Synthesis of β2-microglobulin-free, disulphide-linked HLA-G5 homodimers in human placental villous cytotrophoblast cells
Summary
Human leucocyte antigen-G (HLA-G) is a natural immunosuppressant produced in human placentas that binds differently to the inhibitory leucocyte immunoglobulin-like receptors LILRB1 (ILT2) and LILRB2 (ILT4) according to its biochemical structure. To predict the binding functions of the HLA-G5 soluble isoform synthesized in placental villous cytotrophoblast (vCTB) cells, we investigated structural features of this protein. Biochemical and immunological studies showed that vCTB cell HLA-G5 heavy (H)-chain proteins are disulphide-bonded homodimers unassociated with β2-microglobulin (β2m) light-chain proteins. Although comparatively low levels of β2m messenger RNA (mRNA) were identified by real-time reverse transcription–polymerase chain reaction, immunoprecipitation studies failed to detect β2m protein even when specific mRNA was doubled by transduction of a lentivirus-β2m complementary DNA into vCTB cells. No abnormalities were identified in the translational start codon of vCTB cell β2m mRNA and differentiation into syncytium did not promote β2m synthesis. The failure of vCTB cells to exhibit β2m in vitro was paralleled by a lack of detectable β2m in vCTB cells in vivo. Lack of the β2m protein could be the result of low levels of β2m transcripts or of as yet unidentified translational defects. Experiments with recombinant ectodomains of LILRB indicate that β2m-free HLA-G binds strongly to LILRB2, a receptor that is expressed by macrophages. This potentially immunosuppressive cell type is abundant in the pregnant uterus. Thus, our findings are consistent with the postulate that the natural β2m-free homodimeric form of HLA-G5 synthesized in primary vCTB cells could comprise a particularly effective tolerogenic molecule at the maternal–fetal interface.
Abbreviations:
-
- β2m
-
- β2-microglobulin
-
- H-chain
-
- heavy-chain
-
- HLA-G
-
- human leucocyte antigen-G
-
- LILRB
-
- inhibitory leucocyte immunoglobulin-like receptors
-
- mAb
-
- monoclonal antibody
-
- MC
-
- mesenchymal cell
-
- Treg
-
- regulatory T cells
-
- sTB
-
- syncytial trophoblast
-
- vCTB
-
- villous cytotrophoblast.
Introduction
Reprogramming of immune cell functions into suppressive modes is believed to be a central feature of maternal tolerance to the semiallogeneic fetus.1,2 Support for this idea is found in studies showing that high levels of human leucocyte antigen-G (HLA-G) are associated with successful implantation of in vitro cultured embryos and the finding that low levels produced from specific HLA-G alleles are associated with fertility disorders.3–7 Recent studies indicate that suppression may be promoted by binding of soluble or membrane-bound HLA-G produced in placentas to inhibitory receptors on leucocytes known as LILRB1 (the inhibitory leucocyte immunoglobulin-like receptor 1; also called ILT2, CD85j) and LILRB2 (also called ILT4, CD85d).1,2,8 Immune cells driven into suppressive modes by HLA-G include CD8+ lymphocytes, natural killer (NK) cells, activated macrophages and CD4+ CD25+ cells.9–12
The HLA-G gene differs in significant ways from other HLA class I genes. In particular, it is characterized by alternative splicing of its single transcript to yield seven different messenger RNAs (mRNAs), four of which encode membrane proteins (HLA-G1, -G2, -G3, -G4) and three of which encode soluble proteins (HLA-G5, -G6 and -G7).1,2 HLA-G was first identified in placental trophoblast cells, the unique lineage of cells derived from the trophectoderm layer of the blastocyst that interfaces directly with maternal uterine and blood cells. Subsequently, specific HLA-G isoforms derived from the array of messages were discovered to be differentially distributed according to cell type and anatomic location. In particular, it has been learned that although membrane isoforms are present on invading cytotrophoblast cells, they are absent on both villous cytotrophoblast (vCTB) cells and syncytiotrophoblast (sTB) comprising the placental villi.1,2
Regarding soluble isoforms, vCTB cells underlying the sTB synthesize one of these proteins, HLA-G5, but not a second, HLA-G6.10 Several specific biochemical features of recombinant HLA-G5 produced in HEK293 cells are known10 but those of the HLA-G5 produced in primary vCTB cells in normal placentas have not been reported. The question of whether the cells produce monomers or disulphide-bonded dimers may be critical; Shiroishi et al.13 have demonstrated that the avidity of binding of HLA-G1 dimers to LILRB1 and LILRB2 is significantly greater than the binding of HLA-G1 monomers. In the same study, binding of HLA-G1 dimers to LILRB1 enhanced signals leading to immunosuppression. HLA-G1 dimerization is achieved via disulphide bonding in the α1 region of the molecule.14 The same structure may occur in HLA-G5, which is identical to HLA-G1 in the α1, α2 and α3 domains1,2 and is produced by cells in placental villi.15
Not only is the question of heavy-chain : heavy-chain (H:H) dimer formation via disulphide bonding uninvestigated in HLA-G5-producing primary placental vCTB cells, there is also no information on whether these cells produce the HLA class I light-chain, β2-microglobulin (β2m), which is required for forming heavy-chain : chain (H : L) heterodimers. Production of β2m-free H-chains would not be unique to placentas; free H-chains of other class I HLAs that include HLA-B2716 are found in activated and neoplastic cells. The question of association of β2m is important because very recently, Shiroishi et al.17 demonstrated that association of β2m with HLA-G1 is not required for recognition by LILRB2.
