Progress in the molecular biology of inherited bleeding disorders
The authors stated that they had no interests which might be perceived as posing a conflict or bias.
Since the advent of recombinant DNA technology in the early 1980s, the application of molecular biological approaches to haemophilia treatment has shown progressive advancement. More recently, the addition of various forms of cellular therapies to complement gene and protein-based therapeutics has further enhanced the potential for innovative treatments of these conditions.
In this chapter, we have summarized the recent trends pertaining to several aspects of novel protein, gene, and cell-based therapeutic strategies for haemophilia.
Coagulation proteins with enhanced biological propertiesS. W. Pipe
The ability to produce biological ‘facsimiles’ of plasma-derived coagulation factors within fermenting mammalian cell culture systems promised a supply of haemophilia replacement products liberated from the uncertainties of securing source plasma (a critical driver of this technology during the era of HIV and hepatitis contamination of blood derivatives) and a potentially limitless supply of replacement products that would lead to reduced costs of therapy. This would in turn facilitate an expanded application of prophylaxis strategies and open up therapy to the developing world. However, efficient production of recombinant clotting factors required overcoming significant challenges because of the complexity of both their protein structure and post-translational modifications that are critical for their hemostatic activity. Although they have proven their efficacy in clinical practice, therapeutic costs remain high and such biologicals have not greatly impacted the developing world where as many as 80% of the world’s haemophiliacs do not receive adequate therapy.
Nevertheless, recombinant DNA technology remains a promising platform to reduce the costs of therapy, increase the availability to the developing world and further enhance the quality of life of patients with haemophilia. Following the initial successful expression of recombinant factor VIII (rFVIII) and IX (rFIX), ongoing research has provided detailed structural and functional characterizations for each phase of their life cycle: biosynthesis, macromolecular interactions, activation/inactivation, and clearance. This has come through insights from the study of haemophilia mutations, site-directed mutagenesis, detailed structural models and an expanded repertoire of animal models through molecular biology advances. This has opened up new frontiers for bioengineering strategies to overcome some of the remaining limitations inherent to current clotting factor concentrates (Fig. 1).
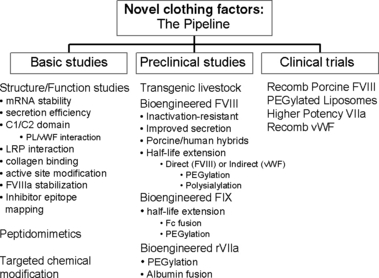
Novel coagulation proteins.
Bioengineered FVIII
Strategies to increase the efficiency of bioproduction
Basic science studies have provided insights into why the expression of rFVIII in heterologous mammalian cell lines is so poor [1]. There is an inefficient expression of mRNA, inefficient folding of the primary translation product, and a requirement for a facilitated transport mechanism from the endoplasmic reticulum to the Golgi apparatus. Targeted bioengineering strategies to overcome these limitations have led to novel FVIII molecules with enhanced secretion efficiency up to 30-fold higher secretion rates compared with that of wild-type FVIII. Such high efficiency expression molecules can now be partnered with novel recombinant production systems such as enhanced mammalian expression systems and transgenic animals. This brings the possibility of producing rFVIII at a significantly lower cost. The higher yields may even open up research into alternative therapeutic strategies such as oral therapy. Partnering such technology with gene therapy has the potential to yield plasma levels that are several fold higher than those achieved in clinical trials to date.
Strategies to increase the potency and stability
Elucidation of the mechanisms involved in the activation and inactivation of FVIII has led to targeted strategies to increase its potency and stability through bioengineering. FVIII molecules that are resistant to inactivation are in preclinical evaluation and have demonstrated increased thrombin generation potential and effective hemostasis in animal models despite lower protein doses. Such molecules could provide effective hemostasis in gene therapy strategies even when expression is limited.
