α2-Antiplasmin and its deficiency: fibrinolysis out of balance
Abstract
Summary. Fibrinolysis serves an important role in the process of coagulation, ensuring that clots that are formed in response to injury resolve after the injured tissue is repaired. Fibrinolysis occurs because the protein plasminogen is converted to the active serine protease plasmin by its activating molecules (primarily tissue plasminogen activator). One of the inhibitors of fibrinolysis is α2-antiplasmin, which acts as the primary inhibitor of plasmin(ogen). Congenital deficiency of α2-antiplasmin causes a rare bleeding disorder because of increased fibrinolysis. Despite the rare nature of this disorder, understanding of the actions of α2-antiplasmin and the results of its deficiency has provided the opportunity for better understanding of the fibrinolytic system in both how it affects the risk of bleeding and its impact on other bodily systems. Here, we review the history of the discovery of α2-antiplasmin, our understanding of its genetics and function, and our current knowledge of its congenital deficiency. We also discuss some of the current avenues of investigation into its impact on other diseases and physiological states.
Introduction
Plasmin is generated from its progenitor protein plasminogen to initiate fibrinolysis. To prevent excess bleeding and tissue damage, it is important that the process of fibrinolysis be precisely coordinated. Inhibitors of fibrinolysis include plasminogen activator inhibitor (PAI-1), which is the main inhibitor of tissue plasminogen activator (tPA), thrombin activated fibrinolysis inhibitor (TAFI) and α2-antiplasmin (α2-AP), which acts as the primary inhibitor of plasmin(ogen). This natural inhibitor of plasmin found in humans has been known variously as α2-AP, α2-plasmin inhibitor and primary plasmin inhibitor.
The congenital deficiency was initially described in 1969 by Masateru Kohakura in a 16-year-old boy with repeated bleeding. Unable to establish haemophilia from laboratory testing, it was noted that the blood clot formed during the test for whole blood clotting time lysed in 12 h at room temperature, a shorter time than expected. This phenomenon led to the consideration of abnormalities in the process of fibrinolysis as the cause of this young man’s bleeding complaints. Subsequent study after many years of evaluation eventually identified, in 1977, the specific abnormality in this patient as being a deficiency of α2-AP. This inhibitor had been isolated from other inhibitors of fibrinolysis such as α2-macroglobulin and α2-antitrypsin, by three independent groups in 1976 [1,2].
Although congenital deficiency of α2-AP (once called Miyasato disease [3]) is a rare disorder, it is important to rule out this disease in the evaluation of patients with an unknown bleeding disorder because of the absence of abnormalities on typical screening laboratory test results. In addition, investigation of the disease state of α2-AP deficiency has improved our understanding of fibrinolysis as a whole. Here, we seek to review the current status of the knowledge related to α2-AP deficiency.
Materials and methods
An OVID search was conducted for the term antiplasmin, which yielded 1423 citations. From these citations, those related to bleeding and fibrinolysis were selected and then visually scanned for the most recent and appropriate articles. References from these articles were also evaluated to ensure that the most recent and pertinent articles were obtained.
Pathophysiology
In conjunction with thrombin activatable fibrinolysis inhibitor (TAFI) and plasminogen activator inhibitor (PAI-1), α2-AP acts as the principal regulator of fibrinolysis [4]. Fibrinolysis is regulated by α2-AP in three ways: (i) by forming a complex with plasmin; (ii) by inhibiting adsorption of plasminogen to fibrin; and (iii) by making fibrin more resistant to local plasmin through cross-linking via factor XIIIa (FXIIIa) [5]. Both thrombus-associated and plasma α2-AP act in regulating fibrinolysis. α2-AP rapidly inactivates plasmin resulting in the formation of a stable inactive complex, plasmin-α2-AP. Two forms of α2-AP circulate in human plasma: a 464-residue protein with methionine as the amino-terminus (Met-α2-AP) and an N-terminally shortened 452-residue form with asparagine as the amino-terminus (Asn-α2-AP). Human plasma-α2-AP consists of approximately 30% Met-α2-AP and 70% Asn-α2-AP [6]. In regulating fibrinolysis, the C-terminal end of α2-AP binds with strong affinity to the lysine-binding site of plasminogen, where fibrin is also non-covalently bound. In this way, α2-AP competitively inhibits the binding of fibrin to plasminogen. After binding to the lysine-binding site, α2-AP is rapidly cleaved by plasmin at the reactive site of the molecule, resulting in release of a peptide and formation of a covalent plasmin-α2-AP complex. The reaction time between plasminogen and α2-AP is very fast, in the order of 2 × 10−7 mol−1 s−1. Fibrin-bound plasmin is protected from this rapid interaction with α2-AP, with a rate of inhibition 10 times slower than that of free plasmin [6]. In addition, during the process of blood clotting, a portion of circulating plasma α2-AP is rapidly covalently bound to fibrin by FXIIIa, resulting in increased resistance of the fibrin to fibrinolysis (Fig. 1). It is in this mechanism of action that the Asn form gains increased inhibitory capacity when compared with the Met form, in that the site where cross-linking takes place is at the second residue from the amino terminus of the Asn form (Gln 14 in the Met form), and this form is cross-linked 3–13 times more quickly than the Met form [7]. Plasminogen activators tPA and urokinase are also inhibited by α2-AP. Recently, it has also been shown that lysine residues in the C-terminus of α2-AP interact with the endothelial cells, although the details of this interaction are still being elucidated [8].