The experiments reported here aimed to establish the biochemical characteristics of the HLA-G5 produced in normal human villous placentas. The results show clearly that normal vCTB cells synthesize β2m-free, disulphide-bonded HLA-G5 H : H-chain homodimers. Taken together with reports of preferential binding of β2m-free H : H dimers to LILRB2, which are abundant on decidual macrophages,18 these findings strongly support the idea that HLA-G5 synthesized in the villous placenta may be particularly effective in driving mononuclear phagocytes11 and other LILRB2-expressing cells into secretory profiles that benefit semiallogeneic human pregnancy.
Materials and methods
Antibodies and control reagents
Table 1 lists the antibodies used in this study. It includes concentrations utilized for immunohistology and immunoblotting, species of origin, target antigen, immunoglobulin subclass when appropriate and source or reference. Purified β2m protein from human urine (Sigma-Aldrich, St Louis, MO) was used as a positive control in some experiments.
| Antibodies | Species | Reference or source | Target | Concentration (μg/ml) | ||
|---|---|---|---|---|---|---|
| IHC | ELISA | IB | ||||
| 3H36 | mouse IgG1 | US Biologicals, Boston, MA | β2m | 6 | – | 1 |
| 2M2 | mouse IgG1 | BioLegend, San Diego, CA | β2m | – | – | 1 |
| W6/32 | mouse IgG2a | ref. 39 | HLA class I | – | – | – |
| OV-TL 12/30 | mouse IgG1 | DAKO, Carpentaria, CA | cytokeratin-7 | 0·5 | – | – |
| 16G1 | mouse IgG1 | ref. 40 | HLA-G5 and -G6 | – | – | 1 |
| Anti-actin | rabbit polyclonal | Sigma-Aldrich, St. Louis MO | Actin | – | – | 0·5 |
| Anti-V5-HRP | mouse IgG2a | Invitrogen, Carlsbad, CA | V5 viral peptide | – | – | 1 |
| Anti-mouse-IgG-HRP | goat polyclonal | Jackson ImmunoResearch, West Grove, PA | mouse H + L IgG | – | – | 0·04 |
| Anti-rabbit-IgG-HRP | goat polyclonal | Jackson ImmunoResearch, West Grove, PA | Rabbit H + L IgG | – | – | 0·04 |
| Anti-mouse-IgG-biotin | horse polyclonal | Vector, Burlingame, CA | mouse H + L IgG | 10 | – | – |
| Control IgG1 | mouse IgG1 | BD Biosciences, San Diego, CA | unknown | 20 | – | 1 |
| Control IgG2a | mouse IgG2a | BD Biosciences, San Diego, CA | unknown | 20 | – | – |
- IHC, immunohistochemistry; ELISA, enzyme-linked immunosorbent assay; IB, immunoblot.
Acquisition of tissues, purification of cells and reagents
Samples of placentas were obtained from normal deliveries under a protocol approved by the Human Subjects Committee of this institution. First- and second-trimester samples were obtained from elective pregnancy terminations, and third-trimester (term) placentas were obtained from Caesarean sections performed to relieve or avoid fetal distress. For immunohistochemistry, which was performed as previously described,10 tissues were embedded in tissue-freezing medium (Triangle Biomedical Sciences, Durham NC), and stored at − 80° until sectioned by cryostat (5-μm sections onto glass slides). Villous CTB cells were purified using a previously described protocol19 that included removal of HLA class I-positive cells by magnetic bead technology (Miltenyi Biotec Inc., Auburn, CA) using the monoclonal antibody (mAb) W6/32. Purity was consistently > 95% as assessed by cytokeratin-7 positivity. In some experiments, W6/32+ placental mesenchymal cells (MCs) that had bound to the beads were released according to the manufacturer's instructions.
