDCs using antibodies against CD172a. However, because CD172a is also expressed on monocytes, these enriched BDC populations contained not only different DC subtypes but monocytes as well.17 In the present study, we adapt previous protocols by initially depleting monocytes and subsequently enriching for CD172a to achieve a purer BDC population. These BDCs were compared with MoDCs in terms of antigen uptake, activation and maturation.
DC maturation occurs upon recognition of microbe-associated molecule patterns and is characterized by up-regulation of co-stimulatory molecules such as CD80/86 and MHC II, various cytokines and the chemokine receptor CCR7.18,19 The process of maturation occurs as DCs migrate towards the lymph nodes where they encounter naive or primed T cells. In porcine MoDCs, stimulation with lipopolysaccharide (LPS) was demonstrated to decrease the expression of CD16, up-regulate the expression of CD80/866,20 and either increase7 or have no effect6,20 on expression of MHC II. Uptake of fluorescein isothiocyanate (FITC) -dextran or bovine serum albumin FITC7 was decreased. Expression of cytokines including IL-6 and tumour necrosis factor-α (TNF-α)21 was increased. Interestingly, transcripts for IL-10, IL-13, interferon-γ (IFN-γ) and IL-12p35 were increased but no production at the protein level was detected.10,21 Furthermore, LPS stimulation did not induce a change in IL-4 gene expression.20 However, T cells that had been exposed to antigen-pulsed MoDCs produced protein for both IL-4 and IFN-γ.6 In contrast to MoDCs, however, very little information is available on maturation and activation of isolated BDCs following stimulation with LPS.
Following their activation and maturation, DCs are known to drive T-cell proliferation and to modulate the immune response towards a Th1, Th2, Th17 or T regulatory type of response.1,2 As a result of the limitations of studying T-cell proliferation in outbred species, most studies in pigs have used mixed lymphocyte reactions6,10,12 and few have used autologous cells.16,21,22 In the present study, both MoDCs and BDCs were isolated from vaccinated pigs and co-cultured with autologous T cells to assess the induction of antigen-specific T-cell activation. We found that both MoDCs and BDCs were equally able to induce T-cell proliferation. However, when stimulated with LPS, BDCs that were directly isolated from blood showed a greater increase in cytokine and chemokine expression, when compared with MoDCs. This study therefore provides further evidence that directly isolated BDCs represent an important cell population for studying DC biology in pigs. Further studies, however, are required to identify the specific role of pDCs within the BDC population.
Materials and methods
Experimental design and animals
Eight-week-old Dutch Landrace pigs purchased from Saskatoon Prairie Swine Centre, University of Saskatchewan were used in this study. The goal of this study was to directly compare MoDCs with isolated BDCs both phenotypically and functionally. Phenotypically, DC morphology was examined by Giemsa staining and the expression of cell surface markers was examined by flow cytometry. Functionally, endocytic ability was examined by flow cytometry, changes in transcript expression and the production of cytokines in response to stimulation with LPS were investigated using quantitative real-time polymerase chain reaction (qRT-PCR) and enzyme-linked immunsorbent assay (ELISA), respectively, and lastly for their ability to stimulate autologous T-cell proliferation, thymidine uptake assays were performed. Studies were performed as per the ethical guidelines of the University of Saskatchewan and the Canadian Council for Animal Care.
BDC and T-cell isolation and generation of MoDCs
Blood was collected by heart puncture using ethylenediaminetetraacetic acid (EDTA) -coated syringes and blood mononuclear cells were isolated using a 60% Ficoll-Paque™ Plus gradient (GE Healthcare, Uppsala, Sweden). Monocytes were isolated using magnetic beads [magnetic antibody cell sorting (MACS); Miltenyi Biotec, Auburn, CA] and human anti-CD14 (TÜK4) microbeads (Miltenyi Biotec).12,13 The cross-reactivity of this antibody was confirmed by testing it against an anti-porcine CD14 (MIL-2) homologue. Flow cytometry was used to verify the purity of the separated cells.