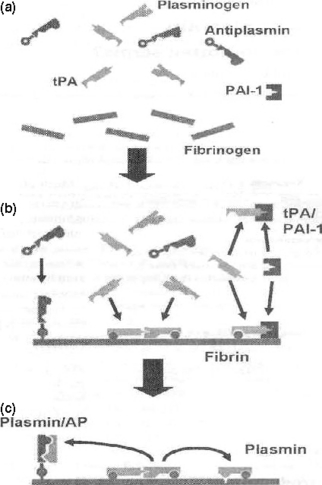
Schematic representation of the assembly of fibrinolytic proteins. (a) In the absence of fibrin clot formation, the principal fibrinolytic proteins are free in plasma. (b) Activation of coagulation results in the fibrin clot formation. Antiplasmin is cross-linked by its N-terminus to fibrin. Tissue plasminogen activator (tPA) and plasminogen assemble on fibrin leading to the generation of plasmin. tPA can be inhibited by plasminogen activator inhibitor (PAI-1) either in solution or at the fibrin surface. Kringle domains on plasmin allow binding to lysine residues on fibrin or alternatively in the C-terminus of antiplasmin. (c) While plasmin remains bound to fibrin, it is relatively protected from antiplasmin and fibrinolysis occurs. Antiplasmin is cross linked to the fibrin surface and is well placed to inactivate free plasmin in this microenvironment.
The bleeding associated with a deficiency in α2-AP is because of the premature dissolution of haemostatic plugs before tissue and vessel repair. Bleeding may be delayed after trauma or invasive procedures. Acquired deficiency of α2-AP may be seen in patients with severe liver disease with plasma levels falling as low as 8% [9]. Decreased levels have also been reported in patients with renal disease and disseminated intravascular coagulation, as well as in patients undergoing thrombolytic therapy [10]. In addition, low levels of α2-AP have been found in a patient with a congenital disorder of glycosylation and intracranial haemorrhage. In this case, the level of antiplasmin was found to be 41% and was thought to contribute to the occurrence of the intracranial haemorrhage but cannot be attributed as the primary cause. This is of interest because α2-AP is a glycosylated molecule [11].
Genetics
A member of the serpin family of enzyme inhibitors, α2-AP is synthesized in the liver as a single-chain glycoprotein with a molecular weight of 51 000 Da. It circulates in the body either bound to plasminogen or in an unbound free form. The typical plasma concentration is 0.7 mg mL−1, with a half-life of 2–6 days. Paediatric reference ranges have been established and remain fairly consistent with adult values [12].
The gene for α2-AP has been mapped to chromosome 17 (17pter-p12) and contains 10 exons and nine introns [13]. Polymorphisms of the gene have been described. The mature protein has been found to have 464 amino acids with three functional regions. The amino acid structure of the serpin family of proteins is highly conserved among species. The human α2-AP molecule shares 80% homology with the bovine protein and 74% with the murine molecule [14]. After the protein is formed, antiplasmin-cleaving enzyme shortens the N-terminal to convert the 464 amino acid form (Met form) to the 452 amino acid form (Asn form), which comprises 60–70% of circulating antiplasmin and is more physiologically active than the Met form [7,15].
Clinical manifestations
Congenital deficiency of α2-AP is a rare disorder that is inherited in an autosomal recessive manner. The real prevalence of the disease is unknown; approximately 40 cases have been reported to date [16–33]. In the few patients for whom the molecular defect has been defined, mutations have variously resulted in impaired intracellular transport, decreased activity because of an abnormal protein, and absence of the protein [6,34]. Consanguinity is common in families with homozygous deficiency.
