Impaired expression of indoleamine 2, 3-dioxygenase in monocyte-derived dendritic cells in response to Toll-like receptor-7/8 ligands
Summary
The effects of immunostimulatory RNAs (isRNAs) on the expression of immuno-suppressive factors are largely unknown. Indoleamine 2,3-dioxygenase (IDO) is a key negative regulator of immune responses and it has been implicated in hampering immunity against tumours. Here we show that the activation of Toll-like receptors (TLR)-7/8 with isRNAs or R848, a specific ligand for TLR7/8, can induce IDO expression in human monocytes, but not in monocyte-derived dendritic cells (moDC). In contrast to TLR7/8 agnosists, treatment of the same moDC with interferon-γ-induced IDO expression. Treatment of monocytes with 2′-O-methyl uridine-modified isRNAs alone does not induce IDO, but totally abrogated the effects of unmodified isRNAs. Like isRNAs, synthetic viral RNAs and cytomegalovirus (CMV) induced IDO in monocytes, whereas TLR2 ligand lipopeptide Pam3Cys exhibited no effect. Furthermore, IDO positive monocytes suppressed autologous T-cell activation. Collectively, these data indicate for first time that the potency of TLR7/8 signalling pathways to induce IDO expression in monocytes is silenced when the cells are programmed to differentiate into dendritic cells. The immunosuppressive properties of IDO might confer an advantage to CMV-infected monocytes to escape T-cell responses. The findings that 2′-O-methyl modified RNAs can block isRNA-induced IDO expression would facilitate the design of new TLR inhibitors.
Introduction
Toll-like receptors (TLR) recognize specific structural motifs expressed by microbes.1 On interaction with their ligands, TLR trigger a rapid induction of inflammatory cytokines (e.g. tumour necrosis factor-α (TNF-α), interleukin-6 (IL-6)) and type I interferon (IFN-α and IFN-β), that are required for the efficient induction of a T helper 1 (Th1) immune response in humans. Notably, the major characteristics that distinguish individual TLR are ligand specificity, signal transduction pathways, expression profiles and cellular localization.1 TLR ligands include lipopolysaccharide (LPS, TLR4), lipopeptides such as Pam3Cys (TLR2), double-stranded (ds) RNAs (TLR3), bacterial flagellin (TLR5), single-stranded (ss) RNA (TLR7/8) and unmethylated CpG DNA motifs (TLR9).2–5 In addition to natural ligands, synthetic antiviral compounds imiquinod and resiquimod (R-848) are also recognized by TLR7 and TLR8.6
As indicated above, TLRs are expressed in cell-type-specific patterns, with the most abundant expression levels found on dendritic cells, monocytes and macrophages.1,5 For example, all TLR, with the exception of TLR8 and TLR9, are expressed in human myeloid dendritic cells (DC), whereas TLR7 and TLR9 are selectively expressed in plasmacytoid DC.7 TLR8 and TLR9 are also expressed in human monocytes, and TLR2, TLR3, TLR5 and TLR9 are expressed in T lymphocytes.8 Such differential expression of TLR might confer distinct maturation signals, therefore yielding distinct types of immune response. While most TLR are expressed on the cell surface, TLR7, TLR8, and TLR9 are restricted to the endosomal compartments.
Engagement of TLR on DC induces their activation, maturation, and expression of a variety of cytokines and costimulatory molecules, including CD80 and CD86.9 Recently, we and others have found that certain RNA sequences, including small interfering RNAs (siRNAs) can activate innate immunity through TLR7 and TLR8, leading to the production of proinflammatory cytokines and type I interferon.10–12 By testing several ss and ds siRNA sequences, we found that chemically synthesized self-RNAs, either ss or ds, can trigger TLR activation when localized in the endosomes.10 These findings suggest that immune cells have an additional level of self–non-self discrimination that operates at the endosome level. The sequences of the most immunostimulatory RNAs that we have identified contain uridines. Interestingly, simple replacement of the 2′-hydroxyl uridines with 2′-fluoro, 2′-deoxy, or 2′-O-methyl uridines abrogated the RNA immune-stimulatory properties, indicating that RNA containing 2′-modified uridines are not sensed by TLR.13 These findings are in agreement with the recent work by Karikó and colleagues who showed that naturally modified host RNAs are not sensed by TLR.14
Upon maturation following TLR ligation, dendritic cells migrate to the draining lymph nodes, where they initiate the activation of naïve T cells. In contrast to microbe antigens, capture of self antigens does not activate immature DC in the absence of inflammation, but rather induce the generation of tolerogenic DC.9 Recent studies showed that indoleamine 2,3-dioxygenease (IDO)-expressing antigen-presenting cells, including DC are involved in maintaining peripheral tolerance to self-antigens.15 IDO is the first and rate limiting enzyme in the l-tryptophan-kynurenine pathway. Tryptophan (trp) catabolism mediated by IDO is an immunoregulatory mechanism that inhibit T-cell activation by depletion of trp and the production of catabolites with immune suppressive activity (kynurenines, kyn).15,16 Given the crucial role of IDO in immune tolerance and the potential use of RNA as therapeutics, we sought to examine whether isRNA modulate IDO expression in human monocytes and monocyte-derived dendritic cells. Also, we studied the impact of IDO up-regulation on T cell activation as well as the impact of 2′-uridine modifications on IDO induction by isRNAs.