Strategies to prolong the clotting factor half-life
Technologies, such as the addition of polyethylene glycol (PEGylation) or polysialic acid polymer conjugates to prolong the half-life of biological proteins has been applied to a number of therapeutics with success [2]. However, the complexities of clotting factor proteins have limited this application for haemophilia replacement products. New technological advancements with more targeted conjugation techniques have increased the enthusiasm for such strategies. Direct chemical modification of FVIII by such techniques is being investigated in preclinical trials. However, indirect strategies have also been explored. Chemical modification of FVIII’s carrier partner in plasma, von Willebrand factor (VWF) could extend the half-life of VWF and in turn extend the half-life of co-infused FVIII. This would avoid many of the limitations of this technology whereby direct chemical modification of FVIII compromises its macromolecular interactions and reduces its specific activity. An alternative indirect strategy is the application of PEGylated liposomes as an alternative plasma carrier for FVIII. With this technique, FVIII is associated with synthetic lipid bilayer spheres that have been chemically modified to extend their half-life in vivo, and designated sterically stabilized liposomes. These have shown promise in small animal models with enhanced duration of hemostatic activity. Clinical trials with this FVIII carrier system have provided some evidence for enhanced duration of haemostatic activity in patients with haemophilia even though classical pharmacokinetic parameters were not enhanced. The efficacy of this strategy will be investigated further in a phase II trial.
These strategies, however, will only impact FVIII half-life as an infusate. Extended half-life molecules for gene therapy purposes will require insights into the mechanism of clearance of FVIII from plasma. Experimental evidence has suggested that FVIII interacts initially with heparan sulphate proteoglycans on the cell surface and then interacts with at least two receptors of the low-density lipoprotein receptor family – low-density receptor-related protein (LRP) and low-density lipoprotein receptor (LDLR). Receptor blockade in animal models is effective at enhancing FVIII half-life up to five-fold. However, these receptors have affinities for a number of plasma proteins that may preclude using this strategy in humans. Potential LRP and LDLR receptor binding sites have been identified within FVIII. These regions of FVIII may be targeted for site-directed mutagenesis to reduce receptor affinity. This has been challenging as these regions overlap with sites critical for FVIII functional interactions. If a FVIII mutant can be identified with limited compromise of FVIII function yet reduced receptor-mediated clearance, this could enhance gene therapy strategies by yielding higher plasma levels.
Strategies to reduce clotting factor immunogenicity
With up to a quarter of patients with severe haemophilia A at risk for inhibitor development to FVIII, this is an important area to apply bioengineering strategies. Human FVIII inhibitor antibodies have limited cross-reactivity with porcine FVIII. A recombinant form of porcine FVIII [3] is being investigated in clinical trials and promises to be an effective hemostatic agent in patients with FVIII inhibitors. Ongoing research has identified critical regions of FVIII that contribute to its immunogenicity. Bioengineering strategies have included the substitution of major inhibitory epitopes in human FVIII with porcine sequences resulting in recombinant porcine/human hybrids with markedly reduced reactivity with inhibitor antibodies. Clinical questions remain as to whether such molecules would also reduce the risk for inhibitor formation in certain individuals with severe haemophilia A.
Bioengineered FIX
Expression of FIX is significantly more efficient than FVIII. Preclinical studies have demonstrated long-term expression of FIX at therapeutic levels within haemophilia B animal models with current gene therapy strategies [1]. However, expression has been limited and transient in early human trials. While new vector strategies are being explored, investigators are also testing FIX molecules that have been modified to enhance mRNA levels, increase potency, and extend plasma half-life. These enhanced FIX molecules may also allow gene therapy protocols to proceed at lower vector dosages, thereby limiting vector-related toxicities.
Direct modification of rFIX to extend its half-life as an infusate is also a key area of investigation. While chemical modifications such as PEGylation, as described for FVIII are possible, other novel technologies are being explored. In one example, FIX is fused with the Fc portion of an antibody to greatly prolong the plasma half-life. In addition, Fc-fusion proteins have also been utilized to facilitate alternative delivery strategies such as oral and intrapulmonary routes [4].
