The importance of B-cells and ecto-5′nucleotidase in Mycoplasma fermentans infection and the relevance to rheumatoid arthritis
Summary
The aim of this work was to discover if Mycoplasma fermentans, which is known to infect B cells, could be the cause of the raised ecto-5′-nucleotidase observed in the synovial fluid of rheumatoid arthritis patients. The ecto-5′-nucleotidase activity in the patients' serum has been shown to correlate with the erythrocyte sedimentation rate and DNA from the mycoplasma has been found in the synovial fluid. B lymphoblastoid cell lines were exposed to 16 strains of Mycoplasma fermentans and their ecto-5′-nucleotidase, CD73, was measured both biochemically and by mouse antibodies to human ecto 5′-nucleotidase using the fluorescence activated cell sorter. The type strain, PG 18, did not grow with the B cells. Some of the mycoplasma strains (9/15) increased the cellular ecto-5′-nucleotidase activity from twice to 17 fold, and usually showed 5′-nucleotidase activity themselves. At least one strain, M106, induced human 5′-nucleotidase on the normally 5′-nucleotidase negative Daudi and Raji Burkitt's lymphoma cell lines, and increased sevenfold the 5′-nucleotidase on the monocyte/macrophage cell line THP-1. Growing the cells in aged medium increased the level of mycoplasma infection. This mycoplasma-induced enzyme showed a conformational change and an increase in activity with a glycosylation change involving mannose groups. The other group of strains, mostly of respiratory or cell culture origin, usually did not have any 5′-nucleotidase of their own and decreased the B-cell enzyme activity by about half. Electron microscopy and flow cytometry showed that the strain M106 was filamentous and could be found inside the B-cells. The 5′-nucleotidase-inducing strains of M. fermentans may be important in the aetiology of rheumatoid arthritis.
Introduction
The removal of B lymphocytes has been shown to cause a temporary abatement of the symptoms of rheumatoid arthritis1 although these recur when the B cells regrow.
Mycoplasma fermentans has been shown to selectively infect B-cells in the peripheral blood.2 About half (10/16) stains of M. fermentans from various sources were found to express activity of the enzyme ecto-5′-nucleotidase (EC 3.1.3.5 and CD 73) (5′N).3 Although this enzyme has very rarely been reported from bacteria, it is commonly found as a glycosylphosphatidylinositol-linked dimer on the plasma membrane of a wide variety of mammalian cells, and as a monomer in the serum. The enzyme specifically removes the phosphate group from AMP and the other nucleoside monophosphates, with a Km in the low µm range for AMP.4 The enzyme is competitively inhibited by ATP, ADP and the non-metabolizable analogue of ADP, a,β-methylene adenosine diphosphate (AMPCP);5 it is also inhibited by concanavalin A, indicating the presence of mannose groups.6 The enzyme cDNA has been sequenced7 and it is known to be a glycoprotein dimer with a molecular weight about 150 000. The molecular structure and function are discussed by Zimmermann.4 One bacterial 5′N from Vibrio parahaemolyticus has been sequenced8 and another has been reported from the type strain of Mycoplasma pulmonis.3
In circulating blood cells 5′N occurs only on relatively mature B cells, although it decreases on plasma cells.9 It is also found on a subgroup of T lymphocytes10 where it is believed to be involved in cytotoxic killing,10 T-cell activation11 and proliferation.12 It is not found on monocytes, but is present on mature macrophages that lose the enzyme when they become activated.13 The enzyme is not present on polymorphs, erythrocytes or platelets. 5′N is found on Epstein–Barr virus (EBV) transformed B-cell lines, but not on the less mature Burkitt's lymphoma cells.14
The 5′N activity in the serum comes mainly from the liver. In rheumatoid arthritis (RA) patients the 5′N enzyme activity in the serum has been shown to correlate strongly with the erythrocyte sedimentation rate (ESR), linking it to inflammation.15 There was no significant correlation between the 5′N in the serum and synovial fluid for RA patients, in marked contrast to the strong correlation between the two activities shown by osteoarthritis (OA) patients. The 5′N activity was greater in the synovial fluid than in the serum for virtually all the RA patients, showing that it was being made locally in the joints. Two groups have reported a raised 5′N activity on the synovial lining cells of rheumatoid arthritis (RA) patients compared to those with osteoarthritis16 or non-arthritic patients.17 This is rather unexpected because the joints of RA patients are inflamed, and their macrophage-like synoviocytes would be activated and would therefore be likely to lose 5′N activity13 to the synovial fluid.
