New assays for monitoring haemophilia treatment
The authors stated that they had no interests which might be perceived as posing a conflict or bias.
Abstract
Summary. Precise measurements of factor VIII (FVIII) or factor IX (FIX) activity are believed to be essential for clinical management in haemophilia, although discrepancies between factor levels and clinical severity have been recognized. Clot wave form analysis has demonstrated that different wave form patterns may be evident in severe haemophilia A patients with levels of FVIII activity <1 IU dL−1, and this might explain, in part, the phenotypic heterogeneity seen in these patients. In addition, the relatively new technique of computer-assisted thrombelastography (TEG), in which coagulation is initiated by tissue factor, has revealed a considerable degree of variability in different patients in the presence FVIII levels, which are sufficient to normalize TEG parameters. In contrast, a global thrombin generation test (TGT) has been proposed as a sensitive and reliable method for assessing overall clotting function in haemophilia patients. Several studies have demonstrated a significant correlation between TGT and FVIII/FIX levels, and these measurements also appear to correlate with the clinical phenotype. The TGT may be very useful, therefore, for evaluating overall haemostasis in different clinical situations, although substantial inter-assay and inter-individual variations have been reported. Both the TEG and TGT have been found to be particularly helpful for monitoring haemostatic therapy with bypassing agents or conventional FVIII or FIX concentrates in patients with inhibitors. These global tests enable the selection of appropriate therapeutic agents in individual circumstances and offer the opportunity to tailor the most effective haemostatic treatment even during severe bleeding or major surgery.
Introduction
Laboratory assays play an essential role in the clinical management of patients with haemophilia. Precise measurements of the clotting factors, factor VIII (FVIII) and factor IX (FIX), are essential for the accurate diagnosis, assessment of clinical severity, evaluation of risk of inhibitor development and haemostatic monitoring of on-demand and regular prophylactic therapy. In an emergency, simple and rapid methods are highly desirable. Furthermore, the test data should reflect the physiological and clinical status in vivo. It has been well recognized, however, that there may be considerable heterogeneity between the levels of clotting factor and clinical severity or the haemostatic effect of treatment [1]. Furthermore, monitoring of patients with inhibitors may be complicated, and there has been neither standardization nor guidelines for therapeutic monitoring in these cases. In this context, the development of reliable and sensitive global coagulation tests may be especially useful.
In recent years, several new assays have been introduced for evaluating global clotting function, reflecting both pathological hyper- and hypo-coagulation. In particular, clot waveform analysis, automated thromboelastography and thrombin generation tests (TGTs) have been described. All these techniques are based on the quantitative measurement of various parameters using real time monitoring of the overall clotting process. Numerous studies have now reported the application of these new tests to monitoring and evaluating patients with haemophilia in various circumstances. In this review, the usefulness, possible applications and limitations of these methods will be discussed from a practical, clinical viewpoint.
Clot waveform analysis
Clot waveform analysis is a recently developed technique using the MDA-II system (bioMerieux, Durham, NC, USA). This instrument is a fully automated coagulometer basically performing clinical laboratory coagulation assays using a variable wavelength photo-optical detection system. During the performance of routine clotting assay such as activated partial thromboplastin time (aPTT) and prothrombin time (PT), it is possible to obtain a continuous measurement of the changes in light transmittance that occur as the test citrated plasma sample clots. The resultant photo-optical data profiles obtained by continuous monitoring are called waveforms caused by their sigmoid patterns [2]. The waveform is mathematically processed by a software algorithm to derive several parameters such as coagulation velocity and acceleration. Clot waveform analysis reflects the whole clotting process, but it can also monitor fibrinolytic activity. Very conveniently, clot waveform analysis can be performed at the same time as regular routine assays.
