Prostaglandin E2 modulates dendritic cell function during chlamydial genital infection
Summary
Inflammatory responses mediated by antigen-presenting dendritic cells (DCs), can be modulated by the presence of prostaglandins (PG), including prostaglandin E2 (PGE2). PGE2 modifies the production of an immune response by altering DC function through PGE2 receptors. PGE2 is produced by epithelial cells lining the murine female reproductive tract during Chlamydia muridarum infection and likely manipulates the antichlamydial immune response during antigen uptake in the genital mucosa. Our data demonstrate that the PGE2 present locally in the genital tract upon chlamydial genital infection enhanced the recruitment of CD11b+ conventional DCs, but not CD45R+ plasmacytoid DCs, to infected genital tract tissue and draining lymph nodes in vivo. Furthermore, exposure to PGE2in vitro during infection of murine bone-marrow-derived conventional DCs (cDCs) boosted interleukin-10 mRNA and protein while not influencing interleukin-12p40 production. Infection of cDCs markedly increased mRNA production of the costimulatory molecules CD86, CD40 and a member of the C-type lectin family, DEC-205, but addition of PGE2 increased other costimulatory molecules and C-type lectins. Also, exposure of PGE2 to infected cDCs increased FcγRIII and FcγRIIb, suggesting that PGE2 enhances the uptake and presentation of C. muridarum and augments production of the antichlamydial adaptive immune response. Taken together, the data suggest that exposure of infected cDCs to PGE2 drives production of a diverse adaptive immune response with implications for regulating tissue inflammation.
Introduction
Chlamydia trachomatis is an obligate intracellular bacterium responsible for the most cases of bacterial sexually transmitted infections. Three million new cases occur in the USA each year1 and the majority of cases (70%) are asymptomatic and not treated.2 Untreated, persistent infection or reinfection results in pelvic inflammatory disease, ectopic pregnancy and tubal infertility, which cost the health-care industry billions of dollars annually. In addition, chlamydial sexually transmitted infections enhance the transmission of human immunodeficiency virus and contribute to human papillomavirus-induced cervical neoplasia. Control programmes can reduce the sequelae of chlamydial genital infection in females by abbreviating the length of infection. However, they do not appear to cause an overall reduction in the number of infections and instead increase reinfection by possibly preventing development of antichlamydial immunity.3 A viable means for preventing an infection or reinfection of the genital infection is to induce immunity through vaccination.
The type of cell-mediated immunity produced during a chlamydial genital infection alters the outcome of infection in humans and mice.4 For instance, the murine model of genital infection with Chlamydia muridarum (MoPn), which has a genetic composition similar to human strains of C. trachomatis,5 has provided information on the immune mechanisms of clearance of infection. It is well-recognized that CD4+ T cells dominate the lymphocytic infiltrate.6,7 Furthermore, CD4+ T helper type 1 (Th1) cells are necessary for eradication of Chlamydia from the genital mucosa.8 Since dendritic cells (DCs), the most potent antigen-presenting cells, have the potential to polarize naive T-cell subsets and the subsequent outcome of infection, it is important to study DC biology during chlamydial infection.
Dendritic cells have been found in mouse vaginal and cervical mucosae.9 They are recruited to the site of inflammation in response to infection with MoPn10 and are necessary for protection from chlamydial infection.11 Incorporation of chlamydiae and/or exposure to inflammatory cytokines induce immature DCs to undergo rapid phenotypic and functional changes that culminate in the complete transition from an antigen-capturing cell to an antigen-presenting cell.12–15 This maturation process is associated with several co-ordinated events characterized by the loss of endocytotic activity,16 increase of surface expression of major histocompatibility complex (MHC) class I–peptide and class II–peptide complexes,17,18 up-regulation of surface expression of adhesion and costimulatory molecules (including CD80 and CD86),17,19 and secretion of the proinflammatory cytokines interleukin-12 (IL-12), IL-2 and tumour necrosis factor-α (TNF-α).18,20 Maturation also induces the mobilization of DCs to draining lymph nodes where they can activate naive T cells. Studies have shown that matured DCs are necessary for priming naive T cells. Also, matured DCs dictate the result of infection and control the induction of tolerance and chronic inflammation.21–24
The DC system contains two broad categories of DCs; the myeloid or conventional dendritic cells (cDCs) and plasmacytoid dendritic cells (pDCs). These two subsets have distinctive properties including phenotype, trafficking pattern and roles in T-cell polarization.25–27 Following DC maturation, DC subsets boost migration to draining lymph nodes. The cDCs rapidly up-regulate surface expression of the homing chemokine receptor CCR7, which drives cDC migration from tissues into the lymphatics through the CCR7 ligand, CCL21, and then to draining lymph nodes through another CCR7 ligand, CCL19.28–32 In contrast, pDCs migrate through the blood to draining lymph nodes by up-regulation of chemokine receptors associated with inflammation.33,34 Thus, the proportions of cDCs and pDCs that reach the draining lymph node and interact with T lymphocytes shape the type of adaptive immune response produced.34
Prostaglandin E2 (PGE2), a well-known immune regulator, is induced by a chlamydial genital infection within epithelial cells.35 Synthesis of PGE2 begins with the liberation of arachidonic acid from cell membranes, and then the rate-liming enzyme cyclo-oxygenase (COX) converts arachidonic acid into prostaglandin PGH2. Cell-specific PG synthases are responsible for the conversion of PGH2 into different PGs, including PGE2.36 Prostaglandin E2 is well-reported to have both stimulatory and inhibitory effects on the activation of DC subsets depending on the order of exposure to PGE2 and DC-activating stimulants.37 Also, PGE2 regulates the production of cytokines by DCs, thus shaping the subsequent T-cell response by favouring non-Th1 subset development.38 To understand how PGE2 influences DC functioning during chlamydial genital infection, we investigated the effect of exogenous PGE2 on migration, cytokine production, expression of costimulatory molecules and the expression of receptors associated with microbial detection by DCs.