Materials and methods
Reagents
Recombinant human granulocyte–macrophage colony-stimulating factor (GM-CSF) and IL-4 were purchased from R & D system. The IDO inhibitor 1-methyl-d,l tryptophan was purchased from Aldrich Chemical (Milwaukee, WI). Neutralizing antibodies against TNF-α and IL-1β and IFN-α were purchased from Pharmingen (San Diego, CA). Monoclonal antibody against IDO was purchased from US Biological (Nordic Biosite AS, Taby, Sweden) and monoclonal antibodies against Stat-1 and β actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). CD4 Dynabeads and CD14 microbeads were purchased from Invitrogen (San Diego, CA) and Miltenyi Biotech (Auburn, CA), respectively. Unmodified and 2′-modidied RNA oligonucleotides were purchased from Eurogentec or Ambion. The sequences of the used RNAs are:
27S, 5′-GUCCGGGCAGGUCUACUUUTT-3 ′;
19S, 5′-GAGGCAAUCACCAAUAGCATT-3′;
19A, 5′-UGCUAUUGGUGAUUGCCUCTT-3′;
CMV-UL37, 5′-GUAGCAUGUUUUAGGAAUG-3′.
Cell isolation and culture
Peripheral blood mononuclear cells (PBMC) were isolated by density gradient centrifugation (Lymphoprep, Nycomed Pharm, Oslo, Norway) from buffy coats obtained from healthy adult donors. Monocytes were isolated by plastic adherence. Also, they were isolated using CD14 microBeads (Miltenyi Biotec) according to the manufacturer's instructions. Monocyte-derived immature (imoDC) and mature (mmoDC) DC were generated by culturing adherent monocytes in the presence of GM-CSF (25 ng/ml) and IL-4 (50 ng/ml). After 4–5 days in culture, imoDC were stimulated with TNF-α (50 ng/ml) to generate mmoDC. Blood myeloid and plasmacytoid DC were purified using the BDCA-1 or BDCA-4 cell isolation kit, respectively, according to the manufacturer's instructions (Miltenyi Biotec).
Cell stimulation and cytokine determination
Monocytes were plated in 96-well tissue culture dishes (2 × 105 cells/200 µl) in RMPI-1640, supplemented with fetal calf serum (FCS) and antibiotics. The cells were incubated with no stimulus or with RNA molecules. The cultures were incubated for 16 hr at 37°, and the levels of TNF-α, IL-β and IL-6 were determined by enzyme-linked immunosorbent assay (ELISA) of culture supernatants. For protein preparations, cells were plated in 6-, 12- or 24-well plates at concentration of 106/ml, and then transfected with the test molecules for 16 hr. Subsequently, cells were harvested, washed with phosphate-buffered saline (PBS) and suspended in lysis buffer (PBS + 1% NP40) and protease inhibitors. After incubation at 4° for 30 min, samples were centrifuged at 16 000 g for 10 min at 4°. The resultant supernatants (protein extracts) were stored at −20°. In all experiments, isRNAs, R848, LPS and PamCys3 were used at 500 ng/ml, 5 µm, 100 ng/ml, and 10 µg/ml, respectively. Clinical isolate of HCMV (HCMV2006) used in this study was kindly provided by the Dr Halvor Rollag (Department of Microbiology, Rikshospitalet National Hospital, Oslo, Norway). HCMV2006 was added to the monocytes at a multiplicity of infection of 5.