Thrombelastography
Thrombelastography (TEG) detects changes in viscosity and elasticity during the clotting process. The technique itself is very old and was developed over half a century ago [3]. The method is simple and has been widely used for evaluating general clotting function. Furthermore, it can be performed on native whole blood, more analogous to clotting in vivo, where cellular blood components such as platelets contribute greatly to thrombin generation (TG) at the site of bleeding. Several disadvantages, including instability and poor reproducibility, have hampered clinical use, however, and the basic method provides relatively qualitative data, of limited clinical value. More recently, computerized automated TEG or rotating thromboelastometry (ROTEM) has been developed. In addition to the classical TEG parameters, such as clotting time (CT), maximum clot firmness (MCF) and clot formation time (CFT), changes in elasticity during whole blood coagulation are continuously analysed and the raw data are transformed into new parameters such as coagulation velocity, maximum velocity (Max Vel) and the time to Max Vel (t) [4]. In the original TEG/ROTEM assay, clotting was initiated by recalcification alone. The addition of a minimal amount of tissue factor (TF) is now recommended, however, to accommodate the current concept of sequential cell-based clotting mechanisms, initiated by TF and promoting robust TG [4]. Furthermore, a novel enhanced ROTEM system, using an extrinsic activator (EXTEM) and an intrinsic activator (INTEM) has been developed and utilized for haemostasis management in emergency medical care [5,6]. Thus, interest in TEG/ROTEM technology has been revived in recent years, not only for monitoring therapeutic agents but also for patient screening and the assessment of global haemostasis in various hyper- and hypo-coagulable pathological situations [7].
Thrombin generation test
Thrombin is a potent and the terminal enzyme in the clotting process. According to the cell based TG model, vessel wall injury leads to TF exposure and the formation of a TF-FVIIa complex on TF-bearing cells. This activates factor X (FX) and results in the conversion of prothrombin to thrombin. Initially, only trace amounts of thrombin are generated, which are not sufficient for stable fibrin formation, and the effect on haemostasis in vivo at this time is minimal. The thrombin that is produced, however, stimulates platelets and activates factor V (FV), FVIII and FXI by positive feedback mechanisms, resulting in the generation of relatively large quantities of thrombin on activated platelets [8]. This platelet dependent, TF-independent burst of thrombin promotes full fibrin plug formation and contributes to the inhibition of fibrinolysis by activating thrombin activatable fibrinolysis inhibitor (TAFI) [9]. On this basis, therefore, measurements of TG appear likely to be a highly appropriate way to determine overall haemostatic potential.
The TGT was described for clinical use in 1953 [10,11]. The original manual method utilized defibrinated plasma and the calculation of thrombin generated was not straightforward. Over the last 15 years, however, various technological advances have substantially improved the assay. In particular, specific fluorescent substrates and highly sensitive detection instruments have been developed. Measurements of enzymatic activity are not impaired by turbidity, and accurate TG can be determined in non-defibrinated platelet poor (PPP) as well as platelet rich plasma (PRP) [12]. In addition, a calibrated automated thrombography (CAT) technique has been developed [13] based on computer-assisted real time monitoring of endogenous thrombin potential (ETP). Using this CAT system, changes in thrombin generated can be visualized continuously as a thrombogram. In addition, the real time data obtained from the thrombogram are mathematically processed to derive several novel parameters, including lag time, time to peak, peak thrombin level, and ETP.
Discussion
Detection of low levels of factor activity
There is no doubt that the accurate measurement of FVIII or FIX activity is a basic requirement for the diagnosis and management of haemophilia A and haemophilia B. Conventionally, classification of the expected clinical severity is based on the determination of baseline clotting factor activity, and there is generally a good correlation between factor levels and clinical phenotype. Occasionally, however, some patients classed as severe on the basis of laboratory assays exhibit milder clinical symptoms. Similarly, some patients who may be initially identified in the moderate-mild category demonstrate a severe clinical phenotype with frequent episodes of spontaneous bleeding.
It seems to be very important, therefore, to consider the methodology used for quantifying clotting factor activity. The sensitivity and reproducibility of FVIII or FIX activity assays are heavily dependent on pre-analytical variables such as the source of the FVIII or FIX deficient plasma, the nature of the phospholipid reagent and the detection system employed for analysis. Furthermore, it may be that the methods in general use do not fully reflect overall haemostatic potential. It is especially noteworthy in this respect that current specific assays based on plasma CTs such as the aPTT can be very insensitive to the presence of low levels of activity. For example, several clinical studies have indicated that a beneficial effect of FVIII replacement therapy is sometimes apparent in the absence of significant FVIII response measured in conventional clotting assays. Furthermore, a highly significant haemostatic effect was noted in haemophilia B following gene therapy in association with elevation of the FIX level to only 0.8 IU dL−1 [14].
