Ammonia may play an important role in the succession of cyanobacterial blooms and the distribution of common algal species in shallow freshwater lakes
Abstract
With the human intensification of agricultural and industrial activities, large amount of reduced nitrogen enter into the biosphere, which consequently results in the development of global eutrophication and cyanobacterial blooms. However, no research had reported the effect of ammonia toxicity on the algal succession. In this study, we investigated the ammonia toxicity to 19 algal species or strains to test the hypothesis that ammonia may regulate the succession of cyanobacterial blooms and the distribution of common algal species in freshwater lakes. The bloom-forming cyanobacterium Microcystis aeruginosa PCC 7806 suffered from ammonia toxicity at high pH value and light intensity conditions. Low NH4Cl concentration (0.06 mmol L−1) resulted in the decrease of operational PSII quantum yield by 50% compared with the control exposed to 1000 μmol photons m−2 s−1 for 1 h at pH 9.0 ± 0.2, which can be reached in freshwater lakes. Furthermore, the tolerant abilities to NH3 toxicity of 18 freshwater algal species or strains were as follows: hypertrophication species > eutrophication species > mesotrophication species > oligotrophication species. The different sensitivities of NH3 toxicity in this study could well explain the distributing rule of common algal species in the freshwater lakes of different trophic states. Meanwhile, the cyanobacterial bloom (e.g. M. aeruginosa) always happened at the low concentration of ammonia in summer, and disappeared with the decrease of ammonia. This may be attributed to the toxic effect of ammonia to M. aeruginosa in spring (the average and maximum ammonia concentration were 0.08 and 0.72 mmol L−1 in 33 Chinese lakes), and the low level of NH3-N in summer and fall in the lakes might be used as preferred nitrogen nutrition by M. aeruginosa, rather than with toxicity. Therefore, ammonia could be a key factor to determine the distribution of common algal species and cyanobacterial bloom in the freshwater systems.
Introduction
The increase of human population and the consequent intensification of agricultural and industrial activities along with the deficiency of water management have led to the development of global eutrophication, and cyanobacterial mass occurrences are a frequent phenomenon worldwide. Eutrophic and hypertrophic lakes have accounted for 44% and 22%, respectively, in over 50 main Chinese lakes (Jin, 2003), and cyanobacteria have accounted for 78.6% of yearly overall phytoplankton in contaminated freshwaters, but only for 28.7% in uncontaminated waters (Padhi, 1995). The formation of cyanobacterial bloom in freshwater is a serious problem for the management of drinking water, as a wide range of toxic compounds including neurotoxins and hepatotoxins are produced (de Figueiredo et al., 2004). A survey of the blooms in freshwaters has shown that on average, 59% contain toxins, with hepatotoxic blooms being more common than neurotoxic blooms (Rantala et al., 2006). The major bloom-forming cyanobacterial species Microcystis aeruginosa forms noxious blooms in many eutrophic freshwater lakes, ponds, and reservoirs, which can produce potent hepatotoxins called microcystins (Yoshida et al., 2008). Microcystins have been paid special attention, not only due to their ability to cause acute poisonings but also due to their cancer promotion potential by chronic exposure of humans to low microcystin concentrations in drinking water (Zhou et al., 2002; de Figueiredo et al., 2004), making the production of these toxins a serious public health issue.
Environmental conditions such as high temperature and pH value, low turbulence, and high nutrient inputs (particularly phosphorus as well as nitrogen) enhance the development of planktonic cyanobacteria in lakes and reservoirs, leading to the formation of surface blooms that may accumulate as scum (de Figueiredo et al., 2004). The dominance of certain cyanobacteria at the surface is due to some advantageous characteristics such as their preference for elevated water temperature (Varis, 1993; Xu et al., 2011), low nutrient requirements (low N/P ratio) (Schindler, 1977; Tilman et al., 1982; Smith, 1983), enduring high pH and/or low carbon dioxide concentration (Shapiro, 1973, 1984; Zhang et al., 2011), and buoyancy regulation in the water column for achieving better light and nutrient conditions (Oliver & Ganf, 2000).
World-wide shallow water ecosystems are much more numerous than deep lakes and important for population as drinking water and nutritional resources (Chen et al., 2003). There are a large number of freshwater ecosystems in China, and many Chinese lakes are shallow, situated in the densely populated lowlands, and are used for drinking and irrigation among several other purposes (Chen et al., 2003). However, freshwater resources of good quality are not abundant in China, and water availability per capita is only a quarter of the world's average (Dokulil et al., 2000; Chen et al., 2003). Historically, human populations have always lived close to lakes, which resulted in increased water consumption and, as a consequence, water pollution and water shortage. For example, Lake Taihu, the third largest freshwater lake in China, is situated in the Yangtze delta, the most industrialized area in China with high population density, urbanization, and economic development. It is one of the main sources for nearby residents’ drinking water, and one of the most severely polluted freshwater lakes in China, where the Microcystis blooms break out every year (Qin et al., 2007b; Duan et al., 2009). Over the last decade, information has accumulated regarding seasonal succession of phytoplankton in freshwater lakes. Phytoplankton succession varied in freshwater according to their limnological features and trophic states (Jensen et al., 1994). Although general hypotheses and patterns of plankton succession are well described in temperate eutrophic lakes (Sommer et al., 1986), the mechanism of the plankton succession in shallow subtropical lakes still remains unclear .