To generate MoDCs, monocytes were cultured in RPMI-1640 (Gibco, Grand Island, NY) supplemented with 10% fetal bovine serum, 0·5 mmβ-mercaptoethanol, 10% antibiotic/antimycotic (Gibco, Grand Island, NY), 10% HEPES (Gibco), 10% minimal essential medium non-essential amino acids (Gibco), 100 ng/ml of recombinant porcine (rp) IL-4 (Biosource, Camarillo, CA) and 20 ng/ml of rpGM-CSF (Biosource) for 6 days at 37° with 5% carbon dioxide. Half of the medium was changed every 3 days. The MoDCs were used between days 4 and 6, at which time non-adherent MoDCs6,23,24 were washed, counted and used in subsequent assays.
To isolate BDCs, which are described as CD172+ CD14−,16,24 CD14− cells were labelled with a CD172 antibody (Serotec, Oxford, UK) and rat anti-mouse immunoglobulin G1 (IgG1) Microbeads (Miltenyi Biotec) and positively selected using MACS. The purity of CD172+ expression was consistently > 95%. CD172+ cells were rested overnight and then used in the assays. This procedure is slightly modified from Summerfield et al.,16 in which PBMCs were rested overnight and the non-adherent cells were depleted of CD3, CD8 and CD45RA, and then sorted for CD172.
To isolate T cells, the CD172– population was positively sorted for CD4+ and CD8+ cells by labelling the cells with anti-CD4 (VMRD Inc., Pullmann, WA) and anti-CD8 antibody (VMRD Inc.) followed by incubation with rat anti-mouse IgG1 microbeads (MACS; Miltenyi Biotec).
Cell stimulation
For stimulation with LPS, day 6 MoDCs and day 1 BDCs were cultured at 1 × 106 cells/ml and stimulated with 100 ng/ml of LPS (Escherichia coli O55:B5; Cambrex Bioscience, Walkersville, MD) for 6-hr for gene expression studies or for 24-hr for ELISA and flow cytometry. Expression of TNF-α was analysed by ELISA following an 8-hr incubation because of its early release.25
Morphology
To evaluate morphology, 1 × 105 cells in medium were centrifuged at 150 g for 4 min, incubated with methanol for 5 min, air-dried and stained with Giemsa stain (Sigma, St Louis, MO) for 15–60 min. Cells were then washed with deionized water, air-dried and fixed for morphological examination by microscopy.
Antibodies for phenotyping
The following anti-porcine antibodies were used for defining the cell types: CD172 (BL1H7, Serotec), CD1 (76-7-4, Southern Biotech, Birmingham, AL), CD3 (PPT3, Southern Biotech, Birmingham, AL), CD4 (74-12-4, VMRD Inc.), CD8 (PT36B, VMRD Inc.), CD14 (MIL-2, Serotec), CD16 (G7, Serotec), CD21 (BB6-11C9.6, Southern Biotech, Birmingham, AL), MHC II (K274.3G8, Serotec), MHC I (SLA-I, Serotec) and human CD152 (CTLA-4 fusion protein) (4 501-020, Ancell, Bayport, MN). FITC anti-mouse immunoglobulins IgG1, IgG2a and IgG2b (Southern Biotech) were used for detection by flow cytometry. The FITC-conjugated anti-mouse immunoglobulins IgG1, IgG2a and IgG2b (Southern Biotech) were used for detection by flow cytometry.
Flow cytometry
Immunofluorescence staining was performed by incubating 1 × 106 cells for 20 min at 4° with each antibody. Cells were washed three times with cold phosphate-buffered saline (1×) (pH 7·2) (Gibco) containing sodium azide (0·03%) and gelatin (0·02%) and incubated with FITC-conjugated secondary antibody for 20 min at 4°, washed three times and fixed with paraformaldehyde (2%). Ten thousand events were collected and analysed by flow cytometry (FACScalibur™ using cellquest™ software; Becton Dickinson, BD Biosciences, Mountain View, CA).
Endocytosis by MoDCs and BDCs
To evaluate endocytosis, 2 × 105 MoDCs or BDCs were incubated with 200 μl FITC-dextran (1 mg/ml) (Sigma) or DQ™ red bovine serum albumin (BSA) (1 mg/ml) (Invitrogen, Carlsbad, CA) for 1-hr at either 0° or 37°.7 Cells were washed three times with cold phosphate-buffered saline and centrifuged at 350 g for 5 min. The uptake of the labelled particles was visualized by confocal microscopy and quantified by flow cytometry using 10 000 cells/event. Endocytosis is inhibited at 0°, so cells incubated at this temperature served as controls for non-specific fluorescence. The endocytic activity of MoDCs was examined from days 0 to 7 and that of BDCs was examined on day 1.