Bioengineered factor VIIa
Recombinant factor VIIa (rFVIIa) has been an important addition to the therapeutic portfolio for haemophiliacs with inhibitors to either FVIII or FIX. However, its clinical application is compromised in part because of a short half-life and high costs for therapy. PEGylation strategies are being applied to extend its plasma half-life. Recently, a rFVII-albumin fusion protein was generated in mammalian cell lines and exhibited a 6- to 9-fold longer plasma half-life in rats and rabbits. In addition, site-directed mutagenesis has yielded a rFVIIa molecule with greatly enhanced potency [5]. Clinical trials with these agents are now being launched. Finally, rFVIIa has also been explored within gene therapy strategies to potentially enhance hemostasis in the presence of FVIII or IX inhibitors [6]. If therapeutic expression levels prove elusive in preclinical trials, a rFVIIa bioengineered for higher potency may be useful.
Bringing haemophilia gene therapy to the clinicK. A. High
One of the most compelling concepts in molecular medicine is gene therapy (Fig. 2). Its ultimate goal is to edit a defective gene sequence in situ to achieve complete reversion of a disease phenotype for the lifetime of the individual. Despite recent successes in site-specific correction of defective gene sequences in the mammalian genome most current gene therapy strategies rely on gene addition rather than gene correction methodologies. Gene addition strategies use vectors as delivery vehicles to provide a wild-type copy of the defective gene to a physiologically relevant target tissue. The most efficient vectors are engineered from viruses which have evolved mechanisms for entering eukaryotic cells and harnessing their synthetic machinery to produce foreign proteins.
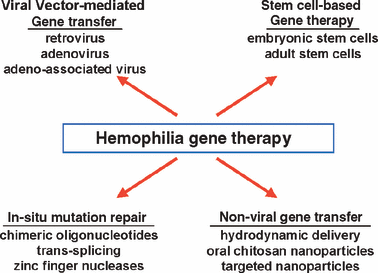
Haemophilia gene therapy.
Despite a strong record of success in animal models, a range of problems has limited the scope of successful application of gene therapy in humans to a few severe immunodeficiency disorders. Clinical studies over the past decade have identified several major obstacles to gene therapy, including: (i) gene silencing; (ii) insertional mutagenesis; (iii) phenotoxicity caused by overexpression or ectopic expression of the donated gene; (iv) immunotoxicity, i.e. harmful immune responses to either vector or transgene product; (v) risks of horizontal transmission of the donated DNA; (vi) risks of vertical transmission, i.e. inadvertent germline transmission of the donated DNA. Typically, for each specific combination of vector, transgene, and target tissue, one or two of these problems predominate as the major clinical obstacle(s).
Haemophilia is a disorder for which gene therapy could prove extremely useful. Patients with factor levels ≥5% normal are only mildly affected, demonstrating that for severe haemophilia patients, increasing factor levels by a relatively small increment would substantially improve disease phenotype [7]. These observations, combined with the high disease prevalence, the width of the therapeutic window, the ability to accommodate the wild-type cDNA sequence in most gene transfer vectors, the reliability and availability of animal models of the disease and the continued lack of access to treatment for 80% of the world’s haemophilia population have generated substantial interest in developing gene therapy approaches for haemophilia.
For genetic disease, where long-term expression of the donated gene is the goal, two potential strategies can be pursued. The first is to introduce the transgene into a stem cell via an integrating vector so that all progeny carry and express the donated gene. Because integration occurs throughout the genome, the major obstacle that has arisen in this setting is insertional mutagenesis and resulting malignant transformation [8]. The second strategy is to introduce the gene of interest into a long-lived postmitotic cell, such as cardiac or skeletal muscle, nerve cells, or hepatocytes. In these settings, for obvious reasons, gene transfer must occur in vivo, but because the cell has a long lifespan, expression can be achieved for prolonged periods even if the vector does not integrate into the host genome. Both types of strategies have been pursued for haemophilia; trials have included i.v. infusion of retroviral vectors [9], plasmid transfection of autologous fibroblasts [10], infusion of adenoviral vectors [11], and the use of adeno-associated viral (AAV) vectors.
