Factor XI deficiency
FXI deficiency mutation database: http://www.factorxi.org/
Abstract
Summary. Although factor XI (FXI) deficiency has a particularly high incidence in Ashkenazi Jews, it is now frequently diagnosed in other ethnic groups. This review gives an overview of the basic pathophysiology, clinical manifestations, and management of FXI deficiency. The correlation between FXI levels and the bleeding phenotype is much less clear than in the haemophilias, and consequently the bleeding risk can be difficult to predict. Two well-characterized mutations in the F11 gene are responsible for the majority of Jewish cases, but new mutations are becoming increasingly recognized. The publication of the crystal structure has greatly enhanced our understanding of the structure–function relationship in FXI. The impact of recent studies on our understanding of the role of FXI in coagulation is discussed.
Introduction
Factor XI (FXI) deficiency was first described in the early 1950s [1]. In contrast with the well-characterized haemophilias, the bleeding disorder was mild, affected both genders, and spontaneous haemorrhage was not a feature, with bleeding generally related to surgery or trauma. The disorder was sometimes referred to as haemophilia C. As further kindred were described, it became apparent that the bleeding phenotype was much more variable than with the haemophilias. In particular, there is often a poor correlation between bleeding and the baseline FXI clotting activity [2]. In some people with severe FXI deficiency (e.g. levels <20 IU/dL), there is no excessive bleeding, whereas other individuals with levels only moderately below the normal range bleed after surgery. On account of its heterogeneity, FXI deficiency presents a variety of management issues to the clinician.
Materials and methods
The core material for this review was found by searching PubMed using the following terms: factor XI, factor XIa, factor XI deficiency, contact system and factor XI inhibitor. Additional material was sourced from the authors’ unpublished observations.
Incidence, racial/ethnic predilection
Refer to the section on Genetics and molecular basis of disorder.
Pathophysiology and the role of FXI in coagulation
Factor XI is unique among serine proteases in that the zymogen circulates in the plasma as a homodimer. It is stabilized in the circulation by formation of a complex with high-molecular-weight kininogen (HMWK). The FXI homodimer has an apparent molecular mass of 160 kDa, and the monomer subunits are linked by a disulfide bond. Current evidence indicates that the physiologically important mechanism for FXI activation is proteolysis by thrombin on the platelet membrane [3]. The active enzyme consists of two peptide chains joined by a single disulfide bridge.
The light chain contains the C-terminal catalytic domain and the heavy chain consists of four apple domains (A1–4) that mediate the various bindings that are essential for the proper functioning of the enzyme (Fig. 1). A1 contains the binding sites for HMWK and thrombin exosite 1, FIX binds between A2 and A3 and A3 also contains binding sites for GpIb and heparin [4]. A4 contains one residue of the Cys362-Cys482 disulfide bond that links the heavy and light chains and the Cys321-Cys321 disulfide bond that links the monomer subunits. FXIa catalyses the activation of FIX by cleavage of the two scissile bonds: Arg145-Val146 and Arg180-Ile181.
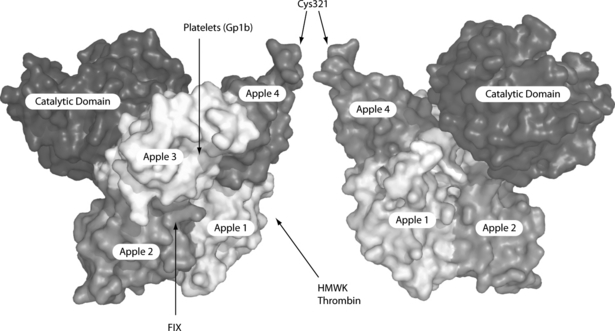
Molecular representation of the FXI dimer based on the crystal structure of the FXI monomer (PDB ID: 2F83). The four apple domains form a saucer-shaped platform for the catalytic domain. In this orientation, apple 3 is at the back of the molecule in the right monomer subunit. The Cys321 residues that form the intermolecular disulfide bridge are arrowed. The binding of certain ligands to respective domains is shown by arrows.
The reason why FXI circulates as a homodimer rather than as a monomer is unclear. There are conflicting data on whether dimerization is required for FIX activation or not [5,6]. However, the absence of the monomer from the circulation suggests that dimerization is important. It may be that in the monomer, the proximity of binding sites prevents efficient interaction with different ligands simultaneously.
The revised waterfall hypothesis contains two mechanisms for the generation of thrombin (Fig. 2). These are the tissue factor (TF)-dependent initiation pathway and the FXI-containing amplification loop. The former is able to generate only trace amounts of thrombin in vivo before it is rapidly shut down by tissue factor pathway inhibitor [7]. If low amounts of TF are used to initiate thrombin generation assays, the amount of thrombin generated is dependent on the concentration of FXI [8,9]. Recent data indicate that when coagulation factors accumulate at the site of thrombus formation, there is competitive inhibition between them that limits thrombin generation. In this situation, continued thrombin generation is dependent on the concentration of FXI and the availability of activated platelets [10].