The human B-lymphoblastoid CESS cell line, which was known to have a very high 5′N activity, was imported into the laboratory, and decontaminated with antibiotics to remove the mycoplasma with which it was infected. This caused a marked decrease in its 5′N activity. The mycoplasma was identified as Mycoplasma fermentans, and designated strain number M106 by the Public Health Laboratory at Colindale, UK. M. fermentans is a common human parasite. Most strains of M. fermentans infecting humans are difficult to cultivate in cell-free media, and the development of the polymerase chain reaction (PCR) with suitable primers has made its detection much easier. M. fermentans has been discovered in the throat, urine or peripheral blood cells of up to 36% of human immunodeficiency virus (HIV) patients;18–20 the organism was not detected in healthy controls but Katseni et al.21 found it in 30% of HIV seronegative patients attending a clinic for sexually transmitted diseases and Kovacic et al.22 reported it in 11% of the peripheral blood mononuclear cells from HIV seronegative subjects. Chingbingyong and Hughes23 found the organism in the saliva of 44% of normal people. Previous to this the mycoplasma had been found infrequently in the human urogenital tract24 and has been isolated from the joints of arthritic patients.25,26 It has also been detected by PCR in the synovial fluid of 14% of patients with rheumatoid and other inflammatory arthritis27,28 although 40% of the biopsies of the RA patients' synovial lining cells showed the organism. More recent results using a nested PCR reaction detected the organism in the synovial fluid of 23/25 (92%) of rheumatoid arthritis patients, and in 6/7 patients with other inflammatory arthritides, but not in 10 osteoarthritis patients, who do not have an inflammatory cell infiltrate.29
Noda et al. and Shibata et al.30,31 found that M. fermentans also had an acid phosphatase which would hydrolyse p-nitrophenol phosphate; its pH optimum was 5·0.
Materials and methods
Cell culture
Established cell lines and cloned cells from EBV-transformed peripheral blood cultures were routinely grown in RPMI-1640 with 2 mg/l l-glutamine and 10% inactivated ‘Myoclone’ fetal calf serum (FCS), without antibiotics unless specified. Cell culture reagents were obtained from Gibco (Paisley, UK) or Flowgen (Nottingham, UK). The cells were grown in 24-well Costar trays (Sigma-Aldrich, Poole, UK) at 37° in a humidified incubator, with an atmosphere supplemented with 5% CO2.
Mycoplasma cultivation
The mycoplasma was grown on modified Friis agar plates in the presence of 10% pig and 10% horse serum. The plate was washed with 10 mm tris buffered saline pH 7·6 containing 2 mm MgCl2 (TBS). The saline suspension was centrifuged at 30 000 g for 20 min, and the pellet retained. It was washed twice with TBS under similar conditions, and resuspended in TBS. Aliquots (10–50 µl) of the M. fermentans suspension were tested for phosphatase activity and mycoplasmal content.
Elimination of mycoplasma infection
The mycoplasma was eliminated from the originally infected CESS cell line by four cycles of alternate culture in BM cyclins 1 and 2 (Boehringer Mannheim, Mannheim, Germany), as described by Johnson.32 The cell line was free of mycoplasma when it was tested with the Genprobe kit 7 weeks after the antibiotic treatment and the mycoplasma no longer grew when cultured on Friis agar.
Cell clones
Cloned cells were obtained from some of the more vigorously growing lymphoblastoid cultures as described in Johnson.32 Others were obtained by transforming peripheral blood lymphocytes with EBV in 96 well plates in the presence of 2 µg/ml cyclosporin A. Only about 20% of the wells produced cultures, and 4% persisted; these secreted immunoglobulin of only one heavy and light chain type, and were regarded as monoclonal. No non-secreting clones were obtained.
Infection with M. fermentans
Infected CESS cells were grown for 7 days without antibiotics. The supernatant was passed through a 0·45 µm filter, and 0·5 ml portions frozen at −70°. Cells to be infected were suspended in antibiotic-free medium, the thawed mycoplasma-containing supernatant was added, the mixture was centrifuged for 8 min at 250 g, and the infected cells resuspended in fresh antibiotic-free medium. To allow a steady state to be reached cells had to be infected at least 10 days before use. However not all the cells were infected under these circumstances.