Materials and methods
Mice and infection
Female BALB/c mice were obtained from Harlan Sprague-Dawley (Indianapolis, ID) and were housed according to the American Association of Accreditation of Laboratory Animal Care guidelines. Experimental procedures were approved by the UCLA Institutional Animal Care. All mice, 5–7 weeks of age, were first injected subcutaneously with 2·5 mg medroxyprogesterone acetate (Upjohn, Kalamazoo, MI) in 100 μl sterile phosphate-buffered saline. Medroxyprogesterone acetate drives mice into a state of anoestrous thus eliminating the variability in the rate and severity of infection caused by the oestrous cycle. Seven days later, anaesthetized mice were vaginally inoculated with 1·5 × 105 inclusion-forming units (IFUs) of C. muridarum (MoPn) Nigg strain, grown in McCoy cells (50% infective dose = 2·5 × 103 IFU). Infection was monitored by obtaining cervical–vaginal swabs (Dacroswab Type 1, Spectrum Laboratories, Houston, TX) every 3 days and describing the findings.39 Swabs were stored in sucrose-phosphate buffer at − 80° until analysed.
Subcutaneous pellet implantation
Pellets were implanted as previously described.35 Briefly, pellets designed to continually release 17–25 μg/mouse PGE2 or placebo daily for 60 days (1·0 mg PGE2 and 1·5 mg placebo) were purchased from Innovative Research of America (Sarasota, FL). Mice were anaesthetized and fur was shaved over the shoulder/back with a no. 40 blade clipper (WAHL Clipper Corporation, Sterling, IL). The skin was prepared with 70% ethanol and a small fold of skin was tented using Adson forceps and pierced with a no. 11 scalpel blade (Feather Safety Razor Co. Ltd, Osaka, Japan). The opening was enlarged to keyhole size using blunt-end scissors (Fisher Scientific, Pittsburgh, PA). A 10 G trochar (Innovative Research of America) was inserted and the pellets were delivered to the subcutaneous pocket. A drop of tissue glue (Abbott Laboratories, North Chicago, IL) was applied and recovery was observed for 7 days after the pellet implantation. No apparent inflammation or infection was observed at the incision site.
Genital tract homogenates
Genital tracts (GT) were divided into the cervical–vaginal region and oviducts with the ovaries removed. Tissue sections were placed in 1·0 ml protease-inhibitor buffer (1 μg each of antipain, aprotinin, leupeptin and pepstatin and 2 mm phenylmethylsulphonyl fluoride in sterile phosphate-buffered saline) (Sigma, St Louis, MO) and homogenized as previously described39 using a hand-held homogenizer (Omni International, Marietta, GA). Supernatants were stored at − 70° until analysis.
Serum
Cardiac puncture was performed to obtain the peripheral blood which was collected into a 2·0 ml sterile centrifuge tube. The tubes sat at room temperature for at least 30 min until a clot was formed. The peripheral blood was centrifuged at 1000 g for 10 min and the serum was collected. The serum was stored at − 70° and PGE2 was measured with a competitive PGE2 enzyme-linked immunosorbent assay (ELISA).
PGE2 measurement
Prostaglandin E2 concentration was measured for each condition with enzyme immunoassay using a PGE2 Immunoassay kit by following the manufacturer's instruction (Cayman Chemical, Ann Arbor, MI). All measurements were made in duplicate. Briefly, the pH of all the serum and homogenate samples was adjusted to 4·0 and then the samples were applied onto a PGE2 affinity column (Cayman Chemical). The eluate was dried with a vacuum-centrifuge and immediately dissolved in 0·5 ml of enzyme immunoassay buffer. The cell culture supernatant (50 μl), acetylcholiesterase tracer, and PGE2 monoclonal antibody were added to a 96-well plate precoated with anti-PGE2 antibody and incubated at 4° for 18 hr. Then, 200 μl Ellmans' reagent was added and the plate was developed at room temperature. The optical density was read at 405 nm using a microplate reader (model 550; Bio-Rad, Hercules, CA).
Enrichment of DCs from the GT and inguinal lymph nodes
Whole GT and iliac lymph nodes (ILN) from all groups of mice were harvested. Tissues were pooled for each group (7–14 mice each) and single cell suspensions were prepared by mincing with scissors and then subjected to digestion with 5 mg collagenase (type I; 5 mg/ml in Hanks' balanced salt solution; Sigma), followed by treatment with 1 mg DNAse (Sigma) in 4·5 ml calcium/magnesium-free Hanks' buffer (CMF Hanks') (Gibco BRL, Gaithersburg, MD) for 20 min at 37°. After the incubation, 1 ml of 180 mm EDTA was added to each tube and the tubes were then placed on a tube rotator for 5 min at room temperature. Single cell suspensions were prepared by expressing the digests through a 70-μm pore-size filter (Falcon, Becton Dickinson, Franklin Lakes, NJ) in CMF Hanks'. The single cell suspension was laid on top of a 4 ml 15% Nycodenz gradient (Accurate Chemical, Westbury, NY) and then centrifuged at 400 g for 20 min at room temperature. The low-density cells that appear at the interface between the medium and the Nycodenz were collected and washed. The washed cells were resuspended in Dulbecco's modified Eagle's medium (DMEM; Gibco BRL, Gaithersburg, MD) containing 1% bovine serum albumin (Sigma) and 0·1% sodium azide to identify DCs and separate them into subsets, cDC and pDC, using flow cytometric techniques (see method description below). DCs were identified by staining with CD11c (a marker on all mouse DCs), CD3 and CD19 (to exclude T and B cells because some cells express low levels of CD11c). After gating on CD11c+ CD3– CD19– cells the DCs could then be separated into subsets by the mutual exclusion of two markers; CD11b (cDC) and CD45R (former name is B220, pDC).25,40,41
Antibodies
The following rat anti-mouse monoclonal antibodies were purchased from eBioscience (San Diego, CA): phycoerythrin-Cy5 (PE-Cy5)-conjugated CD19 (clone MB19-1), fluorescein isothiocyanate (FITC)-conjugated CD45R (clone RA3–6B2), allophycocyanin (APC)-conjugated CD45R (clone RA3–6B2), and APC-conjugated CCR7 (clone 4B12), hamster anti-mouse CD3-PE-Cy5 and streptavidin-conjugated with APC. The following anti-mouse monoclonal antibodies were purchased from Pharmingen (San Diego, CA): rat anti-mouse FITC-conjugated CD11b (clone M1/70), rat anti-mouse APC-conjugated CD45R (clone RA3–6B2), hamster anti-mouse PE-conjugated CD11c (clone HL3), and mouse anti-mouse biotin-conjugated I-Ad. Isotype control antibodies were purchased from the same vendor as each primary antibody with the exception of the isotype controls for CD11b-FITC and CD45R-APC, which were purchased from eBioscience.