Immunoblots and reducing experiments
For immunoblots of cell proteins, the cells were solubilized in radioimmuno-precipitation assay (RIPA) buffer (phosphate-buffered saline containing 1% nonidet-P40 and a complete protease inhibitor cocktail; Sigma-Aldrich). Proteins were separated by polyacrylamide gel electrophoresis (PAGE) then transferred to nitrocellulose membranes. The blots were developed with anti-mouse immunoglobulin G–horseradish peroxidase (IgG–HRP; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) and SuperSignal® West Dura (Pierce Biotechnology Inc., Rockford, IL), and visualized using Hyperfilm™ enhanced chemiluminescence (Amersham Biosciences, Little Chalfont, UK). To establish the effects of reducing and non-reducing conditions, the protein samples were diluted in Laemmli buffer in the absence or presence of 100 mm dithiothreitol and boiled for 5 min before loading onto sodium dodecyl sulphate (SDS)–PAGE as described previosuly for HEK293 cells.10
Real-time polymerase chain reaction
Total RNA was isolated from HeLa cells (American Type Culture Collection, Cat. No. CCL-2, Rockville, MD), purified MCs were isolated from term placentas and matching harvests of vCTB cells were made from the same placentas. HeLa cells were cultured in Eagle's minimal essential medium with 2 mm l-glutamine and Earle's basic salt solution adjusted to contain 1·5 g/l sodium bicarbonate, 0·1 mm non-essential amino acids, 1·0 mm sodium pyruvate, and 10% fetal bovine serum (all from Cellgro®, Mediatech, Inc., Herndon, VA) at 37° in 5% CO2. For these experiments, vCTB cells were affinity column-purified twice. Complementary DNA was synthesized from 1·0 μg RNA as previously described20 and was subjected to real-time polymerase chain reaction (PCR; Applied Biosystems, Foster City, CA) to determine the levels of β2m mRNA in the samples. The relative quantification method was used to estimate the levels of β2m in each sample, with glyceraldehyde-6-phosphate dehydrogenase (GAPDH) mRNA serving as the endogenous normalization control and HeLa cell mRNA serving as the calibrator. Before using this method, a validation study was conducted using serial dilutions of HeLa complementary DNA (cDNA). The results showed that the amplification efficiencies of GAPDH and β2m mRNA messages were equal and that a dilution of 1 : 25 of cDNA was optimal. Real-time PCR was performed in triplicate in 96-well MicroAmp™ optical reaction plates (Applied Biosystems, Foster City, CA). Each 25-μl reaction contained 1 × 6-carboxylfluorescein (FAM)-labelled β2m Taqman® Gene Expression Assay or GAPDH endogenous control, Taqman® Universal PCR Master Mix (Applied Biosystems) and cDNA diluted 1 : 25 in water. The PCR was performed using a 7500 Real-Time PCR System (Applied Biosystems) with an initial AmpErase uracyl-N-glycosylase (UNG) activation step conducted at 50° for 2 min followed by a 10-min incubation at 95° to activate the Amplitaq Gold® polymerase enzyme. This was followed by 40 cycles of denaturation at 95° for 15 seconds and annealing/extension at 60° for 1 min. The data were collected and analysed using the ABI Sequence Detection Software, version 1·3.1 (Applied Biosystems).
Immunoprecipitation
Purified vCTB or peripheral blood mononuclear cells (PBMCs) (20 × 106 cells per immunoprecipitation reaction) were lysed in RIPA buffer as previously described.20 Approximately 5 mg total protein from a single harvest of vCTB or 0·5 mg total protein from PBMCs from a single donor, each contained in 0·5 ml RIPA buffer, were incubated with a mixture of 1·0 μg control immunoglobulin (IgG1 and/or IgG2a) or a mixture of two primary antibodies, 16G1, anti-HLA-G intron 4, a gift of D. Geraghty, Fred Hutchinson Cancer Research Center, Seattle, WA, and W6/32, anti-HLA class I, for 1 hr at 4°. Thereafter, 50 μl of beads (EZview™ Red Protein G Affinity Gel, Sigma-Aldrich) was added and the mixture was incubated for an additional 1 hr at 4°. The mixture was centrifuged for 30 seconds at 8200 g, unbound protein solution was discarded and beads were washed three times with 750 μl RIPA lysis buffer. The bound antigen–antibody complexes were eluted by adding 25 μl non-reducing Laemmli buffer and boiling the samples for 5 min. The entire sample was subjected to SDS–PAGE and transferred to nitrocellulose membranes (Schleicher & Schuell, Keene, NH). Immunoblots were performed to identify β2m as described above but employing a second mouse anti-human β2m mAb (2M2, BioLegend, San Diego, CA).