Reverse transcription–polymerase chain reaction (RT–PCR)
Total RNA was extracted using Trizol (Invitrogen), and cDNA was synthesized from 0.5 to 2 µg total RNA using the first strand cDNA synthesis kit and oligo-dT primer according to the manufacturer's instructions. IDO-specific mRNA is amplified using the following primers: IDO forward, 5′-GGAAATAGCAGCTGCTTCTGCA-3′; IDO reverse, 5′-CTCCTCAGGGAGACCAGAGCTT3′; As an internal control β-actin mRNA was also amplified using the following primers: β-actin forward, 5′-ATCTGGCACCACACCTTCTACAATGAGCTGCG-3′; β-actin reverse, 5′-CGTCATACTCCTGCTTGCTGATCCACATCTGC-3′. PCR products were analysed by agarose gels.
Immunoblot analysis
Equal amounts of protein extracts were analysed by polyacrylamide gel electrophoresis (PAGE) and then transferred to nitrocellulose membrane by electrophoresis. Subsequent to blocking with 5% dry milk in PBS/Tween (0.1%) for 16 hr at 4°, membranes were probed with antibodies specific to IDO or signal transducer and activator of transcription-1 (STAT-1), followed by horseradish peroxidase-conjugated rabbit or mouse secondary antibodies. Signals were visualized with autoradiography using ECL system. Blots were stripped and reprobed with antibodies against β-actin.
T-cell isolation and activation
CD4+ T cells were isolated from PBMC with CD4 Dynabeads® in combination with Detachabead®reagent (Dynal, Oslo, Norway) to >99% purity as determined by flow cytometry. Subsequently freshly isolated T cells were labelled in PBS with 1.5 µm carboxyl flurescein succinimidyl ester (CFSE) for 5 min at 37°. CFSE-labelled T cells (105 cells/well) were washed and cocultured with autologous isRNA-stimulated monocytes (5 × 104/cells/well) along with phyohaemagglutinin (PHA) mitogen (5 µg/ml). As control, unstimulated autologous monocytes were added to T cells as non-suppressive accessory cells. T-cell responses were analysed by flow cytometry after 3 days of culture.
Statistical analysis
anova with Bonferroni t-test was used to compare the data between treated and untreated cells. Data are expressed as means ± SD. P-values of <0.05 were considered to indicate statistical significance.
Results
Immunostimulatory RNAs induce IDO expression in human monocytes
Monocytes are circulating peripheral blood cells that can be differentiated by cytokines into macrophages, dendritic cells as well as into several other cell types such as osteoclasts and microglia-like cells.17 Furthermore, they represent a major source of cytokines that modulate innate and adaptive immune responses. Because IDO enzyme plays a key role in immune regulation, we first investigated its expression in human monocytes after being exposed to TLR7/8 ligands. Our main prototype for TLR7/8 activator is the sense strand of a siRNA, known as 27S. This sequence efficiently induced the production of inflammatory cytokines and type I IFN in human immune cells.10,13 Consistent with their expression of TLR8, monocytes responded to 27S as shown by significant production of proinflammatory cytokines such as TNF-α13 (data not shown).
To evaluate the effects of 27S molecule on IDO expression, Western blot analysis was performed (Fig. 1a). The data showed that human monocytes do not constitutively express IDO, but a substantial amount of IDO protein is induced in transfected monocytes. To determine the time frame for IDO expression following TLR ligation, a kinetic study was performed. The cells were stimulated with siRNA 27S for 16 hr, followed by cell sampling at various time points. A significant up-regulation of IDO occurred at 16 hr incubation time (Fig. 1b). In some cases, a very weak expression of IDO was seen in untreated monocytes. This is more likely caused by donor variation.