Wind-driven sediment resuspension and shallow depth prevent nutrient stratification in shallow lakes, and therefore, they are relatively dynamic systems compared with deep lakes. The eutrophication process and forming mechanism of algal blooms are particularly complicated in shallow lakes due to the strong lake–land, air–water, and water–sediment interactions (Qin et al., 2007a). Previous studies have focused on the relationships between the dynamics of cyanobacterial bloom and the changes in physicochemical factors (e.g. nutrient supply, light, and temperature) that influence cyanobacterial growth in the aquatic environment (Smith, 1983; Reynolds et al., 1987; Scheffer et al., 1997; Ke et al., 2008). Meanwhile, it had showed that the enlarged nitrogen deposition was strongly negatively correlated with plant species richness in a wide range of ecosystem types (Stevens et al., 2006; Maskell et al., 2010; Mcclean et al., 2011). However, none of them considered the toxic effects of ammonia on the cyanobacterial bloom and seasonal succession, and the sensitivity of ammonia toxicity has so far not been systematically investigated in algal species. In this study, we examined the ammonia toxicity on M. aeruginosa associated with light intensity and pH value, and compared the sensitivity of ammonia toxicity among 18 algal species or strains that are usually common species in freshwater systems of different trophic states. Our results suggested that the ammonia level in freshwater lakes could be a key factor to regulate the succession of cyanobacterial blooms and the distribution of common algal species.
Materials and methods
Materials and culture conditions
Microcystis aeruginosa PCC 7806 was obtained from the Freshwater Algae Culture Collection of the Institute of Hydrobiology, the Chinese Academy of Sciences (Wuhan, Hubei, China). It was grown in BG11 medium with 20 mmol L−1 N-[tris(hydroxymethyl)methyl-3-amino]propanesulfonic acid (TAPS, pKa(25 °C) = 8.4, useful pH range 7.7–9.1) to maintain the pH value at 8.0 ± 0.1 by titration with 2 mmol L−1 NaOH, and aerated with sterile filtered ambient air at 25 °C (Stanier et al., 1971). Irradiance was provided at 40 μmol photons m−2 s−1 using cool white fluorescent lamps with a 14 : 10 light/dark cycle. Exponentially growing cultures were used for the following experiments.
All other species or strains used in this study, which are common algal species in freshwater systems with different trophic states, were chosen as follows: Hypertrophication, Chlamydomonas microsphaera FACHB 52, Chlamydomonas reinhardtii CC 125, Chlorella pyrenoidosa FACHB 9, Scenedesmus obliquus FACHB 416, Scenedesmus obliquus CPCC 5, Pseudokirchneriella subcapitata (formerly known as Selenastrum capricornutum) CPCC 37, Cyclotella meneghiniana FACHB 1031 (Palmer, 1969; Alvarez Cobelas & Jacobsen, 1992; Lepistö & Rosenström, 1998); Eutrophication, Microcystis aeruginosa FACHB 912, Microcystis aeruginosa CPCC 299, Microcystis aeruginosa FACHB 469, Microcystis aeruginosa CPCC 632, Microcystis flos-aquae FACHB 1028, Microcystis wesenbergii FACHB 929, Melosira granulate FACHB 1060 (Rawson, 1956; Palmer, 1969; Jao & Zhang, 1980; Lepistö & Rosenström, 1998; Naselli-Flores & Barone, 2000); Mesotrophication, Fragilaria sp. FACHB 1130, Coelastrum sp. FACHB 1176, Cosmarium botrytis FACHB 295 (Jao & Zhang, 1980; Naselli-Flores & Barone, 2000); Oligotrophication, Tabellaria sp. FACHB 1231 (Rawson, 1956; Lepistö & Rosenström, 1998; Reynolds, 1998). They were obtained from the Freshwater Algae Culture Collection of the Institute of Hydrobiology, the Chinese Academy of Sciences (FACHB), Canadian Phycological Culture Collection (CPCC), and Chlamydomonas Collection (CC). All species or strains used in this study were incubated as Microcystis aeruginosa PCC 7806 in BG11 medium, except that Chlamydomonas reinhardtii CC 125 and Tabellaria sp. FACHB 1231 were grown, respectively, in HSM and D1 medium, and Cyclotella meneghiniana FACHB 1031, Melosira granulate FACHB 1060, and Fragilaria sp. FACHB 1130 were grown in BG11 medium added with Na2SiO3·9H2O. Exponentially growing cultures with the OD750 value of 0.6 were used for the following experiments (Jiang & Qiu, 2005; Schlebusch & Forchhammer, 2010).