Modern automated analyzers have greatly improved the results of coagulation assays in recent years by utilizing two standard curves covering the lower and higher ranges of clotting factor activity. In addition, sophisticated end-point detection systems have been devised so that CTs are determined by dynamic analysis of clot formation, so called clot waveforms. This type of instrumentation has also facilitated novel measurements. For example, qualitative analysis of clot waveforms in severely affected haemophilia patients that had been previously assigned to having FVIII:C values <1 IU dL−1 by conventional clotting assays, demonstrated different patterns (Fig. 1). Generally, shorter aPTT CTs were associated with a steeper waveform slope, although there were notable exceptions. In particular, some patients showed a relatively steeper slope with a higher value of Min2 corresponding to accelerated fibrin formation [15]. Furthermore, in a total of 36 patients categorized as severe, FVIII:C levels closely correlated with the Min2. The FVIII:C level was <0.2 IU dL−1 in 23 cases and between 0.2 and 1.0 IU dL−1 in 13 patients, and interestingly, correlation between waveform and aPTT CT was poor. These data suggest that the aPTT is variably affected by other plasma factors. Other studies have also indicated that clot waveform analysis and especially Min2 values may have greater discriminatory power in assessing low clotting factor activity [2].
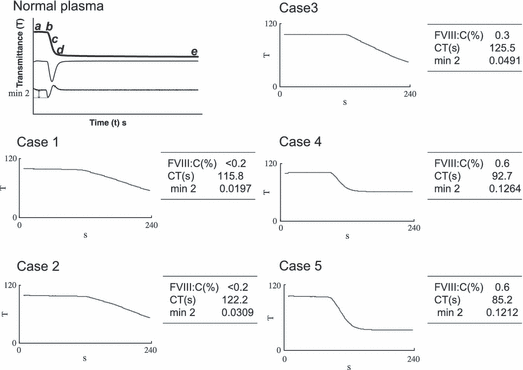
APTT clot waveforms of a normal subject and those of patients with severe haemophilia A. The panel shows the aPTT waveform obtained in one normal subject and five cases of severe haemophilia A (FVIII < 1.0 IU dL−1). In the normal waveform, the upper trace shows the changes in transmittance (T) observed over time (t) during the performance of an aPTT. The middle trace shows the first derivative (dT/dt) from the transmittance data. The lower trace shows the second derivative (d2T/dt2). Point b indicates the initiation of coagulation. The absolute value of Min2 shows the maximum coagulation acceleration. In haemophilia waveforms, coagulation time, Min2 and the level of FVIII:C are illustrated.
Assessment of overall clotting function in haemophilia A
In some of the circumstances described above, the discrepancy between levels of clotting factor activity and clinical symptoms might centre on the presence of trace amounts of circulating FVIII or FIX (<1 IU dL−1) that could support a milder clinical phenotype. In these circumstances, global tests of haemostasis might be especially helpful, in addition to FVIII/FIX activity assays. In most cases of severe haemophilia A with FVIII <0.2 IU dL−1, a dysfunctional waveform pattern, i.e., low Min2 associated with a longer CT appears to define the severe clinical phenotype (Table 1). Scoring clinical severity has not been standardized, however, and in the 27 cases listed in Table 1, a spontaneous bleeding episode at the age of <1 year, the onset of joint or muscular bleeding before the age of 3 years old, or the presence of severe bleeding such as intracranial bleeding or refractory oral bleeding were used as the criteria for the severe phenotype. In each case, the CT was prolonged and Min2 value was decreased, and 24 out of the 27 cases were defined as clinically severe. Although the number of patients with FVIII level 0.2–1.0 IU dL−1 is not sufficient for evaluation, these findings confirmed the importance of precise and highly sensitive measurements at low FVIII levels.