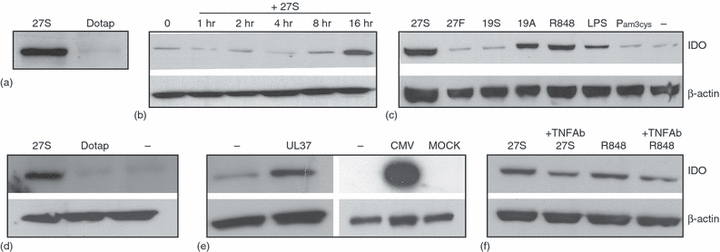
Immunostimulatory RNA induce IDO expression in human monocytes. (a) Effect of isRNA 27S on IDO expression. Monocytes were transfected with 27S (500 ng/ml) for 16 hr. Control cells received only DOTAP. Subsequently, IDO expression was analysed by Western blots. (b) Time course of IDO induction in freshly isolated monocytes. (c) Effect of various TLR ligands on IDO expression. Monocytes were treated for 16 hr with the indicated TLR ligands. Subsequently, IDO expression was analysed by Western blots. (d) Expression of IDO in CD14+ purified cell population. CD14+ purified monocytes were treated with 27S molecule for 16 hr and then IDO expression was analysed by Western blots (b). (e) Effect of viral RNAs on IDO expression in monocytes. Cells were transfected with either chemically synthesized RNA derived from UL37 mRNA or infected with CMV particles for 24 hr. Subsequently, IDO expression was analysed by Western blots. (f) Neutralizing antibodies against TNF-α inhibited TLR7/8 ligand-induced IDO in monocytes. Cells were treated with neutralizing antibodies against TNF-α and then with TLR7/8 ligands for 16 hr. Subsequently, IDO expression was analysed by Western blots. In all cases, blots were stripped and reprobed with polyclonal antibodies against β-actin. The data are representative of at least two independent experiments.
Having shown that monocytes can up-regulate the expression of IDO in response to isRNA 27S, in the next experiments we investigated their response to TLR2 and TLR4 ligands. To generalize our data, the following TLR7/8 ligands and control molecules were also tested: R848, a specific ligand for TLR7/8, a 2′-fluoro uridine modified 27S (27F), a second isRNA (19A), and a non isRNA (19S). Under our experimental conditions, all TLR7/8 ligands induced the expression of IDO (Fig. 1c, shows a representative example). Monocytes did show a significant up-regulation of IDO in response to TLR4 ligand LPS. However, when compared with TLR7/8 ligands, the effect was nearly threefold lesser. TLR2 ligand Pam3Cys exhibited no stimulatory effect. Cells treated with either 27F or the non isRNA 19A did not up-regulate IDO when compared to untreated cells.
In the next set of experiments, we have tested the effects of 27S on positively selected monocytes. In this respect, monocytes were isolated from PBMC with CD14-beads to >99 purity as determined by flow cytometry (data not shown). Cells stimulated with 27S molecule showed a dramatic up-regulation of IDO protein levels as assessed by Western blots (Fig. 1d). IsRNA-treated monocytes produced a large amount of inflammatory cytokines (e.g. TNF-α, IL-6), but not IFN-α and -γ (data not shown).
Having shown that monocytes can respond to synthetic isRNAs by up-regulating IDO and producing proinflammatory cytokines, next we evaluated whether they respond to viral RNAs. It should be noted that monocytes are a major cytomegalovirus (CMV) target cells in vivo, and are responsible for dissemination of the virus throughout the body during acute and late phase of infection.18 As shown in Fig. 1(e), transfection of human monocytes with a synthetic viral RNA derived from the UL37 gene or infection with CMV induced IDO. Notably, a huge amount of IDO protein was induced by CMV when compared to mock-treated cells.
Potential mechanism of TLR-induction of IDO in freshly isolated monocytes
In response to TLR, monocytes produce proinflammatory cytokines such as TNF-α, IL-1β and IL-6.13 In the present study, we have investigated the involvement of TNF-α in IDO induction by 27S and R848. Neutralizing antibodies against TNF-α reduced IDO induction by approximately 50 ± 5% (P < 0.002) as estimated by densitometric scanning (Fig. 1f). Thus, it seems likely that IDO induction by TLR7/8 signalling in freshly isolated monocytes is mediated in part by TNF-α in an autocrine pathway. Neutralizing antibodies against either IL-1β or IFN-α did not affect IDO induction in monocytes (data not shown).
Expression of IDO in immature monocyte-derived DC
Previous studies have shown that DC can up-regulate the expression of IDO in responses to several signals such as interferon-γ.14 Therefore, we explored in additional experiments whether monocyte-derived DC up-regulate the IDO expression in response to isRNAs. In these experiments, freshly isolated monocytes were treated with IL-4 and GM-CSF for 5 days. Subsequently, they were treated with 27S molecule for 16 hr. Surprisingly, we could not detect any induction of IDO in imoDC obtained from several donors (Fig. 2a, shows a representative example). To assess whether the IDO protein levels were reflected at mRNA levels, RT–PCR was performed. As shown, a large amount of IDO mRNA was detected in only 27S-treated monocytes, but not in controls or 27-treated imoDCs (Fig. 2a lower panels).