One-hour NH4Cl treatments for M. aeruginosa PCC 7806
Sampled 1 mL exponential cultures with the cell number of about 107 cells mL−1, and diluted with BG11 medium with different pH buffers to obtain the final concentration of 106 cells mL−1. The 25 mmol L−1 Bis-tris propane (BTP, pKa(25 °C) = 6.8, useful pH range 6.3–9.5), 20 mmol L−1 TAPS, 25 mmol L−1 N-cyclohexyl-2-aminoethanesulfonic acid (CHES, pKa(25 °C) = 9.3, useful pH range 8.6–10.0), and 25 mmol L−1 N-cyclohexyl-3-aminopropanesulfonic acid (CAPS, pKa(25 °C) = 10.4, useful pH range 9.7–11.1) were used to maintain the pH value to 7.0 ± 0.1, 8.0 ± 0.1, 9.0 ± 0.2, and 10.0 ± 0.2, respectively. Samples were treated with various concentrations of NH4Cl and different light intensities (100, 500, and 1000 μmol photons m−2 s−1) in pH 7.0, 8.0, 9.0 BG11 medium or (50, 100, and 200 μmol photons m−2 s−1) in pH 10.0 BG11 medium for 1 h. All treatments were operated in the laboratory-constructed reaction chambers with a water jacket, which is made of transparent glass, and connected with a polystat refrigerated bath (Cole-Parmer Instrument Co., Vernon Hills, IL, USA) to maintain temperature at 25 °C.
Ninety-six-hours NH4Cl treatments for M. aeruginosa PCC 7806
The stored solutions of NH4Cl were sterilized by filtering with 0.22 μm mixed cellulose ester membrane, and NH4Cl was added to each culture to obtain various concentrations. Those without NH4Cl addition were used as control. Exponentially grown samples were transferred into 100 mL BG11 medium with different pH buffers to maintain the pH value at 7.0 ± 0.1, 8.0 ± 0.1, 9.0 ± 0.2, during the following 96 h incubation under 25 °C and 40 μmol photons m−2 s−1 with a 14 : 10 light/dark cycle, and aerated with sterile filtered ambient air. The inoculum amounts were 1 × 106 cells mL−1 for pH value at 7.0 ± 0.1 or 8.0 ± 0.1, and 4 × 106 cells mL−1 for pH value at 9.0 ± 0.2.
Growth measurement
Cell number was monitored using Multisizer™ 3 Coulter Counter® (Beckman Coulter Inc., Brea, CA, USA). Samples were harvested by gentle filtration (< 10 mm Hg) on 0.45 μm nylon filters. Chlorophyll a content was determined spectrophotometrically in 95% ethanol extracts (Lichtenthaler & Buschmann, 2001).
Measurement of chlorophyll fluorescence
The parameter of the operational PSII quantum yield was obtained by the measurement of induction curve using a WATER-PAM Chlorophyll Fluorometer (Walz GmbH, Effeltrich, Germany). For 1-h NH4Cl treatments, samples were treated under 100, 500, or 1000 μmol photons m−2 s−1 at the pH value of 7.0 ± 0.1, 8.0 ± 0.1, and 9.0 ± 0.2, and actinic lights were, respectively, 108 μmol photons m−2 s−1, 551 μmol photons m−2 s−1, or 1098 μmol photons m−2 s−1. The actinic lights were, respectively, 49 μmol photons m−2 s−1, 108 μmol photons m−2 s−1, or 239 μmol photons m−2 s−1 for the treatment under 50, 100, and 200 μmol photons m−2 s−1 at the pH value of 10.0 ± 0.2. For 96-h NH4Cl treatments, the actinic light was 49 μmol photons m−2 s−1. The other settings were given as follows: PM-gain 4, Output-gain 2, Measuring Light Frequency 3, Saturation Pulse intensity 7, and Saturation Pulse width 0.8 s.
Comparison of ammonia toxicity sensitivities among 18 algal species or strains
Exponentially growing cultures at the OD750 value of 0.6 were harvested by gentle filtration (< 10 mm Hg) on 0.45 μm nylon filters, and then resuspended with corresponding growth media contained 25 mmol L−1 CHES buffer at pH 9.0. All species were treated with various concentrations of NH4Cl under 300 μmol photons m−2 s−1 for 1 h. The operational PSII quantum yield was determined using WATER-PAM Chlorophyll Fluorometer with the actinic light of 370 μmol photons m−2 s−1, which was used to calculate the NH4Cl concentration at which 50% inhibition occurred (EC50) and 95% confidence intervals for EC50 values with nonlinear logistic regression generated using GraphPad Prism Version 4.00 for Windows (GraphPad Software, San Diego, CA, USA).