| No. | FVIII: C(IU dL−1) | Clot time(s) | Min2 (d2T/dt2) | Clinical severity |
|---|---|---|---|---|
| 1 | <0.2 | 88.8 | 0.0732 | Severe |
| 2 | <0.2 | 89.8 | 0.0454 | Severe |
| 3 | <0.2 | 95.7 | 0.0535 | Severe |
| 4 | <0.2 | 96.9 | 0.0850 | Severe |
| 5 | <0.2 | 98.5 | 0.0939 | Severe |
| 6 | <0.2 | 102.2 | 0.0574 | Severe |
| 7 | <0.2 | 103.3 | 0.1215 | Severe |
| 8 | <0.2 | 107.2 | 0.0408 | Severe |
| 9 | <0.2 | 108.1 | 0.0507 | Moderate |
| 10 | <0.2 | 115.7 | 0.0471 | Severe |
| 11 | <0.2 | 115.8 | 0.0197 | Severe |
| 12 | <0.2 | 116.3 | 0.0322 | Severe |
| 13 | <0.2 | 117.5 | 0.0561 | Severe |
| 14 | <0.2 | 122.2 | 0.0309 | Severe |
| 15 | <0.2 | 126.7 | 0.0322 | Severe |
| 16 | <0.2 | 129.8 | 0.0460 | Severe |
| 17 | <0.2 | 134.9 | 0.0316 | Moderate |
| 18 | <0.2 | 135.7 | 0.0364 | Severe |
| 19 | <0.2 | 137.2 | 0.0147 | Severe |
| 20 | <0.2 | 139.7 | 0.0291 | Severe |
| 21 | <0.2 | 150.2 | 0.0270 | Severe |
| 22 | <0.2 | 151.5 | 0.0267 | Severe |
| 23 | <0.2 | 153.4 | 0.0156 | Severe |
| 24 | <0.2 | 154.1 | 0.0280 | Severe |
| 25 | <0.2 | 160.6 | 0.0501 | Moderate |
| 26 | <0.2 | 179.0 | 0.0195 | Severe |
| 27 | <0.2 | 199.7 | 0.0203 | Severe |
| 28 | 0.2 | 95.9 | 0.1532 | Severe |
| 29 | 0.2 | 101.3 | 0.0992 | Severe |
| 30 | 0.5 | 84.4 | 0.1115 | Moderate |
| 31 | 0.6 | 81.7 | 0.1403 | Moderate |
| 32 | 0.6 | 82.5 | 0.1630 | Moderate |
| NP(mean ± 2SD, n = 30) | 98.4(±9.4) | 34.0(±3.7) | 0.4375(±0.016) |
The new computer-assisted thrombelastographic method, in which coagulation is activated by small amounts of TF, has extended the range of tests available for assessing global clotting function in haemophilia. Using decreasing amounts of TF, a considerable increase in the inter-individual variation was observed [4]. Ingerslev et al. [16] utilized this test in 11 patients with severe and 11 patients with moderate haemophilia A and found a considerable degree of heterogeneity in the coagulation signal. Wide variations in the CT, corresponding to initiation phase, and in the values of t and Max Vel parameters, corresponding to the propagation phase, were evident in patients with severe haemophilia [17]. Furthermore, ex vivo experiments revealed that there was an additional substantial variation in the FVIII levels, which were sufficient to normalize the thrombelastographic coagulation profile, indicating a difference in response to added FVIII related to different clinical and biochemical parameters [16].
Several reports have described the usefulness of the TGT for evaluating haemophilia patients. Dargaud et al. [18] examined the TGT in 46 haemophilia A and B patients. They found that the parameters, ETP, peak thrombin level, and time to peak correlated well with FVIII/FIX levels. Furthermore, a significant correlation was found between the severe clinical bleeding phenotype and ETP. In addition, Beltran-Miranda et al. [19] investigated the relationship between TGT and clinical severity in 23 patients in nine families with haemophilia. The ETP and the peak thrombin level were significantly inversely related to the severity of haemophilia. Peak thrombin correlated better with FVIII:C level whilst the ETP correlated better with clinical severity. As in earlier studies, there were marked differences in response to therapeutic amounts of FVIII added in vitro especially at levels between 0.5 and 1.0 IU mL−1, suggesting that FVIII is less limiting at these levels, and again indicating that other plasma factors contribute to the differences.