Lymphocyte proliferation assay
Pigs were vaccinated at 4 weeks of age with 10 μg genetically detoxified pertussis toxoid (PTd; Novartis, Sienna, Italy) in 30% emulsigen (MPV Laboratories, Omaha, NE), and boosted every 2 weeks for a total of three vaccinations. Blood was collected from these pigs to isolate MoDCs, BDCs and T cells.
Once generated, MoDCs and BDCs were respectively pulsed with PTd (1 μg/ml in a total of 1 ml) or OVA (100 μg/ml in a total of 1 ml) for 3-hr and washed three times. Then, 3 × 104 MoDCs or BDCs were co-cultured in 200 μl of culture medium with a total of 3 × 105 MACS-purified CD4 and CD8 autologous T cells for 72-hr in 96-well U-bottom plates (Corning, NY). During the last 8-hr of culture 1 μCi [3H]thymidine (Amersham Pharmacia Biotech, Baie de Urfe, PQ) was added and proliferative responses were determined. Results are expressed as a stimulation index and analysed by a Mann–Whitney U-test.
Quantitative RT-PCR assay for messenger RNA expression
To evaluate differential messenger RNA (mRNA) expression, 1 × 106 MoDCs or BDCs were lysed in TRIzol (Invitrogen) and stored at − 80° until further processing. For RNA extraction, 200 μl chloroform was added per 1 ml TRIzol. The sample was incubated at room temperature for 3 min and centrifuged at 12 000 g for 10 min at 4°. The aqueous phase was collected and 500 μl isopropanol was added. The sample was incubated for 5 min at room temperature and then applied to a mini-column (Qiagen RNeasy®, Mississauga, ON) and centrifuged for 15 seconds at 8000 g. The sample was washed as per the manufacturer’s instructions and DNAse I treatment was performed. The optical density at 260 nm (OD260) was used to quantify RNA and the ratio of OD260 : OD280 was used to determine purity. Complementary DNA was generated and RT-PCR was performed using the SuperScript™ III Platinum® Two-Step qRT-PCR Kit as per the manufacturer’s recommendations (Invitrogen). Table 1 lists the primers that were used for mRNA quantification. Samples were analysed using a Bio-Rad iCycler iQ (Bio-Rad, Hercules, CA). Changes in gene expression were determined by calculating the Δ cycle threshold (Ct) by subtracting the Ct for ribosomal protein L19 (RPL19) (reference gene) from the Ct of the gene of interest for each sample.26 The ΔCt of the control was subtracted from the corresponding treated sample giving rise to the ΔΔCt. The fold change was derived from the equation 2−[ΔΔ]Ct. To confirm that the reference gene ribosomal protein L19 was stably expressed in MoDCs and BDCs, a comparison was performed using either glyceraldehyde 3-phosphate dehydrogenase (GAPDH) or RPL19 as the reference gene. Similar trends in fold change were observed. Complementary DNA was diluted to generate a standard curve whose correlation coefficient was > 0·99. The efficiency of qPCR was determined from the slope using the equation (10[−1/M] − 1) × 100 and ranged between 90% and 110%.
| Primer sequences (5′–3′) | Accession number | ||
|---|---|---|---|
| Sense | Anti-sense | ||
| CCR7 | CCCTTCCCTTCTGGGCATAC | CGGTCGATGCTGATGCAGAG | AB116555 |
| IFN-α | CCACCTCAGCCAGGACAGAAGC | GGTCACAGCCCAGAGAGCAGATG | NM_214393.1 |
| IFN-γ | CGAAAAGCTGATTAAAATTCCGGTA | TCTTAGGTTAGATCTTGGTGACAGA | NM_213948.1 |
| IL-12(p40) | GAAATTCAGTGTCAAAAGCAGCAG | TCCACTCTGTACTTCTTATACTCCC | NM_214013 |
| IL-6 | ACCCAGCTATGAACTCCCTCTC | GCATCACCTTTGGCATCT TCTTC | NM_214399.1 |
| IL-8 | AGAAGCAACAACAACAGCAGTAACAAC | CCAGCACAGGAATGAGGCATAGATG | AB057440.1 |
| TNF-α | CCCTTCCACCAACGTTTTCCT | TGATGGCAGAGAGGAGGTTG | EU682384 |
| CCL-2 | GCGGCTGATGAGCTACAGAAG | CCCGCGATGGTCTTGAAG | NM_214214 |
| CCL-4 | CCTCTCCCTCCTGGTCCTG | GGCTGCTGGTCTCATAGTAATC | EF107667.1 |
| CCL-20 | TGCTCCTGGCTGCTTTGATGTC | TCATTGGCGAGCTGCTGTGTG | AJ577084.1 |
| CXCL2 | GCTGCTCCTGCTTCTAGTG | ACTTCCTGACCATTCTTGAGAG | NM_001001861.1 |
| RPL19 | AACTCCCGTCAGCAGATCC | AGTACCCTTCCGCTTACCG | AF435591 |
| GAPDH | CTCAACGGGAAGCTCACTGG | TGATCTCATCATACTTGGCAGG | DQ845173 |
- GAPDH, glyceraldehyde 3-phosphate dehydrogenase; IFN, interferon; IL, interleukin; TNF, tumour necrosis factor.