Super infected cells were produced by culturing the cells in a mixture of 4/5 RPMI-1640 10% FCS medium prepared at least 6 months earlier and stored at 4°, and 1/5 fresh medium. The infected cells were grown in a small quantity of medium to which a little more medium was added but none removed every day for 5 days. This tilted the balance in favour of the mycoplasma. Control cells were treated similarly but without the mycoplasma.
Cell counting
Cell samples were put into Isoton I, in the presence of Zapoglobin at the concentration recommended by the manufacturers. All cell suspensions were counted using a Coulter Counter ZBl (Coulter Electronics, Hialeah, FL).
Measurement of 5′N
The cells were harvested and centrifuged at 330 g for 10 min, washed in 25 ml TBS, centrifuged again and finally resuspended in TBS and counted. The enzyme 5′N was measured on intact cells, as described by Rowe et al.33 For the reactions, 0·5 ml of a mixture containing 40 µm AMP with 0·4 µCi (1·48 × 104 Bq) ml−1[2−3H] AMP, 20 mm MgCl2 and 200 mm glycine/NaOH, pH 8·5, was diluted with an equal volume of TBS containing the (20 µl) sample and the relevant inhibitor. 5′N has a broad pH maximum, and a high pH value was chosen to avoid interference by acid phosphatase. The mixture was incubated at 37° for 15 min. After incubation, the reaction was stopped by chilling to 4° and adding 0·15 ml of 0·15 m ZnSO4. Any unreacted substrate was precipitated by adding 0·5 ml of 0·5 m Ba(OH)2, leaving the 3H-adenosine in the supernatant, for scintillation counting. The reaction mixture contained 1 mm levamisole, to inhibit alkaline phosphatase, and 60 µmβ-glycerophosphate to inhibit non-specific phosphatases. However, for these experiments the precautions proved unnecessary as no other phosphatase activity was detected at pH 8·5. In the enzyme inhibition tests the concentrations of putative inhibitor added to the AMP were up to 200 µmβ-glycerophosphate or AMPCP or 34 µg/ml concanavalin A.
Measurement of mycoplasmal acid phosphatase
To measure the mycoplasmal acid phosphatase 5 ml of the medium (without inhibitors) was adjusted to pH 5·0 with one drop of concentrated HCl and 0·28 g anhydrous sodium acetate. Disodium nitrophenyl phosphate was added at 1 mg/ml, and the reaction was stopped with an equal volume of 3 N NaOH.
Identification of mycoplasma
Mycoplasma strain M106 was cultured and identified as M. fermentans at the Mycoplasma Reference Facility, NCTC, on the basis of its biochemical properties and by species-specific serological tests.
M. fermentanscolony immunofluorescence
M. fermentans strain M106 was grown for 4 days on Friis agar plates, to give many small discrete colonies. Pieces of the agar bearing colonies were treated for 0·5 hr at room temperature with the Flow Cytometry concentrations of the two mouse anti-human 5′N antibodies, the irrelevant mouse monoclonal antibody or left untreated; they were then washed three times with 10 ml phosphate-buffered saline (PBS) with gentle shaking, stained with the second fluoroscein isothiocyanate (FITC) goat anti-mouse antibody, washed again, and studied under the fluorescent microscope. Colonies were also treated with rabbit anti-M. fermentans antibody, then with a FITC swine anti-rabbit antibody.
The binding of human 5′N and human immunoglobulins to the mycoplasma was tested by incubating the mycoplasma plates overnight with human serum, washing with PBS, and retesting for immunoglobulin with the mouse monoclonal antibodies, or with fluorescein-conjugated rabbit anti-human immunoglobulin G (IgG), IgM and IgA.
Flow cytometry
The cells (2 × 105), suspended in PBS containing 0·5% bovine serum albumin (BSA), were labelled at 0° with saturating concentrations of two mouse monoclonal anti-human 5′N antibodies 1E934 or IFH 5N1;35 they were then washed and labelled with a second antibody, fluorescein-conjugated Fab goat anti-mouse immunoglobulin (Dako, High Wycombe, UK). Controls were labelled with an irrelevant mouse antibody and then the fluorescein conjugated goat anti-mouse second antibody or with the fluorescein-conjugated antibody alone.
The size, granularity and fluorescence of 10 000 cells were studied using a FACScan (Becton Dickinson, San Jose, CA). The machine was calibrated with beads of known fluorescence, obtained from Carribbean Microparticles Corp. (Hato Rey, Puerto Rico).