Flow cytometry
Single cell suspensions (3 × 105 to 5 × 105 cells) were stained in DMEM containing 1% bovine serum albumin (Sigma) and 0·1% sodium azide, using the microplate method as previously described.6 For single-colour staining in this paper, isolated cells were first incubated with 10 μg anti-mouse cell surface markers/ml (see the Antibodies section above) for 25 min on ice and then washed twice with DMEM containing 10% bovine serum albumin. The cells were then resuspended in streptavidin-conjugated to APC (Pharmingen) at a concentration of 0·2 μg/ml for 25 min on ice. Following the washing step described above, the cells were fixed in phosphate-buffered saline containing 1% paraformaldehyde and kept at 4° until analysed.
Production of bone-marrow-derived cDCs in vitro
Bone marrow cell cultures were prepared from 6- to 8-week-old BALB/c mice as described previously.42 Briefly, at day 0, 2 × 106 bone marrow cells were seeded per 100-mm dish in 10 ml R10 medium (Fisher Scientific) and cells were fed with 10 ml R10 medium at day 3. The R10 medium was RPMI-1640 supplemented with 10% heat-inactivated fetal calf serum (Fisher Scientific), 2 mm l-glutamine (Fisher Scientific), 50 μm 2-mercaptoethanol (Fisher Scientific), 100 U/ml penicillin (Fisher Scientific), streptomycin (Fisher Scientific), and 20 ng/ml (200 U/ml) recombinant mouse granulocyte–macrophage colony-stimulating factor (Invitrogen Corp., Carlsbad, CA). At day 6, the cells were enriched by positive selection using magnetic microbeads conjugated with anti-mouse CD11c mAb (Miltenyi Biotec, Auburn, CA), following the manufacturer's protocol. Purity of approximately 94% was achieved as assessed by flow cytometry.
RNA harvest
The purified bone-marrow-derived cDCs were incubated with live MoPn at a multiplicity of infection of 3·0 in sucrose-potassium glutamate (SPG) buffer as mock-infected at 35° for 30 min. The cells were washed three times with 1 × phosphate-buffered saline, and then placed into a six-well culture plate. The PGE2 (Cayman Chemical, Ann Arbor, MI) and its solvent control, dimethyl sulphoxide (DMSO), at 1 μm, were added to the cells and incubated at 35° for 24 hr. The RNA was isolated using RNA-bee reagent (Tel.Test Inc., Friendswood, TX). As stipulated in the manufacturer's protocol, 1·0 ml RNA-bee solution was mixed with 5 × 106 cells and then chloroform (0·2 ml, Fisher Scientific) was added per 1·0 ml RNA-Bee. The sample was stored on ice for 5 min and then centrifuged at 1000 g for 15 min at 4°. Following centrifugation, the aqueous phase was transferred to a clean tube, and 0·5 ml isopropanol (Fisher Scientific) was added. After the sample had been rested for 5–10 min at room temperature, the sample was centrifuged at 1000 g for 5 min at room temperature to precipitate the RNA. The supernatant was removed and the RNA pellet was washed once with 75% ethanol. After centrifugation, the ethanol was removed and the RNA pellet was briefly air-dried for 5–10 min. The RNA was dissolved in diethylpyrocarbonate (DEPC) water. The sample was stored at − 80° until further use.
SuperArray analysis
The RNA (5–10 μg) was sent to SuperArray Bioscience Corp. (Frederick, MD) for non-rad-GEArray assay specific for Mouse Dendritic & Antigen Presenting Cell Gene Array analysis. Each array provides a matched set of membranes containing 96 genes encoding the proteins or molecules involved in the DC antigen uptake, antigen presentation, chemokine and cytokine synthesis, signalling transducing pathways and controls. SuperArray converted our experimental RNA to cDNA labelled with their trademarked chemoluminescent substance, TrueLabeling AMP 2·0. The labelled cDNA (3–5 μg) was hybridized to a set of oligo DNA probes dotted onto a membrane representing 96 genes encoded by mouse DCs and other antigen-presenting cells plus controls. Overnight hybridization with the array membrane and subsequent washing were performed in the hybridization oven. Following substrate addition, membranes were exposed to X-ray film for 5 to 10 min. Each cDNA fragment was printed with tetraspot format. Data were quantified using a laser densitometer and ImageQuaNT software (Molecular Dynamics, Sunnyvale, CA) to calculate the average integrated volumes of spots. Data were expressed as the average integrated volume of a sample relative to the average integrated volume of a positive control, β-actin. These data were supplied in Excel sheet format along with the gels (see supplemental data Fig. S03).
Quantitative real-time polymerase chain reaction
The total RNA of each sample was reverse-transcribed with an iScript™cDNA Synthesis kit by following the manufacture's instruction (Bio-Rad, Hercules, CA). Primers for amplifying IL-10 and IL-12p40 have been published previously.43 The quantitative real-time polymerase chain reaction (PCR) contained SYBR green SuperMix (Bio-Rad), cytokine gene-specific primers (0·3 μm) and 0·75 μg cDNA templates of individual samples or 10-fold serial dilutions of the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) standards, with concentrations ranging from 1 × 102 to 1 × 106 copies. Total reaction volume was 25 μl. The thermal profile for all SYBR Green PCRs was 95° for 5 min, followed by 40 cycles of 95° for 45 seconds and 60° for 45 seconds. The quantity of each transcript was calculated by the standard curve method43 using iCycler iQTM Optical System Software version 3·1. The GAPDH mRNA was used for each experimental sample as an endogenous control to account for differences in the amount and quality of total RNAs added to each reaction. Each sample was performed in triplicate. The nucleotide sequences of the primers used in this study were as follows: IL-10: forward, 5′-GGTTGCCAAGCCTTATCGGA-3′, reverse, 5′-ACCTGCTCCACTGCCTTGCT-3′; IL-12p40: forward, 5′-GGAAGCACGGCAGCAGAATA′, reverse, 5′-AACTTGAGGGAGAAGTAGGAATGG′; and GAPDH: forward, 5′-TCCTGCACCACCAACTGCTTAG-3′, reverse 5′-GATGACCTTGCCCACAGCCTTG-3′.