ELISA for cytokines
To evaluate changes in cytokine secretion, 1 × 106 MoDCs or BDCs were incubated in 1 ml culture medium for 24-hr in six-well plates (Corning) and culture supernatants were collected. Concentrations of IL-6, IL-8 and IL-10 were assayed using commercial kits as per the manufacturer’s instructions (R&D Systems, Minneapolis, MN). The ELISA for IFN-α, TNF-α and IL-12 were performed as previously described.27
Statistical analysis
Statistical analysis was performed by non-parametric Mann–Whitney U-tests (P-value < 0·05) using the statistical software programme graphpad prism 5 (GraphPad Software, Inc., La Jolla, CA).
Results
Monocyte-derived DC generation and blood DC isolation
In this study, 800 ml of EDTA blood yielded approximately 2 × 109 PBMCs. Following CD14+ selection, an average of 2 × 108 monocytes were cultured in the presence of IL-4 and GM-CSF to generate MoDCs. On day 6, approximately 2 × 107 MoDCs were harvested and cultured for use. The CD14− population was positively selected for cells expressing CD172, which equates to the BDC (CD14− CD172+) population. Approximately 3 × 107 BDCs were therefore isolated and rested overnight. In contrast to other studies, the protocol used in this study resulted in lower numbers of MoDCs compared with BDCs from an equal amount of blood.28
Phenotypic characterization of porcine DCs
Dendritic cell morphology is characterized by a large cytoplasmic cell mass and extrusion of dendrites which increase the surface area available to sample and take up antigens. In this study, the morphologies of Giemsa-stained MoDCs (Fig. 1a) and BDCs (Fig. 1b) were compared. Both DC populations displayed a typical DC morphology, characterized by an irregular cell border with a large cytoplasmic cell mass. Expression of cell surface markers CD172, MHC II, CD16, CD1, CD80/86 and CD14 was assessed by flow cytometry in 6-day-old MoDCs and BDCs (Table 2). Both MoDCs and BDCs expressed all of these markers; however, BDCs showed similar expression of CD172 and MHC II, higher expression of CD16 and lower expression of CD80/86 and CD1. CD14 was absent from the BDC population as indicated in the cell isolation procedure.
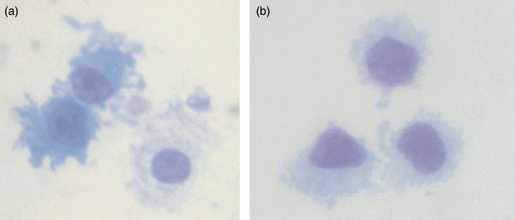
Giemsa-stained pig monocyte-derived dendritic cells (MoDCs) and blood dendritic cells (BDCs). MoDCs at day 6 (a) and BDCs at day 1 (b). Magnification 1000 ×.
| Cell surface markers | MoDCs | BDCs |
|---|---|---|
| CD172 | 92 ± 3% | 96 ± 5% |
| MHC II | 95 ± 2% | 94 ± 10% |
| CD16 | 85 ± 8% | 92 ± 9% |
| CD1 | 61 ± 10% | 17 ± 12% |
| CD80/86 | 43 ± 10% | 14 ± 7% |
| CD14 | 81 ± 7% | ND1 |
- Data shown are as a percentage of the mean ± SD of positive cells. Data for the MoDCs are representative of six pigs and data for the BDCs are representative of four pigs from a different litter.