Electron microscopy
CESS cells both uninfected and infected with M. fermentans strain M106 were grown in RPMI-1640 with 10% inactivated FCS. The cells were fixed with 3% glutaraldehyde in 0·2 m cacodylate buffer, pH 7·2 at room temperature for 2 hr, then gently centrifuged in Eppendorf tubes at 685 g to form a pellet. The pellet was subsequently rinsed 2 × 30 min in cacodylate buffer and then postfixed in 1% osmium tetroxide in cacodylate buffer pH 7·4 for 2 hr. The pelleted cells were re-suspended by shaking then rinsed 2 × 30 min in cacodylate buffer. After centrifugation the pellet was dehydrated with ascending ethanol concentrations (35–70–90–100%) with 2 × 30 minute change in dried absolute ethanol. Embedding was performed using Spur's resin with a 50/50 mix of propylene oxide/resin for 12 hr and a 2 × 2 hr final resin mix with polymerization overnight at 60°.
For transmission electron microscopy tissue sections (80–100 nm) were cut on a diamond knife using a Reichert OMU 4 ultramicrotome and picked up on 200 mesh copper grids. The sections were first stained with aqueous uranyl acetate, washed with H2O followed by Sato's lead citrate for 20 min each. Grids were then examined using a Zeiss 900 transmission electron microscope at 50 kV.
Subsequent analysis was done using negatives scanned into digital format using Adobe Photoshop.
Results
M. fermentans strain M106 induces 5′N on Daudi and CESS cell lines
Figure 1shows the time course for the induction or increase in 5′N activity after M106 M. fermentans infection of the Daudi Burkitt's lymphoma cell line, which does not normally express any 5′N, and for the CESS lymphoblastoid cell line that does express 5′N. It shows that at least 8–10 days are required before a steady state is reached under normal cell culture conditions.
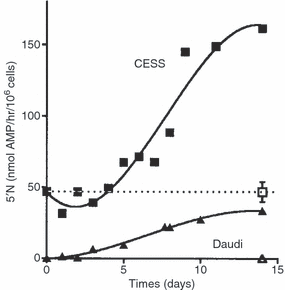
The time course for the development or increase in 5′N activity after infection with M. fermentans strain M106. □, ▮ Uninfected and infected CESS lymphoblastoid cells; , Uninfected and infected Daudi Burkitt's lymphoma cells.
Figure 2shows the fluorescence-activated cell sorting (FACS) studies on the Daudi Burkitt's lymphoma cells shown in Fig. 1. M. fermentans induced the human 5′N epitope on a group of the Daudi cells, as shown by the fluorescence data and the 5′N enzyme activity but one group of cells was apparently uninfected. It appears that some cells were able to prevent the infection.
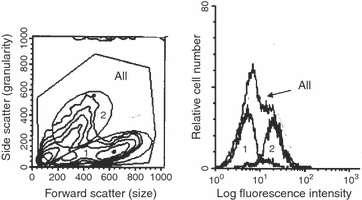
FACS studies on the Daudi cells shown in Fig. 1. M. fermentans infected Daudi Burkitt's lymphoma cells labelled with a mouse monoclonal anti-human 5′N antibody and a fluorescent anti-mouse antibody. The regions are defined in the left-hand diagram, and their fluorescence is shown on the histogram. The histogram from all the cells shows a marked shoulder. This is shown to come from the granular cells in region 2, whereas the cells in region 1 show no. 5′N and do not appear to be infected.
The cell lines were then superinfected with mycoplasma by growing them (and the controls) in old media as described in Methods. Figure 3shows uninfected (a) and infected (b) CESS and Burkitt's lymphoma (Daudi) cells labelled with the fluorescein-conjugated second antibody only (black control), a neutral mouse antibody (almost coincident with, or a little to the left of the second antibody control) and two monoclonal mouse anti-human 5′N antibodies 1E9 and 1FH 5Ν1 (arrowed) all of which were treated with the second fluorescein-conjugated antibody. The 5′N activities and the fluorescence of the two cell lines are shown in Table 1. M. fermentans induced the human 5′N epitope on the Daudi cells, as shown both by the fluorescence data and the 5′N enzyme activity. The CESS cell line showed only a small increase in fluorescence on infection with M. fermentans, but a threefold increase in 5′N activity as shown in Fig. 1 and Table 1. The clones from the EBV-transformed B cells all showed 5′N epitopes whether infected or not, but in most cases the infected cells had more epitopes.