ELISA
The recombinant protein and antibodies used for ELISA against IL-10 and IL-12p70 were purchased from R & D Systems (Minneapolis, MN). Supernatants from the cultures of MoPn-infected or mock-infected bone-marrow-derived DCs (BMDCs) treated with PGE2 or DMSO (1 μm) were collected after a 24-hr incubation at 35°. Supernatants were added to duplicate wells of microtitre enzyme immunoassay plates (Costar/Corning, Acton, MA) and assayed according to the manufacturer's protocol. The recommended substrate was replaced with TMB 1-step substrate (Dako, Carpinteria, CA). The optical densities were read at 450 nm with a microplate reader and cytokine levels were determined using microplate reader software (model 550; Bio-Rad).
Statistics
Student's t-test was performed using SigmaStat 2·03 (SPSS Inc. Chicago, IL). Groups were considered statistically different at P < 0·05. Data are presented as mean ± SD.
Results
Exogenous PGE2 elevates genital tract tissue levels of PGE2 during MoPn infection
We have previously shown that PGE2 was produced within epithelial cells infected with MoPn and was also necessary for the organism's growth.35 To examine the effect of PGE2 on the immune response in vivo, mice were subjected to greater levels of PGE2 throughout a genital infection. Mice were subcutaneously implanted with pellets that released continuous levels of PGE2 or the carrier alone (Placebo) 1 week before the mice were given a genital infection with MoPn because of the short half-life of PGE2. We measured the systemic (serum) and local GT tissue (GT homogenates) PGE2 levels achieved during a chlamydial genital infection by ELISA. As anticipated, PGE2 serum levels were higher in mice that received PGE2 pellets, indicating that PGE2 pellet implantation is the primary contributing factor to the PGE2 level in the serum (Fig. 1a) and that a genital infection with MoPn itself did not dramatically affect the serum PGE2 level.
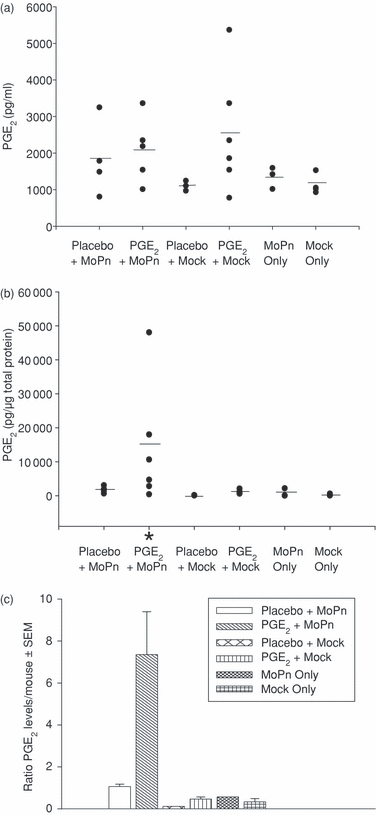
Prostaglandin E2 is concentrated in GT tissues upon infection. PGE2 levels were measured within the serum and GT tissues in MoPn-infected or mock-infected mice on day 8 post infection. Mice were also implanted with a PGE2 or placebo pellet. (a) PGE2 levels in serum; (b) PGE2 levels in GT homogenates. *P < 0·05, compared with placebo + mock infection group by Student's t-test. (c) Ratio of PGE2 levels in GT homogenates to those in serum. PGE2 values of individual mice are an average of two independent experiments in (a) and (b). The short horizontal line represents the average level of each group.
In contrast, we found a statistically significant increase of PGE2 levels only within GT tissues from MoPn-infected mice given PGE2 pellets. This indicates that a local MoPn infection is needed to absorb systemically derived PGE2 and significantly raise levels of PGE2 in the GT tissues. This is appreciated by comparing the ratio of PGE2 levels in GT homogenates to those in serum (Fig. 1c) and implies that PGE2 pellets are the determining factor for increased serum PGE2 levels in MoPn-infected mice, while GT infection is the determining factor for increased PGE2 levels observed in locally challenged tissue.
Elevated PGE2 levels increase the number of DCs in the GT of MoPn-infected mice
Dendritic cells migrate from the peripheral blood into local tissues upon infection and PGE2 is reported to increase DC migration.26,40 To investigate if PGE2 alters the migration pattern of DCs following chlamydial genital infection, we determined the number of total DCs within GT tissue at early time-points after MoPn infection. In addition, adaptive immune responses are influenced by the type of DC subset present, thus we also examined functionally different DC subsets. Total DCs were defined as CD11c+ CD3– CD19– and DC subsets were defined by the mutual exclusion of CD11b (cDC; CD11b+) and CD45R (pDC; CD45R+) (Supplement Fig. S1). Very few DCs were found in the GT of uninfected mice (day 0) despite elevated PGE2 serum levels (Fig. 1a). However, DCs increased in number within the first week of MoPn genital infection (Fig. 2). The availability of additional PGE2in vivo, supplied by the continuous release pellets, caused a rapid elevation of DCs in the GT tissue compared with the placebo control. The cDCs showed similar kinetics of recruitment during infection and also responded to increased PGE2 levels by markedly increasing the number of cDCs in a manner similar to that seen for total DCs (Fig. 2b). Interestingly, excess PGE2 had only a slight influence on the migration of pDCs to the GT tissues as compared to controls (Fig. 2c). These data show that PGE2 preferentially enhances the migration of cDCs over pDCs to infected GT tissues early after infection and has the potential to modulate the adaptive immune response.