Lentivirus transduction and RT-PCR
For primary cell transductions, reagents were purchased from Invitrogen (Carlsbad, CA). To obtain a β2m lentiviral construct, the full-length human β2m cDNA was cloned into pENTRY™/D-TOPO®, then inserted into pLenti6/V5-DEST by recombination using the Gateway® LR Clonase II enzyme mix. The viral stocks were generated using HEK293FT cells and the ViraPower™ Lentiviral Expression System. The vCTB cells were seeded into six-well plates (2 × 106 cells/well) in culture medium. After 24 hr the cells were rinsed and infected with LacZ-V5 or β2m-lentivirus (10−1 to 10−5 dilutions) containing 6 μg/ml Polybrene® (Sigma-Aldrich). After 24 hr the medium was exchanged with fresh medium and the cells were cultured for 3 days to allow protein expression. To distinguish endogenous from lentivirus-derived β2m mRNA the following primer sets were designed (Lasergene, DNAStar Inc., Madison, WI). For endogenous β2m, the forward primer was 5′-CGC TGG CGG GCA TTC CTG-3′ and the reverse primer was 5′-TGC GGC ATC TTC AAA CCT CCA T-3′ (57·8° annealing temperature, 431-base-pair product). For the lentivirus-derived β2m, the forward primer, which followed the transcriptional start site of the vector, was 5′-CCA CGC TGT TTT GAC CTC CAT AGA-3′ and the reverse primer was within the β2m coding region, 5′-GTT CAC ACG GCA GGC ATA CTC ATC-3′ (57·4° annealing temperature, 434-base-pair product). The PCR primers and conditions for HLA-G5 and HLA-G6 mRNA published previously20 were used in these and all other experiments where HLA-G mRNAs were identified. PCR products were analysed by electrophoresis in agarose gels and were stained using ethidium bromide. All PCR products were sequenced for authenticity. The amplicons were subjected to densitometric evaluation.
Sequencing to test for mutations in the β2m gene
Endogenous and lentivirus β2m mRNA derived from vCTB were investigated for mutations. Corresponding cDNAs were extracted from agarose gels, purified (Qiagen, Valencia, CA) and TA-cloned into pGEM-Teasy vector (Promega, Madison WI). Plasmid DNA was extracted from positive E. coli DH5α transformants and both strands were sequenced using the ABII PRISM XL sequencing system (Biotechnology Support Facility, University of Kansas Medical Center). For analyses, the nucleotide sequences obtained from vCTB cells were aligned to human β2m mRNA (accession number NM_004048) using Lasergene (DNAStar).
Epidermal growth factor (EGF) experiments
To test for the effects of EGF, 6 × 106 vCTB cells harvested as described above were seeded into 60-mm dishes in 3 ml Iscove's Dulbecco's modified Eagle's medium containing antibiotics, 2 mm glutamine and 10% fetal bovine serum. Cultures were incubated for 4 hr to allow for adherence, washed to remove non-adherent cells and cultured for the indicated times in the presence or absence of 100 ng/ml EGF (PeproTech, Rocky Hill, NJ) as described earlier.21 The methods for performing semiquantitative reverse transcription (RT) PCR and immunoblots of cell lysates are described above.
Results
vCTB cells produce HLA-G5 disulphide-bonded H-chain dimers
In the first set of experiments we investigated the ability of primary vCTB cells from term placentas to synthesize monomeric and dimeric forms of HLA-G5 and tested disulphide bonding. Proteins were obtained by lysis of vCTB cells that had been maintained in culture medium for 6 days. Figure 1(a) shows that the vCTB cells produced dimers migrating to a molecular weight (MW) of ∼ 74 000 under non-reducing conditions. Disulphide bonding was demonstrated by conducting the experiment under reducing conditions. Under these conditions, the dimers readily dissociated to yield monomers at ∼ 37 000 MW (Fig. 1b).

Villous CTB cell HLA-G5 consists of disulphide-bonded H-chains. Villous CTB cells were cultured for 6 days to promote synthesis of HLA-G5. (a) Non-reducing conditions. HLA-G5 in vCTB cell lysates consists of ∼74 000 MW dimers. (b) Reducing conditions. vCTB cell HLA-G5 consists of ∼37 000 MW dimers. Fifty micrograms of cell lysate protein was loaded into each lane. Signals were detected by immunoblotting with the mAb to HLA-G intron 4 sequences present only in HLA-G5 and HLA-G6, 16G1, as described in the Materials and methods.