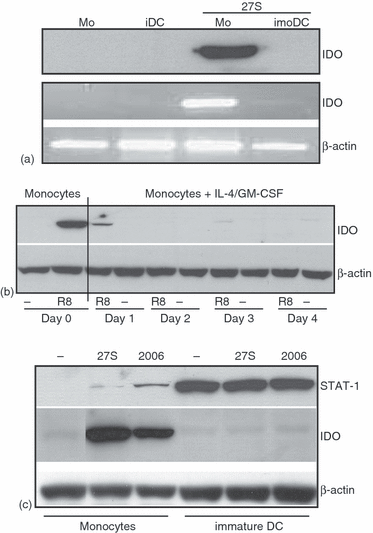
Expression of IDO in immature and mature DC in response to TLR7/8 ligands. (a) ImoDC were prepared by incubating the monocytes with GM-CSF and IL-4 for 5 days. Subsequently, the cells were transfected with 27S for 16 hr and then IDO expression was analysed by Western blots (upper panel) and RT–PCR (lower panel). (b) Time course of IDO silencing during DC maturation. Monocytes were incubated with GM-CSF and IL-4. Subsequently, samples were taken at day 1, day 2, day 3 or day 4 and then treated with TLR7/8 ligand R848 for 16 h in order to induce IDO expression. (c) Expression of STAT-1 in imoDC. Cells were treated with the indicated TLR ligands for 16 hr. Subsequently, IDO expression was analysed by Western blots. Blots were stripped and reprobed with antibodies to STAT-1 and subsequently with antibodies to β-actin.
The kinetics of IDO gene silencing in imoDC were measured by coincubating monocytes with IL-4 and GM-CSF for various times points and then treating the cells with R848 in order to trigger IDO expression (Fig. 2b). By one day incubation with IL-4 and GM-CSF prior to treatment with R848, nearly 95% inhibition of IDO gene expression had already occurred in IL-4/G-CSF-treated monocytes compared to their untreated counterparts (day 0). Thus, the induction of a DC maturation programme is more likely to be responsible for IDO gene silencing. It should noted that cotreatment of monocytes with isRNAs along with IL-4 and/or GM-CSF did not block IDO up-regulation (data not shown).
Monocyte-derived DC express STAT-1
Among the transcription factors that activate IDO expression in DC are STAT-1 and IFR-1. We have previously shown that signalling via TLR in PBMC in response to isRNA induced the activation of several transcription factors, including STATs and IRFs.19 Therefore, we have asked whether a comparable effect occurred when monocytes are activated with isRNAs. Again, a significant induction of IDO was seen in monocytes, but not in imoDC (Fig. 2c). Interestingly, CpG oligo 2006 (TLR9 ligand) also induced IDO expression in monocytes. To examine the expression of STAT-1, the nitrocellulose membrane was stripped and then probed with polyclonal antibodies against STAT-1. As shown, imoDC expressed a huge amount of STAT-1 protein. Monocytes expressed very low levels of STA-1 protein. Collectively, these data suggest that the suppression of IDO expression in human imoDC is not caused by the absence of a key transcription factor such as STAT-1, but it is more likely the result of alteration in chromatin structures, which needs further investigation.
TLR7/8 signalling does not induce IDO expression in mature monocyte-derived DCs
Recent reports reveal that IDO expression is up-regulated in response to cytokines and IFN-γ. Also, its expression can be induced by ligands (e.g. CD28, CTLA4) or antibodies that bind and cross-link surface molecules expressed by antigen-presenting cells (APC) such as CD80/CD86.20,21 Also, regulatory T cells (T reg) have been shown to induce IDO expression in target cells via cell surface expression of CTLA4.22 To evaluate TLR7/8 signalling on IDO expression in DC, monocytes were differentiated into mature DC (mmoDC) and then treated with 27S molecule, R848 or IFN-γ(Fig. 3a). Again, TLR7/8 ligands such as R848 and 27S did not induce IDO expression in mmoDC but IFN-γ did.