Results
Ammonia toxicity to M. aeruginosa PCC 7806
Effects of 1-h exposure to different concentrations of NH4Cl on the operational PSII quantum yield of M. aeruginosa PCC 7806 under different light intensities and pH values were shown in Fig. 1. At the pH value of 7.0 ± 0.1, 5 mmol L−1 NH4Cl caused the operational PSII quantum yield to decrease by 17.7% and 8.1% compared with control, under 1000 and 500 μmolphotons m−2 s−1, respectively. However, 5 mmol L−1 NH4Cl had little effect on the operational PSII quantum yield of samples exposed to 100 μmol photons m−2 s−1. It indicated that higher light intensities could aggravate the sensitivity of M. aeruginosa PCC 7806 to ammonia toxicity. For pH 8.0 ± 0.1 and 9.0 ± 0.2, M. aeruginosa PCC 7806 was also more sensitive to ammonia toxicity under 1000 μmol photons m−2 s−1 than lower light intensities. At the pH value of 10.0 ± 0.2, 0.2 mmol L−1 NH4Cl caused the operational PSII quantum yield to decrease by 35.6% and 66.6% compared with control under 50 and 200 μmol photons m−2 s−1, respectively. From these NH4Cl dose–response curves, the 1-h EC50 value of NH4Cl for M. aeruginosa PCC 7806 under different pH values and light intensities were shown in Table 1. It could conclude that M. aeruginosa PCC 7806 suffered from ammonia toxicity at high pH values and high light intensities. Low NH4Cl concentration (0.06 mmol L−1) resulted in the decrease of operational PSII quantum yield by 50% compared with control at pH 9.0 ± 0.2, exposed to 1000 μmol photons m−2 s−1 for 1 h.
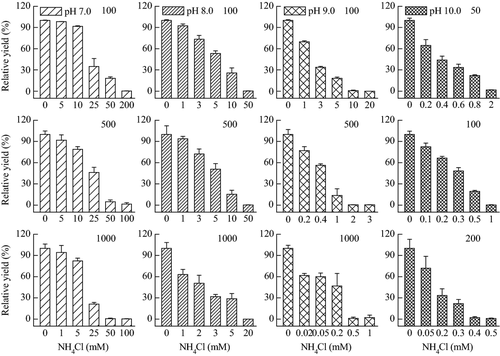
| Light intensity (μmol photons m−2 s−1) | 1h-EC50 (95% CIs) (mM) | |||
|---|---|---|---|---|
| pH 7.0 | pH 8.0 | pH 9.0 | pH 10.0 | |
| 50 | ND | ND | ND | 0.32 (0.27–0.38) |
| 100 | 21.35 (17.71–25.73) | 5.35 (5.05–5.67) | 1.80 (1.53–2.13) | 0.27 (0.23–0.31) |
| 200 | ND | ND | ND | 0.13 (0.09–0.19) |
| 500 | 19.00 (14.83–24.34) | 4.83 (4.43–5.27) | 0.43 (0.37–0.50) | ND |
| 1000 | 16.49 (12.45–21.84) | 1.77 (1.29–2.44) | 0.06 (0.02–0.21) | ND |
- M. aeruginosa PCC 7806 were grown in BG11 medium at pH 8.0 under 25 °C and 40 μmol photons m−2 s−1. Cultures in exponential phase were treated with BG11 medium containing different concentrations of NH4Cl at pH 7.0–10.0 and 50–1000 μmol photons m−2 s−1 for 1 h, and the operational PSII quantum yield was determined for the calculation of EC50 value and 95% confidence intervals. ND, not detected.
Effects of 96-h exposure to NH4Cl on the operational PSII quantum yield of M. aeruginosa PCC 7806 under 40 μmol photons m−2 s−1 and different pH values were shown in Fig. 2. The operational PSII quantum yield of samples treated with 1 mmol L−1 NH4Cl decreased by 29.7% and 16.7%, compared with control, at pH 9.0 ± 0.2 and 8.0 ± 0.1, respectively. However, it had no inhibitory effect on the operational PSII quantum yield at pH 7.0 ± 0.1. According to NH4Cl dose-response curve, the 96-h EC50 values of NH4Cl for M. aeruginosa PCC 7806 were 35.04 mmol L−1 (95% CI: 29.82–41.17 mmol L−1), 5.75 mmol L−1 (95% CI: 4.34–7.62 mmol L−1), and 1.16 mmol L−1 (95% CI: 0.77–1.75 mmol L−1), at pH 7.0 ± 0.1, 8.0 ± 0.1, and 9.0 ± 0.2, respectively (Table 2). Therefore, M. aeruginosa PCC 7806 was more susceptible to suffer from ammonia toxicity at high pH values, even at low light for prolonged exposure.