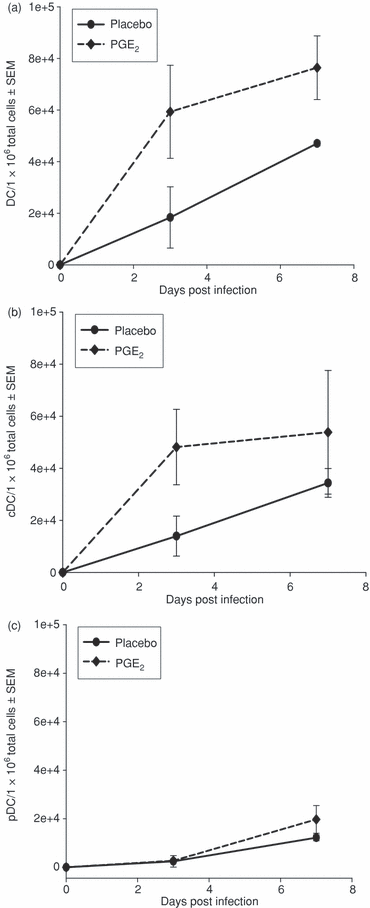
Influence of PGE2 on recruitment of DC subsets during chlamydial infection. DCs were isolated from GT tissue from mice infected with MoPn and implanted with PGE2 or placebo pellets. A Nycodenz gradient was applied to enrich low-density DCs and cDCs were identified by flow cytometry as described in the Materials and methods section. (a) Total number of DCs, (b) number of conventional or myeloid DCs (cDC), (c) number of plasmacytoid DCs (pDC). The results were obtained from two independent experiments.
Elevated PGE2 levels in vivo increase the appearance of cDCs within local draining lymph nodes
PGE2 has been documented to influence the migratory potential in vitro of certain DC subsets during maturation and one of many mechanisms which control leucocyte migration is the up-regulation of CCR7.37,44–46 To determine whether CCR7 expression was increased in our model, we examined the expression of CCR7 on DCs in the GT. We found that the numbers of DCs and cDCs expressing high levels of CCR7 were greater in infected mice treated with PGE2 compared to infected mice treated with placebo (Fig. 3a,b). However, PGE2 did not increase expression of CCR7 on pDCs in the GT after MoPn infection compared to control pDCs. Additionally, PGE2 also increased the number of cDCs expressing only moderate levels of CCR7, suggesting that PGE2 boosts migration by other mechanisms (Fig. 3a). We also examined the number of DCs appearing in the draining lymph node early after infection. Consistent with our findings, more DCs and cDCs were present in MoPn-infected mice implanted with PGE2 pellets compared to MoPn-infected mice implanted with placebo control pellets early after infection (Fig. 3c). Increased expression of CCR7 is not the only factor that enhances DC migration because DC migration can be blocked by treatment with an antagonist to a PGE2 receptor, EP4, even in the presence of CCR7.47 No obvious difference was observed in the numbers of DC subsets between PGE2 and control mice in the spleen early after infection (data not shown) consistent with reports that the ILN is the primary site of T-cell activation early after infection in a genital infection.48,49 These results imply that PGE2 affects the migration of cDCs, but not pDCs, in vivo.
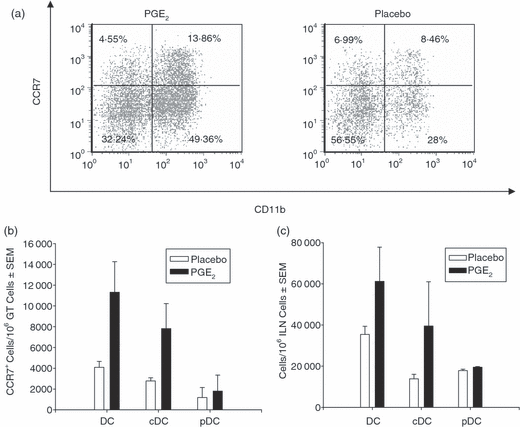
Prostaglandin E2 increases the recruitment of cDC following chlamydial genital infection. Expression of CCR7 on DCs from mice implanted with PGE2 or placebo pellets was analysed 3 days after a chlamydial infection. Low-density DCs were enriched using a Nycodenz gradient. (a) Representative dot plots of CCR7 expression on CD11c+ CD3– CD19– cDCs (CD11b+) and pDCs (CD11b–) within GT. (b) The number of DCs expressing high levels of CCR7 within GT were determined 3 days following infection as described in the Materials and methods. (c) The number of DCs within the ILN was determined 3 days following infection as described in the Materials and methods. The results shown in (b) and (c) were obtained from two independent experiments.
PGE2 alters the expression of mRNA species implicated in T-cell subset development in cDCs
The cDCs are essential for T-cell activation and result in the production of antigen-specific Th1 cells when stimulated with microbial products.21 To further explore the role of PGE2 in modulating functions of DCs important for producing Th1 cells we examined whether exposure of maturing DCs to MoPn was altered by PGE2. As shown in Fig. 4, we analysed various mRNA species of cytokine genes induced in maturing cDCs and chemokine receptors important for migration of cDCs in vitro using immature BMDCs that were > 94% cDCs by phenotypic analysis (Supplement Fig. S02). These particular genes influence the interaction of T cells and DCs. We compared the induction (ratio or fold-increase) of these genes on infection in the absence (Fig. S02, panel A) or presence (Fig. S02, panel B) of PGE2. Some genes require infection to induce expression, thus we examined the influence of PGE2 on infected cells (Fig. S02, panel C). As expected, maturation of cDCs by MoPn infection induced the up-regulation of mRNA species for TNF-α, IL-1α, IL-1β, IL-6 and IL-12p35 (IL-12β gene) consistent with other reports (Fig. 4a and Supplement Fig. S03).12,15 However, MoPn-induced maturation of cDCs exposed to PGE2 increased IL-10 mRNA approximately five-fold compared to mock-infected cDCs treated with PGE2 (Fig. 4b). When the influence of PGE2 was examined on cDCs matured in the presence of MoPn, we found that PGE2 down-regulated mRNA for TNF-α, IL-1α, IL-6 and IL-12p35. The IL-10 mRNA remained elevated and we also found induction of mRNA for interferon-α (IFN-α), CCR7 and CXCR4. This analysis confirmed the increased migration of cDCs observed in vivo (2, 3). IL-10 and IL-12 are important in differentiating T-cell subsets50 and when cytokine modulation was further examined by quantitative real-time PCR, we found that PGE2 had little effect on IL-12p40 mRNA but markedly elevated IL-10 mRNA (Fig. 5a,b). In addition, PGE2 treatment significantly boosted IL-10 protein levels (mean 396 pg/ml) over those induced by infection alone (mean = 209 pg/ml) (Fig. 5c). Thus, cDCs that mature in the presence of PGE2 acquire a cytokine phenotype that favours non-Th1-cell differentiation.