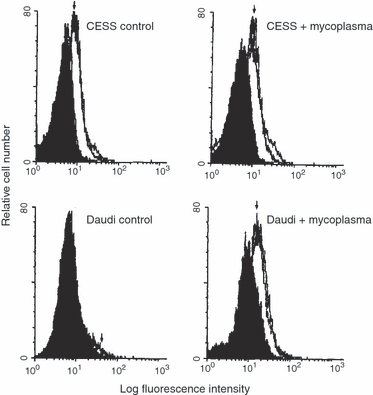
Cytofluorimetry. Daudi and CESS cells superinfected with M. fermentans strain M106. The cells were labelled with the second antibody only (black), a neutral mouse antibody (almost coincident with, or a little to the left of the second antibody control) and two monoclonal mouse anti-human 5′N antibodies 1E9 and 1FH 5N1 (arrowed). There is little change in the amount of 5′N on the CESS cells.
| Cells | 5′N | Fluorescence anti-5′N 1E9 | Fluorescence Anti-5′N 1FH |
|---|---|---|---|
| CESS alone | 42·1 ± 11·7 (5) | 12·9 ± 3·1 (5) | 16·4 ± 5·2 (2) |
| CESS + mycoplasma | 124·2 ± 34·5 (5) | 14·8 ± 1·9 (5) | 18·6 ± 4·9 (2) |
| Daudi alone | 0·2 ± 0·8 (6) | 0·9 | 0·9 |
| Daudi + mycoplasma | 100·7 | 10·9 | 13·8 |
- The 5′N is expressed as nmol AMP hydrolysed/106 cells/hr.
- The fluorescence is the difference in the fluorescence between cells labelled with both antibodies and cells labelled with the second antibody only. When multiplied by 103, it is in molecules of equivalent soluble fluorochrome (MESF).
- Results are expressed as mean ± SD.
Intracellular location of mycoplasmas
2, 4 show the change in light scattering produced by the mycoplasma. Figure 2 shows partially transformed Daudi cells. Cells that were not transformed by the mycoplasma are in the area marked 1, transformed cells are in area 2 and the combined cells are in area ‘all’. The x-axis is related to the host cell size and the y-axis shows the light scattering produced by the host cell granularity. It will be seen that the anti 5′N antibody only sticks to the transformed granular group 2 whereas group 1 shows little fluorescence. 2, 4demonstrate that infected cells, marked B, became much more granular than normal cells, marked A, but do not become larger. This suggests that the mycoplasmas are inside the host cells and producing the granularity change. In these experiments PG 18 grew initially on the Friis agar but would not grow with the B-cells as judged by its inability to grow on Friis agar after cultivation with the B-cells. The other M. fermentans strains did grow after cultivation with the B-cells.
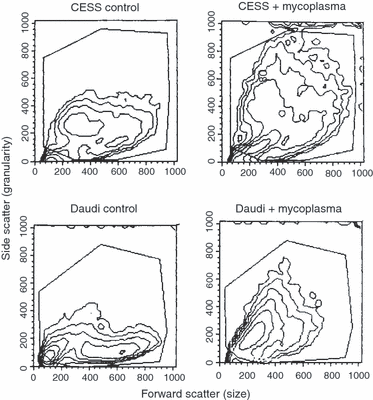
Light scattering by infected and uninfected cell lines. Control and M. fermentans strain M106 super infected CESS and Daudi cells. Cells infected with M. fermentans strain M106 are much more granular. The CESS cells remain the same size but the Daudi cells are smaller when the mycoplasma is present.
The nature of the induced enzyme
Under the fluorescence microscope the 5′N appeared as a diffuse surface fluorescence. A few bright cellular granules were visible on the Raji and some other cells, leading to a little non-specific staining.
It is possible to investigate the nature of the induced 5′N by studying the effect of enzyme inhibitors, as shown in Fig. 5.Figure 5(a) shows that β-glycerophosphate, which may be used to inhibit nonspecific phosphatases37 did not affect the enzyme activity whereas AMPCP, a competitive inhibitor of 5′N5, inhibited the enzyme on both infected and uninfected eucaryotic cells, and on the mycoplasma M106. The induced enzyme was therefore confirmed biochemically to be a 5′N. The eucaryotic cell-free mycoplasma suspension from the agar plates also showed marked phosphatase activity at pH 8·5, which was not inhibited by β-glycerophosphate (Fig. 5a), nor was p-nitrophenyl phosphate hydrolysed. However at pH 5·0 the mycoplasma phosphatase activity against AMP remained almost the same, but was now 11% inhibited by β-glycerophosphate, and the mycoplasma showed some ability to hydrolyse p-nitrophenyl phosphate, indicating that acid phosphatase was present as previously reported.30,31 It is possible that the only 60% inhibition of the mycoplasmal 5′N by AMPCP was a result of some residual acid phosphatase activity against AMP, which may well be a preferred substrate compared to β-glycerophosphate or p-nitrophenyl phosphate.