- 1ND, The isotype of the anti-CD14 antibody was the same as that of the anti-CD172 antibody used to isolate the BDCs and therefore the % of CD14-expressing cells in the BDC population could not be determined.
- MHC, major histocompatibility complex.
Endocytosis by MoDCs and BDCs
Central to DC functioning is their ability to take up antigens. To directly compare the endocytic activity of MoDCs and BDCs, we examined their uptake of FITC-dextran over time from day 0 to day 7. The ability to take up FITC-dextran increased from 29 ± 30% (mean ± SD) on day 1 to 58 ± 24% on day 4 and 57 ± 27% on day 6. In contrast, 16 ± 18% of BDCs on day 1 were endocytically active following their isolation from blood. Laser confocal microscopy confirmed the uptake of particles of FITC-dextran in both MoDCs and BDCs (data not shown). Overall, these results show that BDCs were consistently less endocytic than MoDCs.
Functional characterization of DC maturation following stimulation with LPS
As DCs mature, the expression of co-stimulatory molecules such as CD80 or CD86 increases providing DCs with the ability to activate T cells. Furthermore, up-regulation of the chemokine receptor CCR7 allows DCs to migrate to the lymph node where they encounter lymphocytes.19 To compare the expression of co-stimulatory molecules and CCR7 within each DC population, MoDCs and BDCs were stimulated with LPS (100 ng/ml) for 24-hr. Flow cytometric analysis showed that CD80/86 expression increased from 46% to 67% (median) in MoDCs (stimulation index = 1·5) (Fig. 2a; P < 0·05), and from 14% to 45% in BDCs (stimulation index = 3·8) (Fig. 2b; P < 0·05) as determined by flow cytometry. Within the 6-hr stimulation with LPS, CCR7 gene expression increased by 3·4-fold (median) in BDCs and 2·0-fold in MoDCs (Fig. 3). In summary, in response to stimulation with LPS both MoDCs and BDCs demonstrated the characteristics of mature DCs in terms of co-stimulatory molecule cell surface expression and CCR7 gene expression.
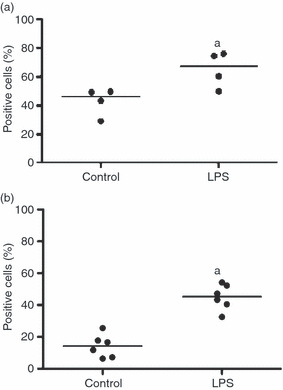
The effect of lipopolysaccharide (LPS) stimulation on CD80/86 cell surface expression in monocyte-derived dendritic cells (MoDCs) (n = 4 animals) and blood dendritic cells (BDCs) (n = 6 animals). MoDCs at day 6 (a) and BDCs (b) were isolated from blood mononuclear cells and rested overnight before being cultured with LPS (100 ng/ml) for 24-hr. The expression of CD80/86 was determined by flow cytometry to examine DCs stimulated with LPS compared with DCs in medium. Results are expressed as the median of the percentages of positive cells. aP < 0·05 versus the control.
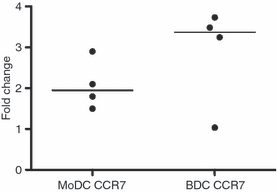
The effect of lipopolysaccharide (LPS) stimulation on CCR7 expression in monocyte-derived dendritic cells (MoDCs) (n = 4 animals) and blood dendritic cells (BDCs) (n = 4 animals). MoDCs at day 6 and BDCs at day 1 were cultured with LPS (100 ng/ml) for 6-hr. Samples were assessed for changes in gene expression of CCR7 by quantitative real-time polymerase chain reaction using ribosomal protein L19 as the reference gene. Results are shown as the median of the fold changes relative to the control.
Chemokine and cytokine production by DCs
At sites of injury, DCs release chemokines that are involved in recruiting innate and adaptive immune cells. The ability of DCs to produce chemokines was examined following a 6-hr stimulation with LPS. Over fourfold up-regulation was observed in CCL-4, CCL-20 and CXCL2 gene expression in both MoDCs and BDCs (Fig. 4a) with the up-regulation observed to be higher in BDCs for all of the genes examined. In BDCs, there was also CCL-2 up-regulation.