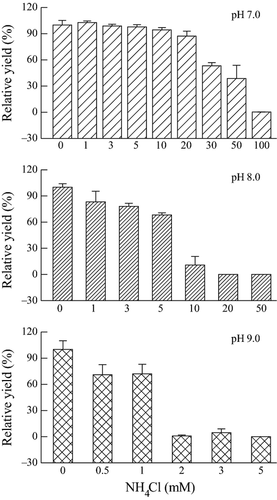
| Parameters | 96h-EC50 (95% CIs) (mM) | ||
|---|---|---|---|
| pH 7.0 | pH 8.0 | pH 9.0 | |
| Operational PSII quantum yield | 35.04 (29.82–41.17) | 5.75 (4.34–7.62) | 1.16 (0.77–1.75) |
| Chl a concentration | 34.04 (25.04–46.24) | 7.20 (5.55–9.33) | 1.30 (1.00–1.68) |
| Cell concentration | 34.65 (22.87–52.49) | 4.14 (1.93–8.86) | 6.22 (1.40–27.66) |
| Total cell volume | 33.57 (24.80–45.44) | 6.72 (5.12–8.83) | 1.41 (1.14–1.76) |
| Total cell surface area | 34.11 (25.18–46.19) | 5.94 (3.93–8.97) | 1.63 (0.90–2.92) |
- M. aeruginosa PCC 7806 was grown in BG11 medium containing different concentrations of NH4Cl for 96 h under 25 °C and 40 μmol photons m−2 s−1, and then various physiologic parameters were determined for the calculation of EC50 value and 95% confidence intervals. The pH value of growth medium was maintained at 7.0, 8.0, or 9.0.
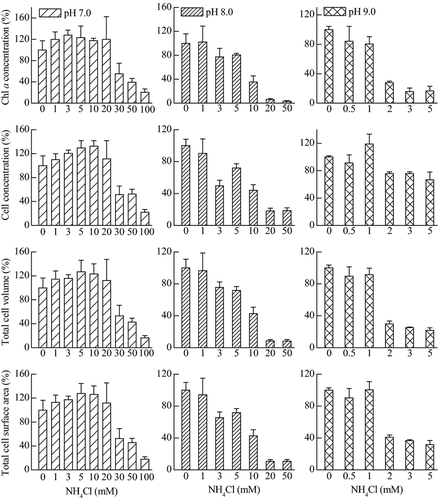
In addition to the operational PSII quantum yield, other parameters (Chl a concentration, cell concentration, total cell volume per mL, and total cell surface area per mL) were investigated in response to NH4Cl treatment (Fig. 3). From the NH4Cl dose-response curves of different parameters, the calculated 96-h EC50 value of NH4Cl for M. aeruginosa PCC 7806 were shown in Table 2. The 96-h EC50 values of NH4Cl calculated with different parameters were consistent, except for a higher value calculated from the parameter of cell concentration at pH 9.0 ± 0.2.
Different sensitivities of common algal species or strains in the freshwater systems of different trophic states to ammonia toxicity
They exhibited a great variation of sensitivity to ammonia toxicity. The 1h-EC50 values for the operational PSII quantum yield with regard to ammonia for 18 algal species or strains were shown in Table 3. The 1h-EC50 of common species from hypertrophic, eutrophic, mesotrophic, and oligotrophic freshwater lakes ranged from 1.54 mmol L−1 to 18.25 mmol L−1, 0.56 mmol L−1 to 1.46 mmol L−1, 0.44 mmol L−1 to 0.94 mmol L−1, and 0.27 mmol L−1, respectively. The average values were, respectively, 6.79, 0.99, 0.61, and 0.27 mmol L−1 for hypertrophic, eutrophic, mesotrophic, and oligotrophic freshwater common species.