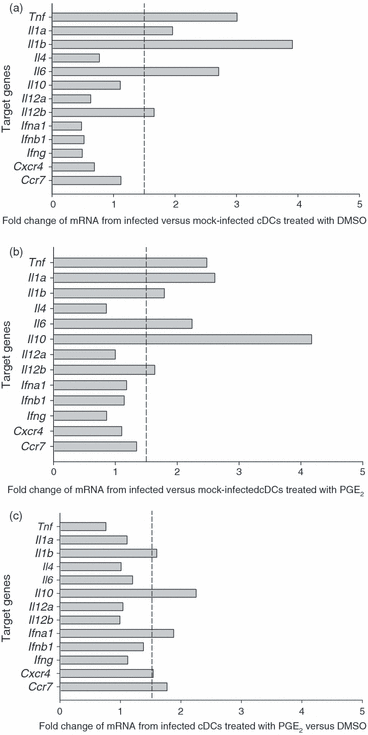
Influence of PGE2 on mRNA expression of selected cytokines and chemokine receptors by infected cDCs. RNA (5–10 μg) was isolated from immature cDCs and infected with MoPn in the presence of PGE2 or DMSO (control) for 24 hr. SuperArray was performed and normalized to β-actin mRNA internal control. The data are expressed as the ratio of spot intensity of groups as indicated below each panel. A vertical dotted line at the ratio of 1·5 was drawn as the criterion for significant increase in mRNA levels. (a) Ratio of various mRNA species from infected versus mock-infected cDCs treated with DMSO. (b) Ratio of various mRNA species from infected versus mock-infected cDCs treated with PGE2. (c) Ratio of various mRNA species from infected cDCs treated with PGE2 versus DMSO. The gene tnf encodes TNF-α. Additional names for genes can be found at http://www.ncbi.nlm.nih.gov/entrez/query.fcgi
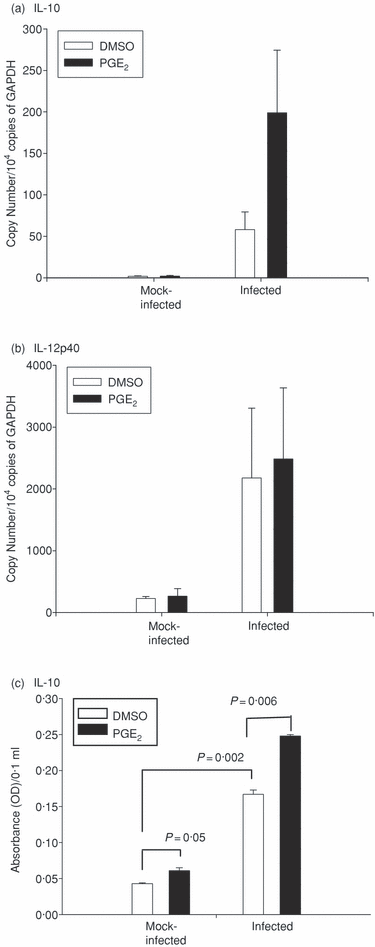
Quantitative real-time PCR and ELISA analysis of IL-10 and IL-12 cytokine expression by cDCs. Groups of BMDCs (5 × 105) were infected with a multiplicity of infection of 3 live C. muridarum or mock-infected with SPG. PGE2 or DMSO control (1 μm) was added at time of infection and the cells were cultured. RNA was isolated 30 min after culture and 1 μg RNA was converted to cDNA for quantitative real-time PCR analysis. The results were normalized with the internal control GAPDH. The PCR results were shown as (a) IL-10, (b) IL-12p40. Supernatants were collected from additional wells cultured for 24 hr and cytokine levels were measured by ELISA from undiluted samples and shown as (c) IL-10. Means correspond to the following protein levels as determine with a standard control: infected cDC + PGE2 = 396 pg/ml; infected cDC + DMSO = 209 pg/ml; mock-infected cDC + PGE2 = 41 pg/ml and mock-infected cDC + DMSO = 23 pg/ml. The panels are representative of two independent experiments. Statistical comparison of two groups was performed using Student's t-test. Significant P-values are indicated.
PGE2 alters the expression of mRNAs for costimulatory molecules and those associated with microbial detection
We also examined various mRNAs that encode the expression of costimulatory molecules or those involved in antigen uptake and DC activation (Fig. 6). Our analysis revealed that infected cDCs up-regulate expression of the costimulatory molecules CD86 and CD40 and a C-type lectin receptor involved in the uptake and activation of DC, DEC-205 (gene ly75) (Fig. 6a and Supplement Fig. S03). Our data suggest that MoPn exposure triggers DCs to extend dendrites into the vaginal lumen because the DEC-205+ DCs have been shown to extend dendrites into the vaginal lumen in response to C. albicans.51 Also, PGE2 was able to boost the expression of FcγRIIb (CD32) and additional costimulatory molecules CD80 and CD52 compared to non-infected cells treated with PGE2 (Fig. 6b). The effects of PGE2 on infected cDCs were examined and found to result in an increase of additional molecules involved in antigen uptake and DC activation: FcRγIII (CD16), DC immunoreceptor (DCIR, gene Clec4a2), macrophage-restricted C-type lectin (MCL, Clec4d gene) and OX40L (Tnfsf4 gene) (Fig. 6c). Our preliminary results found that PGE2 treatment did not elevate the general ability of DCs to perform endocytosis as measured by the uptake of FITC-dextran at 30 min or 1 hr (Supplement Fig. S04) and suggest that PGE2 alters chlamydia-specific uptake mechanisms. These results indicate that PGE2 could further skew the antichlamydial T-cell response and the outcome of genital infection.