In a wider study, Al Dieri et al. [20] demonstrated a correlation between TGT parameters, clotting factor concentrations and clinical severity in patients with congenital deficiencies of prothrombin, FV, FVII, FX, FXI and factor XII (FXII). In all patients with severe bleeding phenotypes, ETP was <20%. There were discrepancies, however, in the different levels of clotting factor affecting the ETP. Half normal ETP was seen at approximately the following concentrations: prothrombin (50%), FV (1%), FVII (2%), FX (5%) and FXI (1%). These results confirmed that the TGT may be influenced by the complete clotting factor profile.
It is clear, therefore, that several features determine the clinical phenotype in patients with haemophilia. Not only levels of coagulation factors but also fibrinolytic potential, other genetic defects, pharmacokinetics and inflammatory processes can all have an impact on clinical outcome. Furthermore, the age at which the first joint bleed occurs appears to be a primary indicator of future bleeding risk [1].
Global tests and thrombin generation
The data provided by clot waveform analysis reflect fibrinogen to fibrin conversion. Fibrinogen is the ultimate substrate for thrombin in the coagulation cascade, and it is likely, therefore that clot waveforms also reflect TG. A comparison of clot waveform analysis and TG in haemophilia A and B demonstrated a significant correlation between the clot waveform and TGT parameters in the presence of both high (0–100 IU dL−1) and low (0–1.0 IU dL−1) levels of FVIII activity in haemophilia A plasma [21]. Similarly, correlation was good in the high range of FIX in haemophilia B, although there was no significant correlation in the low range of FIX activity. The differences most likely reflect the different roles of FVIII and FIX in the generation of tenase activity, but nevertheless, these data confirm that clot waveform analysis does monitor TG.
Modern TEG/ROTEM assays depend on the visco-elastic changes that occur continuously during the whole blood clotting process. These various thromboelastometric measurements are transformed into a dynamic coagulation velocity profile during fibrin formation. The TEG/ROTEM data, therefore, also indirectly reflect TG. In these profiles, the CT corresponds to the initiation phase and the Max Vel of clot formation, and the time from recalcification until Max Vel reflects the propagation phase. Continuous monitoring of coagulation velocity has demonstrated a pattern similar to that of the TGT and Rivard et al. [22] also reported that TG, assessed by the measurement of thrombin/antithrombin complex (TAT), correlated well with total thrombus formation, which was calculated by the first derivative of the TEG waveform.
It has been widely demonstrated that the CT in TEG/ROTEM is prolonged with haemophilic whole blood, in accordance with the aPTT and conventional CT assays. Similarly, the time to peak in the TGT using TF as a trigger is abnormal with haemophiliac plasma but the lag time is not different from normal under the same experimental conditions [19,20]. Dargaud et al. [23] proposed that CTs are governed by the FVIII/FIX dependent propagation phase in addition to the lag time. The aPTT also reflects FVIII/FIX dependent TG, although the addition of ellagic acid maximizes contact activation. Clot waveform analysis is based on the aPTT, and could be expected, therefore to correlate with the TGT. All these findings are consistent with the concept that the global coagulation tests, such as clot waveform analysis and TEG/ROTEM, reflect TG, albeit indirectly.
Standardization of global test for clotting function in haemophilia
As discussed above, TGT parameters are useful for evaluating clotting function in haemophilia A and B. There are several concerns, however, regarding the use of the TGT in such hypocoagulable states. First, TGT assays are heavily dependent on the concentration and selection of test reagents such as phospholipid and trigger materials governing either extrinsic TF/rFVIIa or intrinsic contact activation. In the original method of Macfarlane, [10] the TGT was performed on native whole blood in glass test tubes and coagulation was initiated by intrinsic contact activation in the absence of exogenous additives. McIntosh et al. [24] described a modified TGT for examining FVIII concentrates and utilized contact activation in glass tubes with the addition of FIXa. More recently, however, the concept TF/FVIIa dependent clot initiation has been widely accepted, and TF is now regarded as the most potent trigger of the clotting mechanism. Limited information is available, however, on the optimum concentration of TF necessary for assessing hypocoagulability. TG is amplified by increasing concentrations of TF. Lag time and peak thrombin time are shortened, and both peak thrombin and ETP increased in relation to the TF concentration. The difference in TGT between normal and haemophiliac plasma is less, however, in the presence of higher amounts of TF. This might be a disadvantage in clinical laboratory practice. It may be necessary, therefore, to define appropriate TF concentrations for specific purposes. Some of the variations in TGT results are related to non-specific contact activation by residual platelets, platelet debris and microparticles. To minimize this, venepuncture without the use of a tourniquet [13], double plasma centrifugation, use of filters, and the addition of contact activation inhibitors such as corn trypsin inhibitor (CTI) are recommended [25].