These results indicated that vCTB cells produce primarily HLA-G5 dimers under non-reducing conditions and that the H-chains forming the dimers are disulphide-bonded.
vCTB cells transcribe but do not translate β2m mRNA
Next, we investigated the ability of vCTB cells to produce the light-chains (β2m) required for generating H : L heterodimers. Highly purified vCTB cells from term placentas were tested for β2m mRNA using a semiquantitative RT-PCR and for β2m protein using immunoblotting.
As shown in Fig. 2(a), vCTB cell lysates subjected to semiquantitative RT-PCR contained β2m-specific mRNA. No RT-PCR product was seen when water was substituted for cDNA (Neg). In Fig. 2(b), Real-time PCR was used to determine levels of β2m mRNA in vCTB cells, comparing those levels with levels in matching harvests of placental MCs. Average levels of β2m mRNA in vCTB cells were 13-fold lower than in MCs. As illustrated in Fig. 2(c), proteins from three harvests of placental stromal cells residual from the W6/32 affinity columns that had been used to purify vCTB cell harvests demonstrated readily detectable β2m in immunoblots using a mouse mAb to β2m, 3H36. By contrast, lysates from the three matching harvests of purified vCTB cells failed to demonstrate detectable β2m protein. The positive control consisting of commercially available β2m yielded the ∼ 12 000 MW protein that is expected of authentic β2m protein (Fig. 2c).
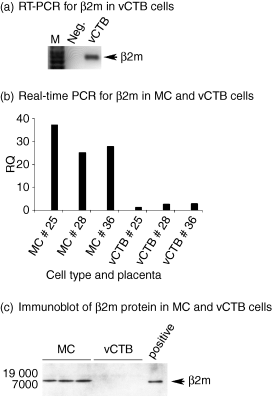
In vitro cultured vCTB cells transcribe the β2m gene but do not demonstrate detectable β2m protein. (a) RT-PCR demonstrates β2m mRNA in lysates of vCTB cells. Semi-quantitative RT-PCR was used to identify β2m mRNA in a lysate of term placental vCTB cells. β2m mRNA was identified based on the generation of a PCR product of expected size (431 bp, arrow). (b) Levels of β2m mRNA in MC and vCTB cells were compared against a standard using real-time PCR. Levels in MC were ∼13-fold greater than levels in matching harvests of term placental vCTB cells. (c) Immunoblotting detected β2m in HLA class I positive placental villous MC cell lysates (lanes 1–3), but failed to detect β2m in three matching lysates of vCTB cells (lanes 4–6). Commercially available purified β2m yielded a positive signal migrating to the same position (∼12 000) on the gel as β2m in placental MC (lane 7). Fifty micrograms of cell lysate protein was loaded into each lane and detected with anti-β2m (3H36) as described in the Materials and methods.
To verify the lack of HLA-G5-associated β2m protein in vCTB cells, immunoprecipitation studies were performed (Fig. 3). Proteins were immunoprecipitated with a combination of anti-HLA class I mAb, W6/32, and anti-HLA-G intron 4, 16G1, then subjected to SDS–PAGE and blotted with a second mouse anti-β2m mAb (2M2). PBMCs served as a positive control (lane 4) and non-specific mouse IgG1 and IgG2a (lanes 1, 2 and 5) served as negative controls. PBMCs contained readily detectable β2m migrating to approximately 12 000 MW, as expected, whereas negative controls did not contain detectable β2m. As with the negative controls, β2m was undetectable in vCTB cells (lane 6).
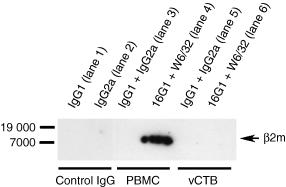
Immunoprecipitation experiments failed to identify β2m in association with HLA class I antigens in vCTB cells. Lysates of PBMC and term placental vCTB cells were immunoprecipitated with W6/32 and 16G1 or control mAb, IgG1 and IgG2a, then subjected to SDS–PAGE and immunoblotted with anti-β2m (2M2). β2m protein was only identified in PBMC.
These experiments showed that vCTB cells synthesize β2m mRNA at comparatively low but readily detectable levels, but do not demonstrate any immunohistochemically detectable β2m protein associated with their HLA-G5 H-chains.