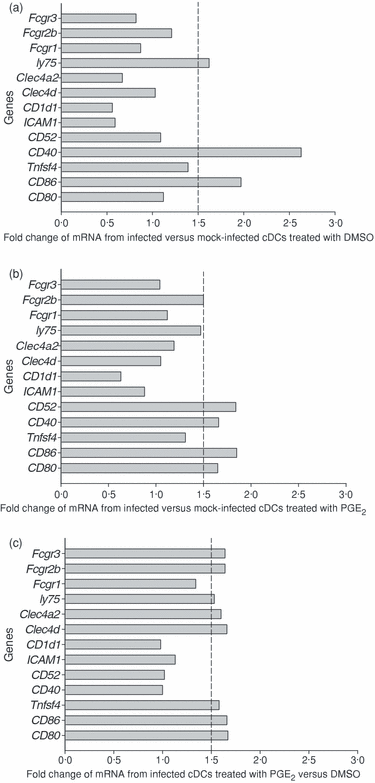
Quantification of mRNA expression of genes involved in antigen uptake and antigen presentation by cDCs infected with MoPn. RNA was isolated from cDCs infected with MoPn in the presence of PGE2 or DMSO control for 24 hr. Between 5 and 10 µg of RNA was used for SuperArray analysis. The data were normalized using the β-actin internal control. The data are expressed as the ratio of spot intensity as indicated in the figure legend. A vertical dotted line at the ratio of 1·5 was drawn as the criterion for significant difference. (a) Ratio of various mRNA species from infected versus mock cDCs treated with DMSO. (b) Ratio of various mRNA species from infected versus mock cDCs treated with PGE2. (c) Ratio of various mRNA species from infected cDCs treated with PGE2 versus DMSO. The gene named ly75 is commonly known as DEC-205, Clec4A2 as DCIR (dendritic cell immunoreceptor), Clec4d as MCL (macrophage restricted C-type lectin). Other gene names can be found at http://www.ncbi.nlm.nih.gov/entrez/query.fcgi
Discussion
Prostaglandin E2 acts on many cell types in addition to immune cells and has varied effects on immune responses, which complicates predictions of its effects on the overall immune response in vivo. Past reports have shown that PGE2 modulates the adaptive immune response by acting directly on T cells and DCs.52 It influences T cells by reducing proliferation and IFN-γ production. This reduces the number of Th1 cells but may also play a role in limiting T effector responses and limiting inflammation within tissues. Prostaglandin E2 also modulates a diversity of DC functions and influences the development of T-cell responses. Here we have shown that increased PGE2 within the GT modulates the migration of cDCs in vivo. Furthermore, exposure of chlamydia-matured cDCs to PGE2 enhances IL-10 secretion and costimulatory molecule expression. Finally, our studies show that PGE2 also alters the ability of DCs to take up and detect microbes by increasing expression of CD16, CD32, DEC-205, DCIR and MCL. Taken together, PGE2 modulates many functional properties of DCs that could impact on the T-cell interactions involved in the generation of the adaptive immune response and modulate the outcome of infection.
In this report, we show that PGE2 alters the in vivo migration of a specific subset of DCs (Fig. 2). Although PGE2 increases vascular permeability,52 our results showed that PGE2 increased the entry of cDCs, but not pDCs, into GT tissues several fold, eliminating a general increase in vascular permeability as a mechanism for the increased tissue migration. Prostaglandin E2 exerts its effect on DC migration via the EP4 receptor53 and it is likely that pDCs either do not express EP4 or cannot signal increased migration through this receptor. It is known that PGE2 destabilizes the podosome in DCs to induce migration but the mechanism is not known. Further studies are needed to specifically identify the lack of migratory influence of PGE2 on pDCs.
The cDCs are important for forming protective immunity following chlamydial genital infection11 and understanding the mechanism by which they enter the GT is vital. In inflammatory circumstances, endothelial adhesion molecules and chemokines are up-regulated together with increased expression of certain DC chemokine receptors, facilitating efficient recruitment of circulating DCs to the affected site.26,28,54 In our model, MoPn exposure did not alter the expression of many of the chemokine receptors involved in DC tissue entry (CCR1, CCR2, CCR3, CCR5, CCR6, CXCR4) (Fig. 4). Others have also shown that exposure of DCs to chlamydiae does not induce the expression of chemokine receptors associated with migration into peripheral tissues such as CCR1, CCR1β, CCR2, CCR3, CCR4, CCR5 and CCR6.15 Although many of these ligands were identified in infected peripheral tissues,12,39,55–57 they appear to control the migration of other cell types. However, exposure to PGE2 slightly boosted CXCR4 mRNA levels (Fig. 4) consistent with an increased migration of cDCs and DCs to infected GT tissue. Our previous work has shown that the ligand for CXCR4, CXCL12, is found within the GT.39 Thus, PGE2 may enhance the formation of an adaptive immune response in vivo by increasing cDC exposure to chlamydiae through increased migration by certain chemokine receptors such as CXCR4.
Consistent with the increased cDC number within infected GT, more cDCs were also present in the ILN of mice treated with PGE2 compared with the placebo group (Fig. 3c). We found that exposure to PGE2 slightly increased the number of cDCs expressing high surface levels of CCR7 and also boosted the number of cDCs expressing intermediate levels of CCR7 (Fig. 3a,b). Although high or intermediate levels of CCR7 expression are needed for DC migration, other cellular events also contribute cells to migration. For instance, exogenous PGE2 induces podosome disassembly within minutes in DCs via the PGE2 receptor, EP4. Podosome disassembly reduces cell adhesion thus enhancing cell migration. The exact mechanism of podosome disassembly is not yet known but it may be mediated by a change in intracellular levels of camp.58 The difference between the effects of PGE2 on cDC versus pDC migration may be the result of differential expression of EP4. Further studies are needed to elucidate this mechanism. The PGE2 also caused expression of the additional costimulatory molecules, CD80 and Ox40L, to compliment CD40 and CD86 expression induced upon MoPn exposure (Fig. 6c). This suggests that PGE2 plays a role in facilitating cDC maturation by enhancing the capacity of antigen presentation with the potential to enhance the adaptive immune response.