An international multicentre study has been undertaken recently in five European centres to assess intra- and inter-assay as well as inter-centre variability of results [26]. The study was conducted in two parts. Study 1 used locally available reagents and conditions. Study 2 used a standardized protocol in which results were normalized. Study 1 revealed a large variability in results because of different sources and concentrations of TF and different phospholipids (PL). The second part of the study demonstrated that this variability could be reduced. The sensitivity of the TGT was dependent on the TF concentration and the clearest differentiation between haemophilia and normal plasma was obtained at 0.5 pmol L−1 of TF. Gerotziafas et al. [27] also demonstrated that in the presence of low TF concentrations, TGT intra-assay and inter assay variations were minimized.
Either PPP or PRP can be utilized in the CAT TGT assay. Platelets would provide a physiological source of PL, and for this reason, PRP might be preferable for clinical purposes. There are, of course, inter-individual variations in platelet counts and this could lead to difficulties with assay standardization, although TG parameters do not seem to be influenced by variations of platelet counts from 50 × 109 to 400 × 109 L−1 [27]. Nevertheless, the use of PPP has merits in that samples can be stored and easily transferred to distant laboratory centres. Synthetic PL adequately mimics the physiological membrane surface and can be especially standardized for TGT parameters. PL concentrations higher than 4 μm appear to minimize the effects of residual platelets, platelet debris and microparticles in the PPP assay system.
Thrombin generation test with intrinsic activation
As noted above, there may be substantial inter-individual variations in TG parameters in the TGT using TF activation, and the assessment of the clotting function in the presence of low levels of FVIII or FIX activity might remain unsatisfactory. McIntosh et al. [24] enhanced the sensitivity of the TGT by adding FIXa to the reaction mixtures and demonstrated that very low levels of FVIII (<1 IU dL−1) can generate significant quantities of thrombin depending upon the amount of exogenous FIXa. This modified assay appears to be effective for evaluating low levels of FVIII activity, but is not likely to be appropriate in haemophilia B.
Matsumoto et al. [21] established a highly sensitive TGT by including small amounts of ellagic acid with TF in the reaction mixtures. The addition of ellagic acid substantially amplifies TG, i.e. shorter lag time and peak thrombin time together with higher peak thrombin levels. Moreover, under these experimental conditions, discrimination between normal plasma and severe haemophilia plasma is significantly more evident (Fig. 2) and the time to peak and peak thrombin parameters correlate well with levels of FVIII activity <1 IU dL−1. In this system, inter-assay and inter-individual variations appear to be minimized and the method seems to be particularly useful for the diagnosis and evaluation of clotting function at low levels of clotting factor activity. These findings suggest that this slightly modified assay is more sensitive to the effects of FVIII and FIX on TG than the methods using TF alone. Furthermore, the technique can be used to evaluate other clotting factor deficiencies.
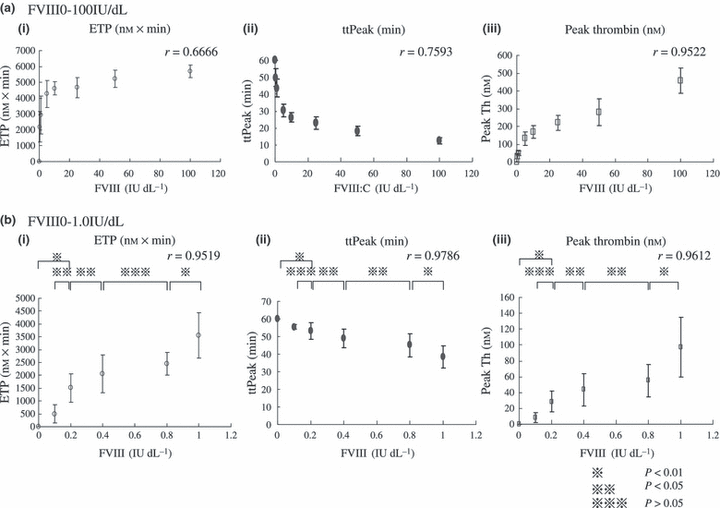
Correlations between FVIII activity and thrombin generation parameters, measured by TGT with ellagic acid. (a) FVIII activity 0–100 IU dL−1, (b) FVIII activity 0–1.0 IU dL−1.