| Species/Strains | 1h-EC50 (mM) | 95% Confidence intervals (mM) | |
|---|---|---|---|
| Hypertrophication | Chlamydomonas microsphaera FACHB 52 | 18.25 | 16.56–20.11 |
| Chlamydomonas reinhardtii CC 125 | 11.43 | 9.82–13.31 | |
| Chlorella pyrenoidosa FACHB 9 | 7.26 | 6.03–8.74 | |
| Scenedesmus obliquus FACHB 416 | 3.63 | 3.22–4.08 | |
| Scenedesmus obliquus CPCC 5 | 2.75 | 1.41–5.37 | |
| Pseudokirchneriella subcapitata CPCC 37 | 2.69 | 2.35–3.07 | |
| Cyclotella meneghiniana FACHB 1031 | 1.54 | 1.45–1.64 | |
| Eutrophication | Microcystis aeruginosa FACHB 912 | 1.46 | 1.20–1.76 |
| Melosira granulata FACHB 1060 | 1.14 | 1.10–1.17 | |
| Microcystis aeruginosa CPCC 299 | 1.08 | 0.91–1.29 | |
| Microcystis aeruginosa FACHB 469 | 1.02 | 0.94–1.10 | |
| Microcystis aeruginosa CPCC 632 | 0.92 | 0.81–1.04 | |
| Microcystis flos-aquae FACHB 1028 | 0.76 | 0.64–0.92 | |
| Microcystis wesenbergii FACHB 929 | 0.56 | 0.45–0.70 | |
| Mesotrophication | Fragilaria sp. FACHB 1130 | 0.44 | 0.36–0.53 |
| Coelastrum sp. FACHB 1176 | 0.45 | 0.37–0.54 | |
| Cosmarium botrytis FACHB 295 | 0.94 | 0.83–1.06 | |
| Oligotrophication | Tabellaria sp. FACHB 1231 | 0.27 | 0.15–0.47 |
- All species were grown in the corresponding medium at pH 8.0 under 25 °C and 40 μmol photons m−2 s−1. Cultures in exponential phase were treated with grown medium containing different concentrations of NH4Cl at pH 9.0 and 300 μmol photons m−2 s−1 for 1 h, and the operational PSII quantum yield was determined for the calculation of EC50 value and 95% confidence intervals.
Discussion
In the freshwater lake ecosystem, NH3-N, NO3-N, and NO2-N are three main forms of soluble nitrogen, and are crucially important to phytoplankton, macrophytes, and microorganisms in nitrogen cycle (Wu et al., 2006). The NH3-N is generated by heterotrophic bacteria as the primary nitrogenous end product of decomposition of organic matter (Quirós, 2003) and the most favorable source of inorganic nitrogen to phytoplankton (Bloom et al., 1992; Britto et al., 2001). It can be converted to NO3-N through nitrification by archaeal and bacterial oxidizers which is a main pathway in the overall nitrogen cycle of freshwater systems (Herrmann et al., 2008). The NH3-N concentrations are usually low in oxygenated waters of oligo- to mesotrophic lakes because of the utilization by plants and nitrification to nitrogen oxidized forms. In the eutrophical and hypertrophical lakes, with relatively low dissolved oxygen, nitrification of ammonia ceases, the absorptive capacity of the sediments is reduced, and a marked increase of NH3-N release from the sediments occurs leading to the increase of NH3-N concentration, especially in shallow lakes.
By comparing the freshwater lakes of different trophic states all over the world, the concentrations of NH3-N increase from oligotrophic and mesotrophic lakes to eutrophic and hypertrophic lakes (Table 4). As shown in Table 3, the ability of tolerance to NH3 toxicity was in this order: hypertrophication species > eutrophication species > mesotrophication species > oligotrophication species. It clearly indicated that the common algal species that lived in high NH3-N content environment were more resistant to NH3 toxicity. Meanwhile, the common algal species in oligotrophic, eutrophic, and hypertrophic freshwater lakes were approximately diatoms, cyanobacteria, and chlorophyta, respectively (Table 3). Cyanobacterial blooms usually occurred in the eutrophic and hypertrophic freshwater lakes, but not in some hypertrophic freshwater lakes. It suggested that NH3-N content in freshwater lakes could be a key factor to control the common algal species and cyanobacterial blooms. In the hypertrophic Lake Little Mere, the maximal concentration of NH3-N occurred in the summer, and there were no cyanobacterial blooms observed (Carvalho, 1994). Over the last 60 years, phytoplankton community structure of Donghu Lake (Wuhan, China), and its trophic status have been studied by many research groups (Jao & Zhang, 1980; Lei et al., 2003, 2005; Tang et al., 2007; Yu et al., 2008). Over this period of time, the concentrations of NH4+-N in Donghu Lake increased significantly (1954–1957, Mesotrophication, 0.06–0.15 mg L−1; 1973–1976, Eutrophication, 0.10–0.16 mg L−1; 1986, Hypertrophication, 0.85 mg L−1; 2001, Hypertrophication, 1.40 mg L−1) (Cheng & Li, 2006; Tang et al., 2007). From the late 1970s to 1985, blooms of species such as Microcystis spp. and Anabaena spp. were frequently observed (Jao & Zhang, 1980; Lei et al., 2003). Although the initiative of Wuhan government over the past few years was to decrease NH4+-N concentration in Donghu Lake (Tang et al., 2007), cyanobacterial blooms can be again observed (Yu et al., 2008). This could be explained by the toxic effect of high concentrations of NH3-N. King (1970) also found some shallow lakes and ponds, particularly those that are organically rich, and tended to favor green algae over cyanobacteria. On the other side, phytoplankton in the ultra-oligotrophic Lake Tahoe is dominated by diatoms and chrysophytes (Tilzer & Horne, 1979). The huge population of diatom Stephanodiscus hantzschii (Grun.), Rhodomonas sp., and Cryptomonas sp. developed in the spring with low concentration of NH3-N in Lake Little Mere (Carvalho, 1994). The distribution of common algal species in the freshwater lakes was consistent with the results of ammonia toxicity sensitivities among 18 algal species or strains (Table 3).