β2m protein in vCTB cells cannot be induced by doubling vCTB cell β2m RNA
Because lack of β2m protein in vCTB cells could be the result of the low levels of β2m mRNA shown in Fig. 2(b), a lentivirus transduction system was used to increase their β2m mRNA. As illustrated in Fig. 4(a), transduction was successful, indicated by the immunoblotting of V5-β-galactosidase. Signal intensity was proportional to the input dose of infectious virus. Figure 4(b) shows that both transduced β2m mRNA (upper panel) and endogenous β2m mRNA (lower panel) were readily detected in the vCTB cells by RT-PCR. Signals were not the result of recognition of β2m genomic DNA, as shown by negative results when the mock reverse-transcribed samples (no reverse transcriptase used) were subjected to PCR. Mock transduction did not yield any signals in the Lenti6-β2m-transduced cells whereas endogenous β2m mRNA was present and unchanged by mock transduction. Comparisons of the strength of the signals of Lenti6-β2m mRNA and endogenous β2m mRNA indicated that levels of β2m mRNA were more than doubled at a virus dilution of 1 : 10 and were doubled at a dilution of 1 : 100.
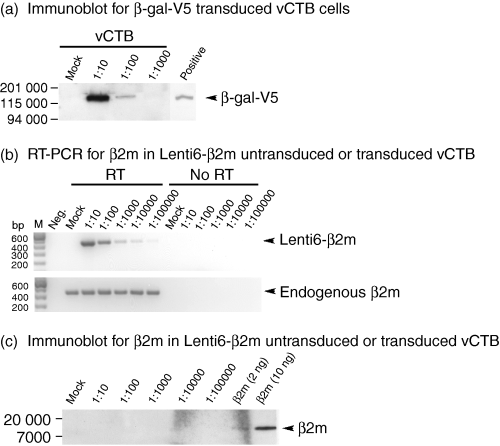
Transduction of Lenti6-β2m into vCTB cells does not induce detectable β2m. (a) Successful transduction into both placental W6/32 + MC and primary vCTB cells is shown by the presence of β-gal-V5 in immunoblots using anti-V5. The positive control consisted of a lysate of HEK293-FT cells transduced with lentivirus β-gal-V5. (b) RT-PCR identified both transduced Lenti6-β2m (upper panel, signals detectable through 1 : 100 000 dilution of virus) and endogenous β2m (lower panel, signals detected in all samples) mRNAs in vCTB cells. In the absence of reverse transcriptase (no RT), no signals were detected. (c) Immunoblots conducted on vCTB cell lysates using anti-β2m (3H36) failed to reveal β2m at any concentration of Lenti6-β2m virus. Human β2m yielded a positive signal at 2 ng. Fifty micrograms of cell lysate protein was loaded into each lane and signals were detected as described in the Materials and methods.
Despite doubling of vCTB cell β2m mRNA, no β2m protein was detectable by immunoblotting at any level of virus input (Fig. 4c). In the same experiment, purified β2m used as a positive control yielded a positive signal at 2 ng, indicating that should any β2m protein be produced, its concentration was likely to be below this level.
These experiments suggested that conditions other than low levels of β2m are required to explain the lack of β2m protein in vCTB cells.
Sequencing of β2m mRNA
Impaired β2m translation as a result of mutations has been reported in tumour cells.22–24 When endogenous and lentivirus-introduced vCTB cell β2m mRNA were sequenced and compared with the known sequence, no mutations were identified (data not shown).
Culture of vCTB cells with EGF does not promote synthesis of β2m
To ascertain whether or not stimulation of vCTB cells into syncytium21 might be required for translation of β2m mRNA, vCTB cells were cultured for 6 days in medium alone or in medium containing EGF to allow for protein synthesis, then were tested by semiquantitative RT-PCR and immunoblotting. The effects of EGF on induction of syncytialization were observed using an inverted microscope (data not shown).
Figure 5(a) shows RT-PCR results and Fig. 5(b) shows scanning densitometer values for mRNAs compared to β-actin mRNA. Culture of vCTB cells in medium containing EGF slightly diminished both β2m and HLA-G5 message levels when comparisons were drawn with β-actin mRNA (Fig. 5b). By contrast, HLA-G6 mRNA dramatically declined. No β2m signal appeared on immunoblots of vCTB cells incubated for 6 days in medium alone or medium containing EGF (upper panel, Fig. 5c) whereas β-actin was readily detected in both lysates (lower panel, Fig. 5c).