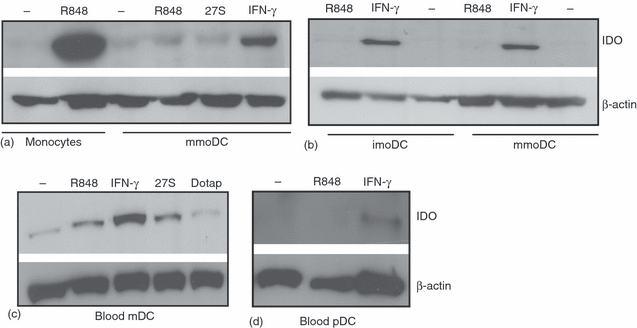
DC uregulated IDO expression in response to INF-γ, but not TL7/8 ligands. (a) Effects of TLR7/8 ligands and IFN-γ on IDO expression in mmoDC. To generate mmoDC, imoDC were treated with TNF-α (50 ng/ml) for 2 days. The cells were washed and then treated for 16 hr with R848, siRNA 27S, or IFN-γ. Subsequently, IDO expression was analysed by Western blots. (b) Analysis of IDO expression in imoDC and mmoDC. Monocytes were treated with GM-CSF and IL-4 in order to obtain imoDC. To generate mmoDC, half of the culture was treated with TNF-α. Both cell populations were treated and processed as in (a). (c and d) Analysis of IDO expression in blood myeloid and plasmacytoid DC in response to 27S molecule, R848 and IFN-γ. Cells were stimulated for 16 hr and then IDO expression was analysed by Western blots. In all cases, the blots were stripped and reprobed with polyclonal antibodies against β actin. The data are representative of at least two independent experiments.
To assess whether TLR7/8-induced IDO pathway is functional in imoDC, monocytes were differentiated into imoDC and mmDC and then IDO expression was investigated by Western blots (Fig. 3b). Similar to mmDC, imoDC did not up-regulate IDO in response to R848 ligand, whereas addition of IFN-γ to cultures had a strong effect on IDO expression. It should be noted that the incubation of imoDC or mmoDC with TLR7/8 ligands induced cytokines production, including TNF-α and IFN-α, thus confirming the functionality of the TLR7/8 signalling pathways in these cells.10
Having demonstrated that the expression of IDO in imoDC or mmoDC was not under the TLR7/8 signalling pathways, in the next experiments we have tested the effects of TLR7/8 ligands on IDO expression in fleshly isolated blood myeloid (m) and plasmacytoid (p) DCs. Although a small up-regulation of IDO expression was seen in mDC treated with either R848 or 27S, only IFN-γ significantly up-regulated the expression of IDO in mDC compared to untreated cells (Figs 3b, P < 0.002). Also, pDC responded only to IFN γ, but the effect was very low (Fig. 3c).
2′-O-modified isRNAs suppress isRNA-induced IDO expression in monocytes
As shown in Fig. 1(c), the 27F molecule did not induce IDO expression. Also, our previous data showed that most of the genes, if not all, that are induced by isRNA can be abrogated by 2′-modifications of isRNAs.19 In the next experiments, we have evaluated whether 2′-modified isRNAs would suppress the production of TNF-α and/or IDO expression elicited by their unmodified version. When 2′-modified isRNA were cocomplexed with unmodified isRNA 27S using DOTAP, the effects on IDO and TNF-α expression were significantly reduced, with 2′-O-methyl-bearing RNA (27M) being the most effective inhibitors (Fig. 4a, b). Indeed, TNF-α and IDO expression were completely inhibited. These observations suggest that 2′-O-methyl modified RNAs can compete with isRNA to bind TLR7/8. It should be noted that most, if not all, the downstream signalling events activated by various isRNA sequences are inhibited by inhibitory 27M molecule at a very low molar ratio, indicating that 27M has a high affinity to TLR7/8 and can function as a general TLR7/8 antagonist (Sioud M. unpublished data).
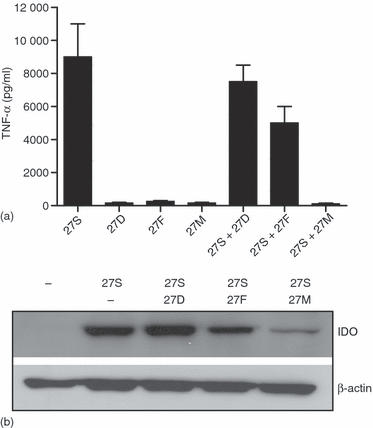
2′ uridine-modified isRNA block isRNA inducced TNF-α and IDO. Freshly isolated monocytes were treated for 16 hr with unmodified 27S, 2′-deoxy (27D), 2′-fluoro (27F), or 2′-O-methyl (27M) uridine-modified versions. Cells were also transfected with 27S in combination with 27D, 27F, or 27M. In these experiments, the modified molecules were cocomplexed with 27S molecule using DOTAP, and then added to the cells. Subsequently, secreted TNF-α was measured by ELISA. Protein extracts from cells treated with the cocomplexed molecules were prepared and then analysed for IDO expression by Western blots. The membrane was stripped and reprobed with antibodies against β-actin. Equal amount the unmodified and modified RNAs were used (500 ng/ml).