| Lakes | Trophic state | Maximal NH3-N (mM) | pH value | Reference |
|---|---|---|---|---|
| Lake Little Mere | Hypertrophic | 0.564 | 7.3–9.0 | Carvalho, 1994; |
| Lake Taihu: Meiliang Bay | Hypertrophic | 0.334 | 6.9–10.1 | Chen et al., 2003; Wu et al., 2007; |
| Lake Hjarbæk Fjord | Hypertrophic | 0.105 | 8.4–11.0 | Olrik & Nauwerck, 1993; |
| Lake Taihu | Eutrophic | 0.025 | 7.0–9.7 | Chen et al., 2003; |
| Lake McIlwaine | Eutrophic | 0.015 | 8.5–9.6 | Marshall & Falconer, 1973; |
| Lake Sammamish | Mesotrophic | 0.007 | Johnston & Jacoby, 2003; | |
| Lake Sariyar Dam | Mesotrophic | 0.002 | 7.1–10.4 | Atici et al., 2008; |
| Lake Windermere | Oligotrophic | 0.001 | 7.0–7.9 | Whitby et al., 2001; |
| Lake Kalgaard | Oligotrophic | 0.005 | 6.5–8.2 | Sand-Jensen & SØndergaard, 1979 |
For phytoplankton, nutrients have been invoked as one of the variables controlling its community structure and biomass (Tilman et al., 1982). Numerous bioassay experiments have demonstrated that nitrogen, phosphorus, and silicon are usually limiting resources (Dillon & Rigler, 1974; Tilman et al., 1982). Lake enrichment experiments demonstrated clearly that nitrogen, phosphorus, and their proportion could increase algal biomass, and have a dramatic effect on the algal species composition (Blomqvist et al., 1994; Moisander et al., 2009; Xu et al., 2010). Therefore, the appearance of cyanobacterial blooms could be explained by nutrients limitation theory. In Lake Taihu of China, the algal blooms occurred every year in the past two decades, and Microcystis spp. blooms were the main species in the summer and fall (Duan et al., 2009). Total phytoplankton biomass and growth rates increased significantly with the addition of phosphorus, but no primary effects from nitrogen in the spring and winter, which suggested that phosphorus was a limited factor to phytoplankton growth, but abundance of nitrogen could be toxic to phytoplankton (Dai et al., 2008; Drath et al., 2008). During the summer and fall, however, nitrogen addition alone revealed a significant positive effect on phytoplankton growth, and phosphorus addition only stimulated phytoplankton growth once nitrogen had been added, which suggested that nitrogen was the primary limiting nutrient, with phosphorus being a secondarily limiting nutrient. Thus, the availability of nitrogen during the summer could be a key growth-limiting factor for the proliferation and maintenance of toxic Microcystis spp. blooms (Xu et al., 2010).
Previous studies also reported that soluble nitrogen was highly variable during the augmentation of trophic state or seasonal cycles of lakes, especially NH3-N. The changes of different dissolved inorganic nitrogen and total phosphorus concentrations were studied during growth and non-growth season in 33 subtropical shallow lakes in the middle and lower reaches of the Yangtze River in China (Wu et al., 2006). The NH3-N increased sharply in nongrowth season in hypertrophic lakes, and mean concentrations of NH3-N were much higher in nongrowth season than growth season. The average ammonia concentrations in 33 Chinese lakes were 0.02 mmol L−1 (0.00–0.38 mmol L−1) and 0.08 mmol L−1 (0.00–0.72 mmol L−1), respectively, in growth season and nongrowth season (Wu et al., 2006). In the large Chinese shallow lake, Lake Taihu, the TN (total nitrogen): TP and TDN (total dissolved nitrogen): TDP (total dissolved phosphorus) mass ratios showed high seasonal variation and changed from 33 to 80 : 1 and 52 to 212 : 1, respectively, in the winter and the spring, and both decreased to below 20 : 1 in the summer, which is accordant with the change of NH3-N concentation (Xu et al., 2010). The same variable rule of NH3-N seasonal cycles was also found in Tuusulanjärvi Lake in southern Finland (Varis, 1993) and Hjarbæk Fjord Lake in Denmark (Olrik & Nauwerck, 1993). Ke et al. (2008) studied the spring–summer successions of phytoplankton in Meiliang Bay of Lake Taihu, and found that cyanobacteria were mainly promoted by decreased concentrations of nitrogen compounds, and chlorophyta showed positive correlations with NH3-N. Therefore, a clear succession in phytoplankton community was observed: the phytoplankton was dominated by chlorophyta in the spring, but rapidly replaced by cyanobacteria in the early summer. This succession rule could be well explained with the present study. Low NH4Cl concentration (0.06 mmol L−1) could result in the decrease of operational PSII quantum yield for M. aeruginosa PCC 7806 by 50% compared