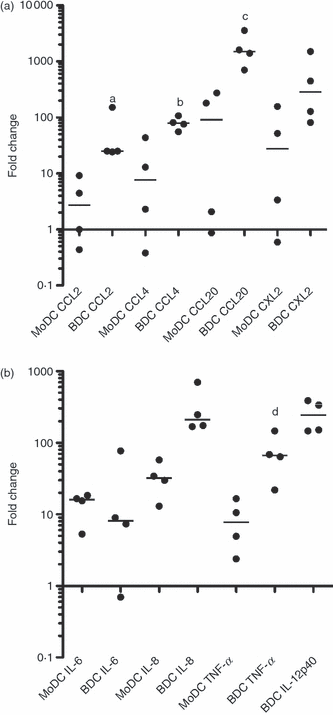
Changes in gene expression of (a) CCL-2, CCL-4, CCL-20 and CXCL2 and (b) interleukin-6 (IL-6), IL-8 and tumour necrosis factor-α (TNF-α) in monocyte-derived dendritic cells (MoDCs) and IL-12 in blood dendritic cells (BDCs) following a 6-hr stimulation with lipopolysaccharide (LPS). MoDCs at day 6 and BDCs at day 1 were cultured with LPS (100 ng/ml). Samples were assessed for changes in gene expression by quantitative real-time polymerase chain reaction using ribosomal protein L19 as the reference gene. Results are shown as the median fold change relative to the control (n = 4 animals). a,b,c,dP < 0·05 MoDC versus BDC.
In lymph nodes, DCs interact with T cells by delivering different types of signals including cytokines. The expression of cytokines in MoDCs and BDCs was compared by qRT-PCR following a 6-hr stimulation with LPS. No changes were observed in IFN-α and IFN-γ, whereas a greater than threefold up-regulation was observed in IL-12 in BDCs and in IL-6, IL-8 and TNF-α in both MoDCs and BDCs (Fig. 4b). No IL-12 was detected in MoDCs.
Cytokine secretion was examined by ELISA following a 24-hr stimulation with LPS. Production of IL-6, IL-8, IL-12 and TNF-α was significantly increased in BDCs (Table 3).
| MoDCs | BDCs | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | LPS | P-value | Stimulation index | Control | LPS | P-value | Stimulation index | |
| IL-6 | 426 ± 186 | 940 ± 277 | 0·2 | 2 | ND | 229 ± 260 | 0·03 | |
| IL-8 | 5261 ± 5756 | 10 586 ± 1673 | 0·4 | 6 | 1114 ± 496 | 9557 ± 3925 | 0·03 | 10 |
| TNF-α1 | 402 ± 138 | 1277 ± 896 | 0·057 | 3 | 277 ± 74 | 833 ± 511 | 0·057 | 3 |
| IL-12 | ND | ND | 1982 ± 1453 | 8381 ± 3101 | 0·03 | 5 | ||
- MoDCs at day 5 and BDCs at day 1 were either cultured with LPS (100 ng/ml) or were unstimulated (Control; n = 4 animals). Supernatants were assayed by enzyme-linked immunosorbent assay for protein detection. Results are expressed as mean (pg/ml) ± SD and analysed by a Mann–Whitney U-test.
- The stimulation index for each sample was determined by comparing the value of the LPS-treated sample with that of the medium control. The average stimulation index of all samples is shown.
- 1At 8-hr of culture.
- BDCs, blood dendritic cells; MoDCs, monocyte-derived dendritic cells; ND, not detectable.
Expression of IL-6, IL-8 and TNF-α was increased in MoDCs although the change was not statistically significant. Higher baseline values (control) were observed in MoDCs compared with BDCs. Interleukin-12 expression was not enhanced in MoDCs so there was a high correlation between the results obtained from qRT-PCR and ELISA.
Basal concentrations of IL-6, IL-8 and TNF-α were higher in MoDCs. Interestingly, when MoDCs and BDCs were stimulated with LPS, the fold increase, but not the absolute concentration, was higher in BDCs than MoDCs. The same trend was observed for changes in chemokine expression.