Our studies show that PGE2 boosts the synthesis of IL-10 from cDCs maturing by exposure to chlamydiae in vitro (4, 5) while not altering IL-12p40 mRNA (Fig. 5b). This finding supports our preliminary work showing that mice treated with PGE2 pellets in vivo had significantly increased levels of IL-10 secretion in response to whole elementary bodies (EBs) (placebo 7·4 pg/ml versus 51·4 pg/ml, P < 0·001) in the ILN 7 days after MoPn infection (data not shown). In addition, a recent study of humans with chlamydial vaginal infections showed that IL-10 levels, but not IL-12 levels, were elevated in cervical lymph nodes in response to the major outer membrane protein (MOMP).59 Although a previous report by Son et al.60 showed that human cDCs matured with lipopolysaccharide and exposed to an equal amount of PGE2 (1·0 μm) for 24 hr increased secretion of IL-12, we did not observe a reduction IL-12p40 mRNA or IL-12p70 protein in the presence of increased PGE2 levels. However, chlamydial lipopolysaccharide has been reported to be 100 times less stimulatory for cytokines such as TNF-α61 and this may explain these differences. IL-10 is a pleiotrophic immunomodulatory cytokine that functions at different levels of the immune response. IL-10 has been shown to inhibit the full maturation of DCs by down-regulating MHC class II expression, to reduce the capacity to produce a Th1 polarizing cytokine, IL-12, and to compromise bacterial eradication.62–64 Determining the influence of PGE2 secreted by local epithelial cells during infection is essential to fully understanding the developing immune response.
In addition, IL-10 supports the differentiation of tolerogenic and anergic T cells by directly inhibiting the phosphorylation of CD28 and subsequent downstream signalling and efficiently inhibits the proliferation and cytokine production of those cells.65 Interleukin-10 is also recognized as stimulating the production of T cells with regulatory function that can suppress effector T-cell proliferation.63In vitro studies show that IL-10 can drive the development of T regulatory (Treg) cells (CD4+ CD25+) because human naive CD4 cells that have been cultured with IL-10, with or without IFN-α, become Treg cells that have the capacity to suppress the activation and proliferation of other bystander T cells.66 Recently, Baratelli, F et al.67 showed that PGE2 can induce the expression of FoxP3 and impart Treg function to CD4 cells. FoxP3 is a transcription factor that is found within murine and human CD4 cells that has the ability to suppress T-cell proliferation and cytokine production.68In vivo, administration of an inhibitor of PGE2 production was also shown to diminish lung tumour growth in mice and the number of FoxP3-expressing T cells.69 We noted that PGE2-treated mice had increased numbers of FoxP3+ cells in the GT after MoPn infection compared to placebo controls and only a slightly prolonged course of infection (data not shown). Local PGE2 may stimulate Treg cells and interfere with T-effector function. Alternatively, PGE2 may stimulate the Treg cells necessary to reduce GT inflammation. Ongoing experiments are aimed at examining the effect of PGE2 on chlamydial genital infection in vivo.
Cell-mediated immunity is essential for the clearance of primary infection, but recent evidence shows that humoral immunity is required for bacterial resolution because the transfer of serum from immune mice facilitates the eradication of chlamydiae. Specific antimicrobial antibodies mediate antimicrobial effector function by directly binding and neutralizing infectious agents, and indirectly activating cells via Fc receptors (FcRs). Dendritic cells can be activated or inhibited through the type and balance of FcRs engaged. Two general classes of FcγR exist on DCs: activating receptors, FcγRI (CD64), and FcγRIII (CD16), containing a tyrosine-based activation motif (ITAM) and an inhibitory receptor, FcγRIIb (CD32), which contains a tyrosine-based inhibitory motif (ITIM).70 It is interesting to find the up-regulation of FcRγIII and FcγRIIb mRNA expression by PGE2 on cDCs that matured in the presence of MoPn. FcR-mediated uptake of immune complexes promotes efficient MHC class I, as well as class II-restricted antigen presentation, by various DC subsets and dramatically lowers the dose of antigen required for T-cell activation.71,72 Recently, Boonyarattanakalin et al. has found that FcγRIII greatly increases antigen uptake over FcγRI or FcγRII.73 Finally, because FcR uptake increases antigen-specific immune responses, it is currently considered as a viable approach for vaccine design.74,75 In chlamydial genital infection in vivo, FcRγIII/FcγRIIb-deficient mice suffered a greater secondary infection marked by a higher number of chlamydiae in the GT and consequently greater ascending disease.76 As such, the PGE2-mediated FcRγIII/FcγRIIb increase might result in a shortened course of secondary infection. This hypothesis is in need of further investigation.
In conclusion, our report shows that PGE2 alters aspects of the adaptive immune response in vivo and in vitro. It enhances DC functions that facilitate the induction of an adaptive immune response by increasing the interaction of DCs with T cells in draining lymph nodes, increasing costimulatory molecule expression and increasing the expression of molecules associated with the uptake and detection of microbes. However, PGE2 also induces the expression of IL-10, which is known to worsen the outcome of a chlamydial infection.64,77 In addition, PGE2 encourages Treg function67 which may interfere with the delivery of antichlamydial Th1 responses and encourage or minimize GT tissue inflammation. Thus, it is difficult to predict what the overall outcome of local PGE2 production is on chlamydial genital infection in vivo. The PGE2-induced IL-10 secretion may promote but also inhibit the host response. Potentially, the enigma of the presence of increased DC migration, antigen uptake and the increase in IL-10 could be a means of promoting protective immunity while not generating destructive inflammatory responses. Understanding how natural infection influences the immune response elicited by a vaccine is crucial because a vaccine is unlikely to elicit sterilizing immunity and natural infection may modulate the adaptive immune response and potentially lessen the effectiveness of vaccination.
Acknowledgements
We thank Su-yin Kok for excellent technical assistance, Steven Dubinett for consultations and Pete Sieling for his critical review. This study was supported by National Institutes of Health grant R01-AI26328.