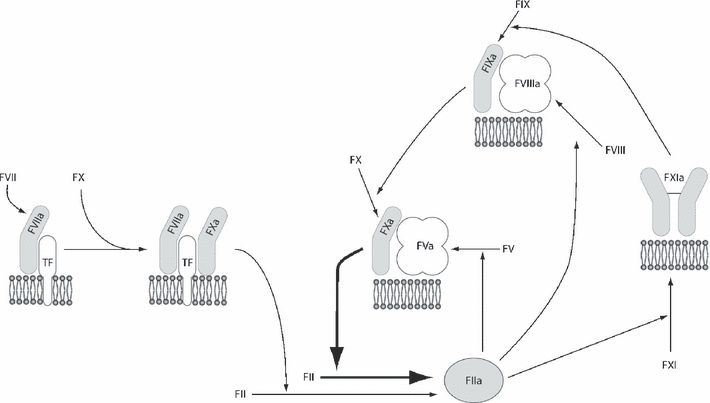
Role of FXI in tissue factor (TF)-dependent thrombin generation. Coagulation enzymes are shown as shades of gray and cofactors in shades of white. All reactions take place on negatively charged phospholipid surfaces, such as those provided by the outer membrane of activated platelets. The formation of the initiating complex, TF•FVIIa•FXa, results in the generation of a small amount of thrombin (FIIa). This is insufficient to produce a large amount of fibrin clot but stimulates a number of reactions in the amplification loop, including the activation of FXI. Subsequent formation of the tenase complex, FIXa•FVIIIa, followed by the prothrombinase complex, FXa•FVa, leads to production of a large burst of thrombin.
Studies of clot lysis suggest that FXI indirectly inhibits fibrinolysis. The mechanism appears to be increased production of thrombin-activatable fibrinolysis inhibitor (TAFI) in a FXI-dependent process [11]. As thrombin inhibits fibrinolysis by a number of mechanisms, it is possible that the FXI-dependent inhibition of fibrinolysis may not be solely dependent on TAFI.
A number of serine protease inhibitors (serpins) have been shown to inhibit FXIa in vitro, including α1-antitrypsin, C1 esterase inhibitor, antithrombin and α2-antiplasmin. However, it appears that the most significant inhibitor of FXIa in vivo is the Kunitz-type inhibitor protease nexin 2, found in the α-granules of platelets [12]. A small amount of FXI is also carried in the platelet α-granule. The relevance of platelet-derived FXI remains a matter for debate, but it has been suggested that it may be a determinant of the bleeding phenotype [13].
Genetics and molecular basis of disorder
Inherited FXI deficiency (OMIM 264900) demonstrates an autosomal pattern of inheritance with variable penetrance. It has now been described in a wide variety of population groups but remains most common in Ashkenazi Jews. In this group, it is estimated that one in eight individuals are heterozygous and one in 190 homozygous for mutations in the F11 gene [14,15]. Most cases of FXI deficiency in Ashkenazi Jews are caused by two distinct mutations, each accounting for 40–50% of abnormal alleles. These are Glu117Stop, referred to as the type II mutation and Phe283Leu, the type III mutation. The type III mutation occurs almost exclusively in Ashkenazi Jews, but the type II mutation is also found in Iraqi Jews and Arabs, suggesting an earlier ancestral origin [16,17]. Phe283Leu results in impaired dimer formation producing a quantitative deficiency [18].
In other population groups, the causative mutations are much more varied, although a few mutations occur with increased frequency. These mutations include Cys38Arg in the Basque region of France [19] and Cys128Ter, which accounts for 10–15% of abnormal alleles in non-Jewish patients in the UK [20,21]. Most mutations result in a concomitant decrease in FXI activity and antigen, with only 4% of mutations producing significantly lower activity than antigen values [22].
Clinical manifestations
Relationship to level of deficiency
Early descriptions of patients with FXI deficiency indicated that bleeding was most frequently seen after surgery or trauma [1]. This finding has been confirmed by larger studies [2,23,24]. Even in individuals with FXI levels <20 IU/dL, serious spontaneous haemorrhage is not common. Figure 3 demonstrates the wide variation in FXI levels among individuals with haemorrhagic symptoms from UK families transmitting FXI deficiency. This means that separation of patients into distinct clinical phenotypes is less clear-cut than with other bleeding disorders. Severe FXI deficiency is defined by levels <15–20 IU/dL, and these individuals have a high probability of postoperative haemorrhage. Individuals with levels between 20 IU/dL and the lower limit of the normal range, generally 65–80 IU/dL, are generally classified as having partial or mild deficiency with a lower risk of postoperative bleeding. However, some patients with levels in the severe range are asymptomatic even after surgery, including tonsillectomy, whereas others with partial deficiency have bleeding symptoms.