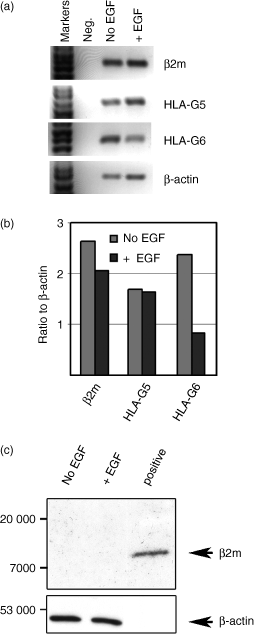
Culture of vCTB cells in medium containing EGF does not increase β2m mRNA or induce β2m protein. (a) Semi-quantitative RT-PCR shows that β2m, HLA-G5 and HLA-G6 as well as control β-actin mRNAs were readily detected in both vCTB cells cultured in medium alone (No EGF) and in vCTB cells cultured in medium containing EGF (+ EGF). (b) As assessed by scanning densitometry with the data expressed as a ratio against β-actin (which was slightly increased in EGF), all of the messages declined, with HLA-G6 demonstrating a ∼60% decrease. (c) Immunoblots using 3H36 failed to detect any β2m signal in medium-cultured or EGF-cultured cells although following stripping and re-probing, β-actin protein was detected in both. Fifty micrograms of cell lysate protein was loaded into each lane and signals were detected as described in the Materials and methods.
Thus, syncytialization achieved by EGF did not result in detectable β2m.
Absence of detectable β2m chain in human placental trophoblast cells in situ
The final group of experiments was designed to learn whether vCTB cells and/or sTB in situ contained immunohistochemically detectable β2m. Whether or not the HLA-G5 H-chain is associated with β2m-chain in vivo was uncertain as the scientific literature contains conflicting evidence on the presence of β2m in sTB.25,26
Immunohistology was used to investigate the expression of β2m in placental villous trophoblast cells in situ during early (Fig. 6a), middle (Fig. 6b) and late (Fig. 6c) stages of pregnancy. Villous CTB cells did not contain detectable protein at any stage although light staining that could have been ingested β2m or neonatal FcR-associated β2m was occasionally observed in sTB (not shown). By contrast, the placental stromal cells were positive for β2m and the intensity of staining increased as gestation proceeded to term. No immunostaining signals were detected in the isotype-specific controls (Fig. 6d).
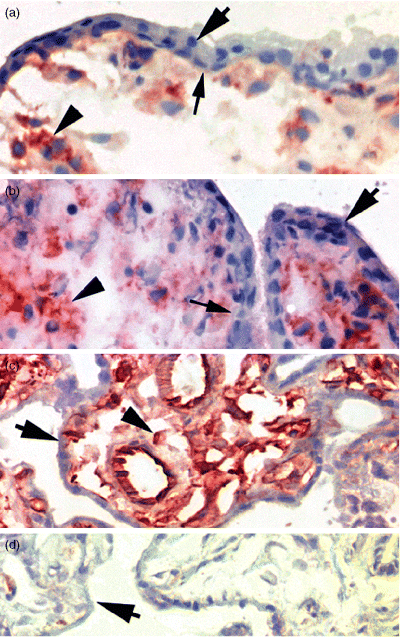
Trophoblast cells in placental villi lack detectable β2m. Immunohistochemistry using 3H36 demonstrates that sTB (large arrows) and underlying vCTB cells (small arrows) do not contain detectable β2m but villous MC (arrowheads) do exhibit the protein. (a) Gestation week 9, first trimester; (b) gestation week 13, second trimester; (c) term placenta, third trimester; (d) isotype-specific control, third trimester. Original magnification, × 400.
These data demonstrated that in situ, villous trophoblast cells uniquely fail to contain detectable levels of β2m for associating with HLA-G5 or other HLA H-chains in H : L-chain heterodimers.
Discussion
The results of the experiments described here show definitively that primary vCTB cells produce β2m-free, disulphide-linked HLA-G5 H : H homodimers. To the best of our knowledge, this study is the first to identify a type of cell that produces HLA-G5 class I H-chains but does not produce the light-chains. We present evidence indicating that one potential reason for production of H : H homodimers rather than H : L heterodimers in this trophoblast cell subpopulation is low transcription of the β2m gene, and a second may be an inability to translate β2m mRNA into protein. Our preliminary conclusions are in accord with those of Capps et al. for other types of cells lacking β2m27 and the results of experiments on HLA-B27 conducted by Allen et al.16
The HLA-G5 protein produced in vCTB cells is a disulphide-bonded dimer that is dissociated under reducing conditions when disulphide bonds are interrupted. This finding was in agreement with the results of a previous study conducted on supernatant culture media from HEK293 cells stably transfected with HLA-G5.10 Disulphide bonding of the H-chains to form dimers was not entirely unexpected as site-directed mutagenesis has indicated that the two extra cysteine residues that characterize HLA-G, cys 42 and cys 147, are associated with disulphide-linked oligomerization.8,14 However, these studies were limited to transfected cells and cell lines. Since there are no cysteines within the 21 amino acids encoded by HLA-G5 intron 4, it is highly likely that cys 42 and cys 147 form the disulphide bonds identified in this study.