Our work has focussed on AAV vectors because they mediate high-level stable transduction of both skeletal muscle and liver following a single administration in small and large animals [12], and have a strong safety profile among viral vectors. They are also the simplest of all viral vectors containing only the transgene expression cassette flanked by two non-coding viral inverted terminal repeats enclosed in a capsid composed of three structural proteins, VP1, 2, and 3. Wild-type AAV is not associated with any disease pathology in humans and requires a helper virus such as adenovirus to replicate. Moreover, the vector is completely devoid of viral coding sequences, reducing any risk of immune response to transduced cells on that basis. A wealth of experience with these vectors in animals and in over 500 human subjects has failed to show any association with oncogenesis, save in a single mouse disease model [13,14]. Finally, expression is mediated primarily through episomally stabilized transgene copies further reducing risks related to insertional mutagenesis.
Multiple serotypes of AAV have been isolated with differences among capsid sequences giving rise to distinct tissue tropisms for each serotype. Majority of clinical gene transfer experience has been with AAV serotype 2.
AAV-mediated gene transfer to skeletal muscle
In haemophilia, the option to use skeletal muscle as the target tissue for gene delivery is critical for the substantial numbers of adult patients whose liver disease renders them ineligible for liver-directed gene therapy. Based on preclinical studies demonstrating the safety and efficacy of direct i.m. injection of an AAV vector expressing FIX, a clinical trial of parenterally administered AAV vectors was carried out [15]. The study showed long-term (>3 years) expression of human FIX, as evidenced by immunofluorescence staining of biopsied injected muscle, but the circulating levels of FIX were not adequate to improve disease phenotype. To improve circulating levels of FIX and to address the issue of immune response to FIX, we worked in the haemophilic dog model to devise methods of intravascular delivery to large areas of skeletal muscle and achieved long-term high-level (>10%) FIX expression in these animals [16].
AAV-mediated gene transfer to liver
Multiple groups have reported long-term expression (>3 years) at therapeutic levels (6–8% of normal factor levels) in haemophilic dogs after infusing a single dose of AAV into the portal vein [17,18]. A dose escalation clinical study of hepatic delivery of an AAV-2 vector expressing human FIX from a liver-specific promoter has been carried out [19]. This was the first and currently the only trial of AAV infusion into liver in human (a second clinical trial is now undergoing regulatory review).
The most important discovery arising from our liver study was the identification of human-specific immune responses to AAV-transduced hepatocytes. This represents a challenge to the goal of using recombinant viruses to introduce new genetic material into the liver. Analysis of T cell responses in lymphocytes from one of the subjects in the trial demonstrated a concomitant increase in circulating AAV capsid-specific T cells [20]. Subsequent mapping of the immunodominant epitope within the AAV capsid allowed direct quantitation of a capsid-specific CD8+ T cell population, which was shown to fluctuate with a time course that closely matched the rise and fall of serum transaminases [21]. The most parsimonious hypothesis to account for these observations is a CD8+ T cell response to capsid that recognized and destroyed the transduced hepatocytes.
The clinical protocol for the upcoming trial was therefore amended to include a short course of immunosuppression to block the T cell response to vector capsid until it can be degraded and cleared from the transduced cell. As an alternate approach, Nienhuis et al. have developed a high efficiency double-stranded AAV-8 vector expressing FIX [21]. Based on data suggesting that AAV-8 uncoats rapidly and thus may be degraded more quickly, and that AAV-8 is less likely to trigger an immune response because of decreased binding to dendritic cells, this vector will be administered without accompanying immunosuppression. Long-term expression with either of these vectors would offer a new possibility for treatment of individuals with haemophilia.