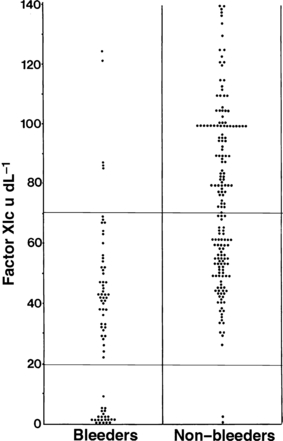
The relationship between FXI:C and bleeding phenotype. Pooled data from 249 individuals in two UK studies [2,23]. Of 128 individuals with partial FXI deficiency, 45 (35%) have bleeding symptoms. The horizontal lines define the lower limit of the normal range (upper line) and the boundary between severe and partial FXI deficiency (lower line).
Although the reason for this discrepancy remains unclear, possible explanations exist. Possibly the bleeding risk is more dependent on the amount of FXI in platelets than the plasma level, but the evidence for this is limited [13]. The total haemostatic potential in an individual is dependent on the contribution of all the procoagulant factors and their inhibitors. Therefore, the bleeding risk may be modified by levels of other coagulation factors, such as low normal von Willebrand factor [2]. In some populations, FXI deficiency may coexist with other bleeding disorders, such as definite von Willebrand’s disease, which determines the bleeding risk [25]. Some functions of FXI, such as indirect inhibition of fibrinolysis, are not measured by conventional tests, because these measure the time taken for initiation of clot formation and are not sensitive to defects in clot stability. Insensitivity of tests to some of the physiologically relevant functions of a coagulation factor may cause a discrepancy between assay results and phenotype.
Generally, surgery that involves mucosal surfaces, such as the oral cavity, nasal cavity or genitourinary tract, is most likely to be associated with bleeding. In a retrospective study of bleeding after surgery in untreated FXI-deficient patients, procedures that involve these sites were associated with excessive bleeding in 40–70% of cases [26]. In comparison, bleeding was unusual in orthopaedic surgery (0/7 cases), gastrointestinal surgery (1/30) or procedures that involved the musculoskeletal system (3/15). Circumcision of male infants with FXI deficiency may carry a significant risk of haemorrhage; four of 19 (21%) in one study [23]. However, others find a smaller risk, with only one of 65 circumcisions associated with excessive bleeding [26].
Timing of presentation
Many patients are asymptomatic until haemostatically challenged by surgery or trauma; so, the diagnosis is often made in late childhood or early adulthood. Partial deficiency is being increasingly recognized as following preoperative tests or as a result of family screening. Thus partial, and occasionally severe, FXI deficiency is often diagnosed in asymptomatic individuals, creating management dilemmas because of the unpredictability of the bleeding risk. Although postoperative bleeding is usually present, easy bruising, epistaxis and menorrhagia are also often described [2].
Diagnosis
The importance of a detailed personal and family bleeding history in the diagnosis of FXI deficiency cannot be understated. Although FXI deficiency is found in all racial groups, it is helpful to establish the racial background, because this may give an indication of the likely molecular defect.
Laboratory
Generally, screening tests will reveal an isolated prolongation of a contact activator-initiated, phospholipid-dependent coagulation test, such as the activated partial thromboplastin time. Different partial thromboplastin reagents vary in sensitivity to FXI and reference ranges should be established locally (the lower limit on package inserts is often stated as 50 IU/dL, which is inappropriate). In cases in which the index of suspicion is high, for example, a Jewish patient with a bleeding history, it is reasonable to proceed directly to measurement of the FXI level. Additional studies should be performed to exclude an FXI inhibitor or lupus coagulant. The diagnosis is confirmed after demonstration of a low FXI level in a clotting assay on two or more occasions with appropriate parallelism at serial dilution. FXI antigen measurements generally correlate with activity levels and are not routinely required or available. Antigen levels are useful in rare cases of a qualitative deficiency.
Healthy infants have low FXI levels at birth and reach adult levels by approximately 6 months of age. This low level is probably related to reduced hepatic synthetic function. Beyond infancy, the FXI level remains unaffected by age. Pregnancy does not have a significant effect on levels in FXI-deficient women [27]. FXI levels are commonly reduced in individuals with liver disease, because plasma FXI is synthesized in hepatocytes. Little is known about the effect of diet or medication on FXI levels, although one study showed a slight increase in patients taking glucocorticoids [28].