with the control at pH 9.0 ± 0.2, exposed to 1000 μmol photons m−2 s−1 for 1 h (Table 1). Meanwhile, NH3 toxicity to M. aeruginosa PCC 7806 depended on the light intensity and pH value, and higher light intensity and pH value led to more sensitive to NH3 toxicity (Table 1; Figs 1, 2). Chen et al. (2003) found that the NH3-N concentration in Meiliang Bay of Lake Taihu could reach 0.33 mmol L−1, and the concentration of NH3-N was in high level in the spring and winter. In addition, the daily photosynthetically available radiation on the lake's surface changed from a minimum of 600 μmol photons m−2 s−1 to a maximum of 2400 μmol photons m−2 s−1, and the pH value ranged from 8.0 to 9.5 (Xu et al., 2010). Therefore, in the Lake Taihu, with the high level of NH3-N in the spring and winter (non-growth season), the bloom-forming Microcystis spp. could suffer from NH3 toxicity, and the growth was inhibited, but chlorophyta that were less sensitive to ammonia could grow well. Nevertheless, bloom-forming Microcystis spp. were fit for growing with the low concentration of NH3-N in the summer and the fall (growth season), and with the aggravation of Microcystis spp. bloom, NH3-N was consumed and became limited to maintain prolific Microcystis spp, and then the bloom disappeared. In this situation, NH3-N was not toxic to Microcystis spp., but limited nutrition. As a result of high competitiveness of non-N-fixing cyanobacteria for ammonium nitrogen sources (Blomqvist et al., 1994), chlorophyta could not be dominant algal species. Thus, the level of NH3-N in the freshwater lake ecosystem could be a key factor to cyanobacterial dominance.
In the present investigation, different parameters were compared to study the response of M. aeruginosa PCC 7806 to NH4Cl treatment (Table 2; Fig. 3). The operational PSII quantum yield characterizes the steady-state PSII photosynthetic electron transport activity (Flameling & Kromkamp, 1998). It is well accepted to reflect the effect of different environmental stresses on the PSII photosynthetic activity (Govindjee et al., 1981; Juneau et al., 2003). Some other four parameters (Chl a concentration, cell concentration, total cell volume per mL, and total cell surface area per mL) that represent biomass were also used to evaluate the NH4Cl toxic effect on M. aeruginosa PCC 7806. The 96-h EC50 values of NH4Cl calculated according to different parameters were approximated, except for a higher value calculated from the parameter of cell concentration at pH 9.0 ± 0.2 (Table 2). Therefore, except for measuring the PSII quantum yield and Chl a concentration, total cell volume and total cell surface per volume are also good indicators for ecotoxicology research.
In conclusion, NH3-N is nutrient for phytoplankton, as well as toxic to them, and a key factor controlling cyanobacterial bloom and common algal distribution. Once a lake became in eutrophic or hypertrophic states, it will be difficult and long-term to recover . For example, the Chinese government started to manage Taihu lake from 1996; the inputs of nitrogen-content from external source are seriously control in Taihu lake. However, cyanobacterial blooms still break out every year, even in some area where seldom had cyanobacterial blooms before the management. Maybe, the reducing NH3-N content in eutrophic or hypertrophic states was more suitable for cyanobacteria growth; cyanobacterial blooms will disappear till the NH3-N content was reduced to be very low, and this become a limiting factor to cyanobacterial growth . Therefore, the management of eutrophic or hypertrophic states costs long time. In addition to NH3-N, temperature is also playing an important role in the phytoplankton composition in shallow lakes, and global warm action has pushed cyanobacterial blooms to break out earlier and more frequently year-by-year. Cyanobacterial recruitment from sediments is also an important process for its bloom, especially in shallow lakes. Integrated researches are needed in future studies to attain effective managements to optimize water quality of eutrophic lakes.
Acknowledgements
We thank Prof. Klaus Hantke (Eberhard-Karls-Universität Tübingen), Prof. Philippe Juneau (Université du Québec à Montréal), and Dr. Jiyong Su (Eberhard-Karls-Universität Tübingen) for critically reading the manuscript. This study was funded by the National Basic Research Program (973 Program, No. 2008CB418004 and No. 2008CB418204) and the Program for New Century Excellent Talents in University (NCET-08-0786).