Cell-based therapies for haemophilia using primary hepatocytesK. Ohashi
Hepatocyte transplantation
Because the hepatocyte is the primary cell type for blood clotting factor productions, hepatocyte-based therapies have been considered as an attractive possibility for congenital bleeding disorders including haemophilia. A pilot transplantation study of hepatocytes from non-haemophilic mice has shown an increase in the clotting activities in the FIX knockout mice. In this study, repeated application of the hepatocyte transplantation procedure resulted in an incremental rise of the coagulation factor suggesting that therapeutic levels could be titrated to a specific level depending on the number of cells and applications. In the clinic, hepatocyte transplantation has been applied to three patients suffering from congenital FVII deficiency by the group of King’s College Hospital, London [22 and Dr Anil Dhawan, Kings College Hospital, London, UK, personal communication]. In each of the three patients, the hepatocyte transplantation provided significant and prolonged therapeutic benefits, which has been attributed to the de novo coagulation factor production from the transplanted cells. In spite of the translational effectiveness of this approach in animals and humans to treat bleeding disorders, there is a limit on the number of hepatocytes that can be transplanted at a single time into the liver. Hepatocytes do not necessary locate within the liver as long as cells remained functional for the clotting factor productions. For these reasons, alternative procedures that utilize the hepatocyte need to be examined. Towards this end, liver tissue engineering experimental technologies have been developed [23–27].
Liver tissue engineering
Liver tissue engineering is an emerging field in which a functional liver system is created at ectopic sites in vivo using isolated hepatocytes and/or other type of cells. One of the major drawbacks in this method has been a transient time frame in which the engineered liver tissues have been functional. To circumvent this problem, functional persistency could be obtained in studies where hepatocytes mixed with laminin and type IV collagen-based extracellular matrix were transplanted under the kidney capsule [24]. The engineered tissues possessed full activity for regenerative growth at similar activity levels found in the naïve livers in response to several different liver regeneration stimuli [24,25]. This data suggested that communication between hepatocytes with non-parenchymal cells could be developed within the engineered tissues, which would be important for the expression of liver-specific protein expression. Moreover, engineering liver tissues at bilateral kidney capsule sites in FVIII-KO mice was found to reconstitute 5–10% of clotting activities [24].
Technologies have also been developed for engineering functional liver tissues at subcutaneous locations. The creation of neovascularized subcutaneous space as a platform for tissue engineering followed by hepatocyte transplantation resulted in engineered liver tissues that could stably persist [23]. Clotting FVIII and FIX mRNA expression levels in the engineered tissues were identical to the naïve livers. More recently, a novel technology to fabricate a hepatic cell sheet in culture has been developed [27]. To create this type of novel hepatic cell sheet, the authors utilized a specialized culture dish on which a temperature-responsive polymer, poly(N-isopropylacrylamide) was grafted [28]. A uniformly continuous hepatic cell sheet including essential intercellular microstructures (bile canaliculi, desmosomes, and gap junctions) was the result. Implantation of the hepatic cell sheet into the subcutaneous space resulted in the creation of a functional liver system within the subcutaneous space [27].
As the methods to engineer functional liver tissues continue to evolve, this technology would be amenable to genetic modification with other types of cells and would not be exclusive to hepatocytes [25,29], which would provide researchers a wide-range of cell sources for cell-based therapy for haemophilia. Cell-based therapy has the advantage of being a less invasive form of treatment for haemophilia. The described findings in the fields of hepatocyte transplantation and liver tissue engineering represent important new steps towards establishing effective therapeutic approaches for the treatment of not only haemophilia, but also other disorders affecting the liver.