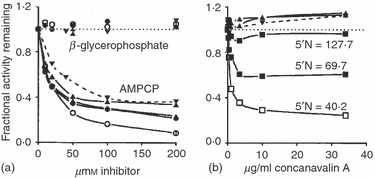
The effect of inhibitors on 5′-nucleotidase activity on cells uninfected or infected with M106. (a) Shows the effect of the specific 5′N inhibitor AMPCP compared to β-glycerophosphate, a competitive inhibitor of non-specific acid and alkaline phosphatases. (b) Shows that concanavalin A looses its power to inhibit 5′N when the cells become more infected and their 5′N increases. ○, • Uninfected and infected IgG-secreting EBV transformed clones. ◊, ◆Uninfected and infected IgM-secreting EBV transformed clones. □, ▮ Uninfected and infected CESS lymphoblastoid cell line. , Uninfected and infected Burkitt's lymphoma cells (Daudi and Raji). M. fermentans strain M106. The 5′N was measured as nmol AMP hydrolysed/hr/106 cells.
Enzyme conformational change
Figure 5(b) shows that concanavalin A inhibited the enzyme on the uninfected cells, but that this inhibition became less as the infection increased. However there was an apparent 10% enhancement of 5′N activity on the infected Burkitt's lymphoma cells and M106. These results indicate a conformational change in the eucaryotic 5′N involving the loss of available mannose groups.
The mouse monoclonal antibody 1FH 5N134 inhibited the 5′N on the uninfected CESS A cells by 75–82%, but this inhibition was decreased to 10% or less if the cells were infected. This again shows a conformation change in the 5′N on the infected cells.
Figure 6shows a plot of 5′N activity against the number of 5′N epitopes, showing the regression lines for the uninfected and super infected clones and cell lines. The correlation coefficient for the uninfected points is 0·86, P = 0·006, and for the infected points r = 0·80, P = 0·02. The ratio of the gradients of the two regression lines is 2·4, showing that for a given number of 5′N molecules on a cell, that on the infected cells is 2·4 times as active. This resolves the apparent contradiction between Fig. 1 and Fig. 3 for the CESS cells. Usually the number of 5′N epitopes increased on infection, except for the CESS cell line and IgM-secreting clone 17, which both showed a high uninfected 5′N activity (46·9 and 44·2 nmol AMP hydrolysed/hr/106 cells, respectively).
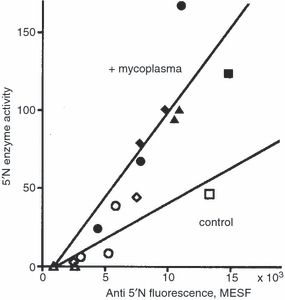
The relation between the epitopes on cells uninfected or infected with M. fermentans strain M106 showing anti5′N fluorescence and 5′N enzyme activity. ○, • Uninfected and infected IgG-secreting EBV transformed clones; ◊, ◆ uninfected and infected IgM-secreting EBV transformed clones; □, ▮ uninfected and infected IgG-secreting CESS lymphoblastoid line; , uninfected and infected non-Ig secreting Daudi and Raji Burkitt’s lymphoma cells. The 5′N is expressed as nmol AMP hydrolysed/106 cells/hr. The fluorescence is the difference in the fluorescence between cells labelled with both antibodies and cells labelled with the second antibody only. When multiplied by 103, it is in molecules of equivalent soluble fluorochrome (MESF).
The apparent Michaelis constant Km[mean ± SD (number of determinations)] for seven uninfected and infected EBV transformed cell lines at pH 8·5 was 9·1 ± 1·0 (7) and 4·6 ± 1·6 (7) µm AMP, respectively. This difference is significant, P < 0·001. Km for the Daudi and Raji cell lines was 3·1 and 5·3 µm AMP, which is not significantly different from that of the other infected cells The Km of the uninfected cells is not significantly different from the Km of 9·6 ± 2·7 (12) µM AMP obtained for normal circulating lymphocytes by Rowe et al.33 using the same assay, and is in good agreement with the results quoted by Zimmermann.4 The Km for the M106 mycoplasmal 5′N was 2·6 ± 1·0 (2) µmAMP.