Application of global assays to monitor bypassing therapy in patients with inhibitors
Treatment of haemophilia patients with high responding inhibitors remains a major challenge, especially in cases of life-threatening bleeding or major surgery. To achieve successful haemostasis, effective and safe therapeutic planning is required. In these circumstances, the coagulation bypassing agents based on activated prothrombin complex (APCC) or recombinant FVIIa (rFVIIa) are generally chosen. However, neither practical guidelines for optimal therapy nor standards for haemostatic monitoring have been adequately defined. TEG has been used for assessing treatment in these difficult cases [28], and the method has provided useful haemostatic information during extensive therapy for severe bleeding episodes [29]. Clot waveform analysis can be performed at the bedside. The evidence indicates, therefore, that new computer-assisted quantitative global assays can facilitate more efficient monitoring of haemostasis.
Ex vivo experiments have demonstrated a clear dose-response relationship between the improvement in clot waveforms and the amount of added rFVIIa [30]. Furthermore, the enhanced reactions are heavily dependent on the concentration of the PL, indicating that these data are more likely to reflect the cell based in vivo mechanisms. Clot waveforms are not fully normalized by rFVIIa and APCC, however, and this appears to be in contrast to their effects on thromboelastograhic assays. For example, Sørensen et al. [17] used thrombelastographic analysis to examine the ex vivo effect of APCC and rFVIIa on the dynamic profiles of whole blood clot formation, and found that, although there was substantial heterogeneity, both agents normalized or nearly normalized clotting profiles in a dose dependent manner,.
Dargaud et al. [31] reported a case of severe haemophilia A with a high responding inhibitor who required bilateral total knee arthroplasty. The patient failed to respond to rFVIIa preoperatively, and the TGT revealed that APCC infusion had limited effect, with 50% recovery of normal ETP and 21% recovery of normal peak thrombin. In view of these findings, they based their treatment protocol, during surgery and for the following postoperative period, on FVIII replacement after immunoadsorption. They concluded that TGT monitoring is useful for evaluating haemostatic control and for tailoring the selection of agents for infusion therapy.
Infusions of rFVIIa every 2 h on the day of operation have been recommended, followed by tapered doses. However, the optimal dose of rFVIIa and frequency of infusion are not clearly established. We recently treated a patient with a high responding FVIII inhibitor who needed major surgery for open reduction and internal fixation of six traumatic bone fractures in both lower legs. Bypass therapy with rFVIIa was selected for haemostatic treatment. Preoperative infusions of rFVIIa improved both ROTEM and clot waveform parameters (Fig. 3, Table 2). Infusions every 2 h on the operation day completely maintained haemostasis. The frequency of infusion was decreased to every 3 h on the day following surgery and to every 4 h for two subsequent days. There was little postoperative bleeding and the dose of rFVIIa was gradually reduced. The haemostatic effects were confirmed by both ROTEM and clot waveform analysis. Interestingly, clear differences between the clot waveform parameters were evident before and after each infusion of rFVIIa, but there was less difference in the ROTEM data before and after infusion during the first 3 days. Nevertheless, both assays confirmed the effectiveness of treatment and proved useful for clinical management.