Cell-based treatment strategies for haemophilic arthropathyA. U. Ural
It has been demonstrated that recurrent haemorrhages as occur in haemophilia result in severe joint destruction. The biological mechanisms of the progression from recurrent haemarthrosis to arthropathy is not precisely known, but is characterized by inflammatory synovitis and cartilage destruction. The erosive changes occurring in haemophilic synovitis are indicative of imbalance between cartilage/bone resorption and formation/repair. Iron appears to play a central role in the development of arthropathy in patients with haemophilia through induction of genes involved in synovial proliferation and stimulation of inflammatory cytokines interleukin-6 (IL-6), IL-1, and tumour necrosis factor-α (TNF-α) [30]. The increase of IL-1 expression in response to tissue injury or trauma initiates a cartilage remodelling programme that includes the expression of enzymes, which degrade extracellular matrix and the formation of dysfunctional matrix. Therefore, chondrocyte function is disrupted by pannus-derived cytokines and other mediators such as IL-1 and TNF-α. IL-1 and TNF-α may also contribute to bone loss by inducing osteoblast apoptosis besides their enhancing effects on the differentiation of osteoclast precursors to mature osteoclasts, thereby retarding reossification of lytic bones.
Angiogenesis, as a consequence of high expression of synovial vascular endothelial growth factor (VEGF), has also been implicated in the development of haemophilic synovitis. Expression of synovial VEGF may be modulated by TNF-α [31].
Adult mesenchymal stem cells (MSCs) can be isolated from marrow aspirates and can be expanded in culture while maintaining their multipotency. MSCs can differentiate into distinctive mesodermal tissues. MSCs can also secrete a broad spectrum of bioactive macromolecules that are both immunoregulatory and provide a regenerative microenvironment for a variety of injured adult tissues to limit the area of damage and to mount a self-regulated regenerative response. During the development of haemophilic synovitis, the high levels of IL-1 and TNF-α can cause functional suppression of MSCs responsible for reparative responses within the joint environment. TNF-α is indeed known to prevent the mesodermal differentiation capacity of MSCs [32]. Thus, in addition to well-known catabolic effects of TNF-α on articular cartilage and bone, TNF-α signalling would decrease the reparative responses of endogenous joint MSCs, thereby limiting cartilage and bone tissue remodelling during arthritis. Likewise, mice lacking TNF-α receptor 1 (TNFR1) form more cartilage and bone and treatment of mice overexpressing the human TNF-α gene with an anti TNF-α mAb antibody resulted in amelioration of polyarthritic disease, periosteal bone erosions, and cartilage destruction [33]. In another study, intra-articular administration of MSCs resulted in the regeneration of a meniscal-like tissue and retarded the progressive destruction of joint disease in osteoarthritis model. MSCs inhibited the T-cell response and modulated the expression of inflammatory cytokines with a significant decrease in the levels of TNF-α [34].
Therefore, blocking TNF-α signalling with MSCs in haemophilic synovitis would not only arrest disease progression by inhibiting the TNFα-mediated inflammatory and catabolic events, but could also contribute to re-establishment of a secondary functional joint homeostasis by restoring the regenerative potential of endogenous and exogenous MSCs and by removing TNF-α-mediated prevention of multilineage differentiation capacity of MSCs. However, there are still controversies about the ambiguous effects of MSCs on joint microenvironment such as MSCs’ direct angiogenic effect, the high propensity of marrow-derived MSCs to induce cartilage hypertrophy and bone formation, and the possible role of MSCs in the initiation and progression of arthritis. Thus, studies in animal models will be necessary to convince the biomedical community that MSCs are a potential cellular therapeutic in haemophilic arthropathy.
Acknowledgements
KO was supported in part by The Leading Projects from the Scientific Research from the Ministry of Education, Science, Sport and Culture of Japan. KAH is a Howard Hughes Medical Institute Investigator. DL holds a Canada Research Chair in Molecular Haemostasis.