Because FXI levels derived from a clotting time assay have a poor correlation with the bleeding risk, global tests of haemostasis are being assessed in the evaluation of the bleeding risk. These assays reflect the role of FXI in the context of other coagulation factor levels. Assays of thrombin generation and thromboelastometry are being increasingly used in research to understand the role of FXI in haemostasis [8,10]. Potentially, these assays offer a much more detailed analysis of the effect of FXI on coagulation in a particular individual but are currently not well standardized and are generally confined to specialized laboratories. Their role in the clinical setting needs further evaluation. In some patients with FXI levels in the severe range, the endogenous thrombin potential and some thromboelastometric parameters may be normalized by high levels of FVIII (K. Gomez, unpublished observations). This raises the possibility that increased levels of other clotting factors may reduce the bleeding risk associated with FXI deficiency.
Molecular
Characterization of the F11 gene on chromosome 4q35.2 in 1987 enabled detection of mutations that cause FXI deficiency. Because primers and conditions for polymerase chain reaction amplification are now well described, genetic analysis is uncomplicated. The preferred method for mutation detection is direct sequencing of the coding regions, splice junctions and promoter of the F11 gene. Molecular diagnosis increases our understanding of the structure–function relationship in FXI. Identification of the mutation in a proband enables accurate detection of other affected individuals in the family. This may be of particular benefit in the screening of asymptomatic family members, because it negates the need for repeated blood tests to measure FXI levels.
Prenatal
Molecular diagnosis is essential for prenatal diagnosis and is becoming more common in severe cases of haemophilia. However, this is expensive, has poor success rates and carries the risk of adverse effects to both mother and foetus. Because the morbidity associated with FXI deficiency is relatively low, prenatal diagnosis is not justified in this condition.
Management
Treatments currently available
In early cases of FXI deficiency, bleeding symptoms were treated with fresh-frozen plasma (FFP). Although this remains widely used, specific FXI concentrates have been available since the 1980s. These have the advantage of shorter infusion times because the volume is reduced and are not associated with unnecessary increases in other coagulation factor levels. For patients with severe deficiency undergoing surgery, FXI replacement is the treatment of choice. In the early 1990s, there were reports of thrombotic complications after the use of both available concentrates. The patients generally were elderly with pre-existing risk factors for thrombotic disease. The FXI concentrate produced in the UK (BPL, Elstree, UK) has a high concentration of antithrombin and now has heparin added to it. A second FXI concentrate, Hemoleven (LFB, Lille, France), is also available, which, in addition to antithrombin and heparin, contains C1 esterase inhibitor. Both concentrates are virally inactivated. On account of the thrombotic risk, current guidelines recommend caution in elderly individuals and those with established thrombotic risk factors. Generally, the aim of the treatment is to raise the FXI level into the lower end of the normal range (60–70 IU/dL). The half-life of FXI in plasma is 45 h; so, if prolonged treatment is required, bolus doses need only be given on alternate days. If these concentrates are not available, FFP may be used, but it should be pathogen inactivated, and large volumes may be required in severely deficient patients [29].
Factor XI replacement is not necessary for some patients with severe deficiency undergoing minor procedures and for most patients with partial deficiency. Treatment with antifibrinolytic agents, such as tranexamic acid and ε-aminocaproic acid, is often effective without the need for plasma products. These agents are structurally similar to the amino acid lysine and competitively inhibit the binding of plasminogen to fibrin, thereby preventing its conversion to plasmin. Antifibrinolytics are effective intravenously, orally and also topically, particularly when used as a mouthwash to cover dental procedures. When administered orally, the medication needs to be given approximately 6 h in advance to allow the drug to enter the circulation. Antifibrinolytics are generally not used alongside FXI concentrate, because this might increase the risk of thrombotic complications. They are also relatively contraindicated in bleeding from the urinary tract because of the danger of clot retention. In a few cases, desmopressin has been shown to produce modest rises in FXI activity in partial deficiency [30], but the evidence for efficacy is unconvincing.
Acquired inhibitors are a rare complication of replacement therapy described in patients with null mutations, particularly the Glu117Stop mutation for which the prevalence in this group after factor replacement therapy was 33% [31]. There are a few case reports describing the successful use of recombinant FVIIa in these cases.
Treatments under research
Neither of the current FXI concentrates is licensed for use in most countries, but clinical trials are under way.
Prognosis
For most patients, little morbidity is associated with FXI deficiency, except after surgery or trauma. With the treatments described herein, prophylactic measures can be instigated to allow surgery to proceed safely. Increased experience in the use of FXI concentrate has led to a reduction in the dosage with a significant lowering of the incidence of thrombotic complications.
Individuals with an interest in area
P. Bolton-Maggs, UKU. Seligsohn, IsraelP. Walsh, USAK. Mann, USAS. J. Perkins, UKJ. Goudemand, FranceO. Salomon, Israel
Disclosures
The authors stated that they had no interests which might be perceived as posing a conflict or bias.