Stimulation of both naive and primed T cells in an autologous proliferation assay
Dendritic cells as key antigen-presenting cells are able to drive T-cell proliferation. We compared the ability of MoDCs and BDCs to drive the proliferation of autologous naive T cells with that of primed T cells. Overall, PTd-stimulated or OVA-stimulated MoDCs and BDCs co-cultured at a ratio of 1 DC to 10 T cells, showed an induction of T-cell proliferation (Fig. 5). However, the stimulation index was higher in PTd-stimulated DCs compared with OVA-stimulated DCs, reflecting the difference between primed and naive T cells. The MoDCs and BDCs stimulated antigen-specific T-cell proliferation in primed cells to the same extent. In contrast, MoDCs were more effective in stimulating naive autologous T cells when pulsed with OVA. Hence, the MoDCs and BDCs differed in their ability to stimulate naive T-cell proliferation but not in their ability to stimulate proliferation of primed T cells.
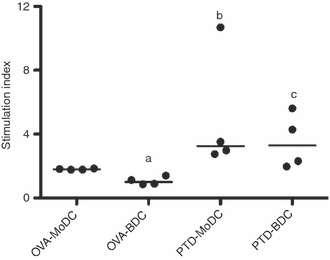
Stimulation of autologous T cells using antigen-pulsed monocyte-derived dendritic cells (MoDCs) and BDCs. MoDCs at day 4 of culture and BDCs rested overnight were pulsed with pertussis toxoid (PTd; 1 μg/ml) or ovalbumin (OVA; 100 μg/ml) for 3-hr then incubated with autologous T cells from four PTd-vaccinated animals at a ratio of 1 DC to 10 T cells. Results are shown as the median of the stimulation index relative to non-pulsed DCs. aP < 0·05 OVA-MoDC versus OVA-BDC, bP < 0·05 OVA-MoDC versus PTd-MoDC, cP < 0·05 OVA-BDC versus PTd-BDC.
Discussion
In the present study, we isolated porcine BDCs and MoDCs and demonstrated that these DC populations differ in their endocytic activity and their response to LPS with regards to cytokine and chemokine gene expression. Also, when we compared BDCs with MoDCs in autologous proliferation assays using T cells from vaccinated and non-vaccinated animals, no difference was observed in their ability to present antigen to primed T cells.
The MoDCs were generated by isolating monocytes via MACS and subsequent culture in the presence of IL-4 and GM-CSF. This isolation technique differs from overnight adherence or CD172 MACS sorting6–8,20,29 and is similar to protocols for generating porcine,12,13 human30 and murine MoDCs.31 The BDCs were generated by using a slightly modified protocol previously described by Summerfield et al.,16 who demonstrated antigen uptake by BDCs using flow cytometric analysis of PBMCs.16 In contrast, we first isolated BDCs from blood by using the negative fraction following CD14 MACS sorting of PBMCs and subsequent positive selection of CD172+ cells. The CD14+ fraction was used to generate MoDCs. Advantages of this isolation procedure include the isolation of a relatively pure population of monocytes which can be generated on the same day without requiring overnight adherence. The purity of isolated BDCs was > 96% combined with only very few or no contaminating monocytes resulting in a yield of approximately 2% of the original PBMC population.32 This is in contrast to previously described 60–75% purity of CD172 cells16 and high numbers of contaminating monocytes.17 However, a limitation of our isolation procedure is that in the absence of IL-3 BDCs display a very short lifespan.16 Interleukin-3 was not available to us in this study so the time that BDCs were kept in culture was limited to a minimum, as previously described by others.32 Despite this limitation, however, this isolation method resulted in functional BDCs, and one can speculate that in the presence of IL-3, such responses would have been enhanced.
Using these isolation methods, we observed that unstimulated MoDCs displayed a more mature phenotype compared with unstimulated BDCs. While a similar percentage of MoDCs and BDCs expressed CD172 and MHC II, BDCs showed a slightly higher expression of CD16 and a lower expression of CD80/86 and CD1. The more mature phenotype of MoDCs may be attributed to culturing artefacts such as disturbing cell–cell contact,33 the presence of serum in the culture medium34 and the effects of IL-435 and GM-CSF.36 Compared with MoDCs, BDCs were only cultured overnight, therefore culturing artefacts were expected to be minimal. This is supported by Fearnley et al.,34 who demonstrated that when human BDCs were cultured for several days they displayed a more mature phenotype similar to that of MoDCs.