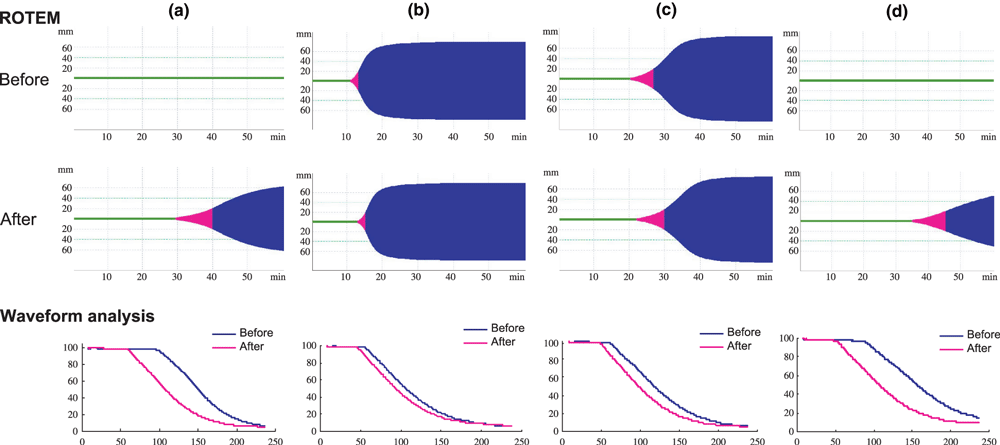
ROTEM and clot waveform analysis during haemostatic therapy for major surgery in a patient with a high-responding FVIII inhibitor. (a) ROTEM and aPTT clot waveform before and 15 min after the infusion of rFVIIa (90 μg kg−1), (b) before and 15 min after infusions every 2-h infusion, (c) before and after infusions every 4 h, (d) before and after infusions every 8 h.
| Rotem | A | B | C | D | ||||
|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | |
| CT (s) | 5431 | 1751 | 669 | 781.5 | 1202 | 1357 | 4523 | 2190.5 |
| CFT (s) | 2843 | 634 | 116 | 138 | 370.5 | 480 | 1542.5 | 613.5 |
| MCF (mm) | >26 | 69 | 78.5 | 79 | 83 | 83 | >59 | 75.5 |
| Alpha (°) | >6 | 30 | 71.5 | 69 | 50 | 45 | 12 | 26.5 |
| MAXV | 1 | 5 | 17 | 15 | 9 | 7.5 | 2 | 3 |
| MAXV-t (s) | 6118 | 2555 | 836 | 953.5 | 1694 | 2159 | 4891 | 2819 |
| Waveform analysis | A | B | C | D | ||||
|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | |
| Clot time (s) | 98.4 | 59.6 | 56.7 | 47.5 | 61.8 | 48.4 | 89.3 | 53.1 |
| Min2(%T s−2) | 0.0737 | 0.0989 | 0.0944 | 0.1076 | 0.0802 | 0.1071 | 0.0533 | 0.0797 |
Application of global assays to monitor conventional replacement therapy in patients with inhibitors
Generally, conventional FVIII or FVIX concentrates are recommended in haemophilia patients with low responding inhibitors, <5 Bethesda Units mL−1, but these products often appear to be ineffective in patients with high responding inhibitors. Assays of FVIII:C or FIX:C do not always adequately reflect haemostatic activity in the presence of inhibitor, however, and global tests may be useful in these circumstances. For example, Kasuda et al. [32] demonstrated an improvement in aPTT clot waveform parameters after FVIII infusions in two haemophilia A patients with high responding inhibitor. In addition, the haemostatic response to FVIII/von Willebrand factor (vWF) concentrates might be different from pure FVIII concentrates in some inhibitor cases [33]. Salvango et al. [34] utilized the TGT to examine inhibitor reactivity in the presence of different kinds of FVIII concentrates and found that higher thrombin levels were achieved when inhibitor plasma was mixed with FVIII concentrates containing vWF than with vWF-free concentrates. These findings are consistent with the suggestion that global assays may assist the evaluation and selection of conventional replacement therapy in patients with inhibitors.
Precise assays of clotting activity remain an essential requirement for the proper diagnosis and haemostatic monitoring of patients with haemophilia. The new global tests of haemostasis have led to substantial advances, however, and it is now clear that there is considerably more heterogeneity between the classical clotting assay and clinical phenotype than previously recognized. The presence of trace amounts of clotting factor activity, undetectable by conventional assays, together with other physiological features, can have a significant impact on haemostasis in vivo, and the global tests can provide an insight into these mechanisms, especially in patients with coagulation inhibitors. Many of these challenging developments remain to be fully clarified, however, and further standardization and optimization of the techniques are required for wider clinical application.
Acknowledgements
This work was supported in part by MEXT KAKENHI Grants 17591110 and by the Bayer Haemophilia Award Programme.