Effects of IDO+ monocytes on T-cell activation
As induction of IDO activity has recently been described as a major T-cell inhibitory mechanism in APC/T-cell interaction.15 we next investigated the effects of IDO positive monocytes on autologous T cell activation in response to the mitogen PHA. In these experiments, T cells were grown in X-vivo-15 medium (Cambrex, Verviers, Belgium) without serum supplementation. Proliferation of T cells was assessed by flow cytometry as CFSE1ow in percentages of CD4+ cells. As shown in Fig. 5, coculture of T cells with IDO+ monocytes did reduce T-cell activation with PHA by 50% (± 10%, P < 0.05), whereas IDO negative monocytes did not. The addition of 1-methyl-tryptophan (1MT), a specific inhibitor of IDO activity, to the coculture suppressed the ability of IDO+ monocytes to suppress T-cell proliferation, suggesting that IDO is involved in suppressing T-cell response. Collectively, these findings support the notion that IDO up-regulation in monocytes may regulate T-cell function in vivo.
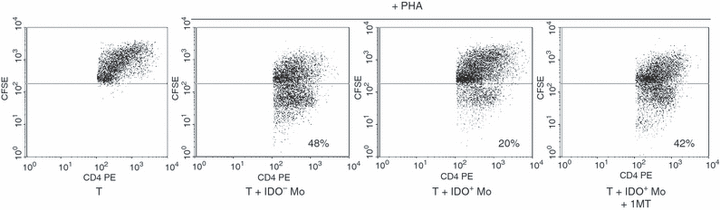
Effect of IDO+ monocytes on mitogen-activated T cells. Freshly isolated monocytes were treated for 16 hr with 27S molecule in order to induce IDO expression (IDO+) and then they were coculture with autologous CFSE-labelled CD4+ T cells in the absence or the presence of 1MT (400 µm), a specific inhibitor for IDO. The cells were stimulated with PHA for 3 days and then analysed by flow cytometry. As control, T cells were incubated with unstimulated monocytes (IDO–).
Discussion
isRNAs stimulate innate and adaptive immunity by binding to TLR7/8 expressed by immune cells. Here we demonstrated that TLR7/8 ligands can induce IDO expression in freshly isolated monocytes, but not in monocyte-derived dendritic cells. Infection of monocytes with CMV also induced IDO expression in monocytes and IDO+ monocytes inhibited T-cell activation when compared to IDO-negative monocytes. In contrast to unmodified isRNAs, chemically 2′-modified isRNA 27S did not induce IDO expression, but competed with the unmodified isRNA to trigger TNF-α production and IDO expression. This latter finding might provide opportunities for the development of suppressive RNA oligonucleotides that can be used to block TLR7/8 signalling. In this respect, previous studies have shown that certain synthetic DNA oligonucleotides inhibited competitively the interaction of CpG ODN with TLR9 and ameliorated autoimmune responses and allergic responses.23,24 Also, TLR-specific inhibitors will be useful in uncovering the specific functions of each receptor as well as in therapies aimed at blocking a specific signalling pathway.
The preferential induction of IDO in monocytes when compared to imoDC and mmoDC indicated distinct signalling requirements for IDO expression. As shown, the ability of monocytes to up-regulate IDO in response to TLR7/8 ligands was suppressed when the cell started to differentiate into imoDC. This observation indicates that monocyte-derived DC cells might rapidly acquire the capacity to differentiate into immunogenic DC, rather than tolerogenic DC. These findings are important because they indicate that isRNAs can be incorporated into vaccine preparations in order to activate dendritic cells.