Nature of M. fermentans strain M106
M. fermentans strain M106 did not bind the mouse antihuman 5′N monoclonal antibodies. Colonies of mycoplasma M106 on Friis agar showed no fluorescence when treated with mouse anti-human or control monoclonal antibodies when stained with the FITC goat anti-mouse second antibody. There was no reason therefore to believe that the mycoplasmal enzyme was of human origin, or would cause artefacts in the flow cytometry. The colonies showed no fluorescence when grown overnight with human serum, and tested (after washing) with FITC rabbit antihuman IgG, IgM and IgA, indicating that the mycoplasma would not bind human immunoglobulins, nor would it bind human 5′N when tested with the appropriate antibodies. However the colonies did show fluorescence when treated with the rabbit anti-M. fermentans antibody and FITC swine anti-rabbit immunoglobulin.
7, 8show electron micrographs of CESS cells grown with M. fermentans strain M106 under normal conditions. It will be seen that this strain is filamentous, and mycoplasma filaments can be seen inside the CESS cells in Fig. 7, particularly those on the left of the photograph. These would make the cells appear more granular.
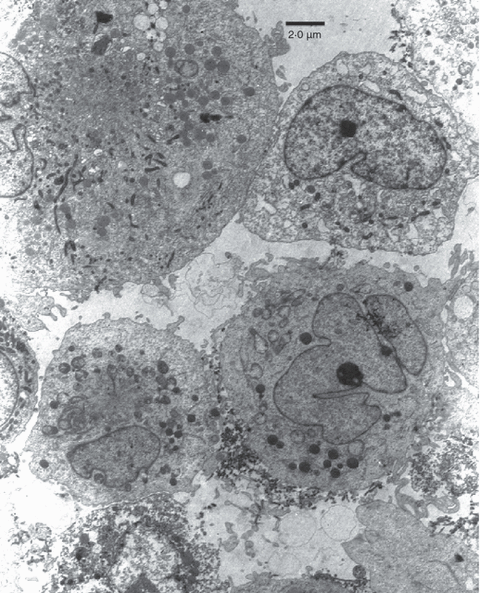
Electron micrograph of CESS cells infected with M. fermentans strain M106. The mycoplasmas are not clustered round the outside of the cell membrane but can be seen as small worm-like filamentous structures inside the two cells on the left of the picture.
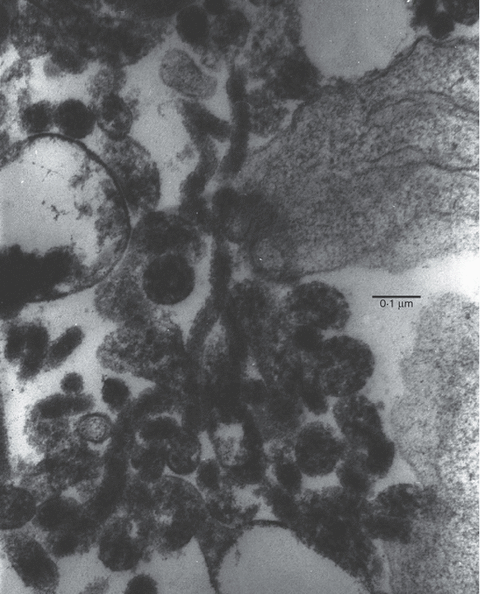
M. fermentans strain M106 at higher magnification showing its filamentous nature.
Other strains of M. fermentans
Table 2shows the effect of 15 strains of M. fermentans on the EBV transformed IgM-secreting cell line P3. PG 18 is omitted as it failed to grow with the B-cells. The cell line P3 had not been previously exposed to mycoplasmas and was grown with the mycoplasma strain for 10 days before use but was not superinfected. The 5′N on the cells was measured as described previously. The strains in Groups I, III and IV and the two strains of leukaemic origin all themselves expressed 5′N. Strains in Group II, which were of respiratory or cell culture origin, did not express the enzyme3 and usually decreased the 5′N on the cell line. The origin of the tissue culture strains is most likely to be respiratory. PG 18 also belongs to Group II and shows no 5′N activity.