The HLA-G5 produced in vCTB cells is also unusual in failing to have detectable β2m associated with H-chains. Biochemical experiments performed earlier on the products of the HLA-G5-transfected HEK293 cells10 indicated that their HLA-G5 H-chains were associated with endogenous β2m, strongly suggesting that HLA-G5 H-chains are fully capable of binding endogenous β2m. To determine whether vCTB cells similarly produced β2m for constructing H : L heterodimers, we examined vCTB cell β2m message and protein. Surprisingly, β2m mRNA was readily detected but protein was undetectable by immunoblotting and immunoprecipitation followed by immunoblotting, suggesting interference between transcription and translation. Since it seemed possible that vCTB cells might produce levels of β2m mRNA that were insufficient for generating detectable protein, levels were increased by delivering additional β2m mRNA using a lentivirus system. Doubling of β2m mRNA in vCTB cells also did not result in any detectable β2m protein, indicating that lack of β2m mRNA may not be the reason for lack of β2m protein in these cells. Whether the HLA-G5 H-chains produced in vCTB cells associate with β2m in sTB in an additional processing step remains to be seen as the question of production of β2m in the syncytium is unresolved.
Because impaired β2m translation has been associated in tumour cells with mutations that affected either the translational start codon or structural codons or with production of a defective message from an intact gene,22–24β2m messages transcribed in vCTB cells were sequenced. No mutations that could have accounted for lack of β2m protein in vCTB cells were identified in either endogenous or lentivirus-introduced β2m mRNA. Presumably, the entirely normal vCTB cells tested in this study are less likely than tumour cells to have mutations that cause failure of translation of β2m mRNA.
Failure of production of β2m in vCTB cells was also unrelated to a requirement for syncytialization. Syncytialization naturally occurs when vCTB cells are placed in culture, and is increased when the culture medium contains EGF. Light-chain, β2m, has been reported in syncytium26 although this remains controversial. However, culturing vCTB cells in EGF again failed to result in production of detectable β2m protein. Potential mechanisms underlying failure of vCTB cells to produce β2m that remain to be investigated include dysregulation of the target of rapamycin (ToR) pathway and interference by micro-RNAs.28–30 Interestingly, lack of β2m synthesis in mammalian trophoblast cells has long been known; mouse placental giant trophoblast cells express H-2 class I H-chains but not light-chains.31 Collectively, the results suggest that that the β2m-free structure has selective functional advantages for trophoblast cells, perhaps in evading immune recognition.
Early studies on HLA-G in placentas concluded that production is entirely confined to the migrating CTB cells that attach to and infiltrate the decidua.32 Even at present, this view is considered viable by some33 despite the fact that several independent laboratories15,34–36 have identified HLA-G5 in the villous placenta. The underlying reason for error may be that the antibody most commonly used for identification of HLA-G in trophoblast cells is W6/32, which preferentially binds to HLA class I H-chains when they are associated with light-chain, β2m. Our observation that the vCTB cell layer produces a form of HLA-G5 that is not associated with light-chain may be the reason for the widespread misunderstanding33 of sources of HLA-G in human placentas.
It seems reasonable to suggest that the disulphide-bonded homodimers of HLA-G5 H-chains produced in the villous placenta are secreted into maternal blood because soluble HLA-G has been identified in pregnancy sera.37 This could be of importance biologically because (1) dimers bind with higher affinity to LILRB than monomers, and (2) association of the H-chain dimers with β2m may also change LILR binding affinities.13,38 It will be of considerable interest to learn whether the homodimers of HLA-G5 produced in the vCTB cell layer reach maternal blood and how their affinity for the LILRB receptors compares with the HLA-G isoforms produced in the trophoblast cell columns and chorion membrane.
In summary, we here document for the first time the novel quaternary structure of HLA-G5 synthesized in primary human vCTB cells. Although the underlying cause for lack of β2m protein in vCTB cells is as yet unexplained, the data collected to date are consistent with the postulate that an intrinsic developmental programme prevents vCTB cells from synthesizing H : L dimers. The results raise several important questions: could recombinant HLA-G5 in its natural homodimeric structure be used to promote tolerance in women with reduced fertility? Are the enzyme-linked immunosorbent assays currently used to identify soluble HLA-G appropriately constructed for maximum sensitivity? These and other questions of translational application remain to be evaluated.
Acknowledgements
These studies are supported by grants from the National Institutes of Health to J.S.H. (PO1 HD39878 Project III; U54 HD33994 Project IV; PO1 HD40984 Project I). The authors appreciate assistance from S. Fernald of the University of Kansas School of Medicine Image Analysis Core, J. S. Platt and the Kansas IDeA Network for Biomedical Research Excellence (P20 RR16475, J. S. Hunt, P.I).