It has been shown that IDO-expressing human DC can function as specialized regulatory cells that could play a role in maintaining tolerance in vivo. IDO positive DC can induce T-cell anergy and/or the generation of adaptive T regulatory cells. Unlike IDO negative monocytes, IDO positive monocytes inhibited T-cell proliferation (P < 0.05). It should be noted that the concentration of tryptophan in culture medium is critically important for the validity of the experimental design. Indeed, if its levels are in great excess in culture medium then the effect of IDO will be underestimated. Using X-vivo-15 medium without serum supplementation, we detected a significant inhibitory effect of IDO+ monocytes on T-cell activation. Furthermore, the data are compatible with IDO activity because the addition of 1MT abrogated the potency of monocytes to suppress T-cell activation. Collectively, these findings provide the first evidence that TLR7/8 induction of IDO in monocytes can affect T-cell function. Previously, Munn and colleagues showed that human monocyte-derived macrophages expressed IDO, and inhibited T-cell proliferation.25 Given that IDO is up-regulated in CMV-infected monocytes, we hypothesize that the in vivo immunosuppressive properties of IDO might confer an advantage to CMV to escape immune response. However, it should be noted that IDO can suppress the growth of a wide range of pathogens. Thus, how both functions can be dissociated needs further investigation.
Previous studies have shown that IFN-γ can induce IDO expression in various cell types including DC, monocyte-derived macrophages, fibroblasts, endothelial cells and tumour cells. Signal transducer and activator of transcription 1 (STAT-1) and IFN-regulator factor (IRF1) function cooperatively to mediate the induction of IDO expression by IFN-γ.26 In accordance with these previous studies, treatment of either imoDC or mmoDC with IFN-γ induced IDO expression (Fig. 3a, b). Because DC did not up-regulate IDO in response to isRNAs and R848, it is tempting to speculate that the presence of IFN-γ in the culture or microenvironment may signal the occurrence of a T-cell response and therefore T-cell priming by immunogenic DC no longer required. IFN-γ is produced by T cells in both innate and adaptive immune responses.27 Furthermore, blood mDC responded much better to IFN-γ than TLR7/8 ligands (see Fig. 3c). On the basis of these findings, we propose a model where TLR7/8 ligation on imoDC mainly induces the generation of immunogenic mmoDC whose key function is to prime naïve T cells. Once T cells are effectively primed, secreted IFN-γ will then induce IDO expression in mmoDC, leading to the induction of tolerogenic DC as illustrated in Fig. 6. This counter-regulatory mechanism is expected to control the magnitude and duration of adaptive immune responses. It has been shown that reverse signalling via B7 molecules (CD80/CD86) was important for IDO expression in DC.14 However, such secondary signal does not appear to be involved in IFN-γ-induced IDO expression in imoDC and mmoDC.
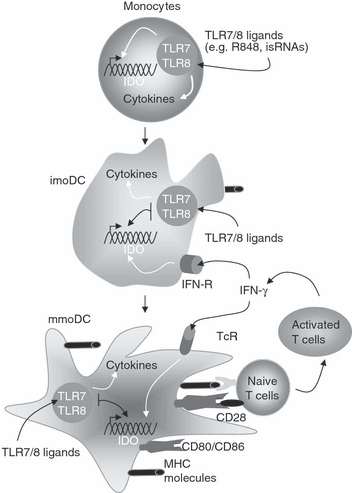
Expression of IDO during monocyte maturation into dendritic cells. Monocytes are able to produce cytokines and IDO in response to TRL7/8 ligands such as R848 and isRNA 27S. Monocyte-derived DC (imoDC and mmDC) can produce a large amount of cytokines, but not IDO in response to TLR7/8 ligands. In contrast to TLR7/8 ligands, IFN-γ plays a determinant role in up-regulating IDO expression in DC. MHC, major histocompatibility complex.
Collectively, the present study provides new understanding of how TLR7/8-induced IDO expression is affected by DC-maturation programme. Furthermore, the findings that 2′-modified RNAs can suppress TLR7/8 signalling in human monocytes would provide important information for the rational design of new TLR7/8 inhibitors. The presence of modified nucleotides in RNAs not only evades immune activation, but might prevent the activation of innate immunity by isRNA derived from microbes or host cells. Thus, host cells need to modify few RNA species in order to block immune activation by all RNA types.
Acknowledgements
We thank Drs Sébastien Wälchli and Anne Dybwad for critical reading of the manuscript. This work was supported in part by the Gene therapy program at the Norwegian Radium Hospital and Helse Sør to M. Sioud.