Despite the more mature phenotype of MoDCs, BDCs displayed lower endocytic activity. Regarding IL-6, IL-8 and TNF-α cytokine production, the basal production of cytokines by MoDCs was over twofold higher than that of BDCs. However, when MoDCs and BDCs were stimulated with LPS, a higher fold change of both cytokine and chemokine expression was observed in BDCs, suggesting that BDCs were more responsive to LPS stimulation. Reasons for these differences remain to be examined but they may be the result of differences in cell signalling pathways. For example, BDCs do not express CD14 and therefore are unable to respond to LPS via a CD14-dependent signalling pathway. However, the presence of CD14-independent signalling in porcine DCs has been previously demonstrated6 and it is known that BDCs respond to LPS stimulation,37 suggesting that BDCs signal via a CD14-independent pathway. Further studies are required to understand the detailed mechanisms of LPS signalling in BDCs.
Another interesting observation in this study was that LPS-stimulated MoDCs did not produce IL-12 whereas BDCs did. This is in contrast to previous observations made by Raymond and Wilkie,20 who found an increase in IL-12p35 mRNA expression in porcine MoDCs following stimulation with LPS. Possible reasons for the observed differences include, cell isolation by plastic adherence, collection of both adherent and non-adherent day 8 MoDCs, and a different concentration of LPS for cell stimulation. However, in a more recent study in which MoDCs were obtained by plastic adherence, no IL-12p40 was detected at the protein level following LPS stimulation at a concentration of 1 μg/ml.10 There is therefore a discrepancy in the literature regarding the ability of porcine MoDCs to produce IL-12 in response to stimulation with LPS and more studies are required to fully address these observations. For human monocytes, it was demonstrated that MoDCs generated from plastic adherence compared with CD14 bead isolation, produced IL-12p70.38
We then determined if the phenotypic and endocytic differences between MoDCs and BDCs translated into differences in their ability to induce T-cell proliferation using autologous T cells. To this end, pigs were vaccinated with PTd and isolated cells were re-stimulated in vitro with two different antigens to be able to compare naive versus primed T cells. When the antigen OVA was used to address stimulation of naive T cells, BDCs induced less proliferation compared with MoDCs. However, when PTd was used for stimulation of autologous primed T cells, the extent of proliferation was the same between MoDCs and BDCs. As the activation threshold for naive T cells is higher because of an uncoupled signalling machinery,39,40 we assume that T cells to which OVA was presented were naive and required more signals that the BDCs were less able to provide. This could be attributed to their lower endocytic ability. With respect to primed T cells, however, BDCs did not differ from MoDCs in their ability to drive T-cell proliferation, which may be a result of a lesser need for additional stimulation. It has also been demonstrated that the pDC population within the BDCs is better able to induce proliferation in antigen-experienced T cells compared with naive T cells.41 Therefore, porcine BDCs differ from MoDCs in their ability to stimulate naive T-cell proliferation but not primed T-cell proliferation. This is in contrast to observations made in mice41 and provides further evidence that BDCs indeed are able to drive T-cell activation in both naive and memory T cells.39
In summary, in the present study we compared two populations of DCs in their phenotype, endocytic ability, response to LPS stimulation and ability to induce an antigen-specific immune response in pigs. The findings suggest that BDCs, which contain both pDCs and cDCs, are less endocytically active than MoDCs and have a lower expression of CD80/86. They also have lower basal cytokine protein concentrations but in response to stimulation with LPS, there is a higher fold increase in response despite the absolute amounts being lower in MoDCs. Furthermore, this is the first time in the pig that chemokines have been examined in response to LPS in both MoDCs and BDCs and it allows for a more comprehensive view of DC behaviour. Lastly, both MoDCs and BDCs are able to induce T-cell proliferation, which is in contrast to observations made in mice,41 and which will further the understanding of these important cells and their role in driving antigen-specific immune responses.
Acknowledgements
We are grateful to all members of the Animal Care Unit at VIDO for their help in isolating large amounts of blood and for housing the pigs. We are especially thankful to Amanda Giesbrecht and Jan Erickson. We also thank Krupal Patel, Stacy Strom and Justin Gawaziuk for their help in isolating PBMCs and DCs. This work was supported by the Natural Science and Engineering Research Council, the Alberta Agriculture Funding Consortium, CIHR and the Bill and Melinda Gates Foundation Grand Challenges in Global Health Initiative grant. The manuscript was published with permission of the Director of VIDO as manuscript number 529.
Disclosures
The authors do not have any conflicting interests.