| RFLP group Reference | Mycoplasma strain grown with B-cell line P3 | Received from: | Effect on human 5′N activity | Isolation site |
|---|---|---|---|---|
| II | M70, M640 | J. Tully ex. R. Dular (Canada) | ×2·9 | Respiratory tract |
| II | M64, M642 | J. Tully ex. R. Dular (Canada) | ×0·53 | Respiratory tract |
| II | M52, M671 | J. Tully ex. R. Dular (Canada) | ×0·45 | Respiratory tract |
| II | PG18TNCTC 10117T | NCTC(UK) | Mycoplasma dies | Male genital ulcer |
| II | M433 | P. Walker (UK) | ×0·62 | Tissue culture |
| II | M148 | S. Hancock (UK) | ×0·41 | Tissue culture |
| IV | KL4, M614 | M.H. Williams | ×0·8, ns | Synovial fluid |
| IV | KL8, M618 | M.H. Williams | ×5·7 | Synovial fluid |
| I | GIM, M670 | C. Bébéar (France) | ×2·3 | Synovial fluid |
| I | BRO, M641 | C. Bébéar (France) | ×2·0 | Urethra |
| I | M106 | S. Johnson | ×8·1 | Tissue culture |
| I | Incognitus M 200 | S-C. Lo (USA) | ×0·47 | Tissue culture* |
| III | #5, M672 | R.B. Kundsin (USA) | ×10·6 | Urine (AIDS) |
| III | #29, M673 | R.B. Kundsin (USA) | ×1·9 | Urine (AIDS) |
| ? | E10 | Murphy (USA) | ×2·4 | Leukaemic bone marrow |
| ? | Z62 | Murphy (USA) | ×1·9 | Leukaemic bone marrow |
Discussion
The cytofluorimetry indicates that M106 is making the B cells more granular but not larger. There appears to be an infected granular population of Daudi cells in Fig. 2 growing with a group of cells not yet affected. This is explained by the electron micrographs (7, 8), which show that M106 is a filamentous form of M. fermentans growing both outside and inside some cells but not particularly adhering to the plasma membrane. Only the granular (mycoplasma-infected) cells develop 5′N. Taylor-Robinson et al.36 showed that the type strain PG 18 was found inside HeLa cells but was not filamentous. Filamentous forms of other species of mycoplasma have been described.38,39M. fermentans has also been reported to induce a number of cytokines, such as interleukin-1 (IL-1), IL-6, granulocyte colony-stimulating factor, prostaglandins and tumour necrosis factor (TNF).40–42 Christensen et al.43 reported that lymphotoxin, prostaglandin E2 (PGE2) and (in some people only) TNF would increase the 5′N activity of lymphocytes. The most effective was PGE2, which gave a threefold increase at the highest concentrations used (3 µm), whereas IL-6 had no effect. M. fermentans (var. incognitus) has also been shown to increase and induce major histocomptibility complex (MHC) expression, at least partly because of an increase in the transcription of the MHC genes.44
Because M. fermentans strain M106 also increased the 5′N on the monocyte/macrophage cell line THP-1, it might be expected to increase the 5′N on the Kupfer cells of the liver. As 5′N has been shown to be released from macrophages by activation,13 which would occur on inflammation, the correlation between the ESR and the serum 5′N could be explained.
M106 appears to be inducing a conformational change in the human 5′N. Both the monoclonal antibodies still bind to the enzyme, but it is no longer inhibited by 1FH 5N1, nor concanavalin A, which is known to bind to mannose groups. The most likely change is to the glycosylation of the enzyme.
The immunological consequences of an altered 5′N are not yet known, as the role of the unaltered enzyme itself is far from clear. It could interfere with the maturation of B cells and their adhesion to follicular dendritic cells or vascular endothelium45,46 and T-cell activation, proliferation and cytotoxic killing.10,11,47
Acknowledgements
I should like to thank Dr R. H. Leach, Mrs D. L. Michelmore and Dr D. Pitcher of the Mycoplasma Reference Facility, NCTC, Colindale, for cultivating and identifying Mycoplasma fermentans strain M106 and for the anti-M. fermentans polyclonal antibody, and Professors Kummer and Thompson for their monoclonal anti-human 5′N antibodies. Mr Raymond Moss kindly did the electron microscopy. I should also like to thank the donors of the cell lines, and gratefully to acknowledge some financial support from the Cancer Research Campaign and more recently St. George's Special Trustees.