Patients with homozygous α2-AP deficiency may exhibit severe bleeding symptoms, often presenting in childhood and appearing similar to those patients with congenital haemophilia. Umbilical bleeding may be the first presentation of the disease. The delayed bleeding associated with the disorder may be reminiscent of bleeding that occurs with congenital deficiency of FXIII. The unusual symptom of intramedullary haemorrhage into the diaphyses of long bones has been described, presenting as pain in the affected limb visualized as homogeneous hyperlucent lesions without marginal sclerosis on radiographs and homogeneous hyperintense signal in the medulla with surrounding hypointense signal on magnetic resonance imaging. These lesions have been reported to occur spontaneously and in response to trauma [16].
Heterozygous individuals, in contrast, may have milder bleeding or may be asymptomatic [31,32]. Bleeding tends to occur in response to trauma, surgery or dental procedures. Intramedullary haematomas have not been described. Symptoms may increase with age as a result of falling plasma levels, sometimes erupting a new in elderly patients with heterozygous deficiency [34].
Diagnosis
Often the results of screening tests such as the prothrombin time, activated partial thromboplastin time, thrombin time, and PFA-100® are normal; therefore, a high index of suspicion is necessary. The euglobulin lysis time may be shortened in patients with α2-AP deficiency [5]. In patients with a history of bleeding in whom all screening test results are normal, a specific assay for α2-AP deficiency should be performed. Patients may either have type I deficiency, wherein the antigen and activity level are equally decreased, or type II deficiency, with lower activity compared with antigen level [9].
Management
Intramedullary haematomas have been successfully treated with surgical evacuation and instillation of a combination of tranexamic acid and fibrin glue [16]. Treatment of bleeding episodes is typically successful with fibrinolytic inhibitors, such as ε-aminocaproic acid or tranexamic acid. These drugs can be used in response to bleeding complications or as prophylaxis before invasive procedures. The oral dose of tranexamic acid that has been recommended is 7.5–10 mg kg−1 every 6 h or i.v. 20 mg kg−1 before an invasive procedure [24]. No specific dose of aminocaproic acid has been suggested in the literature. Antifibrinolytic agents primarily act by preventing the binding of plasminogen to fibrin.
Fresh frozen plasma (FFP) may be used as an alternative to antifibrinolytic agents [17]. Depending on the preparative method of FFP, the α2-AP contained therein may have variable activity. For this reason, and in the interest of reducing the risk of viral transmission that is inherent in FFP administration, antifibrinolytics are the treatment of choice. Desmopressin acetate, which is commonly used to treat bleeding by enhancing platelet activity, should be avoided in α2-AP deficiency because it may induce secretion of plasminogen activator [17].
Future directions
Mice with α2-AP gene deficiency have been developed. These mice are particularly interesting in that although the deficiency leads to increased fibrinolytic activity, no overt bleeding has been observed [35]. The fibrinolytic pathway has also been linked to roles in many different physiological processes, such as ovulation, embryogenesis, atherosclerosis, metastasis and even obesity [36]. However, recent evidence in mice with inactivation of the α2-AP gene shows no increase in adipose tissue development when compared with that of normal mice [36].
Some studies have shown that the levels of plasmin-α2-AP complex in plasma are elevated in acute stroke, myocardial infarction, unstable angina, and atrial fibrillation suggesting a self-defense system against complications of ischemic events [37], but the physiological roles of α2-AP in these conditions are still not understood. However, in a recent study, Matsuno et al. showed in an experimental model that lack of α2-AP promotes acute cor-pulmonale via over-release of vascular endothelial growth factor (VEGF) after acute myocardial infarction [38]. Another recent experimental study showed that lack of α2-AP improved cutaneous wound healing via fibroblast VEGF-induced angiogenesis in wound lesions [39].
Because of its efficacy in preventing fibrinolysis, α2-AP has been used to prevent bleeding complications secondary to thrombolytic therapy without major effect on thrombolysis and to stabilize clots in patients after coiling of patent ductus arteriosi [6]. Efforts are currently being taken to investigate methods of altering the normal α2-AP molecule for therapeutic benefit [40]. More studies will need to be performed in these areas to elucidate the true impact of the fibrinolytic pathway and its components on these phenomena.
Experts in the field
Professor Michael Gallimore, Kent Haemophilia Centre, Kent and Canterbury Hospital, Ethelbert Road, Canterbury, Kent UK CT1 3NG Institute for Surgical Research, Rikshospitalet, Sognvansveien 20, Oslo, Norway.
Tel.: 0044 1227 783 168; fax: 0044 1227 783 157
Dr Nobuo Aoki: e-mail [email protected]
Dr Paul Coughlin: e-mail [email protected]
Links to organizations
National Hemophilia Foundation: http://www.hemophilia.org
Hemostasis and Thrombosis Research Society: http://www.htrs.org
International Society of Thrombosis and Hemostasis: http://www.med.unc.edu/isth
Disclosures
The authors stated that they had no interests which might be perceived as posing a conflict or bias.




